Abstract
Japanese women now account for 43 percent of the labor force. A number of them are involved in construction, agricultural and forestry jobs. The aim of this study was to establish a non-invasive technique for the evaluation of peripheral circulatory functions in women with Raynaud’s phenomenon (RP) and introduce a specific method for the assessment of vascular disturbances in females exposed to hand-transmitted vibration. The subjects of this study were 10 women with primary RP, 7 women with progressive systemic sclerosis (PSS) secondary to RP, and 17 females who were included as the control group. The evaluation of peripheral circulatory functions in all subjects was based on the values of finger blood flow (FBF) and finger skin temperature (FST) measured before, during and following a 5-min recovery period after the hand was immersed in cold water (5°C, 1 min). The measured values of FBF and FST of the primary RP group before and after the immersion test were significantly (p<0.01) lower compared to those of the control group. The technique applied in this study could be used as a non-invasive and tolerable technique to determine the digital circulatory functions in female subjects with RP.
Keywords: Raynaud’s phenomenon, Health examination, Finger blood flow, Finger skin temperature, Cold immersion test, Recovery
Introduction
The strategies for occupational health research plans in Japan in the 21 century aim to effectively and systematically address occupational health problems at work for both male and female working population. It has been reported1) that the number of Japanese female workers increased from about 15.5 million in 1985 to about 24.4 million in 2014. Women now account for 43 percent of the labor force, up from 36 percent three decades ago. Contradictory to this fact, most of the occupational health research plans have predominantly been focused on men. For instance, a large number of investigations on occupational health hazards due to hand-transmitted vibration have been conducted in male workers. Consequently, for both male and female workers, the same protocol and evaluation methods have been used during the annual health examination and/or screening tests. It is noteworthy that, the same physical load may exert greater strain on the average women than on the average men since women’s average lifting strength is only 50% of men’s. However, the difference for pushing and pulling in the horizontal plane is smaller, and there is considerable overlap in size, shape, and strength between the sexes. Both the differences and the degree of overlap are important to minimize repetitive strain injuries especially in female workers.
Before addressing the main hypothesis for this study, the health problems related to hand-arm vibration exposure should be pointed out. Occupational exposure to hand-transmitted vibration of powered hand-held equipment such as grinding tools can give rise to a complex of peripheral neurological, vascular, and musculoskeletal symptoms, called hand-arm vibration syndrome2, 3). The most prominent symptom in workers exposed to hand-arm vibration is periodic ischemic attacks affecting the fingers, known as vibration-induced white finger or Raynaud’s phenomenon (RP) of occupational origin which is a secondary form of RP.
Finger blanching is characterized by recurrent episodes of digital vasospasm triggered by exposure to physical, chemical or emotional stress4). There are two main types of RP—primary and secondary. In primary RP (also called Raynaud’s disease), the cause isn’t known. Primary RP is more common and tends to be less severe than secondary RP. Secondary RP is caused by an underlying disease, condition, or other factors. Secondary RP is a designation usually used in the context of vasospasm associated with another illness, most commonly an autoimmune disease5). Although RP has been described with various autoimmune diseases, the most common association is with progressive systemic sclerosis (PSS) and mixed connective-tissue disease. In subjects suffering from RP, vasospasm of the arteries reduces blood flow to the fingers. As a result, the skin may turn white and then blue for a short time. As blood flow returns, the affected areas may turn red and throb, tingle, or feel numb.
Many objective tests have been developed to detect circulatory impairment in the fingers of RP subjects. Measurement of finger skin temperature (FST) in combination with local cooling of the hand in order to stimulate vasoconstriction is most often used in the surveys of RP. In the last few years, measurement of finger systolic pressure has gained increasing importance as it seems to be of high diagnostic value6, 7). Recently, a new noninvasive technique—laser-Doppler blood flowmetry (LDBF)—has been proposed for the measurement of finger blood flow (FBF) to surface tissues8, 9). Cutaneous LDBF enables monitoring of changes in skin perfusion by quantifying the phase shift of laser light induced by moving red blood cells under a fiber optic probe. It thus can identify the presence of and response to a vasoconstrictive stimulus such as cold water immersion test. It has also been suggested that LDBF could measure changes in FBF through the vessels at different depths by changing the distance between the exciting and receiving by optic fiber probes10, 11, 12). Studies on the finger capillary together with the deeply located vessels (mostly arteriovenous shunts) have been conducted in healthy subjects11) and in subjects suffering from primary RP13).
The present study was undertaken to evaluate the peripheral circulatory functions of subjects with primary or secondary RP, in comparison to a group of symptom-free subjects (control group) by using the simultaneous measurements of FST and deep FBF before, during and for a period after a cold provocation test. The underlying concept for the simultaneous measurements of FST and FBF is that surface temperature at the fingertip is essentially depending on circulation and therefore is able to reflect the volume of peripheral blood flow in an indirect but reliable manner14).
It is noteworthy that primary RP has been reported at a higher rate in females than in males of Japanese general population15, 16, 17). On the basis of this documented issue and in order to have more subjects for the present investigation, female subjects whose age was in the range of 25 to 50 yr were included in this study. It was speculated that the results of this study could be utilized to apply a specific test for the evaluation of peripheral circulatory functions in female workers who are at the risk of developing RP.
