Abstract
The One Health (OH) concept provides an integrated framework for observing and improving health issues involving human, animal, and environmental factors, and has been applied in particular to zoonotic disease problems. We conducted a systematic review of English and Chinese language peer-reviewed and grey literature databases to identify zoonotic endoparasite research utilizing an OH approach in community-based settings. Our review identified 32 articles where specimens collected simultaneously from all three OH domains (people, animals, and the environment) were assessed for endoparasite infection or exposure. Study sites spanned 23 countries, and research teams brought together an average of seven authors from two countries. Surveillance of blood-borne and gastrointestinal protozoa were most frequently reported (19 of 32; 59%), followed by trematodes, nematodes, and cestodes. Laboratory techniques varied greatly between studies, and only 16 identified parasites using Polymerase Chain Reaction (PCR) in all three OH domains. Our review identified important gaps in parasitology research operating under an OH framework. We recommend that investigators working in the realm of zoonotic disease strive to evaluate all three OH domains by integrating modern molecular tools as well as techniques provided by economists and social scientists.
Keywords: Parasite, One Health, Community health, Zoonoses
Highlights
-
•
Few community-based parasitology studies currently operate under an OH framework.
-
•
Studies screening people, animals and the environment by PCR focused on protozoa predominantly.
-
•
OH studies are critical to mapping parasite transmission dynamics and reducing disease incidence.
1. Introduction
Research interest in health issues shared among people, animals and the environment has increased rapidly in recent years, as emerging infectious diseases of zoonotic origin have been recognized as a global public health concern. [1], [2], [3]. ‘One Health’ (OH), ‘One Medicine’, and ‘Ecosystem Health’ are a few of the terms currently used to describe this intersectoral concept, and each advocates for increased collaboration among diverse scientific disciplines in order to mitigate many of the ‘wicked’ problems that impact health. These terms stemmed from the recognition that many emerging infectious diseases arise from complex, diverse, and constantly evolving factors related to the environment (e.g. deforestation, climate change), people (e.g. urbanization, food procurement), and animals (e.g. livestock production intensification, wildlife translocation) [2], [3], [4]. As well, integrating less traditional allies, such as social scientists and economists, in the effort to address such disease problems, may be critical to understanding drivers such as poverty and global trade, which can influence both disease emergence and the success of an intervention [5], [6]. It is now recognized that efforts to mitigate disease outbreaks are more likely to succeed when intervention teams integrate multiple disciplines and work collaboratively with policy makers to tackle an issue from multiple perspectives [7]. Furthermore, both people and animals benefit when clinicians use a shared sentinel approach and communicate unusual cases that might lead to emerging zoonoses, as suggested retrospectively by the early identification of birds infected by West Nile Virus in New York City in 1999 [8]. Such approaches require collaboration and coordination, not just across healthcare sectors, but also between organizations at the regional, national and international levels, which is a tenet central to several OH definitions [9], [10].
Parasitic zoonoses continue to cause significant morbidity and mortality worldwide, demonstrating that mass drug administration and parasite eradication campaigns have not yet successfully mitigated all parasites of public health and veterinary importance [7], [11], [12]. This may be explained by the resilience of some life stages in surviving adverse environmental conditions, or by the low host specificity utilized by some parasites to maximize transmission potential between predators and prey [13], [14]. It may also relate to the complexity of parasite life cycles, some of which involve people, animals, vectors, and/or transmission through the environment (e.g., food, water, surfaces, air). Such life cycles illustrate why OH approaches are appropriate for studying zoonotic parasites, and provide clues to the types of multi-pronged parasite control strategies that might work in scenarios where more simplistic interventions have failed. Interdisciplinary approaches are ideal for investigating zoonotic parasites when they take multiple hosts into account, and present multiple solutions for control that go beyond traditional drug administration to include input from social scientists, sanitation experts, and economists [7]. Modern molecular techniques, such as Polymerase Chain Reaction (PCR), are an important component of the OH ‘toolbox’, as they have facilitated understanding of zoonotic transmission pathways by allowing researchers to identify parasite species with much higher specificity than traditional microscopy.
Although OH research promotes collaboration among three ‘domains’ – animals, people, and environment- the integration of all three domains has been recognized as a major challenge [15]. In particular, it has been pointed out that some studies purporting to use an OH approach appear to neglect or ignore the environmental aspects. The aim of this study was to systematically review the literature to identify articles that characterized endoparasites at the community level using an OH framework, and to describe the methods and geographic distribution of these studies.
2. Methods
2.1. One Health study framework definition
For the purposes of this review, we considered a study to have used an OH framework to explore a zoonotic parasite disease issue only if the investigators collected and reported biological specimens from each of the OH domains (human, animal, environment), and if specimens from each domain were subsequently screened for the presence of endoparasites. Human samples included stool, blood/serum, urine, and post-mortem examination. Animals were defined as terrestrial or aquatic vertebrates, and samples included meat, fecal, blood/serum, urine, and necropsy. Environmental samples included terrestrial and aquatic invertebrates, soil, air, water, and pooled animal/human waste (i.e. latrines, sewage or manure).
2.2. Search strategy and selection criteria
We used PubMed, Embase, Google Scholar, and the Chinese National Knowledge Infrastructure (CNKI) search engines to identify English and Chinese peer-reviewed literature relevant to our topic. In accordance with PRISMA guidelines, two blinded reviewers (JMS and EM) searched the Pubmed database. One author (JM) reviewed Embase and Google Scholar, and another author (CL) reviewed CNKI. As well, we reviewed bibliographies of highly relevant research papers, other OH systematic reviews, and OH or Ecohealth parasitology reviews to capture additional studies suited to our topic. Non-English publications were translated by Google Translate for an initial screening, and then by a native language speaker if the paper was relevant to our topic. Grey literature was searched using GreyLit.org and by manually reviewing the websites of OH focused organizations (i.e., One Health Commission, One Health Initiative, One Health Platform, and EcoHealth Alliance, One Health Global Network). The protocol for this systematic review was registered on PROSPERO (CRD42016033982) and can be accessed online (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016033982).
