Abstract
Myocardial ischaemia results from a direct mismatch between oxygen supply and demand, commonly arising as a result of coronary atherosclerosis, microvascular dysfunction or acute thrombosis and luminal obstruction. However, transient ischaemia may also occur due to coronary spasm leading to acute and unexpected myocardial ischaemia without obvious visible coronary pathology. Aside from symptoms of chest pain, coronary spasm can cause infarction, LV impairment, promote life threatening arrhythmias and ultimately sudden cardiac death. While therapeutic options are available, controversies exist around diagnosis, pathology, management and prognosis. This review summarises some of the common questions in this area. In particular we explore and discuss the available evidence for the pharmacological treatment of coronary spasm, and strategies for identification and management of very high risk patients to try and reduce the incidence of sudden premature death.
Keywords: Coronary spasm, Variant angina, Printzmetal angina
Highlights
-
•
Myocardial ischaemia results from a mismatch between oxygen supply and demand.
-
•
Spasm might lead to myocardial ischaemia without visible coronary pathology.
-
•
Coronary spasm can cause infarction, LV impairment and sudden cardiac death.
1. Introduction
Myocardial ischaemia results from a direct mismatch between oxygen supply and demand, commonly arising as a result of coronary atherosclerosis, microvascular dysfunction or acute thrombosis and luminal obstruction. However, transient ischaemia may also occur due to coronary spasm leading to acute and unexpected myocardial ischaemia without obvious visible coronary pathology. Aside from symptoms of chest pain, coronary spasm can cause infarction, LV impairment, promote life threatening arrhythmias and ultimately sudden cardiac death. While therapeutic options are available, controversies exist around diagnosis, pathology, management and prognosis. This review summarises some of the common questions in this area. In particular we explore and discuss the available evidence for the pharmacological treatment of coronary spasm, and strategies for identification and management of very high risk patients to try and reduce the incidence of sudden premature death.
2. Overview of coronary spasm
Coronary spasm, also known as variant or Printzmetal's angina, represents a syndrome characterised by sudden chest pain due to epicardial coronary artery spasm which usually leads to transient myocardial ischemia, with chest pain and ECG changes. It differs from traditional stable angina pectoris, symptomatically in that it is not directly related to effort and pathologically as it is not driven by atherosclerosis and lumen encroachment within the coronary vasculature (Table 1).
Table 1.
Common differences between coronary spasm and chronic stable angina.
| Coronary spasm | Chronic stable angina | |
|---|---|---|
| Frequency | Less | More frequent |
| Age | Younger | Older |
| Sex | Female | Male |
| Ethnicity | Japanese | No specific prevalence |
| Risk factors | Smoking, drug addiction, alcohol, hyperventilation, beta blockers | Classic cardiovascular risk factor |
| Circadian pattern | Night–early morning | No |
| Exertion/rest | Exertion/rest | Exertion |
| ECG | ST segment elevation | ST segment depression |
The actual prevalence of the condition in the general population remains largely unknown, primarily due to challenges in diagnosis. However, in contrast to traditional angina pectoris, it appears to be less frequent, afflicts younger patients and females more than males [1], [2], [3], [4], [5]. It is also widely recognised that individuals from Japan tend to have significantly greater coronary reactivity and tendency to spasm than those from other nations. This is borne out by a greater incidence and prevalence of the syndrome in this region, with a concurrently lower rate of coronary atherosclerotic disease compared with those in Europe [6], [7], [8], [9], [10], [11], [12], [13], [14]. Coronary spasm is also associated with smoking, cocaine, amphetamine, marijuana and alcohol consumption, which can often explain myocardial infarction in young patients with few traditional cardiovascular risk factors. Notably, chemotherapy and anti-migraine drugs may also induce coronary spasm and cautions are listed for these agents (Table 2) [15], [16], [17], [18], [19], [20], [21].
Table 2.
Recognized predisposing factors for coronary spasm.
|
A key feature of coronary spasm is its temporally transient nature which often leads to difficulties in diagnosis. Intriguingly, spasm occurrences tend to follow a circadian pattern, being most frequent during the night or early morning [1], [2], [3], [4], [5]. This is an important feature of the history and often used in diagnostic criterion [13].
Anatomically, coronary spasm may be focal or diffuse within a vessel, may affect multiple vessels and may migrate from site to site. Mixed type spasm is defined as multi-vessel spasm in which at least one coronary artery has a focal spasm and the other a diffuse spasm. Takagi et al. reported the presence of multi-vessel spasm in 32% of their cohort, and of these the ones with mixed type spasm had the highest risk of Major Adverse Cardiovascular Events (MACE), including myocardial infarction [14]. Additionally, they reported that right coronary spasm was significantly associated with ventricular arrhythmias compared with other vessels [14]. Patients with diffuse spasm are generally less likely to respond to therapy and have a worse outcome, presumably because of advanced and extensive vasomotor dysfunction of the coronary circulation [14], [22], [23]. Although infarction sizes in the context of spasm are usually relatively small, due to early spontaneous reperfusion once spasm has subsided, the risk of fatal arrhythmias remains a feared complication.
3. Pathophysiology
The exact underlying pathology predisposing to spasm is not clearly understood, but a complex interplay of factors is postulated to be responsible for the highly heterogeneous clinical manifestations, natural history and response to therapy. Although a constellation of such precipitating factors have been proposed (genetic background, vagal and sympathetic activity alteration, magnesium deficiency, enhanced oxidative stress, inflammation), the most established and accepted mechanisms are endothelial dysfunction, nitric oxide (NO) release and enhanced vascular smooth muscle cell contractility which all determine vascular tone [15], [22], [23], [24], [25], [26], [27], [28], [29], [30].
3.1. Atherosclerosis and spasm — are they mutually exclusive?
Coronary spasm often occurs in angiographically normal arteries but can also be found in those with mild and more severe atherosclerotic stenosis. With increasing availability of angiography and more patients undergoing testing, presence of concurrent atherosclerotic plaque is not uncommon, raising the question as to whether (1) spasm is the cause of chest pain, (2) plaque promotes spasm and (3) if patients with both fare worse?