Subjects and Methods
The subjects of this study were all females living in a city located in the Central Japan. Primary RP subjects (group A) were selected based on the criteria proposed by LeRoy and Medsger18). These subjects were 10 females whose age was between 25 to 38 yr. Six subjects in group A were office workers and 4 of them were sale representatives. They were asked to fill out a medical questionnaire and then were interviewed regarding the family history of primary RP, the number and the onset, as well as the exact circumstances that they had finger blanching. The first step to include a subject for further investigations was if she answered “yes” to the following questions:
-
1.
Are you more sensitive to the cold than others that you know?
-
2.
Do you notice color changes on the skin of your fingers when you are exposed to the cold?
-
3.
Are the skin color changes white and/or blue?
The subjects in the second group (group B) were 7 female patients suffering from progressive systemic sclerosis (PSS patients) secondary to RP. They were engaged in jobs related to general office working activities, and two of them left the job about 2 yr prior to the time of this study. In this group, age ranged from 35 to 50 yr, and the disease duration was from 5 to 21 yr. In all of them, the existence of RP was confirmed by reviewing their medical history and personal interview.
A group of 17 out of 28 female office workers who had no signs or symptoms related to RP were included in this study and were considered as the control group (group C). The subjects in this group were requested to fill out the same medical questionnaire as those in group A and B. The age range of this group was from 30 to 50 yr. All subjects were given sufficient information on the purpose of the study and they agreed to participate in the entire study procedure. The study was approved by the Ethical Committees of both Gifu Pharmaceutical University and Gifu University School of Medicine.
All subjects in the three groups were non-smokers, they were from the same socioeconomic status, and had no occupational exposure to hand-arm vibration. Table 1 shows characteristics of the three groups.
Table 1. Characteristics of study population. Values are given as the mean and standard deviation.
| Variables | Number of subjects |
p value (one way ANOVA) |
||
|---|---|---|---|---|
| Primary RP group (group A) n=10 |
PSS group (group B) n=7 |
Control group (group C) n=17 |
||
| Age (yr) | 32.8 ± 4.3 | 39.3 ± 6.4 | 37.1 ± 5.7 | F (2, 31)=3.3 p=0.052 |
| RP duration (yr) | 8.5 ± 4.3 | 10.6 ± 5.2 | — |
p>0.05* (group A vs. group B) |
| Height (cm) | 158 ± 4.6 | 158.7 ± 5.1 | 154.9 ± 5.2 | F (2, 31)=1.98 p=0.15 |
| Weight (kg) | 52.7 ± 7.1 | 53.3 ± 7.0 | 53.6 ± 6.9 | F (2, 31)=0.05 p=0.9 |
| BMI (kg/m2) | 20.3 ± 2.1 | 21.4 ± 2.7 | 22.2 ± 2.5 | F (2, 31)=1.9 p=0.16 |
*: The unpaired student’s t-test was used.
Finger skin temperature and finger blood flow
All measurements were made in a quiet air-conditioned room (24 ± 1°C) on lightly clad seated subjects. During the tests, the subjects were not allowed to talk or move. Exercise was avoided for at least 1 h before the tests. For each subject, 30 min was allowed for equilibration to room temperature. The FST was taken by a thermistor (Takara Thermistor D-925) attached to the palmar side of the subject’s most severely affected finger; if all were equally affected then the middle finger was used.
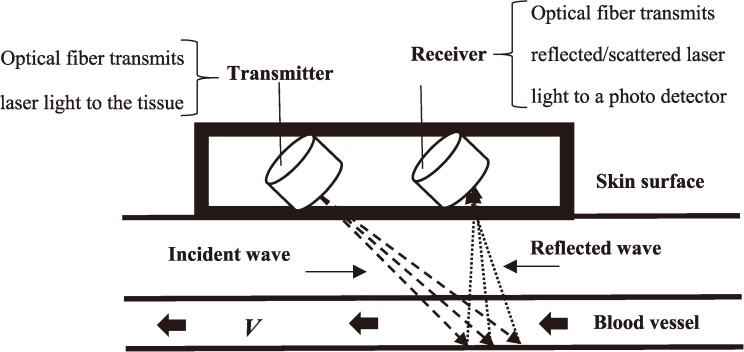
The FBF was measured by the laser Doppler flow-meter, ALF 21 (Advance Co, Tokyo, Japan). The equipment had a low power (2 mW) semiconductor laser source, producing light at 780 nm wavelength. The laser Doppler flowmetry [LDF] is based on dynamic light scattering in tissue. The LDF analyses the frequency spectrum of the light intensity fluctuations observed when laser light is scattered from moving particles (Fig. 1).
Fig. 1.
Block diagram of laser Doppler system.
The probes used here had fiber separation between exciting and receiving fiber of 0.7 mm (LDF-0.7). The maximum sensitivities for LDF-0.7 have been reported to be at depth of 1.2 mm from the tissue surface11). According to Hirata et a1.11) the flow probe applied in this study could mainly measure blood flow through arteriovenous shunts in the human finger. The output signals from the flow-meter were continuously recorded on paper (U-228-2P Pantos, Nippon Denshi Kagaku, Kyoto, Japan). Values of tissue blood flow were expressed in ml blood/min/100 g tissue. The tissue probe of the laser-Doppler flow-meter was lightly attached to the palmar side of the finger (i.e., the same finger which was selected for the measurement of FST) with a circular, open-centered, double-sided adhesive tape of the same diameter. This technique was successfully utilized in our previous research19) in which we detected no inter-influences or adverse effects by attaching the probes of FST and FBF to the same finger. This technique was successfully used in our previous study. AA baseline flow was decided when a steady baseline reading was achieved. Then, the hand was gently immersed in cold water (5°C) and the flows were recorded during a 1-min cold provocation test and in a period of 5-min after immersion.