2.3. Eligibility criteria
Our searches for primary literature had no restrictions with respect to geography or time frame. The inclusion criteria restricted results to studies that: (i) collected biological specimens from people, animals, and the environment at the same location(s) and during the same time period; (ii) focused on zoonotic endoparasites; and (iii) utilized laboratory methods to detect endoparasite species in all biological specimens. We excluded review papers, case reports, randomized clinical trials, retrospective study designs, research conducted on banked clinical samples, and studies with the primary goal of developing diagnostic laboratory tests. Our search for secondary literature was restricted to review papers only (systematic or non-systematic).
2.4. Search
We employed a multifaceted approach to identifying publications of interest by searching both primary and secondary peer-reviewed literature. To capture primary reports of observational studies, we searched PubMed and Embase databases using two sets of search terms, where search terms in [All Fields] were included. The first focused on capturing environmental surveillance:
(water OR air OR manure OR soil OR latrine OR sewage OR cesspool OR snail OR vector OR food).
AND zoono* AND (parasite OR protozoan OR helmint* OR metazoo*) AND (sample OR survey OR specimen OR detection OR prevalence OR isolation OR occurrence) AND (communit* OR town OR state OR province OR village OR district OR city OR farm).
The second set of terms focused on capturing human and animal surveillance:
(meat OR urine OR fecal OR feces OR stool OR blood OR serum OR necropsy OR post-mortem) AND zoono* AND (parasite OR protozoan OR helmint* OR metazoo*) AND (survey OR sample OR specimen OR detection OR prevalence OR isolation OR occurrence) AND (communit* OR town OR state OR province OR village OR district OR city OR farm).
Our Google Scholar search was limited to articles containing zoonotic, parasite and sample, and one of the following: community, town, state, province, village, district, city, or farm. Patents were excluded while citations were included. One author (JS) reviewed the first 10 pages of results (300 citations), and each page thereafter containing at least one relevant citation.
One set of terms was used to capture human, animal, and environmental research in the CKNI database:
寄生虫 and 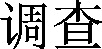 and
and 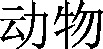 and (城市 or
and (城市 or 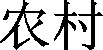 ).
).
To capture secondary reports, one author (JS) searched PubMed using the terms ((One Health [Title]) AND systematic review) as well as ((One Health[Title/Abstract] OR One Medicine[Title/Abstract] OR Ecosystem Health[Title/Abstract] OR Ecohealth[Title/Abstract]) AND parasit*[Any field]). Results were screened for citations relevant to this review; however, only papers satisfying two or three OH domains were extracted.
Pubmed was searched for primary literature on December 12–14, 2015 (JMS) and December 27–31 (EM); secondary literature was reviewed on February 11th, 2016 (JMS). Embase searches occurred on January 15th, 2016 (JMS) and Chinese language publications were searched January 19th 2016 (CL).
2.5. Study selection and data collection
An initial screen of the search results included reading all titles and abstracts. Articles with potential to meet the criteria of community level parasite sampling in at least two of the three OH domains were read in entirety. If a publication reported sampling one domain, we recorded the citation and categorized the domain (people, animals, or environment). If a paper met criteria for two OH domains, we extracted data relating to publication date, parasite species, and study location. Research studies that met all three domains were assessed for bias (see next section), and were mined for additional data: animal species, human population characteristics, diagnostic tests (people, animals, environment), biological specimens collected (people, animals, environment), study design, and study aims.
2.6. Bias and quality assessment
Publications that satisfied all search criteria were qualitatively assessed by two authors (EM and JS) using a pre-established set of criteria (Table 1). We modified previously published grading criteria to create an appropriate tool for evaluating primarily cross-sectional OH studies [16]. The mean quality score of the two assessments for each individual paper was calculated and translated to a ranking of ‘low’, ‘moderate’ or ‘high’.
Table 1.
Assessment tool used to grade bias and quality of individual papers that met all inclusion criteria for One Health studies.
| Criteria | Scorea |
|---|---|
| 1. Was the research question or objective in this paper clearly stated? | |
| 2. Was the study population clearly specified and defined? | |
| 3. Were sample size calculations reported? | |
| 4. Was the study sample selected to be representative of the study population? | |
| 5. Was ethical approval reported for both humans and animals? | |
| 6. Were inclusion and exclusion criteria for being in the study pre-specified and applied uniformly to all participants? | |
| 7. Were the laboratory measures clearly defined, valid, reliable, and implemented consistently across all study participants? | |
| 8. Were any of the environmental, human, or animal samples measured at multiple time points? | |
| 9. Were molecular detection methods used for all sample types? | |
| 10. Was the overall participation percentage reported? | |
| 11. Did statistical tests include variance and effect estimates? | |
| Total score (out of 11)b |
Yes = 1 point; no = 0 points.
Low = 0–3; moderate = 4–7; high = 8–11.
2.7. Synthesis of results
We summarized studies that met all OH inclusion criteria by country, endoparasite focus, specimen type for each OH domain (people, animals, environment), diagnostic tools, use of PCR to identify endoparasites, and use of risk factor analysis. We also described authorship, multilateral collaboration, and the diversity of disciplines for these studies. For publications that satisfied all inclusion criteria but spanned only two OH domains, we described the parasite focus and the number of publications categorized as human-animal, human-environment, and animal-environment studies.
We illustrated the distribution of research studies that assessed two or three OH domains by extracting field site locations (latitude and longitude) and entering them into a spreadsheet. If field coordinates were not reported, we entered site location names into the Harvard University Centre for Geographic Analysis online mapping tool (http://maps.cga.harvard.edu/gpf/; Cambridge, MA) to determine the coordinates. If several study sites were named, we used coordinates that represented a central point at the next highest organizational unit (e.g. district versus village level). Geographic coordinates were mapped using ArcGIS (v10.3.1; Esri, Redlands, California, USA).
