Abstract
Visuospatial attention depends on the integration of multiple processes, and people with right hemisphere lesions after a stroke may exhibit severe or no visuospatial deficits. The anatomy of core components of visuospatial attention is an area of intense interest. Here we examine the relationship between the disruption of core components of attention and lesion distribution in a heterogeneous group (N=70) of patients with right hemisphere strokes regardless of the presence of clinical neglect. Deficits of lateralized spatial orienting, measured as the difference in reaction times for responding to visual targets in the contralesional or ipsilesional visual field, and deficits in re-orienting attention, as measured by the difference in reaction times for invalidly vs. validly cued targets, were measured using a computerized spatial orienting task. Both measures were related through logistic regression and a novel ridge regression method to anatomical damage measured with magnetic resonance imaging. While many regions were common to both deficit maps, a deficit in lateralized spatial orienting was more associated with lesions in the white matter underlying the posterior parietal cortex, and middle and inferior frontal gyri. A deficit in re-orienting of attention toward unattended locations was associated with lesions in the white matter of the posterior parietal cortex, insular cortex and less so with white matter involvement of the anterior frontal lobe. An hodological analysis also supports this partial dissociation between the white matter tracts that are damaged in lateralized spatial biases versus impaired re-orienting. Our results underscore that the integrity of fronto-parietal white matter tracts is crucial for visuospatial attention and that different attention components are mediated by partially distinct neuronal substrates.
1. Introduction
Visuospatial attention depends on the integration of multiple processes including mechanisms for arousal/vigilance, orienting to relevant stimuli in the environment, and target detection in both the spatial and temporal dimensions (Husain, Shapiro, Martin, & Kennard, 1997). It is currently believed that these processes are mediated by different and distributed cortico-subcortical systems (Corbetta & Shulman, 2002, 2011; Kastner & Ungerleider, 2000; Mesulam, 1981; Posner & Petersen, 1990; Ptak, 2012). In this study, we examine the effects of right hemisphere stroke lesions in a large prospective cohort of stroke patients on two specific processes underlying orienting to visual stimuli: i. ‘goal-driven’ lateralized spatial attention; and ii. ‘stimulus-driven’ re-orienting (Corbetta, Kincade, & Shulman, 2002; Corbetta & Shulman, 2002; Doricchi, Macci, Silvetti, & Macaluso, 2010; Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005). To explore the spectrum of attentional deficits after right hemisphere stroke in a continuous way, from no impairment to severe impairment, our sample was not restricted solely to patients with neglect. Behavioral scores and structural imaging were used to develop statistical maps of symptom-lesion relationships using logistic regression analysis and a novel ridge regression approach (Corbetta et al., 2015; Mah, Husain, Rees, & Nachev, 2014; Phan et al., 2010) in combination with a probabilistic atlas of 40 white matter tracts (Rojkova et al., 2015).
Numerous behavioral tests have been developed to assess deficits in visuospatial attention including line bisection (Schenkenberg, Bradford, & Ajax, 1980), target cancellation (Weintraub & Mesulam, 1987), figure copying (Oxbury, Campbell, & Oxbury, 1974) and test batteries such as the Behavioral Inattention Test (Bailey, Riddoch, & Crome, 2000; Wilson, Cockburn, & Halligan, 1987). Recently, computerized target detection tasks have been shown to be more sensitive measures of visuospatial bias than commonly used pencil-and-paper tests (Bonato & Deouell, 2013; Bonato, Priftis, Umilta, & Zorzi, 2013; Rengachary, d'Avossa, Sapir, Shulman, & Corbetta, 2009). Previous studies have also reported signs of spatial bias in patients with normal performance on paper and pencil tests (Bonato, 2012; Bonato, Priftis, Marenzi, Umilta, & Zorzi, 2012; Rengachary et al., 2009).
The spatial orienting task measures different core components of attention within a single paradigm (Posner, 1980). An ipsilesional egocentric bias (rightward, in the case of right hemisphere stroke) in the distribution of spatial attention is one of the most common and important deficits in hemispatial neglect (Corbetta & Shulman, 2011; Rengachary et al., 2009; Rengachary, He, Shulman, & Corbetta, 2011). It can be quantified by comparing reaction times for targets presented in the contralesional (left) vs. ipsilesional (right) visual field (Field Effect). The Field Effect provides a sensitive and continuous measure of the ipsilesional spatial bias (Rengachary et al., 2009; Rengachary et al., 2011). Goal-driven, lateralized detection of targets may depend on signals in dorsal prefrontal and parietal cortex and their connection with occipital visual cortex. These regions interact with sensory cortex during stimulus selection and with motor regions during response selection (Bressler, Tang, Sylvester, Shulman, & Corbetta, 2008; Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Hopfinger, Buonocore, & Mangun, 2000; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999). Their disruption results in an egocentric spatial bias. In neglect, these regions show an inter-hemispheric imbalance of task-driven activity during the spatial orienting task (Corbetta, Kincade, Lewis, Snyder, & Sapir, 2005) and impaired temporal correlation at rest (He et al., 2007) that are related to the severity of the visual field bias at 1–3 weeks post-stroke (Baldassarre et al., 2014; Carter et al., 2010). Based on the pattern of task co-activation and inter-regional coherence at rest, these regions form a dorsal attention network (DAN) (Corbetta & Shulman, 2002; Fox, Corbetta, Snyder, Vincent, & Raichle, 2006; Goldberg & Bruce, 1985; Gottlieb, Kusunoki, & Goldberg, 1998; Indovina & Macaluso, 2007; Ptak & Schnider, 2010; Sylvester, Shulman, Jack, & Corbetta, 2007). Within the DAN, dorsal prefrontal and parietal cortex are linked by white matter fiber tracts forming the superior longitudinal fasciculus (SLF) (Makris et al., 2005; Schmahmann et al., 2007; Schmahmann, Smith, Eichler, & Filley, 2008; Thiebaut de Schotten et al., 2005; Urbanski et al., 2008). With recent advances in diffusion tensor imaging methods, such as the combination of spherical deconvolution (Dell'Acqua et al., 2007) with adaptive regularization to reject spurious non-physiologic fiber orientations it is possible to tease apart the three components of the SLF (Dell'Acqua & Catani, 2012). It has now been reported that SLF II lateralization is correlated with line bisection bias (Thiebaut de Schotten et al., 2011) and that SLFII damage in particular is associated with spatial neglect (Lunven et al., 2015; Thiebaut de Schotten et al., 2014) refining prior results implicating the SLF (Doricchi & Tomaiuolo, 2003; Leibovitch et al., 1998; Urbanski et al., 2008; Urbanski et al., 2011).
The other core component of attention measured by the spatial orienting task is stimulus-driven re-orienting of attention. The Validity Effect provides a continuous measure of performance by comparing reaction times for stimuli that appear at a correctly cued location (following an arrow cue presented at fixation) vs. stimuli that appear opposite to the cued location. Response times are typically longer for targets at non-cued locations, and this relative delay is increased in stroke patients with hemispatial neglect (Friedrich, Egly, Rafal, & Beck, 1998; Posner, Walker, Friedrich, & Rafal, 1984). Deficits in attention shifts are non-lateralized manifestations of hemispatial neglect as they occur in both sides of space (Corbetta et al., 2005; He et al., 2007; Rengachary et al., 2011), but they are more pronounced when shifting from the ipsilesional field to the contralesional field than vice-versa (Friedrich et al., 1998; Losier & Klein, 2001; Posner et al., 1984). Stimulus-driven shifts of attention recruit right lateralized regions in the temporo-parietal junction (TPJ), including separate foci in the supramarginal gyrus (SMG) and superior temporal gyrus (STG) (Shulman et al., 2010), and ventral frontal cortex (VFC), in conjunction with the DAN. TPJ and VFC cortex form a Ventral Attention Network (VAN) based on their consistent co-activation and coherence at rest (Corbetta & Shulman, 2002; Fox et al., 2006), and damage to this network can cause problems not only with re-orienting, but also target detection and vigilance (Corbetta & Shulman, 2011). Studies suggest that SLF II connects the caudal portion of the VAN (caudal inferior parietal lobe/occipital-parietal area) to the rostral portions of the DAN (dorsolateral prefrontal cortex) (Doricchi, Thiebaut de Schotten, Tomaiuolo, & Bartolomeo, 2008; Schmahmann et al., 2007; Thiebaut de Schotten et al., 2011), an anatomical arrangement consistent with its involvement in spatial neglect. The neighboring arcuate fasciculus (AF), a lateral associative tract composed of long, short anterior and short posterior fibers that connect perisylvian cortex of the frontal, parietal, and temporal lobes (Catani & Thiebaut de Schotten, 2008) is also involved in visuospatial function (Chechlacz, Rotshtein, & Humphreys, 2014; Ciaraffa, Castelli, Parati, Bartolomeo, & Bizzi, 2013; Thiebaut de Schotten et al., 2014).
Numerous other distinct separate frontal lobe projecting white matter tracts have been isolated in the human brain (Forkel et al., 2014; Lawes et al., 2008; Rojkova et al., 2015; Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004). Other tracts whose damage has been associated with neglect include more ventral pathways that could be considered part of the VAN (Umarova et al., 2010), These include the Inferior Longitudinal Fasciculus (ILF) (Bird et al., 2006) connecting occipital and temporal lobes, and the Inferior Fronto-Occipital Fasciculus (IFOF) (Catani & Thiebaut de Schotten, 2008; Urbanski et al., 2008) which connects the lateral orbitofrontal cortex and marginal gyrus with the inferior middle occipital, ligual and inferior occipital gyri (Forkel et al., 2014; Lawes et al., 2008). More recently, damage to the splenium of the corpus callosum has also been shown to be associated with chronic neglect (Lunven et al., 2015).