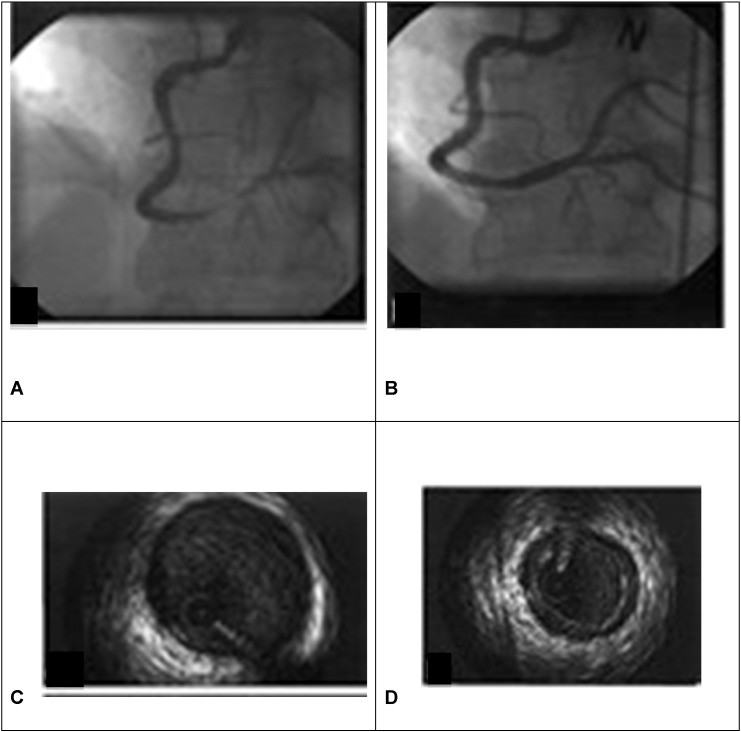
Early studies on coronary spasm patients reported that the presence of co-existent severe coronary disease with coronary spasm was associated with an adverse prognosis [23], [32], [33], [34], [35], [36], [37], [38], [39]. However, long term followup data has also revealed that the presence of even non-significant stenosis impacts unfavourably on patient outcome [14]. Notably, the presence of subtle atherosclerotic infiltration at the site of spasm has been documented by intravascular ultrasound (IVUS) (Fig. 1), in coronary spasm patients with apparently normal coronary angiograms yet this was associated with a faster progression rate of atheroma [14], [40], [41], [42]. This is in line with the idea that spasm is a widespread phenomenon of arterial remodelling. While plaque may itself induce spasm, this is difficult to prove, but what is known is that coronary spasm can itself induce the rupture of stable atherosclerotic plaque, with consequent coronary thrombosis and myocardial infarction. Moreover, in patients with chronic stable angina, myocardial ischaemia may also be related to distal coronary artery spasm [43]. Furthermore, the microvasculature may also demonstrate spasm, although this is more difficult to observe. For example, during myocardial infarction, there is widespread microvascular hyper-reactivity, not necessarily related to the infarct zone, and considered to be responsible in part for the no-reflow phenomenon. Therefore, if a patient suspected to have coronary spasm complaints of chest pain during a provocation test (acetylcholine or ergonovine administration) along with direct or indirect findings of myocardial ischaemia (ECG abnormalities, reduction of coronary blood flow rate, and myocardial lactate production), but angiography is negative for spasm, microvascular spasm should be suspected.
Fig. 1.
Evidence at angiography (A–B) and intra-vascular ultrasound (C–D) evaluation of right coronary artery spasm.
Thus coronary spasm may occur in the presence or absence of coronary atheroma or plaques and the two are not mutually exclusive. Where they do occur concurrently, the prognosis is clearly worse. It is likely that spasm and atherosclerosis share common aetiological processes (endothelial dysfunction, arterial remodelling), but may operate independently in causing myocardial ischaemia.
4. Diagnostic challenges
Coronary spasm may present in a variety of ways and are often subtle. It is for this reason that delays in diagnosis are common, with an estimated 3 months from presentation to diagnosis [29]. In some cases, the first (and only) manifestation may be with a sudden myocardial infarction or cardiac arrest. The spectrum of symptoms, ECG changes, and strong circadian pattern required for a diagnosis of coronary spasm have been described in detail previously [1], [2], [4], [5], [13]. Notably, despite the safety of provocation tests, these are still not commonly performed [14].
Patients with chest pain, ECG ST segment elevation and normal coronary arteries are usually considered to be affected by spasm if the clinical scenario is consistent with the characteristics of coronary spasm (Table 1) [13]. Myocardial infarction can develop if the spasm and ischaemia are sufficiently prolonged to induce myocyte death. Following an acute presentation as described, patients are usually discharged on a medication with anti-spasm properties, along with any other therapies for co-existent atherosclerosis or LV impairment, lifestyle modification advice and subsequent re-evaluation in clinic. If full control of symptoms has been achieved, no further investigations are pursued at this stage.
4.1. Diagnosis in specific clinical settings
-
(1)
Patients with ECG ST segment elevation and coronary stenosis.
If concordance between clinical (ECG and echo) and angiographic findings is present for lesion localisation, stent implantation according to the type of lesion is performed. If there are no culprit lesions on angiography and the clinical presentation (including risk factors) is indicative, coronary spasm treatment is initiated and any observed, and presumed bystander, stenosis is re-evaluated by stress testing.
-
(2)Patients referred to the clinic for chest pain after a stress test.
-
a.If positive, patients usually begin anti-ischaemic treatment or are considered for invasive coronary investigation and management. If spasm is present with atherosclerosis, despite the restored patency of a diseased coronary artery after PCI, the patient may continue to complain of chest pain due to spams episodes.
-
b.If negative, additional testing with invasive angiography or with Holter ECG monitoring can be performed. If symptoms and ischaemic ECG changes correlate the diagnosis is made. Unfortunately symptoms may not occur in a short time frame and longer recordings may be needed if the likelihood of spasm is high. If a patient reports symptoms during ECG monitoring but without ECG changes, and the clinical scenario still fits coronary spasm characteristics, microvascular spasm may be a possibility and empirical therapy started. They are usually re-evaluated in the clinic in order to verify the effects of therapy on symptoms control.
-
a.
-
(3)
Patients with syncope or bradyarrhythmias.