Statistical methods
The data were entered into a computer for statistical analysis, using Statistical Package for Social Sciences (SPSS, Inc., Release 15). The descriptive statistics were used to determine means, standard deviations (SD) and standard errors of means (SEM). One way analysis of variance (ANOVA) was used to test the null hypothesis of no difference in the mean values of FST and FBF during the experimental time among the three groups. Statements of statistical significance were based on a probability level of 5% (p<0.05). If the p-value was less than 5%, then the null hypothesis was rejected in favor of the alternative hypothesis. The multiple comparison tests were conducted using the Tukey’s honestly significant difference (Tukey’s HSD) with 95% confidence interval of the value (95% CI) to indicate which groups were significantly different from which others. The Pearson correlation coefficient between FST and FBF in the mean data was also computed. The independent samples student’s t-test was used when comparing two mean values.
Sensitivity and specificity
To assess the sensitivity and specificity of the method introduced here, analysis of the first set of the collected data were run blindly by a person who had neither special information on the subjects’ characteristics nor on their medical histories. The recorded data of FST and FBF at the fifth min after the immersion time was used to calculate the cut-off values. According to the method described by Ishitake et al.20), the 5th and 30th percentile values were applied. The cut-off value for FST was set at the 5th percentile (15.7°C), and for the FBF test, the cut-off value was set at the 30th percentile (3.4 ml/min/100 g). The sensitivity and the specificity of FST and FBF expressed the incidence of true positive and true negative of the results, respectively. The positive predictive values were also calculated.
Results
The results of sensitivity and specificity with their 95% confidence intervals (95% CI) of FST and FBF are shown in Table 2. The sensitivity and specificity of FST were 90%, and 86%, respectively. The data analysis revealed that the FBF test had a sensitivity of 77%, while its specificity was 81%. The positive predictive values of FST and FBF calculated as 85%, and 82%, respectively.
Table 2. Sensitivity, specificity and positive predictive values of FST and FBF. Values are given as percentage (%) with 95% confidence intervals (95% CI).
| Test | FST | FBF |
|---|---|---|
| Sensitivity 95% CI |
90 (73–97) |
77 (60–89) |
| Specificity 95% CI |
86 (68–94) |
81 (63 –92) |
| Positive predictive value 95% CI |
85 (68–94) |
82 (65–93) |
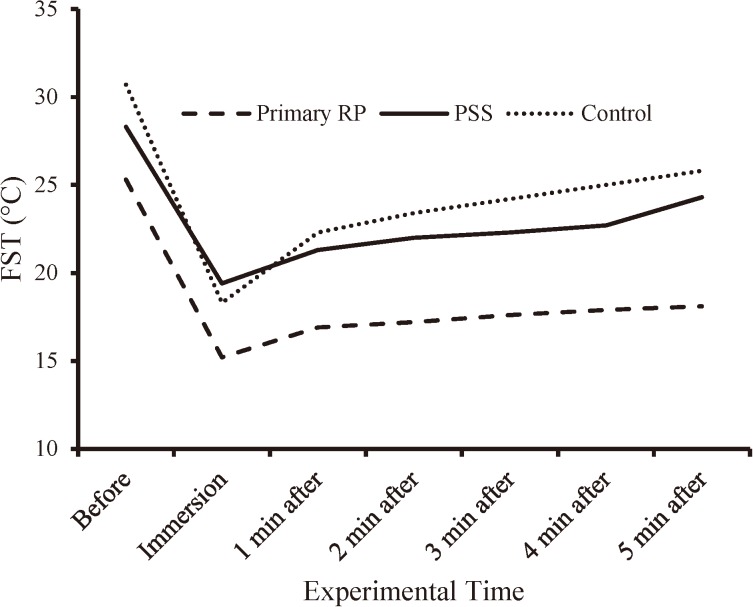
The recovery patterns of FST in the three groups during the experimental procedure are shown in Fig. 2. Although all subjects were given 30 min equilibration to room temperature, surprisingly the initial FST (Mean ± SD) of those in the primary RP group (group A, 25.3 ± 3.5°C) was lower compared with the PSS group (group B, 28.3 ± 4.0°C) and the control group (group C, 30.7 ± 3.6°C), and the results of one-way ANOVA (Table 3) indicated a significant difference among the groups [F (2, 31)=6.9, p=0.003]. By further statistical analysis, the Tukey HSD test revealed that only the primary RP group had significantly lower mean FST compared with the control group (95% CI=1.8–8.9, p=0.002). A decrease of about 10°C in mean FST of the three groups during the 1 min cold immersion test was noticed, and no significant differences could be detected in the correspondence data [F (2, 31=2.4, p=0.1)]. The recovery patterns in the values of FST in the primary RP group and the PSS group were similar over a period of 5-min after the immersion test. The PSS group and the control group tended to have recovery from the second min after the immersion test (Fig. 2), and their FST values recorded about 5–6°C higher compared with the primary RP group. The recovery rate of FST in the three groups at the end of the experiment was 71% in the primary RP group, 84% in the PSS group and 85% in the control group. Statistical analysis (Table 3) showed significant differences (p=0.001) between the primary RP group and the control group for the means of FST from the first to the fifth min after the cold immersion test.