Since the behavioral test chosen may have a profound effect on the results of any lesion-symptom mapping analysis (Saj, Verdon, Vocat, & Vuilleumier, 2012), and test batteries likely reflect multiple cognitive processes, the spatial orienting task was selected to probe these elemental aspects of visuospatial attention in a large group of right hemisphere stroke patients regardless of the presence or absence of neglect. While there are no strict cutoffs for diagnosing neglect using the Field and Validity Effects obtained with a computerized spatial cueing task, these continuous dependent variables are well suited for exploring the full spectrum of impairment, from none to severe.
To map deficits of spatial attention onto lesion anatomy, we employed logistic regression to determine the likelihood that a voxel in the right hemisphere is damaged when a significant Field Effect or Validity Effect is present. Logistic regression does not make any assumptions about the distribution of the independent variables and is well suited for predicting the outcome of a categorical dependent variable (a voxel is lesioned or not) based on a continuous predictor (the behavioral score) and can be applied to a whole group of patients without arbitrary cut-offs (e.g. median split) based on either anatomy or behavior. Logistic regression was therefore used to determine if the location of the greatest lesion burden associated with disrupted attention. Also, if the Field Effect and the Validity Effect represent disruption of dissociable core components of visuospatial attention with distinct neural substrates, we predicted that logistic regression analysis would return different statistical maps of lesion location for these two components of attention. A secondary analysis was performed using ridge regression to confirm the results of the logistic regression. Ridge regression is a novel multivariate method based on machine learning that can control for hidden biases, such as the vascular distribution of damage, that consistently distort lesion-deficit maps computed using commonly used voxel-wise univariate methods (Mah et al., 2014; Phan et al., 2010). Ridge regression models predict behavioral variance based on structural features including volume, location, white matter tracts affected, or functional connectivity. Finally, a tract by tract correlationlal analysis was performed to obtain a qualitative sense of how damage in which white matter tracts was associated with deficit severity.
2. Materials and methods
2.1 Subjects
Seventy stroke patients participated in the study. Candidates were identified through daily monitoring of the neurology stroke inpatient service at Barnes-Jewish Hospital (BJH), and the Neurorehabilitation service of the Rehabilitation Institute of St. Louis. The subjects consisted of patients with a first ever right hemispheric ischemic stroke with or without evidence of hemispatial neglect. To achieve a sufficient sample size data was pooled across multiple smaller studies with the same criteria. Exclusion criteria were: a) prior strokes except for clinically silent lacunes (up to 2, each not greater than 15 mm), b) evidence of periventricular white matter disease grade 3 on the classification of de Groot et al (de Groot et al., 2000) (corresponding to = or >grade 5 of Longstreth (Longstreth et al., 1996), c) dementia, defined as a score greater than 13 on the Short Blessed scale, d) other medical conditions preventing survival for 12 months (e.g. CHF class IV; cancer); e) schizophrenia, bipolar, obsessive-compulsive, personality disorders and major depression and f) visual field cut as demonstrated by bedside exam. Subjects with a clinically diagnosed visual field cut or who missed all the targets on one side were excluded from the study, as were any subjects unable to maintain fixation. Patients with recent depression in the setting of stroke or minor depression were enrolled. All subjects had both complete neuroimaging data and behavioral data set. Clinical, demographic, and time of testing from stroke onset are presented in Table 1. All subjects provided informed consent according to Washington University Institutional Review Board guidelines and were compensated for their participation. A healthy control group (N=20) was matched with the study sample for age, gender, and years of education. They serve as a performance baseline in the computerized spatial orientation test.
Table 1.
Characteristics of right hemisphere lesion subjects.
| Subj # | Age | Gender | Time since stroke (days) | FE (msec) | VE (msec) | Volume (cm3) | Location | Stroke Type |
|---|---|---|---|---|---|---|---|---|
| 1 | 89 | M | 30 | 536.93 | 222.19 | 298.539 | FL, TL, PL | Isch |
| 2 | 63 | F | 232 | 205.68 | −257.11 | 14.067 | MFG, TPJ | Isch |
| 3 | 50 | M | 28 | 68.51 | 33.49 | 27.432 | Thal, Occ, TL | Isch |
| 4 | 69 | M | 42 | 139.38 | 30.78 | 45.522 | TL | Isch |
| 5 | 58 | F | 229 | 462.91 | 216.61 | 2.079 | ALIC | Isch |
| 6 | 66 | M | 39 | 198.65 | 313.15 | 173.394 | FL, TL, PL | Isch |
| 7 | 47 | F | 218 | 839.85 | −339.25 | 45.549 | FL, Ant Ins | Isch |
| 8 | 87 | F | 304 | −89.03 | 241.17 | 0.891 | PLIC | Isch |
| 9 | 50 | M | 312 | 744.13 | 894.53 | 104.733 | FL, TL, PL, IFG | Isch |
| 10 | 48 | M | 94 | 111.67 | −129 | 10.152 | Put, Sub Ins | Isch |
| 11 | 42 | M | 515 | 1238.35 | 1151.29 | 71.577 | FL, TL, Ant Ins, MFG | Isch |
| 12 | 73 | M | 23 | 234.4 | 120.07 | 1.188 | Put | Isch |
| 13 | 55 | F | 603 | 242.27 | 20 | 3.753 | Thal, PLIC | Hem |
| 14 | 60 | F | 256 | −4.79 | 458.99 | 14.877 | SPL, Put, Ins | Isch |
| 15 | 36 | F | 19 | 63.73 | 270.27 | 8.046 | SPL | Hem |
| 16 | 80 | F | 267 | 236.4 | −94.6 | 66.312 | PL, Occ | Hem |
| 17 | 56 | M | 25 | 563.77 | 568.37 | 148.095 | FL, PL, TL | Hem |
| 18 | 43 | F | 59 | 13.27 | −65.93 | 16.227 | FL | Isch |
| 19 | 61 | M | 50 | 482.81 | 361.51 | 36.585 | FL, TL, Ins, Put | Hem |
| 20 | 50 | M | 28 | 274 | 194 | 50.058 | FL, TL, Ins, Put | Isch |
| 21 | 48 | F | 36 | 545 | 435 | 128.736 | TL, Ins, Put | Isch |
| 22 | 44 | M | 19 | 627 | 21 | 59.562 | FL, PL, TL, MFG | Isch |
| 23 | 41 | M | 34 | 1009.04 | 371.54 | 60.804 | FL, PL, TL, TPJ, IFG | Isch |
| 24 | 50 | F | 18 | 104.53 | 360.38 | 29.349 | FL, Caud | Isch |
| 25 | 61 | F | 29 | 710.52 | −114.78 | 17.523 | Caud, Put, Ins, ALIC | Isch |
| 26 | 63 | M | 18 | 541.77 | 392.03 | 45.333 | FL, CC Genu, ACC | Isch |
| 27 | 79 | F | 10 | 287.03 | −57.41 | 6.183 | Caud, Put, GPi, PLIC | Isch |
| 28 | 58 | M | 16 | 537.74 | 86.48 | 21.168 | FL | Isch |
| 29 | 54 | F | 14 | −210.17 | 81.83 | 1.971 | Pons, MCP | Emb |
| 30 | 76 | F | 17 | 535.77 | −369.57 | 8.478 | FL, PLIC | Isch |
| 31 | 77 | F | 9 | −101.63 | 10.79 | 2.538 | Thal, PLIC | Isch |
| 32 | 48 | F | 17 | 333.02 | 267.6 | 39.744 | FL, IFG | Isch |
| 33 | 39 | M | 18 | 210.62 | 152.26 | 27.432 | Cbl | Isch |
| 34 | 50 | F | 7 | 364.99 | −147.43 | 47.763 | FL, Ins | Isch |
| 35 | 76 | M | 26 | 652.86 | 38.56 | 177.309 | FL, TL, PL, Put, IFG | Isch |
| 36 | 27 | M | 17 | 0.56 | 249.6 | 55.161 | FL, Caud, Put, ALIC | Isch |
| 37 | 49 | M | 12 | −45.1 | 99.9 | 2.484 | Thal | Isch |
| 38 | 70 | F | 18 | 25.47 | 57.65 | 2.7 | Put, CR | Isch |
| 39 | 66 | M | 14 | −164.8 | 51.98 | 54.81 | TL, Occ, Thal, PLIC | Diss |
| 40 | 43 | F | 8 | 0.63 | 15.33 | 0.459 | Caud, Put, ALIC | Isch |
| 41 | 63 | F | 7 | 161.05 | 130.43 | 20.79 | FL, Ins, Put | Isch |
| 42 | 56 | F | 12 | 25.37 | 193.57 | 2.079 | Caud, Put | Isch |
| 43 | 53 | M | 10 | 58.23 | −28.77 | 2.889 | Thal | Isch |
| 44 | 60 | M | 9 | −222.5 | 681.44 | 7.965 | Thal, IC | Isch |
| 45 | 63 | F | 16 | 245.99 | −94.37 | 87.615 | FL, TL, Ins,Put, CR | Isch |
| 46 | 43 | F | 11 | −24.93 | −26.93 | 25.974 | Occ | Isch |
| 47 | 57 | M | 8 | −102.81 | 60.13 | 1.323 | PLIC | Isch |