These patients will often require monitoring with Holter monitoring to identify the cause of syncope, during which ECG changes consistent with ischaemia may indicate a diagnosis of coronary spasm. In this case, treatment may be initiated with revaluation thereafter.
-
(4)
Patient surviving a cardiac arrest with no coronary artery disease.
Once other causes of cardiac arrest have been excluded, secondary prevention ICD implantation is usually offered to patients in line with guidelines. If coronary spasm is a possibility, based on previous symptoms or other evidence, medical therapy to reduce spasm is also coronary spasm started.
Cardiac arrest in a patient with coronary artery disease is usually managed with standard revascularisation approaches. Coronary spasm therapy is not considered unless symptoms or signs previously or subsequently indicate that this is a possibility. According to international guidelines, an ICD is generally not implanted in this setting [31].
-
(5)
Asymptomatic coronary spasm.
Occasionally, a diagnosis of spasm may be considered if ECG criteria are identified incidentally during Holter or prolonged ECG monitoring despite a lack of reported symptoms. It is worth noting that in such patients, depending on the site, duration and the severity of the spasm, the first symptomatic manifestation may be a life threatening event (myocardial infarction, cardiac arrest, syncope).
4.2. Invasive diagnosis or non-invasive diagnostic tests?
A diagnosis of coronary spasm is usually made once coronary spasm is suspected from history and investigations have been performed. These tests may be non-invasive or invasive or may not be needed at all. Indeed, as reported in Japanese Circulation Society Guidelines, coronary angiography can be avoided altogether if a patient meets all listed criteria for coronary spasm (Table 1) [13]. In usual clinical practice, with the exception of Japan, myocardial spasm provocation tests are not performed routinely because of the concern of adverse events during drug administration. This is despite studies reporting an acceptable safety profile of acetylcholine and ergonovine intracoronary administration, with provision of useful diagnostic and prognostic information [14]. Moreover, in a recent Korean study, the response to ergonovine stimulation was assessed. Patients with positive response had worse prognosis than patients with intermediate response [32].
5. Management
Pharmacological interventions (Table 3) have the potential to reduce or prevent spasm attacks, symptoms and life-treating arrhythmias. Aside from compliance with treatment, identification and avoidance of precipitants are an essential component of any management plan. In this regard it is essential to advise patients to quit smoking. Despite a wide consensus on the role of nitrates and calcium channel antagonist, in treating patients with coronary spasm, there remain uncertainties about how to best manage these patients, from which agent to use through to when to consider the use of ICD for primary prevention.
Table 3.
Current therapeutic approaches for patients with coronary spasm.
|
5.1. What is the best pharmacological treatment?
Calcium channel antagonists (both dihydropyridine and non-dihydropyridine) and nitrates represent the mainstay of therapy because they reduce, prevent and resolve spasm attacks and thus angina and arrhythmias. These beneficial effects are mediated suppressing Ca2 + inflow into the vascular smooth muscle and through the nitrate metabolisation to NO, both resulting in relaxation of vascular smooth muscle and vasodilation. Prospective double-blind studies with calcium channel antagonists compared with nitrates have reported efficacy for both agents to a similar degree, in reducing spasm occurrence [44]. Some have argued for use of both agents concurrently [13], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. Importantly, the drugs can be used safely, without adverse reactions, and the dosage should be increased up to the maximal tolerated dose. Monotherapy with either drug alone is based on patient choice and circumstance.
In some cases a combination of 2 calcium antagonists (dihydropyridine and non-dihydropyridine) may be required for more severe symptoms, although few data exist for this approach.
Non-selective β-blockers (like propranolol) convert the effects of sympathetic stimulation into a pure a-adrenergic vasoconstrictor response, thus they can exacerbate and prolong vasospasm in these patients [62]. However, since coronary spasm can occur in patients with stable angina as well, concomitant use of calcium channel antagonists and selective β-blockers can be considered in coronary spasm patients with significant coronary artery stenosis, preventing effort related pain, but it should be noted that this can exacerbate spasm recurrence.
Notably, especially when symptoms are under control, medications should not be discontinued. As reported by Takagi et al., discontinuing or reducing medication is associated with symptom recurrence, higher incidence of lethal arrhythmic events and non-fatal myocardial infarction [59], [60], [61]. Drugs should preferably be taken nocturnally because of the reported circadian pattern and risk of events in the early morning.
5.2. The natural history of variant angina
Despite the therapeutic approaches advocated by clinical evidence and endorsed by guidelines, prevention of coronary spasm remains difficult.
In an old survey conducted on 286 patients, the rates of therapeutic success, defined as the resolution of angina attacks, were high (above 85%), and were dependent on the therapy itself [63]. However, the success of coronary spasm treatment should not be constrained to the reduction of symptoms alone but should also aim to prevent the underlying episodes of spasm. Symptoms and spasm may not always correlate and only approximately the 30% of ECG changes consistent with spasm are associated with symptoms, especially when they last for less than 5 min [64]. On the hand, in 35 patients' sample, impaired myocardial fatty acid metabolism was still present after one year of spasm therapy, thought to be related to ongoing persistent silent ischaemic events [65]. Thus, absence of symptoms is not a wholly reliable parameter with which to judge therapeutic efficacy. Persistence of coronary spasm despite optimal therapy exposes patients to potentially life-threatening events (arrhythmias and myocardial infarction) [66].
5.3. What other options exist?
Several studies have evaluated alternative therapies for coronary spasm including nicorandil, statins, aspirin, magnesium, Vitamins C and E, Iloprost, alpha receptor blockade, selective serotonin receptor inhibitors, and selective thromboxane A2 synthetase inhibition. Although considered effective in certain patients, in specific clinical settings, there is a lack of evidence and consensus on their use and further studies are needed to ascertain their effectiveness in daily clinical practice. All of these drugs can be considered if a patient is still symptomatic despite optimal therapy.
5.3.1. Nicorandil
Nicorandil opens ATP-sensitive potassium channels and has a nitrate like effect [67]. As such it would be expected to have beneficial effects on spasm, and indeed Kishida et al. has demonstrated its efficacy when compared with placebo in reducing symptoms and ECG changes in coronary spasm [68]. Lablanche et al., in a comparative study between nifedipine and nicorandil, reported similar efficacy and no significant differences between the two groups for prevention of coronary spasm [69].