Fig. 2.
The FST (°C) of the three groups before, during and following the cold water immersion test (1 min, 5°C).
Table 3. Finger skin temperature (°C) of the subjects before, during and after the cold immersion test. The values are given as the mean ± SD (standard deviation) for each group. The results of one-way ANOVA and the Tukey HSD post-hoc test are also shown.
| Group | Before immersion |
During immersion |
1 min after immersion |
2 min after immersion | 3 min after immersion |
4 min after immersion | 5 min after immersion |
|---|---|---|---|---|---|---|---|
| Primary RP (group A) n=10 |
25.3 ± 3.5 | 15.2 ± 1.7 | 16.9 ± 1.5 | 17.2 ± 1.3 | 17.6 ± 1.4 | 17.9 ± 1.4 | 18.1 ± 1.4 |
| PSS secondary to RP (group B) n=7 |
28.3 ± 4.0 | 19.4 ± 6.0 | 21.3 ± 3.6 | 22.0 ± 4.4 | 22.3 ± 4.5 | 22.7 ± 4.7 | 24.3 ± 4.3 |
| Control (group C) n=17 |
30.7 ± 3.6 | 18.3 ± 4.5 | 22.3 ± 4.0 | 23.4 ± 4.3 | 24.2 ± 4.8 | 25.0 ± 5.2 | 25.8 ± 5.5 |
| Statistical results | |||||||
| One-way ANOVA | F (2, 31)=6.9 p=0.003 |
F (2, 31)=2.4 p=0.1 |
F (2, 31)=8.2 p=0.001 |
F (2, 31)=8.9 p=0.001 |
F (2, 31)=8.4 p=0.001 |
F (2, 31)=8.4 p=0.001 |
F (2, 31)=9.7 p=0.001 |
| Tukey honestly significant difference (HSD) post-hoc test | group A vs group C, 95% CI=1.8–8.9 p=0.002 |
NS* | group A vs group C, 95% CI=2.1–8.7 p=0.001 |
group A vs group C, 95% CI=2.5–9.8 p=0.001 |
group A vs group C, 95% CI=2.6–10.5 p=0.001 |
group A vs group C, 95% CI=2.8–11.3 p=0.001 |
group A vs group C, 95% CI=3.3–12.0 p=0.001 |
NS*: One-way ANOVA could not detect (p>0.05) any significant differences among the correspondence means.
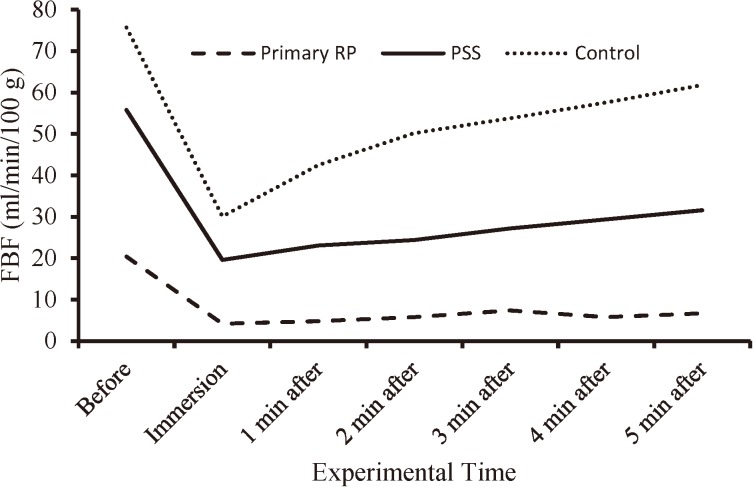
The recorded values of FBF (Mean ± SE) in the three groups are tabulated in Table 4. After the 30 min equilibration time, the mean value of FBF in the primary RP group was recorded at a much lower level (group A, 20.4 ± 3.3 ml/min/100 g) compared to the PSS group (group B, 55.8 ± 15.4 ml/min/100 g) and the control group (group C, 75.7 ± 12.6 ml/min/100 g); and a significant difference among the three groups [F (2, 31)=5.5, p=0.001] could be detected (Table 4). The Tukey HSD test revealed that only the primary RP group had significantly lower mean FBF compared with the control group (95% CI=14.3–96.3, p=0.006). In Fig. 3 the recovery patterns of FBF in the three groups are depicted. During the immersion time and one min after that, the 3 groups showed no significant differences (p≥0.05) in the recorded FBF values. However, from the second to the fifth min after the cold immersion test, the PSS group and the control group gradually had an apparent recovery in their FBF. At the fifth min after the immersion time, the recovery rate of FBF in the primary RP group was lower (32.8%) compared with the PSS group (56.6%) and the control group (81.6%). Statistical analysis (Table 4) showed significant differences (p<0.05) between the primary RP group and the control group for the means of FBF from the second to the fifth min after the cold immersion test. The Tukey HSD could not detect any significant differences in the mean values of FBF between the PSS group and the control group.