3. Results
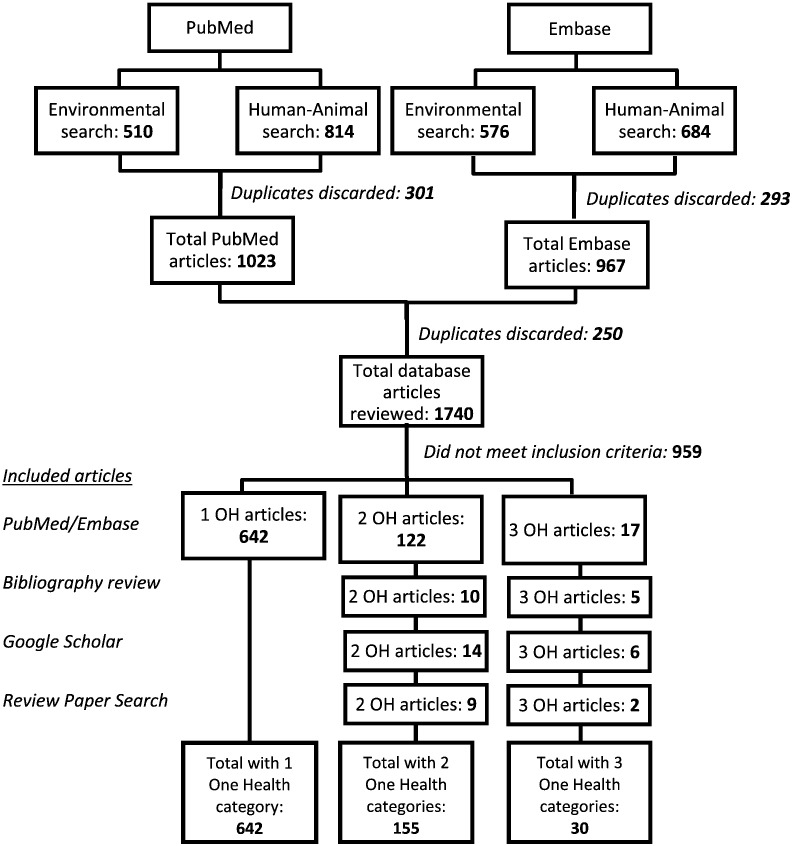
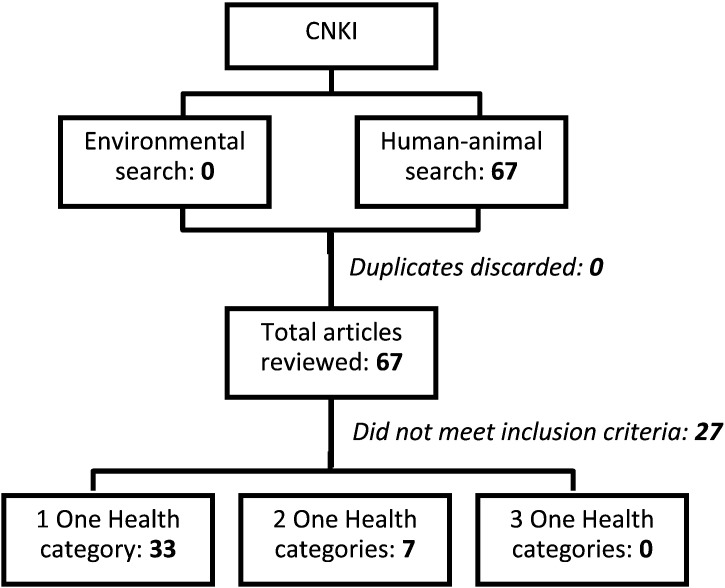
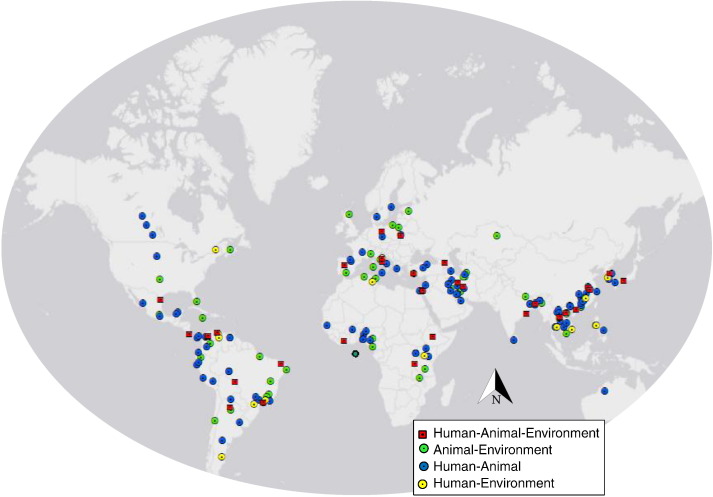
Our review of English and Chinese peer-reviewed literature identified 30 unique publications that met all inclusion criteria for an OH parasitology study, and 162 publications that sampled from two OH domains (Fig. 1, Fig. 2). Our review of the grey literature identified two OH projects that met all OH criteria. The OH studies were geographically diverse, with research conducted in 23 countries in Asia, Europe, Africa, South and Central America, and the Middle East (Fig. 3).
Fig. 1.
Systematic search of One Health framed parasite surveillance in English language databases.
Fig. 2.
Systematic search of One Health framed parasite surveillance in a Chinese language database.
Fig. 3.
Field sites of community-based parasitology research addressing two or three domains of the One Health triad.
Among the primary literature studies that met all inclusion criteria, most were published in English (N = 27); other languages included Spanish (N = 2), Korean (N = 1), and German (N = 1). On average, publications had seven authors (range 1–16), involved three institutions (range 1–6) with three departments (range 1–7), and bridged together authors based in two countries (range 1–4). Except for one outbreak investigation and one 2-year longitudinal study, all other studies used a cross-sectional approach, with human and animal specimens collected at a single time point. Of these, approximately half collected environmental specimens at repeated intervals. The studies spanned 40 years of research (1975–2015), and covered a broad range of endoparasite species. Approximately half of primary OH studies focused on protozoan parasites (17 of 30; 56%), including blood-borne species (Leishmania spp., Trypanosoma spp., Toxoplasma gondii, and Babesia spp.) or gastrointestinal species (Cryptosporidium spp. and Giardia spp.). Research focused on trematodes (fishborne trematodes and Schistosoma spp.) and nematodes (Toxocara canis, Dirofilaria immitis, and Angiostrongylus cantonensis) each comprised 20% (6 of 30) of our dataset. One study focused on a cestode species (3%; Taenia saginata). These parasites were diagnosed using a variety of laboratory techniques (Table 2), which included examination of gross and/or microscopic morphology, xenodiagnosis/bioassay, and/or immunological assays. Approximately half of these studies (16 of 30) utilized PCR to identify parasites collected from at least one domain (human, animal or environment); however, only six used PCR and/or DNA sequencing to characterize parasites in all three domains of the OH triad. One-third of OH studies used questionnaires to obtain qualitative data on human subjects, and two-thirds conducted risk factor analyses, using statistical tools such as logistic regression or chi-square to identify possible associations for at least one of the three specimen sources.