| 48 | 57 | M | 10 | 483.65 | −144.55 | 20.952 | FL,MFG, IFG | Isch |
| 49 | 58 | M | 23 | 127.02 | −28.82 | 2.781 | MidB, Po, MCP | Hem |
| 50 | 58 | M | 9 | 13.54 | 157.6 | 20.601 | Cbl | Isch |
| 51 | 59 | M | 12 | 251.24 | 73.94 | 22.788 | Thal | Hem |
| 52 | 50 | F | 16 | 511.34 | −442.86 | 2.214 | Put | Isch |
| 53 | 44 | M | 15 | −56.21 | −166.41 | 13.068 | Thal | Hem |
| 54 | 62 | M | 12 | 528.78 | 102.7 | 94.311 | FL, TL,PL | Isch |
| 55 | 40 | F | 11 | −141.79 | 605.91 | 28.161 | FL, BG | Isch |
| 56 | 40 | F | 22 | 386.04 | 37.26 | 95.364 | TL, Ins, BG | Isch |
| 57 | 62 | F | 12 | 339.41 | 459.31 | 10.962 | TL, BG | Isch |
| 58 | 40 | M | 27 | 84.59 | 100.59 | 2.322 | Put | Isch |
| 59 | 39 | F | 20 | 124.63 | −14.23 | 2.7 | BG | Isch |
| 60 | 57 | M | 9 | −71.77 | 34.83 | 8.91 | BG | Isch |
| 61 | 51 | M | 10 | 293.53 | −7.11 | 78.462 | BG, IC | Isch |
| 62 | 47 | F | 13 | 13.49 | −19.55 | 36.099 | FL, Ins | Diss |
| 63 | 39 | M | 10 | 301.54 | 1.44 | 65.259 | FL, TL | Isch |
| 64 | 50 | M | 14 | 62.94 | −55.6 | 8.37 | CB | Isch |
| 65 | 39 | F | 15 | 4.81 | 328.21 | 48.006 | FL, TL | Isch |
| 66 | 52 | F | 11 | 113.4 | 14.94 | 26.946 | Thal, PLIC | Hem |
| 67 | 51 | M | 13 | 47.23 | −45.97 | 5.67 | CB | Isch |
| 68 | 52 | F | 18 | 35.53 | 10.13 | 18.144 | Po | Hem |
| 69 | 70 | F | 19 | 75 | −0.06 | 53.892 | PL | Hem |
| 70 | 53 | M | 16 | 99.26 | 63.5 | 48.006 | Thal | Hem |
| Ave | 55.24 | - | 59.14 | 232.79 | 117.06 | 39.87 | - | - |
| St.Dev | 12.58 | - | 114.23 | 293.33 | 267.17 | 51.00 | - | - |
FL: frontal lobe; TL: temporal lobe; PL: parietal lobe; Occ: occipital lobe; CB: cerebellum; BG: basal ganglia; Put: putamen; Caud: caudate; GPi: globus pallidus; Thal: thalamus; Po: pons; MidB: midbrain; MFG: middle frontal gyrus; IFG; inferior frontal gyrus; Ins: insula; Acc: anterior cingulate; PLIC: posterior limb internal capsule; ALIC: anterior limb internal capsule; CC: corpus callosum. Lesions of the corona radiata or centrum semi-ovale were assigned to the corresponding lobe.
2.2 Behavioral measures
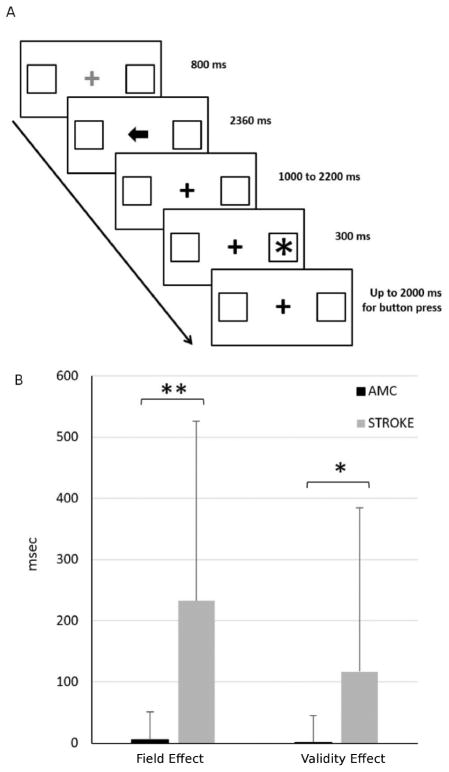
In the spatial orientation task, the subject viewed a computer display containing a centrally positioned fixation cross and two square frames positioned at 3.3º to the left and right of fixation along the horizontal meridian. The onset of a new trial was signaled by a color change, from red to green, of the fixation cross. Eight hundred milliseconds later, an arrow cue pointing left or right appeared at fixation for 2360 milliseconds. After a delay ranging from 1000 to 2000 milliseconds, the target (an asterisk) appeared for 300 milliseconds within 1 of the 2 frames (left or right). On 75% of the trials, the target appeared at the location indicated by the cue (valid condition), while on 25% of the trials, it appeared at the opposite location (invalid condition). Patients had to detect the target as quickly as possible with a right-hand key-press. The RTs were recorded. An inter-trial interval of 2360 milliseconds separated subsequent trials. Blocks contained 40 trials (30 valid, 10 invalid). Each patient completed 2 blocks. Test duration was 15 minutes, including a practice block. We measured lateralized spatial attention as relative delay in RTs for targets presented in the left versus right visual field. The maximum allotted time to respond was 2000ms. If the subject did not respond in time, that trial was eliminated from the RT based results. Subjects who missed every trial for any condition were not included in the analysis.
The four stimulus presentation conditions were:
Contralesional (left) visual field preceded by valid directional cue (contra valid)
Contralesional visual field preceded by invalid directional cue (contra invalid)
Ipsilesional visual field (right) preceded by valid directional cue (ipsi valid)
Ipsilesional visual field preceded by invalid directional cue (ipsi invalid)
For each subject the behavioral effects were calculated as follows based on the RT values:
T-tests were used to investigate differences in reaction time between stroke and healthy patients. The Pearson product-moment correlation coefficient (Pearson's r) was used to investigate correlations between measures.
2.3 MRI structural scanning
2.3.1. Pulse sequences
Thirty-one subjects were scanned with a Siemens 3.0 Tesla Allegra MRI scanner. Structural images for atlas transformation and lesion segmentation were acquired using a T1-weighted MP-RAGE (1x1x1.25 mm voxels; TE=3.93ms, TR=1810ms, TI=1200ms, flip angle=12 deg) and T2-weighted fast spin echo sequence (1.1x1.1x3.0 mm voxels; TE=96ms, TR=8430ms). Thirty-nine subjects were scanned with a Siemens 3.0 Trio MRI scanner. Structural images for atlas transformation and lesion segmentation were acquired using a T1-weighted MP-RAGE (1x1x1 mm voxels; TE=2.26ms, TR=1950ms, TI=900ms, flip angle=9 deg) and T2-weighted fast spin echo sequence (1x1x1 mm voxels; TE=441ms, TR=2500ms).
2.3.2. Atlas transformation and image cross-registration
An atlas representative MP-RAGE target in Talairach coordinates was produced by mutual co-registration (12 parameter affine transformations) of images obtained in twelve normal subjects. An affine transformation then registered the MP-RAGE image to the atlas target. This target atlas was used for all the stroke patients. Data were realigned within and across scanning runs to correct for head motion using an eight parameter (rigid body plus in- plane stretch) cross-modal registration similar to the method described by Andersson (Anderson, 1995). Cross-modal registration was used as most patients also participated in fMRI scans. This method has a sub-millimeter accuracy adequate for the analysis presented. We have also measured the error associated with the atlas transformation of lesions by running the transformation with and without masking of the lesion, and found that the error is less than 2 mm in any axis even for large lesions (Corbetta et al., 2005).
2.3.3. Lesion segmentation
T1-weighted MP-RAGE and T2-weighted spin echo images were acquired as described above. Lesions were segmented in data space by trained personnel using T2-weighted images (where the lesion will be hyperintense) (Supplemental figure) and T1-weighted images (where the lesion will be hypointense) presented next to each other in Analyze AVW image analysis software (Robb et al., 1989) (The Biomedical Imaging Resource at the Mayo Foundation). The co-registered T1W and T2W structural images were segmented into regions corresponding to gray matter, white matter, cerebrospinal fluid and infarct based on a bispectral fuzzy class means semi-automated procedure after gain-field correction as previously described (Sheline et al., 2008). The total number of infarcted voxels in each slice was calculated using Analyze AVW and binary maps representing lesioned space were produced in atlas space. All binary lesion maps were visually inspected and manually optimized by one of two board certified neurologists (MC, ARC). All scans were obtained at the time of testing, and the majority of subjects were scanned within 1 month of their stroke. At that time the borders of the stroke are well defined both on T1 and T2. In the case of conflicting appearances on the T1 and T2, the appearance on T2 was respected to resolve any ambiguity in lesion border. In the case of hemorrhages where significant peri-lesional edema may appear quickly, the edematous region was included in the lesion. In the case of ischemic lesions edema was not prominent in this sample. To address differences in tissue contrasts that resulted from the variability in the time to scan, time since stroke was used as a covariate in the ridge regression to perform the lesion-symptom analysis.