Table 4. Finger blood flow (ml/min/100 g) of the subjects before, during and after the cold immersion test. The values are given as the mean ± SEM (standard error of the mean) for each group. The results of one-way ANOVA and the Tukey HSD post-hoc test are also shown.
| Group | Before immersion |
During immersion |
1 min after immersion |
2 min after immersion | 3 min after immersion |
4 min after immersion | 5 min after immersion |
|---|---|---|---|---|---|---|---|
| Primary RP (group A) n=10 |
20.4 ± 3.3 | 4.2 ± 1.2 | 4.8 ± 3.8 | 5.8 ± 1.4 | 7.4 ± 2.0 | 5.8 ± 1.2 | 6.7 ± 2.0 |
| PSS secondary to RP (group B) n=7 |
55.8 ± 15.4 | 19.6 ± 10.5 | 23.1 ± 11.5 | 24.4 ± 13.1 | 27.2 ± 13.4 | 29.4 ± 14.1 | 31.6 ± 14.2 |
| Control (group C) n=17 |
75.7 ± 12.6 | 30.1 ± 7.8 | 42.5 ± 11.7 | 50.2 ± 12.7 | 53.8 ± 12.3 | 57.6 ± 12.2 | 61.8 ± 12.4 |
| Statistical results | |||||||
| One-way ANOVA | F (2, 31)=5.5 p=0.001 |
F (2, 31)=3.1 p=0.06 |
F (2, 31)=3.2 p=0.05 |
F (2, 31)=3.9 p=0.03 |
F (2, 31)=4.4 p=0.02 |
F (2, 31)=5.5 p=0.01 |
F (2, 31)=6.0 p=0.006 |
| Tukey honestly significant difference (HSD) post-hoc test | group A vs group C, 95% CI=14.3–96.3 p=0.006 |
NS* | NS* | group A vs group C, 95% CI=4.5–84.2 p=0.027 |
group A vs group C, 95% CI=7.4–85.4 p=0.01 |
group A vs group C, 95% CI=12.8–90.8 p=0.007 |
group A vs group C, 95% CI=15.4–94.7 p=0.005 |
NS*: One-way ANOVA could not detect (p>0.05) any significant differences among the correspondence means.
Fig. 3.
The FBF (ml/min/100 g) of the three groups before, during and following the cold water immersion test (1 min, 5°C).
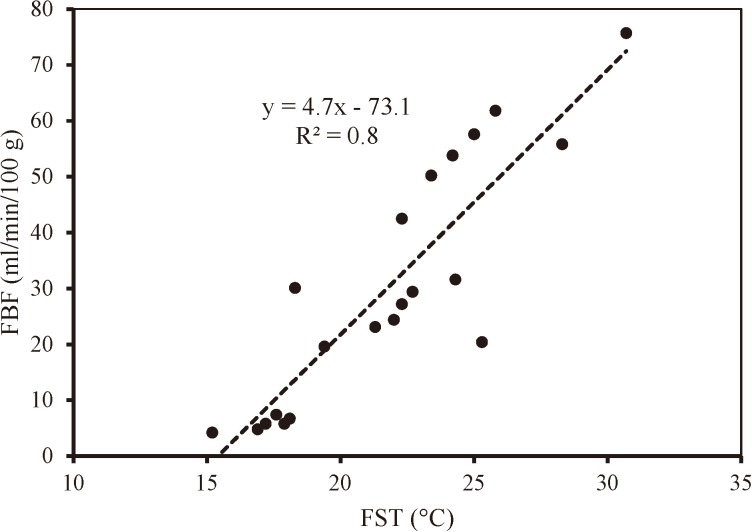
Figure 4 presents the relationship between the mean FST and FBF obtained for the three groups. Coherently with the results presented, FST and FBF values were significantly correlated at p<0.001 (R2=0. 8).
Fig. 4.
Relationship between the FST and FBF of all subjects during the experimental procedure.
Discussion
The aim of this investigation was to introduce a tolerable and simple non-invasive technique in the surveys of RP. In Japan, for the evaluation of peripheral circulatory functions in suspected RP, nail compression on the fingers and FST measurements have generally been used. The FST has been widely performed with different procedures. As for the nail compression test, the disadvantage of variations in results due to the examiner’s skill and experience should be noted. In the present investigation, the simultaneous measurements of FST and FBF were used to evaluate peripheral disturbances in subjects with RP. Intuitively, one question might arise as to why some data on blood pressure of the groups are not shown here. The reason could be explained by the role of blood flow in cold exposure circumstances. When a person is exposed to cold, the body’s normal response is to slow the loss of heat and preserve its core temperature. To maintain this temperature, the blood vessels that control blood flow to the skin surface move blood from arteries near the surface to veins deeper in the body. For people who have either primary or secondary RP, this normal body response is intensified by the sudden spasmodic contractions of the small blood vessels (arterioles) that supply blood to the fingers. As a result, the blood supply to the extremities is greatly decreased, causing a reaction that includes skin discoloration and other changes. Therefore, it was assumed that knowing the exact values of the subjects’ blood pressure could cause unwanted prejudice in data analysis. Thus, we focused on the evaluation of FST and FBF, without any particular concern on the subjects’ blood pressure.