Table 2.
Summary of peer-reviewed parasite surveillance reports identified as using One Health frameworks.
| Country (language) | Study design | Animal species – diagnostic assay | Human study population –diagnostic assay | Environmental specimen – diagnostic assay | Parasite identification by PCR (isolate source) | Risk factor analysis (host) | Quality score | Citation |
|---|---|---|---|---|---|---|---|---|
| Visceral Leishmaniasis (Leishmania infantum) | ||||||||
| Georgia (English) | Cross-sectional | Dogs, jackals, foxes – ELISA (sera) | General public – Leishmanin Skin Test (LST) | Sandflies - midgut dissection | No | Yes (canid & human) | Moderate | [17] |
| Turkey (English) | Cross-sectional | Dogs – PE, IFAT & ELISA (sera), smear/culture (lymph node aspirate) | Village residents – PE, IFAT & ELISA (sera) | Sandflies - midgut dissection | No | No | Moderate | [18] |
| Cutaneous Leishmaniasis (Leishmania major, L. tropica, L. aethiopica) | ||||||||
| Egypt (English) | Cross-sectional | Rodents - Giemsa smear, culture, biochemical typing (skin scraping) | Temporary workers – PE, IHAT, Giemsa smear, culture (skin scraping) | Sandflies - midgut dissection, biochemical typing | No | No | Low | [19] |
| Iran (English) | Cross-sectional | Dogs – PE; Rodents – Giemsa stain, bioassay, culture, RAPD-PCR (skin scraping) | Village residents – PE, Giemsa stain, bioassay, culture, RAPD-PCR (skin scraping) | Sandflies - midgut dissection | Yes (animal & human) | Yes (human) | Moderate | [20] |
| Iran (English) | Outbreak investigation | Rodents - Giemsa stain, culture, RAPD-PCR (skin scraping) | CL patients – Giemsa stain, culture, RAPD-PCR (skin scraping) | Sandflies – midgut dissection, culture, bioassay, RAPD-PCR | Yes (all) | No | Moderate | [21] |
| Ethiopia (English) | Cross-sectional | Rock hyraxes, rodents – culture, bioassay, PCR (skin scraping) | Current/past CL cases – culture, bioassay (skin scraping) | Sandflies - midgut dissection | Yes (hyrax) | Yes (human) | Moderate | [22] |
| African Trypanosomiasis (Trypanosoma spp.) | ||||||||
| Côte d'Ivoire (English) | Cross-sectional | Pigs – HCT, MLEE, bioassay, PCR (sera) | Residents – CATT, MLEE, bioassay, PCR (sera) | Tsetse flies – dissection, PCR | Yes (all) | No | Moderate | [23] |
| American Trypanosomiasis (Trypanosoma cruzi) | ||||||||
| Venezuela (Spanish) | Cross-sectional | Dogs – ELISA, MABA (sera) | Village residents – ELISA, MABA (sera) | Triatomes – gut dissection, PCR | Yes (triatome) | Yes (dog & human) | Moderate | [24] |
| Columbia (English) | Cross-sectional | Dogs, small mammals – IFAT, ELISA, xenodiagnosis, hemoculture (sera) | Community children (< 15 yrs) – IFAT, ELISA (sera) | Triatomes – stool assay, PCR | Yes (animal & triatome) | Yes (dog) | Moderate | [25] |
| Brazil (English) | Cross-sectional | Dogs - IIFA, ELISA (sera); Small mammals – IFAT(sera) | Community residents – IIFA, ELISA, hemoculture, PCR (sera) | Triatomes – stool assay, PCR | Yes (human & triatome) | No | High | [26] |
| Columbia (Spanish) | Cross-sectional | Dogs, small mammals – ELISA, IIFA (sera) | Residents – ELISA, IIFA (sera) | Triatomes – PCR (stool) | Yes (triatome) | Yes (human) | Moderate | [27] |
| Costa Rica (English) | Cross-sectional | Dogs, cats, rats, mice, pigs, cattle, rabbits, opossum - xenodiagnosis, CF, IHA, bioassay (sera and/or necropsy) | Town residents - CF, xenodiagnosis | Triatomes – stool assay | No | Yes (animal & triatome) | Moderate | [28] |
| Babesiosis (Babesia spp.) | ||||||||
| Italy (English) | Cross-sectional | Dogs, cattle, sheep, goats, wild boars, fallow-deer, roe-deer, horses – IFA, PCR (sera) | High risk residents – ELISA (sera) | Ticks - PCR | Yes (animal & tick) | No | Moderate | [29] |
| Toxoplasmosis (Toxoplasma gondii) | ||||||||
| Poland (English) | Cross-sectional | Dogs, cats, cattle, pigs, horses, goats, rabbits, poultry – Giemsa smear, DAT, PCR (sera) | Farm residents – ELFA (sera) | Water – filtration, bioassay, PCR | Yes (water) | Yes (all) | Moderate | [30] |
| Cryptosporidiosis and/or Giardiasis (Cryptosporidium & Giardia spp.) | ||||||||
| Tanzania (English) | Cross-sectional | Dogs, goats, sheep, baboons, chimpanzees – PCR (stool) | Gombe National Park and village residents – PCR (stool) | Water – filtration, PCR | Yes (all) | Yes (animal & human) | High | [31] |
| Bangladesh (English) | Cross-sectional | Cattle – IFAT, PCR (stool) | Village residents, calf handlers – IFAT, PCR (stool) | Water - filtration, IMS, PCR | Yes (all) | Yes (all) | High | [32] |
| India (English) | Cross-sectional | Dogs, cats, cattle, buffalo, goats, sheep, chickens – IMS-DFA (stool) | Diarrhea ward patients - IMS-DFA (stool) | Water – IMS-DFA | Yes (all) | Yes (all) | Moderate | [33] |
| Fishborne Trematodiasis (Heterophyidae, Echinostomatidae, Opisthorchis viverrini, Clonorchis sinensis, Metagonimus spp.) | ||||||||
| Laos (English) | Cross-sectional | Cats, fish – necropsy | Work camp residents, villagers – stool exam | Snails – cercariae shedding, dissection | No | No | Low | [34] |
| Vietnam (English) | Cross-sectional | Dogs, cats, pigs – FECT (stool); fish – necropsy | Village residents – Kato-Katz assay (stool) | Snails – cercariae shedding, dissection | No | Yes (all) | Moderate | [35] |
| Laos (English) | Cross-sectional | Cats - unsure; fish – necropsy | Village residents - MIFC or Kato-Katz (stool) | Snails - dissection | No | No | Low | [36] |