5.3.2. Statins
Statins have also been tested in this context. Fluvostatin was evaluated in a randomised open-label trial of 64 patients with coronary spasm and was associated with a significant reduction in coronary reactivity to intracoronary injection of acetylcholine. This effect was postulated to be driven by the pleiotropic effect of statins, specifically due to improvement of endothelial dysfunction and a reduction of calcium sensitivity [70].
5.3.3. Aspirin
Small doses of aspirin have vasodilating properties and platelet-inhibiting effects, mediated by blocking thromboxane A2, which has powerful vasoconstrictor properties; on the other hand large doses inhibit prostacyclin production aggravating coronary vasospasm and may worsen symptoms and spasm although this is not proven [73].
5.3.4. Magnesium
Long-term supplementation with magnesium had also been shown to prevent coronary spasm and it is particularly beneficial in coronary spasm recurrence during effort and after alcohol consumption [74], [75], [76], [77].
Increased activity of the Rho-kinase system causes hypercontraction of vascular smooth muscle. Fasudil, a selective Rho-kinase inhibitor, has been demonstrated to attenuate coronary vasospasm and may have therapeutic potential [78].
5.3.5. Others
Angiotensin receptor blockers are known to abolish the development of nitrate tolerance [71]. Whether this therapy can reduce the frequency of the attacks with or without nitrate use requires further study. Since autonomic dysfunction is also implicated in the development of spasm, balanced therapy with alpha receptor agonists and antagonists could also be tried [72].
6. Refractory variant angina
When vasospastic attacks cannot be prevented, relieved or suppressed by a combination of at least two coronary vasodilators therapy (calcium channel antagonists and nitrates) the patient is considered to have refractory variant angina. According to a Japanese prevalence study, these patients represent 13% of the coronary spasm population and, since spasm attacks are not fully prevented, they should be considered at high risk for myocardial infarction, malignant arrhythmias, syncope, and sudden death [13]. Patients are usually smokers, normotensive and are younger than those with usual coronary spasm.
In refractory variant angina two classes of patients should be considered: the true non-responder, and those who are refractory secondary to poor compliance with therapy or life style advice (i.e. smoking cessation, alcohol consumption). True non-responders can be further split into those who are symptomatic and those who are asymptomatic.
In both groups efforts still need to be made to decide the most appropriate therapeutic approach and clinical management, and asymptomatic patients are even more difficult to monitor throughout the time and evaluate the therapy efficacy. Once standard management has been attempted other less commonly used drugs can also be tried, such as those listed earlier [66]. Failing these, other non-drug options include the following:
Brachytherapy: at the sites of spasm has been developed as an option for refractory coronary spasm, although it has to be weighed carefully because it exposes the patient to a significant risk of thrombosis in the site of radiation. Immediately after the procedure, a reflex vasoconstriction rise has been documented, which progressively reduces, until there is a complete loss of vasomotion and spasm. Some evidence suggests that it is beneficial, with a study of 18 patients experiencing fewer spasm attacks, who took Clopidogrel for one year followed by aspirin for life to prevent thrombosis [79], [80], [81].
Coronary stenting: In patients with refractory coronary spasm, coronary stenting can be considered as an additional treatment in carefully selected and clinically unstable patients. The long terms results are unpredictable, and further studies are still needed. It has been performed in patients without significant stenosis at the spasm site and in patients with concomitant atherosclerotic lesions as well. In the latter, stent implantation can improve angina of effort but spasm often occurs in different segments of the treated artery, probably due to the diffuse nature of the disease. For this reason, concomitant drug therapy should be maintained. Stent implantation is associated with risk of in-stent thrombosis and restenosis [82], [83], [84], although interestingly, despite their benefit compared with bare metal stents, drug-eluting stents adopted in coronary spasm, have a negative effect because they induce endothelial dysfunction, which leads to a paradoxical coronary vasoconstriction of the adjacent vessel segments [85], [86].
Surgery: Coronary artery bypass surgery for coronary spasm patients without obstructive coronary artery disease has been associated with high morbidity, mortality and recurrence of angina and is not an advocated treatment option. However, in rare cases with medically intractable or even life-threatening variant angina surgical intervention can be considered. Historical case reports of bypass grafting of the spasmodic artery associated with a plexectomy suggest that this may be efficacious for patients with focal spasm. Sympathetic denervation has also been justified by the adrenergic role in the development of the vasoconstriction [87], [88], [89].
7. Risk stratification and ICD therapy
Severe myocardial ischaemia caused by coronary spasm can lead to life threatening ventricular arrhythmias. In addition patients with coronary spasm have increased ventricular irritability and prolonged QT dispersion, compared with controls, particularly during symptom-free periods [90], [91], [92]. For these reasons, coronary spam patients are considered at high risk for malignant arrhythmias and ICD implantation has been advocated in this group.
Coronary spasm patients resuscitated from a cardiac arrest have a poorer prognosis, from further arrhythmias and death when compared with coronary spasm patients with other presentations [59]. In addition a recent study by Matsue et al. retrospectively evaluated records of patients with coronary spasm receiving a device for secondary prevention, and demonstrated a high ICD therapy rate for ventricular arrhythmias [93]. However, even in the absence of this data, current guidelines permit and recommend ICD implantation for anyone with failed sudden death and normal coronary arteries on angiography.
In contrast, ICD implantation for primary prevention of sudden cardiac death (SCD) remains an area of uncertainty. This is primarily so as risk stratification is poorly established or understood for coronary spasm, although several features have been proposed to identify those at greatest risk of sudden death:
Symptoms: Mesiel et al. demonstrated that coronary spasm patients manifesting a run of a dangerous arrhythmia, prior to their event, had persistence of symptoms despite medical treatment. They postulated that ongoing symptoms on medical therapy should be regarded as a marker of inadequate treatment, and thus such individuals should be considered for ICD [94]. Unfortunately symptoms such as chest pain are not reliable parameters to guide risk, especially as episodes may be short, require several minutes before manifesting and 70–80% of individuals are asymptomatic.
Risk behaviour: Several studies have demonstrated higher recurrences of symptoms and sudden death in patients with coronary spasm who smoke, use illicit substances and/or discontinued medications [13], [34], [37], [59], [61], [92], [94].