Noninvasive measurement of blood flow has long been both a clinical and research goal. Laser Doppler is a standard technique for the non-invasive blood flow monitoring and measurement of blood flow21). The advantage of the method used in this study is that blood flow in control subjects can be compared with the flow in those who have suspected lower finger blood flow. Furthermore, a provocation (stimulus) test such as cold water immersion test could be applied to detect even partial changes in the blood flow. The results of this study are in good agreement with those reported by other research groups22, 23). For example, Suichies et al.22), investigated the effects of cooling of a hand on lateral and contralateral digital skin blood flow in 18 patients with primary or secondary Raynaud’s phenomenon by using photoelectrical plethysmography and laser Doppler flowmetry. For cooling they used water from 33°C to 3°C in steps of 3°C, each step lasting four min, followed by a recovery period of ten min in room air of 24°C. They concluded that both methods had consistent and quite comparable results for testing the severity of RP when measured on the cooled hand. However, as compared to the method described here, their technique requires much longer time (about 54 min) to complete for each subject.
The method used here is simple and quick to set up, and a series of measurements can be made rapidly and easily. Only a minimum of experience is required to be effective in its utilization. The results of sensitivity and specificity of both FST and FBF were persuasive evidence (Table 2) to proceed to the main objective parts of this research. The positive predictive values of FST (85%) and FBF (82%) clearly support the hypothesis of this study that simultaneous evaluation of FST and FBF (for the pooled data: R2=0.8) as explained here could be considered as a technique in the surveys of RP.
In the studies of peripheral vascular disorders in the upper extremities of subjects with RP, a number of cold provocation tests have been introduced. For instance, Poole et al.24), used a cold provocation test of 15°C for 5 min. In another study by Harazin25), two cold provocation tests of 14°C for 10 min, and 12°C for 5 min were used to compare the recovery of FST in healthy subjects. Most of the provocation tests require much time to be carried out and are mainly focused on the evaluation of FST. It is assumed that some cold provocation tests with a higher water temperature might not be appropriate to cause vasoconstriction in the affected fingers. The test used in the present study (5°C, 1 min) could be performed within less than 10 min (about 7 min) after the equilibration time. Moreover, as subjects were required to immerse their hands in cold water for just 1 min, all subjects could endure doing that, and no complaints were received about.
During the cold provocation test, a vasoconstriction in all subjects was noticed. After the cold immersion, the primary RP group showed no noticeable recovery in the recorded FST and FBF. The PSS group had the similar pattern in the FST curve compared with that in the healthy subjects. However, the pattern of FBF curve before, during and then after the immersion test could obviously be used to differentiate the control subjects from the other two groups with RP. The almost no-recovery pattern in FBF of the two groups with RP during and after the cold provocation test could be explained as an overreaction of arterioles or small arteries to cold exposure. While cold normally causes the muscle which makes up the walls of arteries to contract, in subjects with RP the degree is extreme. Consequently, blood flow to the area is severely restricted. These subjects should avoid situations that precipitate their attacks, and they should insulate their hands from the cold.
Throughout the course of this investigation, the primary RP group showed lower FST and FBF compared to the PSS group. These results are in good agreement with those reported by Rosato et al.26). They investigated the perfusion and digital artery pulsatility of the hands in patients with primary RP and systemic sclerosis by laser Doppler perfusion imaging and photoplethysmography. They found that the mean value of sphygmic wave amplitude was lower in primary RP group than in systemic sclerosis group, and also in the systemic sclerosis group compared with the healthy controls. In another study by Correa et al.27), conducted among a group of patients with systemic sclerosis and a group of healthy controls, the researchers used laser Doppler imaging to assess the functional aspect of superficial skin blood flow. They demonstrated lower laser Doppler imaging in systemic sclerosis patients when compared with healthy controls, both at baseline and after the cold stimulus.
It should be noted that in the vast majority of patients with primary RP, symptoms do not progress to tissue injury, and the abnormality is thought to be reversible vasospasm. Therefore, most people with primary RP never seek medical advice. However, subjects with systemic sclerosis are aware of their symptoms and they periodically consult with specialized physicians to receive medications as well as precautions advice. This could explain why the primary RP group had lower FST and FBF when compared with those of the subjects suffering from PSS.
The episodic blanching of the fingers in response to cold in subjects with either form of RP can rarely be observed by a physician during annual and/or screening health check-up28). It has been reported that a lower FST is expected to reflect a persistent abnormality of blood flow in patients with RP due to hand-arm vibration exposure29). In either primary or secondary form of RP, the most similar prominent component in affected subject is whitening of the finger(s). Although the pathogenesis of RP is complex, abnormalities of the blood vessel wall, of neural control mechanisms and of intravascular (circulating) factors are generally known30) to interact and contribute. For either form of RP, cold exposure (occupational site and/or climate) increases the risk of white finger31, 32). These similarities in the appearance and occurrence of either form of RP were considered as the basis to propose the technique explained here as a useful method in the evaluation of RP. Accordingly, we recommend our method in the occupational settings concerning surveys of RP in subjects exposed to hand-arm vibration.
Due to the time-constrained schedule we had to complete the experiment for each subject, we could not record FST and FBF for a longer time after removal of the hand from the cold water. However, as we reported in our previous study19), the theory of the defective function of the deeply located vessels in those with RP due to vibration exposure, could be supported by the finding of severely decreased resting blood flow and the very slow rewarming after direct cooling. In our aforementioned study, we demonstrated that FBF measurement during the recovery period could expeditiously detect differences in the vascular capacity of workers with RP due to occupational vibration exposure and the symptom-free workers. We could also show that during the recovery time, FST has generally less pronounced increase and it requires somehow more prolonged recovery time compared with FBF. Therefore, we assume that simultaneous measurement of FST and FBF could further deepen our understanding of the vascular disturbances of subjects with RP.