| South Korea (Korean) | Cross-sectional | Fish – PE, bioassay (necropsy) | School children with parents – formalin-ether MGL (stool) | Snails – dissection | No | No | Low | [37] |
| Schistosomiasis (Schistosoma japonicum) | ||||||||
| China (English) | 2-year longitudinal study | Dogs, cats, cattle, water buffalo, goats, pigs – Miracidia hatching test (stool) | IHAT positive village residents - Miracidia hatching test, Kato-Katz (stool) | Snails - dissection | No | Yes (snail) | Moderate | [38] |
| China (English) | Cross-sectional | Dogs, cats water buffalo, cattle, goats, pigs– Kato-Katz, PCR (stool) | Village residents (3–65 yrs) – Kato-Katz, PCR (stool) | Snails – cercariae shedding, PCR | Yes (all) | No | Moderate | [39] |
| Angiostrongyliasis (Angiostrongylus cantonensis) | ||||||||
| China (English) | Cross-sectional | Rodents – necropsy | City residents – ELISA (sera) | Snails – tissue digest, bioassay | No | Yes (all) | Moderate | [40] |
| Dirofilariasis (Dirofilaria immitis) | ||||||||
| Japan (English) | Cross-sectional | Dogs – hematocrit centrifugation assay/IFAT (sera) | City residents – ELISA (sera) | Mosquitoes - ELISA | No | Yes (dog & mosquito) | Moderate | [41] |
| Toxocariasis (Toxocara canis) | ||||||||
| Spain (English) | Cross-sectional | Dogs – zinc sulphate flotation (stool) | Village or town residents - ELISA (sera) | Soil – magnesium sulphate assay | No | Yes (all) | Low | [42] |
| Italy (English) | Cross-sectional | Dogs – sodium nitrate flotation (stool) | Area residents – ELISA (sera) | Soil - sodium nitrate flotation | No | Yes (dog & soil) | Low | [43] |
| Brazil (English) | Cross-sectional | Dogs – water-ether flotation (stool) | Community children – ELISA (sera) | Soil - flotation assay | No | Yes (dog & human) | Moderate | [44] |
| St. Lucia (English) | Cross-sectional | Dogs – Richie assay (stool) | Village children – ELISA (sera) | Soil – zinc sulfate flotation | No | Yes (human) | Moderate | [45] |
| Taeniasis (Taeniasaginata) | ||||||||
| Germany (German) | Cross-sectional | Cattle – smear test | People- smear test | Waste-water – smear test | No | No | Low | [46] |
Abbreviations: PE = Physical Exam, ELISA = Enzyme-linked Immunosorbent Assay, IFAT = Indirect Fluorescent Antibody Test; PCR = Polymerase Chain Reaction; RAPD-PCR = Random Amplified Polymorphic DNA-Polymerase Chain Reaction; IHAT = Indirect Haemagglutination Test; FSC – Filtration, Sedimentation, Centrifugation; FECT = Formalin-ethyl Acetate Concentration Test; MIFC = Merthiolate-Iodine-Formalin Concentrations; NR = Not reported; IMS = Immunomagnetic Separation; DFA = Direct Immunofluorescence Antibody test; IIFA = Indirect Immune Fluorescence Assay; ELFA = Enzyme Linked Fluorescent Assay; MLEE = Multilocus Enzyme Electrophoresis; HCT = Haematocrit Centrifugation Technique; CATT = Card Agglutination Test for Trypanosomosis; CF = Complement Fixation.
Arthropods were the most frequently collected environmental specimen (N = 14), followed by mollusks (N = 7), water (N = 4), soil (N = 4), and sewage/fodder (N = 1). Approximately two-thirds of OH studies engaged in blood or stool collection from dogs, making them the most frequent choice for animal sampling. Livestock (ruminants, swine, and horses) were second most common followed by domestic cats and wild-caught rodents/small mammals, various fish species, large wildlife (canids, primates, cervids), and poultry. Approximately half of OH studies collected specimens from a broad range of animals, rather than from a single host animal species.
Of the 162 publications that sampled parasites from two of the three OH domains, 102 sampled from people and animals, 44 sampled from animals and the environment, and 16 sampled from people and the environment. Study sites were located in 54 countries across all continents except Antarctica (Fig. 3), and were investigated between 1979 and 2015. These publications reported data on a wide range of endoparasite species, with Cryptosporidium/Giardia spp., Leishmania spp., and Taeniidae (Taenia and Echinococcus spp.) being the most frequent focus, overall. Some endoparasites such as Leishmania, Trypanosoma, Cryptosporidium/Giardia, and fishborne trematodes were investigated using all 2-domain combinations of the OH triad (human-animal, human-environmental, and animal-environmental). Others, such as Trichinella and Capillaria hepatica were studied using one of these three approaches.
Our review of the grey literature identified two additional projects that met our inclusion criteria for OH research. The first was conducted at Lackland Air Force Base (Texas, USA), where Trypanosoma cruzi (aka. Chagas disease) had been diagnosed in a military working dog. In response, public health officials launched a large scale investigation, testing military personnel, dogs, and triatomes for exposure or infection, and then evaluating the results of interventions to minimize infection [47]. The second project was based in Argentina, where researchers conducted long-term T. cruzi surveillance in people, animals, and triatomes, in addition to their physical and biological environments [48]. The project resulted in forty publications (e.g. [49]), some of which were identified in our peer-reviewed searches, but not one paper satisfied all inclusion criteria for three domains of the OH triad.