2.4 Lesion-symptom mapping and quantification
2.4.1. Statistical maps of lesion location by logistic regression
In this study a voxel-wise logistic regression analysis was performed. The software used for the logistic regression analysis is custom software called FIDL (functional independent data language) developed by Mark McAvoy for the analysis of 4 dimensional floating point (4dfp) imaging data. Please see http://www.nil.wustl.edu/labs/fidl/ for additional details regarding FIDL and ftp://imaging.wustl.edu/pub/raichlab/4dfp_tools/4dfp_release.txt for additional information regarding 4dfp. In logistic regression the independent variables (here behavioral scores) differentiate between two classes of the dependent variable (a damaged or undamaged voxel). Logistic regression can map many types of independent variables onto the dependent variable, including continuous, discrete, dichotomous, categorical, and indicator. When a test case is input, the output is the predicted probability that the test case will be classified into one rather than the other of the dependent variable’s two classes (Menard, 1995; Minka, 2003). In the current application, the behavioral score predicts whether a voxel is damaged or undamaged. The logistic regression model is
where y = ± 1 are the two classes of the dependent variable predicted from the independent variables x with estimated weights w(Minka, 2003). Weights are efficiently estimated by Newton’s iteratively reweighted least squares (Minka, 2003). In this study, subjects’ behavioral scores and corresponding stroke lesion maps were entered into a logistic regression. The result for each behavior of interest is a statistical map showing the likelihood that a given voxel will be damaged if the behavior tested is abnormal. Results were controlled for multiple comparisons by performing a Monte Carlo simulation for gaussianized T statistics, as the maps that form the logistic regression analysis are gaussianized chi-square statistics. While the shape of the chi-squared probability density function may not be symmetric when there are few subjects at each voxel it becomes fairly symmetric with ten subjects at each of voxel. In the custom software used for the analysis, p is fixed at 0.05 and there is a fixed relationship between predetermined cluster sizes and the T statistic. A cluster size of 13 was chosen to minimize false positives and because of the available options it was closest to the smallest stroke size (‘resel’) recorded in this sample (17 voxels) which may represent the smallest detectable lesion that leads to clinical deficits.
2.4.2. Ridge regression and tract-wise hodology analysis
Ridge regression was used as a second technique to map lesion-behavior relationships. Ridge regression is a multivariate machine learning method where the independent variable (here distribution of damaged voxels) is used to generate regression models to predict the dependent variable (here behavioral scores). Models were trained and tested using a leave-one-out cross validation loop (Golland & Fischl, 2003). The advantage of the ridge regression is that, unlike logistic regression, it minimizes bias due to vascular distributions (Phan et al., 2010), and it predicts behavior based on lesion (thus we could quantitatively assess deficit prediction), but still retains the ability to plot predictive weights to brain anatomy. However, it also requires high lesion overlap to generate unbiased prediction (Corbetta et al., 2015), thus only subjects with right MCA lesions (n=55) could be included. Lesion maps and behavioral scores were used to train a ridge regression algorithm that minimizes squared loss with a squared regularization term. Time since stroke was included as a covariate and was regressed from behavior measures before running the ridge regression. The algorithm is as follows:
The x vector indicates lesion location. The y vector contains behavioral factor scores for these same patients. The vector is the weight vector that describes the relative importance of each feature in x to the prediction of y. Lambda, a regularization coefficient, is determined empirically using a leave-one-out approach over a range of lambda values. Optimal weights were solved across the entire training set using gradient descent. Then, optimal model weights were applied to the lesion of the left-out subject to predict that subject’s behavioral score. A prediction was generated for all subjects in this way. Predictive weights were then averaged across loops. When visualizing lesion-deficit maps, weights were normalized to have a standard deviation of one, and thresholded so that only weights greater than 1 are shown. This method has been successfully used in a recent publication from our team (Corbetta et al., 2015).
Hodological analysis was performed using a recently published DTI atlas (Rojkova et al., 2015) and tracts obtained from NatBrainLab.com. Tracts were defined using DTI from 40 healthy subjects and reconstructed MNI-registered tracks were aligned to our stroke brain atlas. The resulting probabilistic atlas reflects probability of tract membership across the 40 subjects. White matter tracts that include any right hemisphere voxels (e.g. superior longitudinal fasciculus, corticospinal tract, corpus callosum) were retained, leaving 40 tracts. Lesion maps were projected on to the set of tracts to determine number of voxels damaged per tract for each subject. This approach was previously implemented by Thiebaut de Schotten and colleagues to identify white matter pathways associated with neglect (Thiebaut de Schotten et al., 2014). The correlation between the amount of damage to each white matter tract and the severity of the Field Effect and the Validity Effect was determined by calculating the Spearman's rho correlation coefficient. Both lesion size and time since stroke were regressed from the analysis.
3. Results
3.1. Stroke lesion distribution and characteristics
The distribution and size of stroke lesions in this heterogeneous patient sample are illustrated in Figure 1 and characterized in Table 1. The region of highest degree of lesion overlap was centered in the frontal subcortical white matter near the lateral ventricles underlying the middle frontal gyrus (MFG) and extending inferiorly and posteriorly. There was also significant cortical involvement of the overlying cortex from the inferior and middle frontal gyrus down through the insular cortex to the superior temporal gyrus and inferior parietal lobule. Subcortical nuclei (caudate, putamen, and thalamus) were also often damaged. This distribution is typical of a clinical distribution of prospectively selected right hemisphere strokes (Corbetta et al., 2015).
Figure 1.
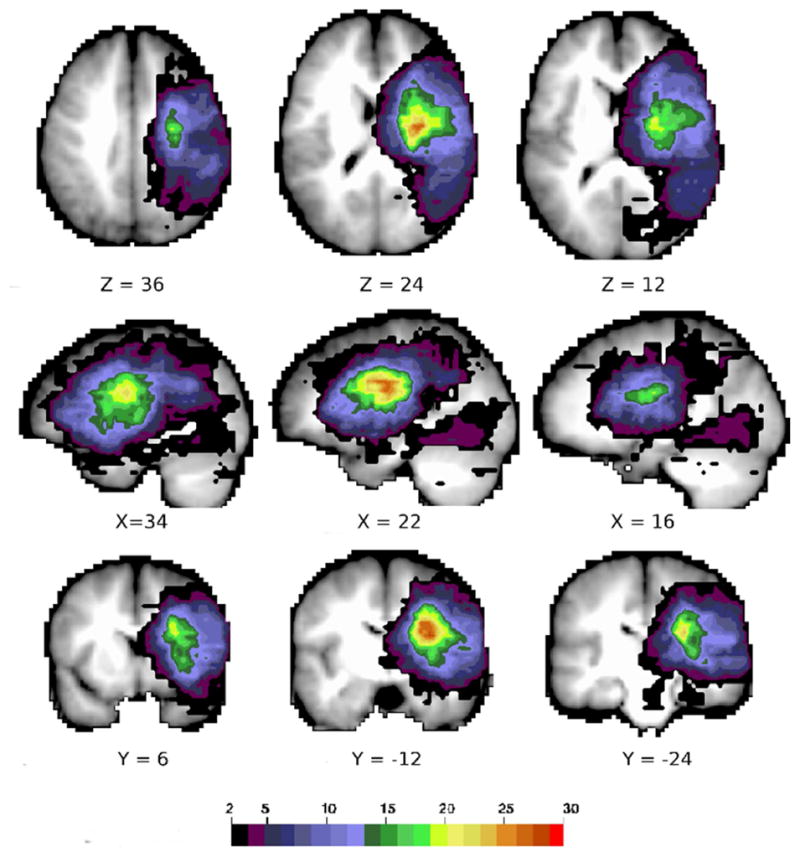
Stroke lesion distribution. A conjunction map of the right hemisphere stroke lesions in 70 subjects. Color scale indicates number of subjects with lesion at that voxel.
3.2. Behavioral performance
Deficits in lateralized attention and in reorienting of attention were determined using a computerized spatial orienting task (Figure 2A). A two group t-test assuming unequal variances was conducted to compare the Field Effect and the Validity Effect in our stroke sample to a group of age-matched healthy controls (Figure 2B). There was a significant difference in the Field Effect (msec) for healthy subjects (mean = 5.77, sd = 45.19) versus stroke subjects (mean = 232.79, sd = 293.34; p <0.001, assuming unequal variances). There was also a significant difference in the Validity Effect (msec) for healthy subjects (mean = 2.08, sd = 43.21) versus stroke subjects (mean = 117.06, sd = 267.17; p <0.001, assuming unequal variances).
Figure 2.
Effect of right hemisphere lesions on core components of attention. A. Timeline of stimulus presentation in the computerized spatial reorienting task. B. Field Effect and Validity Effect in msec in healthy age-matched controls and in stroke; AMC: age-matched controls; * = p < 0.01; ** = p < 0.001.
These results suggest that patients with right hemisphere lesions have, as compared to age matched controls, an impairment in directing attention and detecting visual targets in the contralesional visual field (visual field bias), and in shifting attention to an unattended spatial location. Interestingly, there was no significant correlation between the Field Effect and the Validity Effect (pearson r = 0.2, p = 0.098) (Figure 3). This indicates that these two attention deficits can be dissociated in patients with different lesions.
Figure 3.