We assume that our simple and quick objective test as described here could be a useful technique in identifying subjects who are at the risk of developing disorders in their peripheral circulatory functions leading to finger blanching. Although during the course of this research we were unable to include any female workers with vibration-induced white finger, the aim of our future study is to use the technique explained here during the annual health examination especially in female workers exposed to hand-transmitted vibration.
Even though the data presented here support that in addition to FST, the FBF evaluation could be beneficial in the surveys of RP, some limitation issues on FBF measurement should be pointed out. In a review by Herrick and Clark33), they addressed that even a minute change in position or orientation of the probe can cause significant changes in the estimation of blood flow. However, to some extent, this difficulty can be overcome by incorporating dynamic testing into the microcirculatory assessment, i.e. the probe should remain in a fixed position, and a standard stimulus should be applied. In another study by Bertelink et al.34), changes in laser Doppler flux in response to a finger cooling test in a large number of primary RP, secondary RP, and healthy controls have been reported. Patients with primary and secondary RP had reduced flux, but there was substantial overlap between groups, and patients with primary RP could not be distinguished from those with secondary RP on the basis of the test applied by them. These authors assessed the reproducibility of their technique35), and concluded that the test has little value in the monitoring of individual cases, but might reasonably be used to look for differences between large patient groups. Also, some studies suggested that laser Doppler techniques in the evaluation of response to treatment should be interpreted with caution36, 37).
On the basis of the discussed material and the results presented here, it was concluded that: 1) laser Doppler blood flowmetry can provide useful information in distinguishing subjects with either primary or secondary RP; and 2) the non-invasive technique introduced here could be used as a method for the evaluation of peripheral circulatory functions in female workers being at the risk of developing finger blanching.
Acknowledgement
The authors are grateful to Dr. Ryoichi Inaba for his help in preparing the document for the ethical committee of Gifu University, and his comments on the revised manuscript.
References
- 1.The Japan Times, Editorial, Still a struggle for working women (Online April 8, 2016)
- 2.Taylor W, Brammer AJ (1982) Introduction to proceeding of 3rd international conference on hand arm vibration. In: Vibration effects on the hand and arm in industry, Brammer AJ, Taylor W (Eds.), 1–12, Wiley, New York.
- 3.Mirbod SM, Yoshida H, Komura Y, Fujita S, Nagata C, Miyashita K, Inaba R, Iwata H (1994) Prevalence of Raynaud’s phenomenon in different groups of workers operating hand-held vibrating tools. Int Arch Occup Environ Health 66, 13–22. [DOI] [PubMed] [Google Scholar]
- 4.Gayraud M. (2007) Raynaud’s phenomenon. Joint Bone Spine 74, e1–8. [DOI] [PubMed] [Google Scholar]
- 5.Henness S, Wigley FM (2007) Current drug therapy for scleroderma and secondary Raynaud’s phenomenon: evidence-based review. Curr Opin Rheumatol 19, 611–8. [DOI] [PubMed] [Google Scholar]
- 6.Bovenzi M. (1988) Finger systolic pressure during local cooling in normal subjects aged 20 to 60 years: reference values for the assessment of digital vasospasm in Raynaud’s phenomenon of occupational origin. Int Arch Occup Environ Health 61, 179–81. [DOI] [PubMed] [Google Scholar]
- 7.Olsen N. (1988) Diagnostic tests in Raynaud’s phenomena in workers exposed to vibration: a comparative study. Br J Ind Med 45, 426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloway GA Jr, Watkins DW (1977) Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol 69, 306–9. [DOI] [PubMed] [Google Scholar]
- 9.Oberg PA, Nilsson GE, Tenland T, Holmström A, Lewis DH (1979) Use of a new laser Doppler flowmeter for measurement of capillary blood flow in skeletal muscle after bullet wounding. Acta Chir Scand Suppl 489, 145–50. [PubMed] [Google Scholar]
- 10.Bonner RF, Clem TR, Bowen PD, Bowman RL (1981) Laser-Doppler continuous real-time monitor of pulsatile and mean blood flow in tissue microcirculation. In: Scattering techniques applied to supramolecular and non-equilibrium system, NATO ASI ser B, 73, Chen SH, Chu B, Nossal R (Eds.), 685–702, Plenum, New York. [Google Scholar]
- 11.Hirata K, Nagasaka T, Noda Y (1988) Partitional measurement of capillary and arteriovenous anastomotic blood flow in the human finger by laser-Doppler-flowmeter. Eur J Appl Physiol Occup Physiol 57, 616–21. [DOI] [PubMed] [Google Scholar]
- 12.Hashizume T, Mitsui K (1988) Properties of laser blood flowmeter. Jpn Laser Assoc J 9, 3–7 (in Japanese). [Google Scholar]