Provocation response: Patients with a positive provocation test (ergonovine, acetylcholine) seem to have a worse prognosis when compared with patients with an intermediate response [32]. Moreover, a provocation test identifying multi-vessel spasm is associated with more extensive myocardial ischaemia and has been associated with an adverse prognosis [14].
In summary, there are currently no accepted criteria for recommending an ICD for primary prevention of SCD in patients with coronary spasm. Those with multi-vessel ischaemia on provocation testing may derive benefit but this requires further trial evidence before it can be advocated in guidelines. Decisions are currently made on an individual patient basis, determined by local practice and consensus.
8. Conclusion
Coronary spasm should be considered as resulting from extensive vasomotor and endothelial dysfunction of the coronary circulation. It remains highly challenging to diagnose, but has important symptomatic and prognostic implications. While medications are available and mostly effective for symptom control, suppression of spasm remains a problem. Multiple alternative therapies have been proposed and tested, but currently there is little consensus on their use. The most important prognostic therapy, ICD implantation is expensive and better risk stratification tools are urgently needed to guide their use for primary prevention of SCD.
Funding
RSP is funded by the British Heart Foundation.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Footnotes
The authors take full responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Prinzmetal M., Kennamer R., Merliss R. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am. J. Med. 1959;27:375. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- 2.Hillis L.D., Braunwald E. Coronary artery spasm. N. Engl. J. Med. 1978;299:695–702. doi: 10.1056/NEJM197809282991305. [DOI] [PubMed] [Google Scholar]
- 3.Maseri A., Beltrame J.F., Shimokawa H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ. J. 2009;73:394–403. doi: 10.1253/circj.cj-09-0033. [DOI] [PubMed] [Google Scholar]
- 4.Kusama Y., Kodani E., Nakagomi Variant angina and coronary artery spasm: the clinical spectrum, pathophysiology, and management. J. Nippon Med. Sch. 2011;78:4–12. doi: 10.1272/jnms.78.4. [DOI] [PubMed] [Google Scholar]
- 5.Yasue H., Nakagawa H., Itoh T., Harada E., Mizuno Y. Coronary artery spasm—clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 2008;51:2–17. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Miwa K., Fujita M., Sasayama S. Recent insights into the mechanisms, predisposing factors, and racial differences of coronary vasospasm. Heart Vessel. 2005;20:1–7. doi: 10.1007/s00380-004-0794-4. (Review) [DOI] [PubMed] [Google Scholar]
- 7.Pristipino C., Beltrame J.F., Finocchiaro M.L. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation. 2001;101:1102–1108. doi: 10.1161/01.cir.101.10.1102. [DOI] [PubMed] [Google Scholar]
- 8.Beltrame J.F., Sasayama S., Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J. Am. Coll. Cardiol. 1999;33:1442–1452. doi: 10.1016/s0735-1097(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 9.Sueda S., Kohno H., Fukuda H. Frequency of provoked coronary spasms in patients undergoing coronary arteriography using a spasm provocation test via intracoronary administration of ergonovine. Angiology. 2004;55:403–411. doi: 10.1177/000331970405500407. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand M.E., LaBlanche J.M., Tilmant P.Y. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299–1306. doi: 10.1161/01.cir.65.7.1299. [DOI] [PubMed] [Google Scholar]
- 11.Survivors of out-of-hospital cardiac arrest with apparently normal heart: need for definition and standardized clinical Evaluation. Consensus statement of the Joint Steering Committees of the unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United StatesCirculation. 1997;95:265–272. doi: 10.1161/01.cir.95.1.265. [DOI] [PubMed] [Google Scholar]
- 12.de la Grandmaison G.L. Is there progress in the autopsy diagnosis of sudden unexpected death in adults? Forensic Sci. Int. 2006;156:138–144. doi: 10.1016/j.forsciint.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 13.JCS Joint Working Group Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ. J. 2010;74:1745–1762. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 14.Takagi Y., Yasuda S., Takahashi J., Japanese Coronary Spasm Association Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: Multicentre Registry Study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013;34:258–267. doi: 10.1093/eurheartj/ehs199. [DOI] [PubMed] [Google Scholar]
- 15.Lanza G.A., Careri G., Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 16.Menyar A.A. Drug-induced myocardial infarction secondary to coronary artery spasm in teenagers and young adults. J. Postgrad. Med. 2006;52:51–56. [PubMed] [Google Scholar]
- 17.Wasson S., Jayam V.K. Coronary vasospasm and myocardial infarction induced by oral sumatriptan. Clin. Neuropharmacol. 2004;27:198–200. doi: 10.1097/01.wnf.0000136889.78887.6f. [DOI] [PubMed] [Google Scholar]
- 18.Morrow J.D., Frai B., Longmire A.W. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez D., Rosenthal J.E., Cohen L.S., Hammond G., Wolfson S. Alcohol-induced Prinzmetal variant angina. Am. J. Cardiol. 1973;32:238–239. doi: 10.1016/s0002-9149(73)80128-6. (El) [DOI] [PubMed] [Google Scholar]
- 20.Bathina J.D., Yusuf S.W. 5-Fluorouracil-induced coronary vasospasm. J. Cardiovasc. Med. 2010;11:281–284. doi: 10.2459/JCM.0b013e32832e934b. [DOI] [PubMed] [Google Scholar]