4. Discussion
Although OH is frequently discussed in literature pertaining to zoonotic pathogens, our work demonstrates that endoparasites are not frequently explored by approaches that address all three domains of the OH triad simultaneously. This might be explained by the financial and logistical challenges inherent to interdisciplinary collaboration, especially at the level required for OH research. Potential barriers include communication barriers between languages or disciplines, data sharing, synching research priorities, budget allocations, and ensuring that team members remain engaged throughout the study period. Our review found 162 publications reporting endoparasite research in two of the three OH domains, most of which were conducted in the last 20 years. As well, the use of the terms ‘OH’ and ‘One Medicine’ has grown steadily in the peer-reviewed literature. Together, this suggests that researchers are finding ways to overcome obstacles of ‘team science’, likely aided by policy driven trends such as increased funding for collaborative research [3], [50], [51]. New tools for community-based research, such as citizen science, crowd-sourcing, Photovoice, and digital storytelling have been instrumental for facilitating data collection and analysis in rural and remote areas, and have provided opportunities for continuous sampling and public health education [52], [53]. Our analysis of OH publications identified one such example, where Venezuelan community members participated in Chagas' disease surveillance by collecting the parasite vector (triatome insects) [24].
The field of microbiology has undergone rapid advancement over the last several decades, and it continues to produce novel tools for accurate detection and diagnosis of parasites in people, animals, and the environment. Our search highlighted a wide range of methods, ranging from simple physical exams and blood smears to more complex immunological methods and bioassays. We highlighted PCR as an important diagnostic tool because it can be used on parasite life stages collected from all OH domains, and because it is the only method capable of identifying isolates to species level and beyond (i.e., subtypes, genotypes, or subspecies). Comparing parasite differences at the nucleotide level is a promising technique for identifying primary transmission routes, which in turn contributes to evidence based risk reduction. However, we identified only six OH studies where PCR identified parasites in all three domains, and four where PCR identified parasites in two domains. Similarly, only one-third of the OH research used questionnaires to collect qualitative data on human subjects, and one-fifth compared infection/contamination status to qualitative data using statistical techniques in all domains. This demonstrates a missed opportunity for identifying and quantifying location specific risk factors for parasite exposure or infection.
The overall quality of evidence reporting in papers that we classified as OH was moderate. Some criteria, such as reporting the research aim, specifying the study population, and clearly describing laboratory protocols were high quality across the board. Other criteria, such as describing ethics approval for human and animal subjects, reporting the participation percentage of a study group, including variance or effect estimates, and providing inclusion/exclusion criteria for participant recruiting were infrequently satisfied. At the present time, multiple checklists have been broadly accepted by the scientific community that promote high quality reporting in specific disciplines (e.g. STROBE for observational epidemiology [54], CONSORT for randomized control trials [55]); however, no set of guidelines has yet been established to guide OH research reporting. Our research supports the need for such guidelines, which are currently under development (i.e. the “Checklist for One Health Epidemiological Reporting of Evidence (COHERE)” [56].
Among papers that did not meet our search criteria, we observed ‘publication splitting’, where a project conducted at a single study site was published two or more times with separate papers reporting surveillance results of different pathogens. While there are no rules against this practice, splitting datasets and/or analyses creates barriers against comprehensively and holistically understanding complex disease ecosystems. Our grey literature search identified a long-term project site involving several institutions, various OH disciplines, and a number of students, post-doctoral researchers and veterinarians who contributed to forty publications [49]. In such scenarios, it would be useful to write up at least one comprehensive overview of research aims and key stakeholders, similar to the Health for Animals and Livelihood Project [57]. Ideally, this would be followed by brief updates every five to ten years, in which authors highlighted research progress, lessons learned, and revised research goals.
Our finding that 30 peer-reviewed publications met the criteria for OH research is based on our definitions of the ‘human’, ‘animal’, and ‘environment’ domains, which are not standard across the research community. A definition that classified insect vectors in the animal domain would produce different results. Out of the publications that reported parasite surveillance in two of the three OH domains, we observed a greater number of human-animal collaborations than animal-environment collaborations, or human-environment collaborations (of which there were fewest). This could indicate that our search terms were better suited to detecting clinical data, especially as we focused on zoonotic parasites. Conversely, it might re-enforce a common perspective that OH research is dominated by the veterinary community, which works more frequently with human clinicians than environmental scientists. Our inclusion criteria required that parasites be subject to laboratory analysis in all domains, and as a result, some high quality studies that collected specimens such insect vectors without testing them, were excluded. As well, our search likely underestimated the true number of OH endoparasite investigations, as we used only two languages and four search engines. However, our overall results were widely distributed with respect to geography, and included a range of languages beyond English and Chinese.
Research and interventions that integrate multiple disciplines are valuable within academic and clinical care settings, but may be especially powerful when stakeholders from all levels of a perceived issue are included (e.g., community members, advocacy groups, and policy makers). As well, input from social scientists and economists should continue to be sought, as these disciplines help us to plan prevention programs that are feasible. Our study highlights several excellent examples of multidisciplinary parasite-focused research, and demonstrates a continued need for reporting disease risk factors in addition to infection status. As molecular tools become more refined and interactions among people, animals, and the environment continue to amplify pathogen emergence, it will become increasingly important to use these tools in harmony with OH frameworks to identify and mitigate predominant transmission pathways.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank Sarah Safranek for her guidance during the study design process. As well, we are grateful to Hyoung Frank Ryou and Arcadio Viveros-Guzmán for translating the Korean and Spanish language publications, respectively. Post-doctoral stipend funding for JS was provided by a Natural Sciences and Engineering Research Council (NSERC) Collaborative Research and Training Experience (CREATE) grant to the University of Saskatchewan for an Integrated Training Program in Infectious Diseases, Food Safety and Public Policy (ITraP).
References
- 1.Schwabe C. third ed. Williams and Wilkins; Maryland: 1984. Veterinary Medicine and Human Health. [Google Scholar]
- 2.Jones K., Patel N., Levy M., Storeygard A., Balk D., Gittleman J., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leboef A. Making Sense of One Health: Cooperating at the Human-Animal-Ecosystem Health Interface. 2011. http://onehealthinitiative.com/publications/IFRI_ifrihereport7alineleboeuf.pdf accessed.