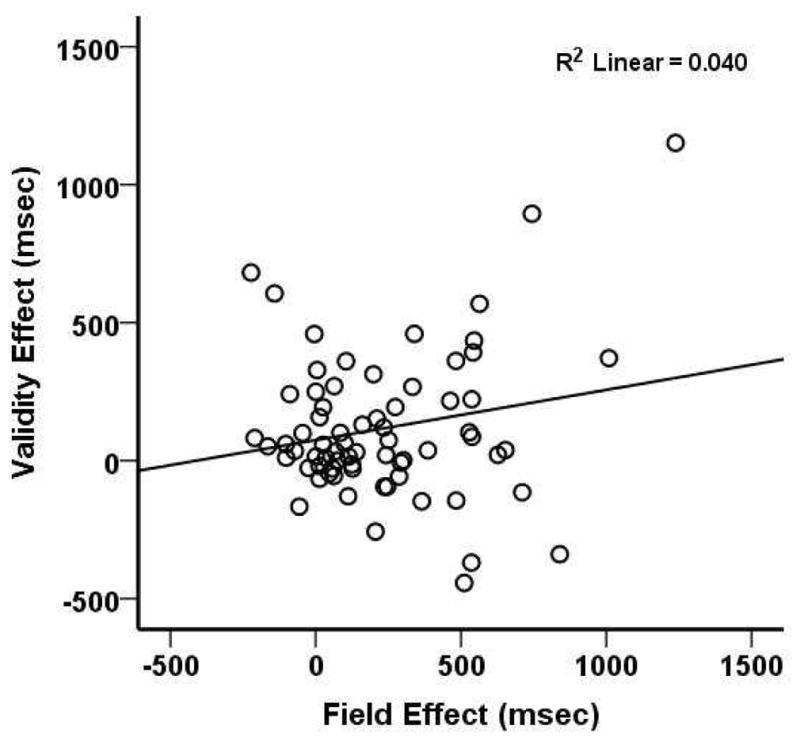
Relationship between Field Effect and Validity Effect.
3.3. Voxel-wise logistic regression
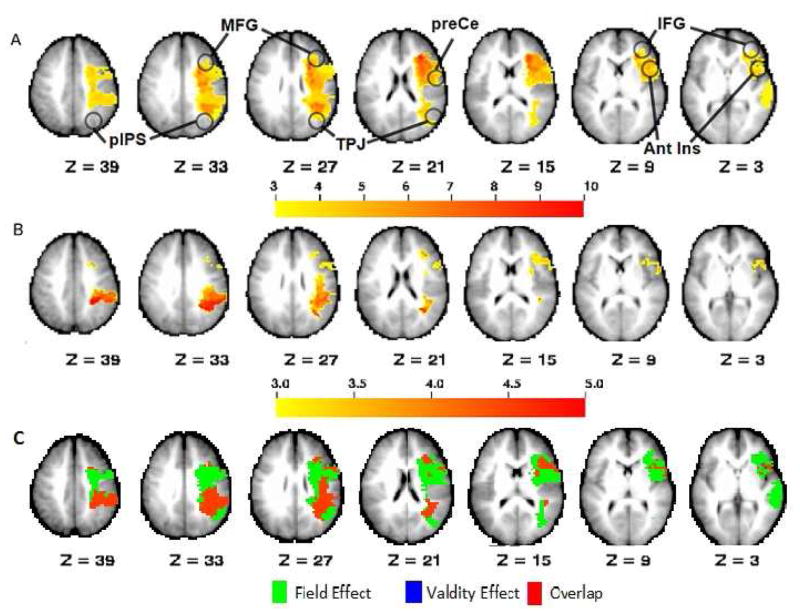
A whole-brain voxel-wise logistic regression was performed to determine which brain regions were predicted to be damaged as function of the severity of the Field Effect and Validity Effect, respectively. These statistical maps were corrected for multiple comparisons via Monte Carlo simulation (see methods) (Figure 4). The Field Effect was associated with a statistical map that predicted lesion load involving the frontal and parietal white matter with two peak loci: one in the frontal subcortical white matter extending from under the MFG ventrally to the inferior frontal gyrus (IFG) and posteriorly to the precentral (preCe) gyrus and anterior and middle insula; the other involving the parietal subcortical white matter between the posterior intraparietal sulcus (pIPS) and the TPJ (Figure 4A). While the areas of statistical significance extended to include some overlying cortical area, the focus was centered in the white matter. The Validity Effect was related with lesions of the parietal subcortical white matter similar to the Field Effect (Figure 4B) and relatively less with frontal lobe lesions. To qualitatively compare the distribution of predictive lesions for the Field Effect and the Validity Effect, the two statistical maps were binarized. A conjunction of the two maps indicates that they overlap primarily in the posterior parietal and inferior parietal white matter, and diverge more in the frontal lobe where lesions predicting the Field Effect alone predominate. There were very few voxels associated exclusively with the Validity Effect (Figure 4C).
Figure 4.
Whole-brain voxel-wise logistic regression maps relating lesion location to behavior. Both Field Effect and Validity Effect were entered into the model and regressed against the stroke lesion distribution. The results are z statistic maps for each behavior showing the likelihood that a given voxel will be damaged if that behavior is impaired. A. Field Effect; B. Validity Effect; C. Conjunction (Green = Field Effect; Blue = Validity Effect; Red = Overlap) Maps are Monte Carlo-corrected for multiple comparisons (p<0.05) with z threshold=3. Ant Ins: anterior insula; IFG: inferior frontal gyrys; MFG: middle frontal gyrus; pIPS: posterior intraparietal sulcus; preCe: pre-central sulcus; TPJ: temporo-parietal junction.
3.4. Ridge Regression
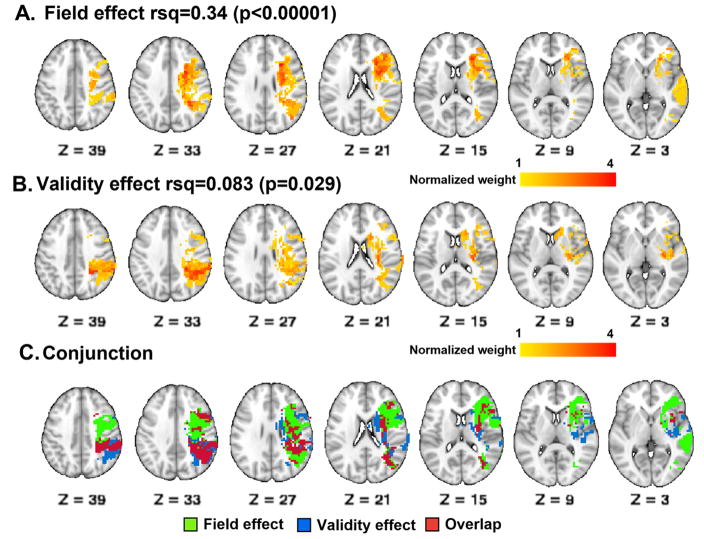
Ridge regression was used to further confirm the lesion-behavior relationships identified by logistic regression (Figure 5). Ridge regression is a novel multivariate machine learning method. The analysis generates a model and the weights (u) from the model are back-projected onto the brain to display the damaged voxels most associated with the behavior. Figure 5 shows the ridge regression (u) maps for the Field Effect (Figure 5A) and the Validity Effect (Figure 5B). The color scale reflects z-scored predictive weights for damage to that voxel as compared to a random distribution. The effect of lesion size has been regressed out. The results show a pattern of predictive weights that is similar to the results obtained via logistic regression with significant subcortical white matter involvement. The Field Effect was associated with damage to two foci including one in the centrum semiovale and the corona radiata in the frontal lobe, extending inferiorly along the white matter underlying the MFG and IFG and anterior to the anterior insula, and the other within the posterior parietal white matter. The Validity Effect was associated with damage to the white matter underlying the superior and inferior parietal lobules, similar to the Field Effect but more strongly so and over a larger area. In addition, the Validity Effect was associated with damage to the basal ganglia and peri-insula region. To qualitatively compare the distribution of predictive lesions for the Field Effect and the Validity Effect, the two statistical maps were binarized. A conjunction of the two maps (Figure 5C) indicates that they overlap primarily in the posterior parietal and inferior parietal white matter, and diverge more in the frontal lobe and peri-insular region where predictive lesion distributions are adjacent and only partially overlapping. This model predicts that damage to some peri-insular areas and the basal ganglia may be associated with the Validity Effect more specifically while more anterior lesions may be associated with the Field Effect more specifically.
Figure 5.
Ridge regression maps relating lesion location to behavior: The color scale indicates weights (u) determined by ridge regression for the Field Effect and the Validity Effect. A. Field Effect; B. Validity Effect; C. Conjunction (Red = Field Effect; Blue = Validity Effect; Gray = Overlap).