- 21.Sestito A., Sgueglia G.A., Pozzo C. Coronary artery spasm induced by capecitabine. J. Cardiovasc. Med. 2006;7:136–138. doi: 10.2459/01.JCM.0000199785.94760.50. [DOI] [PubMed] [Google Scholar]
- 22.Kaski J.C., Tousoulis D., Gavrielides S. Comparison of epicardial coronary artery tone and reactivity in Prinzmetal's variant angina and chronic stable angina pectoris. J. Am. Coll. Cardiol. 1991;17:1058–1062. doi: 10.1016/0735-1097(91)90830-3. [DOI] [PubMed] [Google Scholar]
- 23.Yasue H., Takizawa A., Nagao M. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. doi: 10.1161/01.cir.78.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H., Kawai S., Aizawa T. Histological evaluation of coronary plaque in patients with variant angina: relationship between vasospasm and neointimal hyperplasia in primary coronary lesions. J. Am. Coll. Cardiol. 1999;33:198–205. doi: 10.1016/s0735-1097(98)00520-8. [DOI] [PubMed] [Google Scholar]
- 25.Kaski J.C., Tousoulis D., McFadden E., Crea F., Pereira W.I., Maseri A. Variant angina pectoris. Role of coronary spasm in the development of fixed coronary obstructions. Circulation. 1992;85(2):619–626. doi: 10.1161/01.cir.85.2.619. [DOI] [PubMed] [Google Scholar]
- 26.Crea F., Chierchia S., Kaski J.C. Provocation of CAS by dopamine in patients with active variant angina pectoris. Circulation. 1986;74:262–269. doi: 10.1161/01.cir.74.2.262. [DOI] [PubMed] [Google Scholar]
- 27.McFadden E.P., Clarke J.G., Davies G.J., Kaski J.C., Haider A.W., Maseri A. Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. N. Engl. J. Med. 1991;324:648–654. doi: 10.1056/NEJM199103073241002. [DOI] [PubMed] [Google Scholar]
- 28.Kaski J.C., Maseri A., Vejar M., Crea F., Hackett D. Spontaneous coronary artery spasm in variant angina results from a local hyperreactivity to a generalized constrictor stimulus. J. Am. Coll. Cardiol. 1989;14:1456. doi: 10.1016/0735-1097(89)90382-3. [DOI] [PubMed] [Google Scholar]
- 29.Cox I.D., Kaski J.C., Clague J.R. Endothelial dysfunction in the absence of coronary atheroma causing Prinzmetal's angina. Heart. Jun 1997;77(6):584. doi: 10.1136/hrt.77.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaski J.C., Crea F., Meran D. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. Dec 1986;74(6):1255–1265. doi: 10.1161/01.cir.74.6.1255. [DOI] [PubMed] [Google Scholar]
- 31.Epstein A.E., Di Marco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) J. Am. Coll. Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 32.Shin D.I., Baek S.H., Her S.H. The 24-month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc. Interv. Jun 2015;8(7):914–923. doi: 10.1016/j.jcin.2014.12.249. [DOI] [PubMed] [Google Scholar]
- 33.Walling A., Waters D.D., Miller D.D., Roy D., Pelletier G.B., Théroux P. Long-term prognosis of patients with variant angina. Circulation. 1987;76:990–997. doi: 10.1161/01.cir.76.5.990. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M., Takeshita A., Nose Y. Clinical characteristics associated with myocardial infarction, arrhythmias, and sudden death in patients with vasospastic angina. Circulation. 1987;75:1110–1116. doi: 10.1161/01.cir.75.6.1110. [DOI] [PubMed] [Google Scholar]
- 35.Bory M., Pierron F., Panagides D., Bonnet J.L., Yvorra S., Desfossez L. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur. Heart J. 1996;17:1015–1021. doi: 10.1093/oxfordjournals.eurheartj.a014996. [DOI] [PubMed] [Google Scholar]
- 36.Scholl J.M., Veau P., Benacerraf A., Brau J., Hennetier G., Achard F. Long-term prognosis of medically treated patients with vasospastic angina and no fixed significant coronary atherosclerosis. Am. Heart J. 1988;115:559–564. doi: 10.1016/0002-8703(88)90804-6. [DOI] [PubMed] [Google Scholar]
- 37.Lanza G.A., Sestito A., Sgueglia G.A. Current clinical features, diagnostic assessment and prognostic determinants of patients with variant angina. Int. J. Cardiol. 2007;118:41–47. doi: 10.1016/j.ijcard.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi T., Yokoyama M., Namikawa S. Location of focal vasospasm provoked by ergonovine maleate within coronary arteries in patients with vasospastic angina pectoris. Am. J. Cardiol. 2006;97:1322–1325. doi: 10.1016/j.amjcard.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Heupler F.A., Jr. Syndrome of symptomatic coronary arterial spasm with nearly normal coronary arteriograms. Am. J. Cardiol. 1980;45:873–881. doi: 10.1016/0002-9149(80)90134-4. [DOI] [PubMed] [Google Scholar]
- 40.Yamagishi M., Miyatake K., Tamai J., Nakatani S., Koyama J., Nissen S.E. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J. Am. Coll. Cardiol. 1994;23:352–357. doi: 10.1016/0735-1097(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 41.Beltrame J.F., Crea F., Kaski J.C., Ogawa H., Ong P., Sechtem U., Shimokawa H., Bairey Merz C.N. Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2015 doi: 10.1093/eurheartj/ehv351. (Aug 4) [DOI] [PubMed] [Google Scholar]
- 42.Sueda S., Kohno H., Fukuda H. Limitations of medical therapy in patients with pure coronary spastic angina. Chest. 2003;123:380–386. doi: 10.1378/chest.123.2.380. [DOI] [PubMed] [Google Scholar]
- 43.Pupita G., Maseri A., Kaski J.C. Myocardial ischemia caused by distal coronary-artery constriction in stable angina pectoris. N. Engl. J. Med. 1990;323:514–520. doi: 10.1056/NEJM199008233230804. [DOI] [PubMed] [Google Scholar]
- 44.Hung M.J., Cherng W.J., Cheng C.W., Yang N.I. Effect of antispastic agents (calcium antagonists and/or isosorbide dinitrate) on high-sensitivity C-reactive protein in patients with coronary vasospastic angina pectoris and no hemodynamically significant coronary artery disease. Am. J. Cardiol. 2005;95:84–87. doi: 10.1016/j.amjcard.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 45.Beller G.A. Calcium antagonists in the treatment of Prinzmetal's angina and unstable angina pectoris. Circulation. 1989;80:IV78–IV87. Review. [PubMed] [Google Scholar]