- 13.Coffman JD, Cohen AS (1971) Total and capillary fingertip blood flow in Raynaud’s phenomenon. N Engl J Med 285, 259–63. [DOI] [PubMed] [Google Scholar]
- 14.Welsh CL. (1980) The effect of vibration on digital blood flow. Br J Surg 67, 708–10. [DOI] [PubMed] [Google Scholar]
- 15.Harada N, Ueda A, Takegata S (1991) Prevalence of Raynaud’s phenomenon in Japanese males and females. J Clin Epidemiol 44, 649–55. [DOI] [PubMed] [Google Scholar]
- 16.Mirbod SM, Inaba R, Iwata H (1992) A study on the vibration-dose limit for Japanese workers exposed to hand-arm vibration. Ind Health 30, 1–22. [DOI] [PubMed] [Google Scholar]
- 17.Mirbod SM, Inaba R, Iwata H (1995) Operating vibrating tools and prevalence of subjective complaints in vibration syndrome. Cent Eur J Public Health 3 Suppl, 97–102. [PubMed] [Google Scholar]
- 18.LeRoy EC, Medsger TA Jr (. 1992) Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol 10, 485–8. [PubMed] [Google Scholar]
- 19.Mirbod SM, Yoshida H, Jamali M, Miyashita K, Takada H, Inaba R, Iwata H (1998) Finger skin temperature and laser-Doppler finger blood flow in subjects exposed to hand-arm vibration. Ind Health 36, 171–8. [DOI] [PubMed] [Google Scholar]
- 20.Ishitake T, Sato S, Kume Y, Nagase T, Sakakibara H, Toibana N, Kurozawa Y, Miyahita K, Mahbub H, Harada N (2011) New standard criteria for cold provocation test with hand immersion for cases of HAVS in Japan. Can Acoust 39, 30–1. [Google Scholar]
- 21.Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GA Jr, Keiser HR, Bowman RL (1977) Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol 232, H441–8. [DOI] [PubMed] [Google Scholar]
- 22.Suichies HE, Aarnoudse JG, Wouda AA, Jentink HW, de Mul FF, Greve J (1992) Digital blood flow in cooled and contralateral finger in patients with Raynaud’s phenomenon. Comparative measurements between photoelectrical plethysmography and laser Doppler flowmetry. Angiology 43, 134–41. [DOI] [PubMed] [Google Scholar]
- 23.Engelhart M, Kristensen JK (1986) Raynaud’s phenomenon: blood supply to fingers during indirect cooling, evaluated by laser Doppler flowmetry. Clin Physiol 6, 481–8. [DOI] [PubMed] [Google Scholar]
- 24.Poole K, Elms J, Mason H (2006) Cold-provocation testing for the vascular component of hand-arm vibration syndrome in health surveillance. Ind Health 44, 577–83. [DOI] [PubMed] [Google Scholar]
- 25.Harazin B. (2010) Comparison of recovery time in a cold provocation test performed according to Polish requirements and ISO 14835-1 standard. Med Pr 61, 413–7 (in Polish). [PubMed] [Google Scholar]
- 26.Rosato E, Molinaro I, Rossi C, Pisarri S, Salsano F (2011) The combination of laser Doppler perfusion imaging and photoplethysmography is useful in the characterization of scleroderma and primary Raynaud’s phenomenon. Scand J Rheumatol 40, 292–8. [DOI] [PubMed] [Google Scholar]
- 27.Correa MJ, Andrade LE, Kayser C (2010) Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis Res Ther 12, R157 (doi:10.1186/ar3112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada N. (2002) Cold-stress tests involving finger skin temperature measurement for evaluation of vascular disorders in hand-arm vibration syndrome: review of the literature. Int Arch Occup Environ Health 75, 14–9. [DOI] [PubMed] [Google Scholar]
- 29.Landry GJ. (2013) Current medical and surgical management of Raynaud’s syndrome. J Vasc Surg 57, 1710–6. [DOI] [PubMed] [Google Scholar]
- 30.Herrick AL. (2012) The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol 8, 469–79. [DOI] [PubMed] [Google Scholar]
- 31.Jackson CM. (2006) The patient with cold hands: understanding Raynaud’s disease. JAAPA 19, 34–8. [DOI] [PubMed] [Google Scholar]
- 32.Burström L, Järvholm B, Nilsson T, Wahlström J (2010) White fingers, cold environment, and vibration—exposure among Swedish construction workers. Scand J Work Environ Health 36, 509–13. [DOI] [PubMed] [Google Scholar]
- 33.Herrick AL, Clark S (1998) Quantifying digital vascular disease in patients with primary Raynaud’s phenomenon and systemic sclerosis. Ann Rheum Dis 57, 70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartelink ML, Wollersheim H, Leesmans E, de Boo T, Thien T (1993) A standardized finger cooling test for Raynaud’s phenomenon: diagnostic value and sex differences. Eur Heart J 14, 614–22. [DOI] [PubMed] [Google Scholar]
- 35.Bartelink ML, Wollersheim H, Jansen RWM, Theeuwes A, Thien T (1993) Reproducibility of the finger cooling test. Microvasc Res 45, 65–73. [DOI] [PubMed] [Google Scholar]
- 36.Bunker CB, Reavley C, O’Shaughnessy DJ, Dowd PM (1993) Calcitonin gene-related peptide in treatment of severe peripheral vascular insufficiency in Raynaud’s phenomenon. Lancet 342, 80–3. [DOI] [PubMed] [Google Scholar]
- 37.Whitmore SE, Wigley FM, Wise RA (1995) Acute effect of topical minoxidil on digital blood flow in patients with Raynaud’s phenomenon. J Rheumatol 22, 50–4. [PubMed] [Google Scholar]