- 4.Bengis R., Leighton F., Fischer J., Artois M., Morner T., Tate C. The role of wildlife in emerging and re-emerging zoonoses. Rev. Sci. Tech. Off. Int. Epiz. 2004;23:497–511. [PubMed] [Google Scholar]
- 5.Rock M., Buntain B., Hatfield J., Hallgrimsson B. Animal-human connections, “one health,” and the syndemic approach to prevention. Soc. Sci. Med. 2009;68:991–995. doi: 10.1016/j.socscimed.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 6.The World Bank . Report 69145–GLB. Vol. 65. 2012. The economics of One Health: people, pathogens, and our planet, Washington, DC. [Google Scholar]
- 7.WHO/TDR Disease Reference Group on Zoonoses and Marginalized Infectious Diseases of Poverty . Report 971. World Health Organization; Switzerland: 2012. Research priorities for zoonoses and marginalized infections. [Google Scholar]
- 8.Steele K., Linn M., Schoepp R., Komar N., Geisberg T., Manduca R., Calle P., Raphael B., Clippinger T., Larsen T., Smith J., Lanciotti R., Panella N., McNamara T. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Path. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 9.One Health Global Network What is One Health? 2012. http://www.onehealthglobal.net/what-is-one-health/ accessed.
- 10.One Health Commission Why One Health? 2016. https://www.onehealthcommission.org/en/why_one_health/ accessed.
- 11.Wharam B., Lazarou L. Ethical considerations in an era of mass drug administration. Parasit. Vectors. 2013;6:234. doi: 10.1186/1756-3305-6-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torgerson P., Devleesschauwer B., Praet N., Speybroeck N., Willingham A., Kasuga F. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajadhar A., Allen J. Factors contributing to the public health and economic importance of waterborne zoonotic parasites. Vet. Parasitol. 2004;126:3–14. doi: 10.1016/j.vetpar.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs E. The evolution of One Health: a decade of progress and challenges for the future. Vet. Rec. 2014;174:85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services (National Institutes of Health) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort accessed.
- 17.Babuadze G., Alvar J., Argaw D., de Koning H., Iosava M., Keklidze M. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Töz S., Sakru N., Ertabaklar H., Demir S., Sengul M., Ozbel Y. Serological and entomological survey of zoonotic visceral leishmaniasis in Denizli Province, Aegean Region, Turkey. New Microbiol. 2009;32:93–100. [PubMed] [Google Scholar]
- 19.Morsy T., Naser A., El Gibali M., Anwar A., El Said A. Studies on zoonotic cutaneous leishmaniasis among a group of temporary in North Sinai governorate, Egypt. J. Egypt. Soc. Parasitol. 1995;25:99–106. [PubMed] [Google Scholar]
- 20.Yaghoobi-Ershadi M., Jafari R., Hanafi-Bojd A. A new epidemic focus of zoonotic cutaneous leishmaniasis in central Iran. Ann. Saudi Med. 2004;24:98–101. doi: 10.5144/0256-4947.2004.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doroodgar A., Sadr F., Razavi M., Doroodgar M., Asmar M., Doroodgar M. A new focus of zoonotic cutaneous leishmaniasis in Isfahan Province, Central Iran. Asian Pac. J. Trop. Dis. 2015;5:S54–S58. [Google Scholar]
- 22.Lemma W., Erenso G., Gadisa E., Balkew M., Gebre-Michael T., Hailu A. A zoonotic focus of cutaneous leishmaniasis in Addis Ababa, Ethiopia. Parasit. Vectors. 2009;2:60. doi: 10.1186/1756-3305-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamonneau V., Ravel S., Koffi M., Kaba D., Zeze D., Ndri L. Mixed infections of trypanosomes in tsetse and pigs and their epidemiological significance in a sleeping sickness focus of Côte d'Ivoire. Parasitology. 2004;129:693–702. doi: 10.1017/s0031182004005876. [DOI] [PubMed] [Google Scholar]
- 24.Rojas M., Várquez P., Villarreal M., Velandia C., Vergara L., Morán-Borges Y. Estudio seroepidemiológico y entomológico sobre la enfermedad de Chagas en un área infestada por Triatoma maculata (Erichson 1848) en el centro-occidente de Venezuela. Cad. Saude Publica. 2008;24:2323–2333. doi: 10.1590/s0102-311x2008001000013. [DOI] [PubMed] [Google Scholar]
- 25.Cantillo-Barraza O., Garcés E., Gómez-Palacio A., Cortés L., Pereira A., Marcet P. Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasit. Vectors. 2015;8:482. doi: 10.1186/s13071-015-1100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima M., Sarquis O., de Oliveira T., Gomes T., Coutinho C., Daflon-Teixeira N. Investigation of Chagas disease in four periurban areas in northeastern Brazil: epidemiologic survey in man, vectors, non-human hosts and reservoirs. Trans. R. Soc. Trop. Med. Hyg. 2012;106:143–149. doi: 10.1016/j.trstmh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Vásquez C., Robledo S., Street J., Triana O. Identificación de nuevos escenarios epidemiológicos para la enfermedad de Chagas en la región momposina, norte de Colombia. Biomedica. 2013;33:526–537. doi: 10.7705/biomedica.v33i4.836. [DOI] [PubMed] [Google Scholar]
- 28.Zeledón R., Solano G., Burstin L., Swartzweslder J. Epidemiological pattern of Chagas' disease in an endemic area of Costa Rica. Am.J.Trop. Med. Hyg. 1975;24:214–225. doi: 10.4269/ajtmh.1975.24.214. [DOI] [PubMed] [Google Scholar]
- 29.Pietrobelli M., Cancrini G., Moretti A., Tampieri M. Animal babesiosis: an emerging zoonosis also in Italy? Parassitologia. 2007;49:33–38. [PubMed] [Google Scholar]
- 30.Sroka J., Wojcik-Fatla A., Szymanska J., Dutkiewicz J., Zajac V., Zwolinski J. The occurrence of Toxoplasma gondii infection in people and animals from rural environment of Lublin region - estimate of potential role of water as a source of infection. Ann. Agric. Environ. Med. 2010;17:125–132. [PubMed] [Google Scholar]