- 46.Watanabe K., Izumi T., Miyakita Y. Efficacy of amlodipine besilate therapy for variant angina: evaluation by 24-hour Holter monitoring. Cardiovasc. Drugs Ther. 1993;7:923–928. doi: 10.1007/BF00877728. [DOI] [PubMed] [Google Scholar]
- 47.Ardissino D., Savonitto S., Mussini A. Felodipine (once daily) versus nifedipine (four times daily) for Prinzmetal's angina pectoris. Am. J. Cardiol. 1991;68:1587–1592. doi: 10.1016/0002-9149(91)90314-b. [DOI] [PubMed] [Google Scholar]
- 48.Winniford M.D., Gabliani G., Johnson S.M., Mauritson D.R., Fulton K.L., Hillis L.D. Concomitant calcium antagonist plus isosorbide dinitrate therapy for markedly active variant angina. Am. Heart J. 1984;108:1269–1273. doi: 10.1016/0002-8703(84)90752-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal S.J., Lamb I.H., Schroeder J.S., Ginsburg R. Long-term efficacy of diltiazem for control of symptoms of coronary artery spasm. Circ. Res. 1983;52:I153–I157. [PubMed] [Google Scholar]
- 50.Winniford M.D., Johnson S.M., Mauritson D.R. Verapamil therapy for Prinzmetal's variant angina: comparison with placebo and nifedipine. Am. J. Cardiol. 1982;50:913–918. doi: 10.1016/0002-9149(82)91253-x. [DOI] [PubMed] [Google Scholar]
- 51.Rosenthal S.J., Ginsburg R., Lamb I.H., Baim D.S., Schroeder J.S. Efficacy of diltiazem for control of symptoms of coronary arterial spasm. Am. J. Cardiol. 1980;46:1027–1032. doi: 10.1016/0002-9149(80)90362-8. [DOI] [PubMed] [Google Scholar]
- 52.Conti C.R., Hill J.A., Feldman R.L., Conti J.B., Pepine C.J. Isosorbide dinitrate and nifedipine in variant angina pectoris. Am. Heart J. 1985;110:251–256. doi: 10.1016/0002-8703(85)90495-8. Review. [DOI] [PubMed] [Google Scholar]
- 53.Paolillo V., Marra S., Aquaro G. Sodium nitroprusside in the treatment of Prinzmetal's variant angina. Chest. 1980;77:807–810. doi: 10.1378/chest.77.6.807. [DOI] [PubMed] [Google Scholar]
- 54.Lombardi M., Morales M.A., Michelassi C., Moscarelli E., Distante A., L'Abbate A. Efficacy of isosorbide-5-mononitrate versus nifedipine in preventing spontaneous and ergonovine-induced myocardial ischaemia. A double-blind, placebo-controlled study. Eur. Heart J. 1993;14:845–851. doi: 10.1093/eurheartj/14.6.845. [DOI] [PubMed] [Google Scholar]
- 55.Hoshio A., Shirota K., Sawada Y. Development of nitrate tolerance and usefulness of pulse therapy in a patient with vasospastic angina. Eur. Heart J. Jun 1992;13(6):853–855. doi: 10.1093/oxfordjournals.eurheartj.a060270. [DOI] [PubMed] [Google Scholar]
- 56.Aschermann M., Bultas J., Karetová D., Kölbel F., Kozáková M., Simper D. Randomized double-blind comparison of isosorbide dinitrate and nifedipine in variant angina pectoris. Am. J. Cardiol. 1990;65:46J–49J. doi: 10.1016/0002-9149(90)91312-t. [DOI] [PubMed] [Google Scholar]
- 57.Stazi F., Meloni C., Ballarotto C. An uncommon case of variant angina. G. Ital. Cardiol. 1999;29(10):1208–1211. [PubMed] [Google Scholar]
- 58.Ginsburg R., Lamb I.H., Schroeder J.S., Hu M., Harrison D.C. Randomized double-blind comparison of nifedipine and isosorbide dinitrate therapy in variant angina pectoris due to coronary artery spasm. Am. Heart J. 1982;103:44–49. doi: 10.1016/0002-8703(82)90527-0. [DOI] [PubMed] [Google Scholar]
- 59.Takagi Y., Yasuda S., Tsunoda R., Japanese Coronary Spasm Association Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ. Arrhythm. Electrophysiol. 2011;4:295–302. doi: 10.1161/CIRCEP.110.959809. [DOI] [PubMed] [Google Scholar]
- 60.Myerburg R.J., Kessler K.M., Mallon S.M. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary-artery spasm. N. Engl. J. Med. 1992;326:1451–1455. doi: 10.1056/NEJM199205283262202. [DOI] [PubMed] [Google Scholar]
- 61.Lette J., Gagnon R.M., Lemire J.G., Morissette M. Rebound of vasospastic angina after cessation of long-term treatment with nifedipine. Can. Med. Assoc. J. 1984;130:1169–1171. [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson R.M., Wood A.J., Vaughn W.K., Robertson D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation. 1982;65:281–285. doi: 10.1161/01.cir.65.2.281. [DOI] [PubMed] [Google Scholar]
- 63.Kimura E., Kishida H. Treatment of variant angina with drugs: a survey of 11 cardiology institutes in Japan. Circulation. 1981;63:844–848. doi: 10.1161/01.cir.63.4.844. [DOI] [PubMed] [Google Scholar]
- 64.Yasue H., Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern. Med. 1997;36:760–765. doi: 10.2169/internalmedicine.36.760. [DOI] [PubMed] [Google Scholar]
- 65.Sueda S., Oshita A., Izoe Y. A long-acting calcium antagonist over one year did not improve BMIPP myocardial scintigraphic imagings in patients with pure coronary spastic angina. Ann. Nucl. Med. 2007;21:85–92. doi: 10.1007/BF03033985. [DOI] [PubMed] [Google Scholar]
- 66.Kurata C., Shimane A. Intractable vasospastic angina. Heart. 1997;78:93–94. doi: 10.1136/hrt.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaski J.C. Management of vasospastic angina—role of nicorandil. Cardiovasc. Drugs Ther. 1995;9:221–227. doi: 10.1007/BF00878469. [DOI] [PubMed] [Google Scholar]
- 68.Kishida H., Murao S. Effect of a new coronary vasodilator, nicorandil, on variant angina pectoris. Clin. Pharmacol. Ther. 1987;42:166–174. doi: 10.1038/clpt.1987.127. [DOI] [PubMed] [Google Scholar]
- 69.Lablanche J.M., Bauters C., Leroy F., Bertrand M.E. Prevention of coronary spasm by nicorandil: comparison with nifedipine. J. Cardiovasc. Pharmacol. 1992;20:S82–S85. doi: 10.1097/00005344-199206203-00014. [DOI] [PubMed] [Google Scholar]