3.5. Tract-wise Hodological Analysis
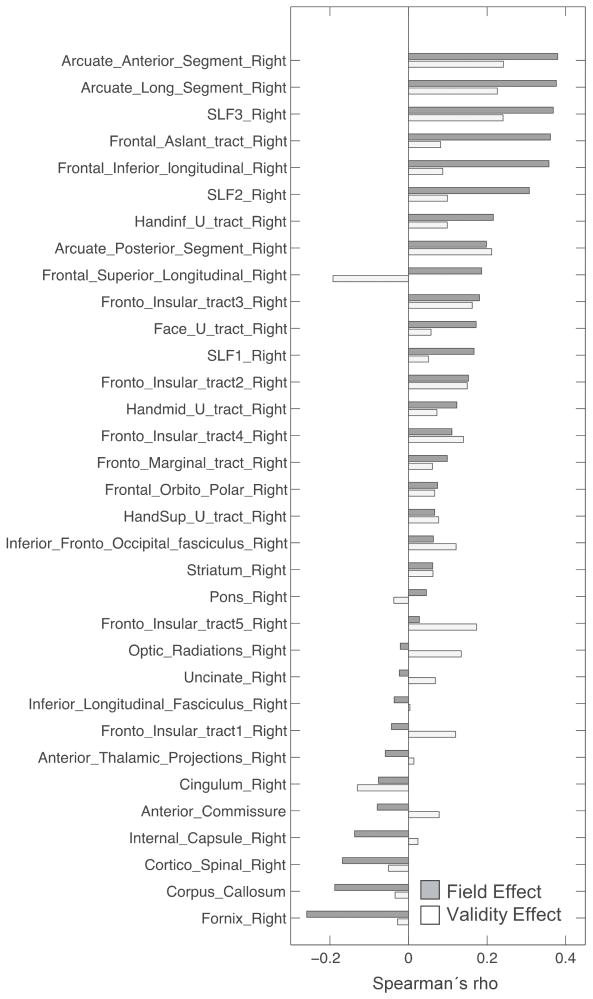
To compare the relative association of damage in different white matter tracts with behavioral deficits a correlational analysis was performed where the lesion distribution was overlapped with each right hemisphere tract obtained from a white matter atlas (Thiebaut de Schotten et al., 2014) and correlated with the Field and Validity Effects. Individual correlations were not significant after correcting for lesion size, time since stroke and multiple comparisons. However, some trends were observed. A qualitative analysis was performed by ranking each tract based on its correlation with the Field Effect score and visually inspecting the results. The strongest correlations with the Field Effect were seen for the anterior and long segments of the AF, SLF III, frontal aslant, frontal inferior longitudinal and SLF II tracts. These tracts also seemed to be more involved in the Field Effect than the Validity Effect although the anterior and long segments of the AF, SLF III returned the highest correlations of all the tracts for the Validity Effect. Among the few tracts that showed a stronger correlation with the Validity Effect than the Field Effect were the Fronto-Insular Tracts 1 and 5, even though the correlation of the Fronto-Inuslar tracts 2,3 and 4 were very closely matched for the Field Effect and the Validity Effect. A small number of negative correlations were observed, indicating the association of damage to the tract with a paradoxical leftward bias on the Field Effect or a tendency to respond more quickly in the invalid condition for the Validity Effect (Thiebaut de Schotten et al., 2014).
4. Discussion
We have used logistic regression and ridge regression to perform a behaviorally-driven lesion-symptom mapping analysis of core components of visuospatial attention in right hemisphere stroke. This study demonstrates that deficits in lateralized detection and re-orienting can occur together or separately along a continuum, with the simultaneous presence of both presumably leading to more severe hemispatial neglect clinically. Both analyses predict that damage to subcortical white matter of the posterior parietal cortex is strongly implicated in both Field Effect and Validity Effect deficits. However, the neuroanatomical substrates for the two deficits differ more anteriorly, where logistic regression indicates a large contribution from white matter underlying the MFG and IFG to the Field Effect, but does not resolve any regions that can be ascribed purely to the Validity Effect. The ridge regression however further refines this picture by suggesting the presence of peri-insular regions of white matter damage as well as basal ganglia damage that contribute more specifically to the Validity Effect. Our qualitative tract-wise lesion-deficit analysis returns tracts whose damage has previously been implicated in the genesis of neglect even though our study was focused on core components of attention and not exclusively neglect, These findings reinforce the suggestion that a differential pattern of white matter involvement is associated with the Field Effect and the Validity Effect.
4.1. Behavioral components of attention
Visuospatial attention is not a single process, and therefore its components may be individually subject to disruption, contributing to the different manifestations of hemineglect. In the original model proposed by Posner, attention consisted of three main components: alerting/arousal, orienting attention to a target stimulus, and executive attention to resolve conflicting inputs (Amso & Scerif, 2015; Posner & Petersen, 1990). More recently our understanding of visuospatial attention has been recast within a neuroanatomical framework of two interacting brain networks, the dorsal attention (DAN) and ventral attentions networks (VAN), defined by both task-based and resting state fMRI studies (Corbetta & Shulman, 2002, 2011). The bi-hemispheric DAN is involved in selectively attending to stimuli in a goal directed manner. Meanwhile, the right hemisphere lateralized VAN is involved in reorienting to salient unexpected stimuli and possibly non-spatial aspects of neglect such as overall level of arousal or temporal processing both of which are suppressed in individuals with neglect (Husain et al., 1997).
In our spatial orienting task, the theory predicts that the Field Effect would be associated with dysfunction of the DAN, as subjects have to voluntarily direct attention to a lateralized spatial location and detect a visual target, while the Validity Effect would be associated with dysfunction of the VAN, or interaction of DAN and VAN, as subjects have to re-direct attention and detect a target at a novel unexpected location. Our behavioral results support this distinction since the Field Effect and the Validity Effect are significantly impaired in right hemisphere stroke patients, but are not correlated with each other suggesting a partially dissociated neural basis. In a recent paper from our group, we have replicated this lack of correlation between Field and Validity effect in a partially overlapping group of subjects (Baldassarre et al., 2014).
4.2. Predicting lesions from behavior
Recently, a few studies have used voxel-wise lesion-symptom mapping to probe the neural correlates of neglect (Karnath, Rennig, Johannsen, & Rorden, 2011; Molenberghs, Gillebert, Peeters, & Vandenberghe, 2008; Ptak & Schnider, 2011; Rengachary et al., 2011; Saj et al., 2012; Sapir, Kaplan, He, & Corbetta, 2007) and of its different cognitive components (Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010).
Our study used two methods, logistic regression and a novel ridge regression (Corbetta et al., 2015) to answer this question. The main result of our analysis is that damage to the white matter, with relative disconnection, is an important mechanism of pathogenesis in stroke (see Corbetta et al., 2015 for a similar conclusion across multiple deficits and domain of impairment). More specifically, here we show that different attention impairments, i.e. the lateralized spatial bias typical of neglect and the inability to shift attention to novel stimuli, are both associated with white matter damage. Furthermore, the pattern of white matter damage is partially overlapping in the parietal lobe, but differs with regard to the involvement of frontal lobe white matter. Accordingly, different identifiable white matter tracts are likely to be lesioned with different behavioral deficits.
These anatomo-clinical correlations cannot be explained completely by the distribution of lesions resulting in a bias in statistical power (Kimberg, Coslett, & Schwartz, 2007). In fact, the area of maximum lesion overlap (Figure 1) is close to - but not the same as - the peak regions identified by the logistic regression or ridge regression analyses. Logistic regression is sensitive to the number of subjects with lesions covering a specific region in the brain, and while the area of maximum lesion overlap in our sample involved the frontal subcortical white matter, the area over which voxels were lesioned in at least 7 subjects for the logistic regression to be reliable (Vittinghoff & McCulloch, 2007) was much larger and included large regions of cortex as well (Figure 1).
Our results are similar to previous voxel-wise lesion-symptom mapping studies (Karnath et al., 2011; Saj et al., 2012; Verdon et al., 2010), but also partly different. Our statistical maps also predict lesions in the paraventricular matter of the superior and inferior parietal gyrus, frontal operculum, middle and inferior frontal gyrus, superior portion of the TPJ, the anterior insula, and basal ganglia. However, the temporal gyri and occipital cortex were not implicated. Our more restricted lesion mapping may result from the use of a very specific behavioral assessment (computerized spatial orienting task) compared to the battery of tests used by Saj et al. However, there is a striking proximity between the areas of white matter involvement we report and the cortical regions reported by Ptak et al (see below) who also employed a target detection paradigm (Ptak & Schnider, 2011). A similar relationship is seen between our results and those of Molenberghs et al in the parietal lobe, though that study did not emphasize any frontal lobe involvement (Molenberghs et al., 2008). In addition, when compared with the factor analysis on behavior performed by Verdon et al (Verdon et al., 2010), our findings of right inferior parietal lobe white matter involvement match areas affected with their component 1, and our findings of right inferior and middle frontal lobe white matter involvement are consistent with areas affected with their component 3.
4.3. Role of disrupted dorsal and ventral attention networks
From task-evoked fMRI studies of attention, Corbetta and Shulman proposed the interaction of the DAN and the VAN in controlling the focus of visuo-spatial attention. In this model, the DAN consists of regions centered on the intraparietal sulcus and frontal eye fields and mediates the allocation of attention (both goal- and stimulus-driven). The VAN consists of the temporo-parietal junction and the IFG and MFG in the ventral prefrontal cortex, and is recruited during stimulus driven re-orienting, i.e. when attention is redirected to novel unattended events. The VFC may play a significant role in VAN to DAN communication (Corbetta & Shulman, 2011).
Clinically, lesions associated with neglect are often located in the territory of the VAN, leaving the DAN structurally intact (Husain & Kennard, 1996; Karnath, Ferber, & Himmelbach, 2001; Karnath, Fruhmann Berger, Kuker, & Rorden, 2004; Karnath, Himmelbach, & Rorden, 2002; Mort et al., 2003; Vallar & Perani, 1986). However, in our analysis the Field Effect is associated with lesions in the white matter undercutting the connections between the frontal eye fields, the preCe and the IPS. Moreover, more ventral lesions (ie affecting the frontal aslant tract in the MFG) are likely to disconnect the VFC from the dorsal prefrontal cortex, in addition to SLF III lesions disconnecting the TPJ from the VFC and frontal eye fields.