- 31.Parsons M., Travis D., Lonsdorf E., Lipende I., Roellig D., Kamenya S. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015;10 doi: 10.1371/journal.pntd.0003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehsan A., Geurden T., Casaert S., Parvin S., Islam T., Ahmed U. Assessment of zoonotic transmission of Giardia and Cryptosporidium between cattle and humans in rural villages in Bangladesh. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels M., Shrivastava A., Smith W., Sahu P., Odagiri M., MIsra P. Cryptosporidium and Giardia in humans, domestic animals, and village water sources in rural India. Am.J.Trop. Med. Hyg. 2015;93:596–600. doi: 10.4269/ajtmh.15-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giboda M., Ditrich O., Scholz T., Viengsay T., Bouaphanh S. Current status of food-borne parasitic zoonoses in Laos. Southeast Asian J. Trop. Med. Public Health. 1991;22:56–61. [PubMed] [Google Scholar]
- 35.Hung N., Dung D., Anh N., Van P., Thanh B., Ha N. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasit. Vectors. 2015;8:21. doi: 10.1186/s13071-015-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ditrich O., Scholz T., Giboda M. Occurrence of some medically important flukes (Trematoda: Opisthorchiidae and Heterophyidae) in Nam Ngum water reservoir, Laos. Southeast Asian J. Trop. Med. Public Health. 1990;21:482–488. [PubMed] [Google Scholar]
- 37.Ahn J., Ryang Y. Epidemiological studies on Metagonimus infection along the Hongcheon river, Kangwon province. Korean J. Parasitol. 1988;26:207–213. doi: 10.3347/kjp.1988.26.3.207. [DOI] [PubMed] [Google Scholar]
- 38.Lu D., Wang T., Rudge J., Donnelly C., Fang G., Webster J. Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China–a two-year longitudinal parasitological survey. Parasitology. 2010;137:99–110. doi: 10.1017/S003118200999103X. [DOI] [PubMed] [Google Scholar]
- 39.Wang T., Shrivastava J., Johansen M., Zhang S., Wang F., Webster J. Does multiple hosts mean multiple parasites? Population genetic structure of Schistosoma japonicum between definitive host species. Int. J. Parasitol. 2006;36:1317–1325. doi: 10.1016/j.ijpara.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Chen D., Zhang Y., Shen H., Wei Y., Huang D., Tan Q. Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol. Res. 2011;109:305–314. doi: 10.1007/s00436-011-2255-1. [DOI] [PubMed] [Google Scholar]
- 41.Konishi E. Culex tritaeniorhynchus and Aedes albopictus (Diptera: Culicidae) as natural vectors of Dirofilaria immitis (Spirurida: Filariidae) in Miki City, Japan. J. Med. Entomol. 1989;26:294–300. doi: 10.1093/jmedent/26.4.294. [DOI] [PubMed] [Google Scholar]
- 42.Conde Garcia L., Muro Alvarez A., Simon Martin F. Epidemiological studies on toxocariasis and visceral larva migrans in a zone of Western Spain. Ann. Trop. Med. Parasitol. 1989;83:615–620. doi: 10.1080/00034983.1989.11812395. [DOI] [PubMed] [Google Scholar]
- 43.Habluetzel A., Traldi G., Ruggieri S., Attili A., Scuppa P., Marchetti R. An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet. Parasitol. 2003;113:243–252. doi: 10.1016/s0304-4017(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 44.Muradian V., Gennari S., Glickman L., Pinheiro S. Epidemiological aspects of visceral larva migrans in children living at Sao Remo community, Sao Paulo (SP), Brazil. Vet. Parasitol. 2005;134:93–97. doi: 10.1016/j.vetpar.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 45.Thompson D., Bundy D., Cooper E., Schantz P. Epidemiological characteristics of Toxocara canis zoonotic infection of children in a Caribbean community. Bull. Wld. Hlth. Org. 1986;64:283–290. [PMC free article] [PubMed] [Google Scholar]
- 46.Engelbrecht H., Mentzel U. Der urbane Wirtswechselzyklus von Taenia saginata im Kreis Wittstock, Bezirk Potsdam (DDR) Angew. Parasitol. 1984;25:126–132. [PubMed] [Google Scholar]
- 47.Smith J. America's War on the Kissing Bug. 2015. http://www.newyorker.com/tech/elements/americas-war-on-the-kissing-bug-and-chagas-disease accessed.
- 48.Gürtler R., Cohen J., Kitron U. Eco-epidemiology and control of Chagas disease in northern Argentina. One Health Newsletter. 2010;3:8–9. [Google Scholar]
- 49.Cardinal M., Castañera M., Lauricella M., Cecere M., Ceballos L., Vazquez-Prokopec G. A prospective study of the effects of sustained vector surveillance following community-wide insecticide application on Trypanosoma cruzi infection of dogs and cats in rural northwestern Argentina. Am.J.Trop. Med. Hyg. 2006;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- 50.Choi B., Pak A. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin. Invest. Med. 2006;29:351–364. [PubMed] [Google Scholar]
- 51.Zinsstag J., Schelling E., Bonfoh B., Fooks A., Kasymbekov J., Waltner-Toews D. Towards a “One Health” research and application tool box. Vet. Ital. 2009;45:121–133. [PubMed] [Google Scholar]
- 52.Wang C., Burris M.-A. Photovoice: concept, methodology, and use for participatory needs assessment. Health Educ. Behav. 1997;24:369–387. doi: 10.1177/109019819702400309. [DOI] [PubMed] [Google Scholar]
- 53.Curtis-Robles R., Wozniak E., Auckland L., Hamer G., Hamer S. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Elm E., Altman D., Egger M., Pocock S., Gøtzsche P., Vandenbroucke J. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Schulz K., Altman D., Moher D., For the CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Brit. Med. J. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.EQUATOR Network Reporting Guidelines under Development. 2016. http://www.equator-network.org/library/reporting-guidelines-under-development/#58 accessed.
- 57.Mazet J., Clifford D., Coppolillo P., Deolalikar A., Erickson J., Kazwala R. A “One Health” approach to address emerging zoonoses: the HALI project in Tanzania. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]