- 70.Yasue H., Mizuno Y., Harada E. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J. Am. Coll. Cardiol. 2008;51:1742–1748. doi: 10.1016/j.jacc.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 71.Münzel T., Daiber A., Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ. Res. Sep 30 2005;97(7):618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 72.Chierchia S. The role of alpha-adrenergic receptors in the pathogenesis of coronary spasm. Clin. Cardiol. Oct 1983;6(10):496–500. doi: 10.1002/clc.4960061005. [DOI] [PubMed] [Google Scholar]
- 73.Miwa K., Kambara H., Kawai C. Effect of aspirin in large doses on attacks of variant angina. Am. Heart J. 1983;105:351–355. doi: 10.1016/0002-8703(83)90548-3. [DOI] [PubMed] [Google Scholar]
- 74.Teragawa H., Kato M., Yamagata T., Matsuura H., Kajiyama G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest. 2000;118:1690–1695. doi: 10.1378/chest.118.6.1690. [DOI] [PubMed] [Google Scholar]
- 75.Turlapaty P.D., Altura B.M. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 76.Miwa K., Igawa A., Miyagi Y., Fujita M. Importance of magnesium deficiency in alcohol-induced variant angina. Am. J. Cardiol. 1994;73:813–816. doi: 10.1016/0002-9149(94)90886-9. [DOI] [PubMed] [Google Scholar]
- 77.Kugiyama K., Yasue H., Okumura K. Suppression of exercise-induced angina by magnesium sulfate in patients with variant angina. J. Am. Coll. Cardiol. 1988;12:1177–1183. doi: 10.1016/0735-1097(88)92597-1. [DOI] [PubMed] [Google Scholar]
- 78.Masumoto A., Mohri M., Shimokawa H., Urakami L., Usui M., Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. Apr 2 2002;105(13):1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 79.Erne P., Jamshidi P., Juelke P., Hafner H.P., Thum P., Resink T. Brachytherapy: potential therapy for refractory coronary spasm. J. Am. Coll. Cardiol. 2004;44:1415–1419. doi: 10.1016/j.jacc.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 80.Basso C., Thiene G., Durrelman S., Erne P. Late coronary thrombosis following brachytherapy for vasospastic angina. Cardiovasc. Pathol. 2008;17:244–245. doi: 10.1016/j.carpath.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Chatterjee T., Juelke P.D., Thum P., Erne P. Successful brachytherapy of coronary vasospasm. Heart. 2003;89:e25. doi: 10.1136/heart.89.9.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanabe Y., Itoh E., Suzuki K., Ito M., Hosaka Y., Nakagawa I., Kumakura M. Limited role of coronary angioplasty and stenting in coronary spastic angina with organic stenosis. J. Am. Coll. Cardiol. 2002;39:1120–1126. doi: 10.1016/s0735-1097(02)01746-1. [DOI] [PubMed] [Google Scholar]
- 83.Gupta S., Schiele F., Vuillemenot A., Appfel F., Bassand J.P. Coronary stent for variant angina: atypical presentation. Catheter. Cardiovasc. Diagn. 1998;45:439–441. doi: 10.1002/(sici)1097-0304(199812)45:4<439::aid-ccd21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 84.Martí V., Ligero C., García J., Kastanis P., Guindo J., Domínguez de Rozas J.M. Stent implantation in variant angina refractory to medical treatment. Clin. Cardiol. 2006;29:530–533. doi: 10.1002/clc.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abe M., Yoshida A., Otsuka Y. Intractable Prinzmetal's angina three months after implantation of sirolimus-eluting stent. J. Invasive Cardiol. 2008;20:E306–E309. [PubMed] [Google Scholar]
- 86.Togni M., Windecker S., Cocchia R. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 2005;46:231–236. doi: 10.1016/j.jacc.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 87.Ono T., Ohashi T., Asakura T., Shin T. Internal mammary revascularization in patients with variant angina and normal coronary arteries. Interact. Cardiovasc. Thorac. Surg. 2005;4:426–428. doi: 10.1510/icvts.2005.107128. [DOI] [PubMed] [Google Scholar]
- 88.DiPaolo C., Kerin N.Z., Rubenfire M., Levine F. Surgical treatment of medically refractory variant angina pectoris: segmental coronary resection with aortocoronary bypass and plexectomy. Am. J. Cardiol. 1985;56:792–793. doi: 10.1016/0002-9149(85)91139-7. [DOI] [PubMed] [Google Scholar]
- 89.Sen R.C., Hitter E., Ranquin R., Cauwelaert V., Lieber S., Van den Branden F. Surgical coronary angioplasty for left main vasospasm. Am. Heart J. 1995;129:399–400. doi: 10.1016/0002-8703(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki M., Nishizaki M., Arita M. Increased QT dispersion in patients with vasospastic angina. Circulation. 1998;98:435–440. doi: 10.1161/01.cir.98.5.435. [DOI] [PubMed] [Google Scholar]
- 91.Nishizaki M., Arita M., Sakurada H. Induction of polymorphic ventricular tachycardia by programmed ventricular stimulation in vasospastic angina pectoris. Am. J. Cardiol. 1996;77:355–360. doi: 10.1016/s0002-9149(97)89363-0. [DOI] [PubMed] [Google Scholar]
- 92.Matsue Y., Suzuki M., Nishizaki M., Hojo R., Hashimoto Y., Sakurada H. Clinical implications of an implantable cardioverter-defibrillator in patients with vasospastic angina and lethal ventricular arrhythmia. J. Am. Coll. Cardiol. 2012;60:908–913. doi: 10.1016/j.jacc.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 93.Meisel S.R., Mazur A., Chetboun I. Usefulness of implantable cardioverter-defibrillators in refractory variant angina pectoris complicated by ventricular fibrillation in patients with angiographically normal coronary arteries. Am. J. Cardiol. 2002;89:1114–1116. doi: 10.1016/s0002-9149(02)02283-x. [DOI] [PubMed] [Google Scholar]
- 94.Miwa K., Fujita M., Miyagi Y. Beneficial effects of smoking cessation on the short-term prognosis for variant angina–validation of the smoking status by urinary cotinine measurements. Int. J. Cardiol. 1994;44:151–156. doi: 10.1016/0167-5273(94)90019-1. [DOI] [PubMed] [Google Scholar]