With regard to the Validity Effect, the statistical lesion maps implicate a more caudal and posterior area of parietal lobe damage (also seen with the Field Effect but to a lesser extent) involving white matter and overlying cortex of the pIPS down through the TPJ. Prior studies have already implicated the TPJ (Chang et al., 2013; Friedrich et al., 1998; Kincade et al., 2005; Macaluso & Doricchi, 2013) and the VFC in reorienting (Arrington, Carr, Mayer, & Rao, 2000; Corbetta & Shulman, 2002; Snyder & Chatterjee, 2006). Our findings are consistent with a recent study by Ptak and Schnider (Ptak & Schnider, 2011) who report lesions in pIPS in association with tests of both target detection and reorienting. Of particular interest is that Ptak et al also observed a differential involvement of the pIPS for their Target Detection and Validity Effect conditions. Significant pIPS voxels were located slightly more rostrally for the Validity Effect condition in a pattern that is very similar to our results. Just as with the DAN, where impaired lateralized detection predicted lesions located inferior to the DAN, impaired stimulus-driven reorienting predicts lesions located superior to the VAN, a surprising paradox which warrants further investigation. However, because of the distributed character of attention networks, local structural damage can cause remote network dysfunction therefore, it is important to bear in mind that the pattern of lesion may not be identical to the pattern of dysfunction that drives the observed behavior (Mah et al., 2014).
In a large study of neglect patients at 2 weeks post-stroke (n=88), we recently reported that, independently of lesion location, patients with visual field biases and overall decrement in general performance, a measure of non-spatial attention, show a common pattern of abnormal cortical synchrony, measured with resting state fMRI. This pattern consists of a loss of inter-hemispheric correlation in DAN, motor, visual and auditory networks, and an abnormal increase of correlation between networks that are normally segregated in the damaged hemisphere (Baldassarre et al., 2014). We relate the loss of inter-hemispheric correlation to the Visual Field Effect, and the loss of right hemisphere segregation to the lower overall performance. It will be interesting in future studies compare the functional correlates of Validity Effect vis-à-vis Field Effect.
4.4. Role of disrupted white matter connections
It has been argued for some time that spatial neglect is a disconnection syndrome resulting from damage to fronto-temporo-parietal white matter tracts (Bartolomeo, Thiebaut de Schotten, & Doricchi, 2007; Catani & ffytche, 2005; Doricchi et al., 2008; Gaffan & Hornak, 1997; Lunven et al., 2015; Mesulam, 1981; Roux et al., 2011; Samuelsson, Jensen, Ekholm, Naver, & Blomstrand, 1997; Schmahmann et al., 2008; Thiebaut de Schotten et al., 2005; Vallar et al., 2014). Commonly implicated tracts include SLF II and III, AF and IFOF. A recent study has shown that decreased fractional anisotropy in SLF II and SLF III are both associated with persistent neglect (Lunven et al., 2015). The IFOF, connecting the (Umarova et al., 2010) fronto-basal cortex with parieto-occipital cortex may also play a significant role in visuospatial attention (Karnath et al., 2011; Umarova et al., 2010; Urbanski et al., 2008) although its existence has been disputed by some (Schmahmann & Pandya, 2007). Few studies have attempted to specific white matter tracts to specific components of spatial attention (Verdon et al., 2010).
Our tract-wise correlational analysis highlights many of the white matter tracts previously reported in neglect studies. Accordingly, our hodological analysis shows that SLF II and arcuate fasciculus damage have a higher correlation with the Field Effect than most other tracts. More interestingly, there is a trend for more anteriorly located tracts to be more correlated with the Field Effect than the Validity Effect, though the anterior and long segments of the AF and SLF III are also correlated with the Validity Effect here. Conversely, tracts whose damage is more correlated with the Validity Effect than the Field Effect tend to have a more parieto-occipital, or ventro/peri-insular location. See for example, fronto-insular tracts 1 and 5. Fronto-insular tracts 2,3, and 4 however do not show any differential involvement with the Field or Validity Effect. These regional differences are consistent with the patchy mosaic in the peri-insular region seen in the ridge regression analysis indicating the presence (in blue in Figure 5C) of regions whose damage predicts the presence of a Validity Effect.
The relationship of SLF II and the frontal aslant with the Field Effect are consistent with an analysis by Thiebaut de Schotten et al who suggested that the leftward bias in spatial attention observed in healthy subjects is due to a greater density of SLF II fibers in the right hemisphere (Thiebaut de Schotten et al., 2011), and that damage to the right SLF II is the best predictor of left neglect (Thiebaut de Schotten et al., 2014). The location of the frontal aslant is also consistent with a region in the middle frontal gyrus that appears to be one of the convergence points between VAN and DAN (Fox et al., 2006). Why then is damage to the frontal aslant not associated with the Validity Effect is unclear. Damage to SLF and arcuate are also associated with the remote patterns of cortical dysynchrony described above (He et al., 2007).
In contrast the association between SLF III and Validity Effect is consistent with the prominent role played by the VAN in stimulus-driven re-orienting, the attention process captured by this performance index. The VAN is strongly lateralized to the right hemisphere (Corbetta, Patel, & Shulman, 2008), and its two core regions TPJ and IFG are connected by SLF III, which is also the most right lateralized branch of the SLF. In conclusion the results of the tract-wise lesion-deficit correlational analysis are generally compatible with the results from the ridge regression and the functional role of the implicated tracts.
4.6. Limitations
We recognize the temporal heterogeneity in our sample with regard to the time since stroke which can affect the lesion-symptom relationship. As a result we have included time since stroke as a covariate in our ridge regression. In previous studies persistent chronic neglect was associated with white matter damage (Karnath et al., 2011) while acute neglect was more associated with cortical and subcortical gray matter damage. (Karnath, Rorden, & Ticini, 2009) Our finding of significant lesion-behavior relationships involving the white matter in a heterogeneous group of subjects that is more heavily weighted toward the subacute end of the temporal spectrum rather than the chronic end, further reinforces the importance of white matter integrity in the two core components of visuo-spatial attention studied here. Our results may be driven by our choice of behavioral task. While we contend that we have targeted core processes in spatial attention, the use of other tests may lead to different mapping results (Saj et al., 2012). Our main finding implicating significant white matter involvement does not preclude the importance of cortical involvement and does not contradict the results of studies showing an association between various cortical lesions and neglect. Also, ideally anatomical connectivity, functional connectivity, and vascular connectivity (Mah et al., 2014) should be taken into account to reduce the errors from hidden biases. When behavior is subserved by set of widely distributed functional networks symptoms may result from dysfunction in the area of structural damage, or the area of diaschisis, or disconnection between the two. Anatomical studies will by definition not include structurally intact but dysfunctional areas that are the true cause of the deficit. Finally, we did not investigate the effects of IV thrombolysis. While the thrombolytic effects of tissue plasminogen activator are well known, its neuroprotective effects on both gray (Grummisch, Jadavji, & Smith, 2016) and white matter (Correa et al., 2011) it is hard to predict its effect on the lesion-symptom mapping relationship.
5. Conclusions
Starting with specific behaviors thought to reflect distinct cognitive components of attention, we have shown, using a voxel-wise approach, that deficits in those behaviors are predictive of different lesion patterns. This approach strongly implicates damage to subcortical white matter in the disruption of specific aspects of attention that may contribute to the emergence of spatial neglect. The results suggest that damage to long-range fronto-parietal white matter tracts needs to be considered in association with cortical lesions when evaluating the disruption of attentional mechanisms.
Supplementary Material
T2-weight MRI scans of subjects even within 10 days of stroke demonstrate clear areas of T2 hyperintensity that allow for segmentation and quantification of the volume of the infracted tissue.
Figure 6.
Tract-wise lesion-deficit correlations. For each tract, the correlation between damage to that tract and the Field Effect and the Validity Effect are shown. After correcting for lesion size, time since stroke and multiple comparisons, correlations are not statistically significant taken individually. Tracts are ordered by strength of the correlation with the Field Effect. Results suggest damage to some tracts may be more highly correlated with one deficit or the other. Only tracts which sustained damage in this sample are shown.
Acknowledgments
This work was supported in parts by grants including R01 NS095741 and R01 MH096482-02 from NIH to MC; K08 NS064365 from NINDS and AMFDP 65592 from the Robert Wood Johnson Foundation to ARC. We also thank Michel Thiebaut de Schotten for assistance in using white matter tracts obtained from the NatBrainLab database (http://www.natbrainlab.co.uk/) and Rojkova et al, 2015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amso D, Scerif G. The attentive brain: insights from developmental cognitive neuroscience. Nat Rev Neurosci. 2015;16(10):606–619. doi: 10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL. A rapid and accurate method to realign PET scans utilizing image edge information. J Nucl Med. 1995;36(4):657–669. [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12(Suppl 2):106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Riddoch MJ, Crome P. Evaluation of a test battery for hemineglect in elderly stroke patients for use by therapists in clinical practice. NeuroRehabilitation. 2000;14(3):139–150. [PubMed] [Google Scholar]
- Baldassarre A, Ramsey L, Hacker CL, Callejas A, Astafiev SV, Metcalf NV, … Corbetta M. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain. 2014;137(Pt 12):3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17(11):2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bird CM, Malhotra P, Parton A, Coulthard E, Rushworth MF, Husain M. Visual neglect after right posterior cerebral artery infarction. J Neurol Neurosurg Psychiatry. 2006;77(9):1008–1012. doi: 10.1136/jnnp.2006.094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M. Neglect and extinction depend greatly on task demands: a review. Front Hum Neurosci. 2012;6:195. doi: 10.3389/fnhum.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M, Deouell LY. Hemispatial neglect: computer-based testing allows more sensitive quantification of attentional disorders and recovery and might lead to better evaluation of rehabilitation. Front Hum Neurosci. 2013;7:162. doi: 10.3389/fnhum.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M, Priftis K, Marenzi R, Umilta C, Zorzi M. Deficits of contralesional awareness: a case study on what paper-and-pencil tests neglect. Neuropsychology. 2012;26(1):20–36. doi: 10.1037/a0025306. [DOI] [PubMed] [Google Scholar]
- Bonato M, Priftis K, Umilta C, Zorzi M. Computer-based attention-demanding testing unveils severe neglect in apparently intact patients. Behav Neurol. 2013;26(3):179–181. doi: 10.3233/ben-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28(40):10056–10061. doi: 10.1523/jneurosci.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, … Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(Pt 10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chang CF, Hsu TY, Tseng P, Liang WK, Tzeng OJ, Hung DL, Juan CH. Right temporoparietal junction and attentional reorienting. Hum Brain Mapp. 2013;34(4):869–877. doi: 10.1002/hbm.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Humphreys GW. Neuronal substrates of Corsi Block span: Lesion symptom mapping analyses in relation to attentional competition and spatial bias. Neuropsychologia. 2014;64:240–251. doi: 10.1016/j.neuropsychologia.2014.09.038. [DOI] [PubMed] [Google Scholar]
- Ciaraffa F, Castelli G, Parati EA, Bartolomeo P, Bizzi A. Visual neglect as a disconnection syndrome? A confirmatory case report. Neurocase. 2013;19(4):351–359. doi: 10.1080/13554794.2012.667130. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8(11):1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, … Shulman GL. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927–941. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, … Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208(6):1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47(2):145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. 2012;25(4):375–383. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F, Rizzo G, Scifo P, Clarke RA, Scotti G, Fazio F. A model-based deconvolution approach to solve fiber crossing in diffusion-weighted MR imaging. IEEE Trans Biomed Eng. 2007;54(3):462–472. doi: 10.1109/tbme.2006.888830. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Macci E, Silvetti M, Macaluso E. Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the Posner task. Cereb Cortex. 2010;20(7):1574–1585. doi: 10.1093/cercor/bhp215. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex. 2008;44(8):983–995. doi: 10.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14(17):2239–2243. doi: 10.1097/01.wnr.0000091132.75061.64. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell'Acqua F, Danek A, Catani M. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2014;56:73–84. doi: 10.1016/j.cortex.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: a comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12(2):193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120(Pt 9):1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bruce CJ. Cerebral cortical activity associated with the orientation of visual attention in the rhesus monkey. Vision Res. 1985;25(3):471–481. doi: 10.1016/0042-6989(85)90072-0. [DOI] [PubMed] [Google Scholar]
- Golland P, Fischl B. Permutation tests for classification: towards statistical significance in image-based studies. Inf Process Med Imaging. 2003;18:330–341. doi: 10.1007/978-3-540-45087-0_28. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391(6666):481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Grummisch JA, Jadavji NM, Smith PD. tPA promotes cortical neuron survival via mTOR-dependent mechanisms. Mol Cell Neurosci. 2016;74:25–33. doi: 10.1016/j.mcn.2016.03.005. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. J Neurol. 1996;243(9):652–657. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385(6612):154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Indovina I, Macaluso E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb Cortex. 2007;17(7):1701–1711. doi: 10.1093/cercor/bhl081. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411(6840):950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex. 2004;14(10):1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125(Pt 2):350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. 2011;134(Pt 3):903–912. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Rorden C, Ticini LF. Damage to white matter fiber tracts in acute spatial neglect. Cereb Cortex. 2009;19(10):2331–2337. doi: 10.1093/cercor/bhn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25(18):4593–4604. doi: 10.1523/jneurosci.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clark CA. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39(1):62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Leibovitch FS, Black SE, Caldwell CB, Ebert PL, Ehrlich LE, Szalai JP. Brain-behavior correlations in hemispatial neglect using CT and SPECT: the Sunnybrook Stroke Study. Neurology. 1998;50(4):901–908. doi: 10.1212/wnl.50.4.901. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, … Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Losier BJ, Klein RM. A review of the evidence for a disengage deficit following parietal lobe damage. Neurosci Biobehav Rev. 2001;25(1):1–13. doi: 10.1016/s0149-7634(00)00046-4. [DOI] [PubMed] [Google Scholar]
- Lunven M, Thiebaut De Schotten M, Bourlon C, Duret C, Migliaccio R, Rode G, Bartolomeo P. White matter lesional predictors of chronic visual neglect: a longitudinal study. Brain. 2015;138(Pt 3):746–760. doi: 10.1093/brain/awu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Doricchi F. Attention and predictions: control of spatial attention beyond the endogenous-exogenous dichotomy. Front Hum Neurosci. 2013;7:685. doi: 10.3389/fnhum.2013.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137(Pt 9):2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Menard SW. Applied logistic regression analysis. Thousand Oaks, California: Sage Publications; 1995. [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10(4):309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Minka T. A comparison of numerical optimizers for logistic regression. 2003 Retrieved from http://research.microsoft.com website: http://researchmicrosoftcom/en-us/um/people/minka/papers/logreg/
- Molenberghs P, Gillebert CR, Peeters R, Vandenberghe R. Convergence between lesion-symptom mapping and functional magnetic resonance imaging of spatially selective attention in the intact brain. J Neurosci. 2008;28(13):3359–3373. doi: 10.1523/jneurosci.5247-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126(Pt 9):1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Oxbury JM, Campbell DC, Oxbury SM. Unilateral spatial neglect and impairments of spatial analysis and visual perception. Brain. 1974;97(3):551–564. doi: 10.1093/brain/97.1.551. [DOI] [PubMed] [Google Scholar]
- Phan TG, Chen J, Donnan G, Srikanth V, Wood A, Reutens DC. Development of a new tool to correlate stroke outcome with infarct topography: a proof-of-concept study. Neuroimage. 2010;49(1):127–133. doi: 10.1016/j.neuroimage.2009.07.067. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18(5):502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci. 2010;30(38):12557–12565. doi: 10.1523/JNEUROSCI.2722-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The attention network of the human brain: relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologia. 2011;49(11):3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Rengachary J, d'Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Arch Phys Med Rehabil. 2009;90(12):2081–2088. doi: 10.1016/j.apmr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M. A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci. 2011;5:29. doi: 10.3389/fnhum.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell'Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Lauwers-Cances V, Boukhatem L, Brauge D, Draper L, … Demonet JF. Electrostimulation mapping of spatial neglect. Neurosurgery. 2011;69(6):1218–1231. doi: 10.1227/NEU.0b013e31822aefd2. [DOI] [PubMed] [Google Scholar]
- Saj A, Verdon V, Vocat R, Vuilleumier P. 'The anatomy underlying acute versus chronic spatial neglect' also depends on clinical tests. Brain. 2012;135(Pt 2):e207. doi: 10.1093/brain/awr227. author reply e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson H, Jensen C, Ekholm S, Naver H, Blomstrand C. Anatomical and neurological correlates of acute and chronic visuospatial neglect following right hemisphere stroke. Cortex. 1997;33(2):271–285. doi: 10.1016/s0010-9452(08)70004-2. [DOI] [PubMed] [Google Scholar]
- Sapir A, Kaplan JB, He BJ, Corbetta M. Anatomical correlates of directional hypokinesia in patients with hemispatial neglect. J Neurosci. 2007;27(15):4045–4051. doi: 10.1523/JNEUROSCI.0041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30(5):509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. J Hist Neurosci. 2007;16(4):362–377. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, … McKinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165(4):524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JJ, Chatterjee A. The frontal cortex and exogenous attentional orienting. J Cogn Neurosci. 2006;18(11):1913–1923. doi: 10.1162/jocn.2006.18.11.1913. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M. Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. J Neurosci. 2007;27(52):14424–14433. doi: 10.1523/JNEUROSCI.3759-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, … Doricchi F. Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex. 2014;24(3):691–706. doi: 10.1093/cercor/bhs351. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309(5744):2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Umarova RM, Saur D, Schnell S, Kaller CP, Vry MS, Glauche V, … Weiller C. Structural connectivity for visuospatial attention: significance of ventral pathways. Cereb Cortex. 2010;20(1):121–129. doi: 10.1093/cercor/bhp086. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touze E, … Bartolomeo P. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2008;79(5):598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Oppenheim C, Touze E, Meder JF, … Bartolomeo P. DTI-MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res. 2011;208(4):491–505. doi: 10.1007/s00221-010-2496-8. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bello L, Bricolo E, Castellano A, Casarotti A, Falini A, … Papagno C. Cerebral correlates of visuospatial neglect: a direct cerebral stimulation study. Hum Brain Mapp. 2014;35(4):1334–1350. doi: 10.1002/hbm.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24(5):609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133(Pt 3):880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM. Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Arch Neurol. 1987;44(6):621–625. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68(2):98–102. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T2-weight MRI scans of subjects even within 10 days of stroke demonstrate clear areas of T2 hyperintensity that allow for segmentation and quantification of the volume of the infracted tissue.