Abstract
The “Asian tiger mosquito”, Aedes albopictus, is highly invasive, an aggressive biter and a major arbovirus vector. It is not currently present on mainland Australia despite being intercepted on numerous occasions at international ports and infesting the Torres Strait of Australia since at least 2004. In the current paper, we describe the invasion and current status of Ae. albopictus in the Torres Strait, as well as research conducted to assess the threat of this species becoming established in arbovirus transmission cycles on the Australian mainland. Genetic analysis of the invading population demonstrated that the Indonesian region was the likely origin of the invasion and not Papua New Guinea (PNG) as initially suspected. There was also intermixing between Torres Strait, PNG and Indonesian populations, indicating that the species could be re-introduced into the Torres Strait compromising any successful eradication programme. Vector competence experiments with endemic and exotic viruses revealed that Ae. albopictus from the Torres Strait are efficient alphavirus vectors, but less efficient flavivirus vectors. Ae.albopictus obtains blood meals from a range of vertebrate hosts (including humans), indicating that it could play a role in both zoonotic and human-mosquito arbovirus transmission cycles in Australia. Predictive models coupled with climate tolerance experiments suggest that a Torres Strait strain of Ae. albopictus could colonise southern Australia by overwintering in the egg stage before proliferating in the warmer months. Cohabitation experiments demonstrated that the presence of Aedes notoscriptus larvae in containers would not prevent the establishment of Ae. albopictus. Evidence from these studies, coupled with global experience suggests that we need to be prepared for the imminent invasion of Australia by Ae. albopictus by thoroughly understanding its biology and being willing to embrace emerging control technologies.
Keywords: Aedes albopictus, Invasive mosquito, Arbovirus transmission, Australia, Ecology, Control
1. Aedes albopictus as an invasive species
The “Asian tiger mosquito”, Aedes albopictus, is a notorious pest mosquito that is a competent laboratory vector of at least 22 arboviruses and a major vector of dengue (DENVs) and chikungunya (CHIKV) viruses [1], [2]. It is a highly invasive species, which has expanded its geographical range from Southeast Asia and India to include North and South America, Europe, Africa and the Pacific region in the past three decades [3]. Ae. albopictus lays desiccation resistant eggs in both natural and man-made containers, which has helped facilitate the rapid movement of this species both within and between countries [4]. An example of its ability to rapidly colonise new locations occurred in the USA where, within two years of its introduction into Texas in 1985, it had spread to 15 states [5]. There has been considerable research conducted on Ae. albopictus, both in its native region of Southeast Asia and in areas where it has recently colonized, such as the USA, Brazil and parts of Europe (reviewed by [1], [6], [7]).
The expansion of Ae. albopictus into Australia was first recognized in 2005, when established populations were identified in the Torres Strait, the region that separates mainland Australia from Papua New Guinea (PNG) [8]. Because Ae. albopictus is only a recent addition to the mosquito fauna of Australia, there had been no research conducted into the ecology and potential disease risk of this species in the Australian context. Thus, we have been in the unique position where we have been able to study an emerging arbovirus vector before it invades and colonises mainland Australia. In the current paper we outline the discovery and status of Ae. albopictus, as well as research that has been conducted in the last 10 years to assess the factors that may influence the colonization and proliferation of this species, and its potential role in arbovirus transmission cycles in Australia. It is important to note that research on Ae. albopictus in Australia is restricted in its scope by regulatory requirements necessitating high level containment facilities on the mainland. Field work is limited to the Torres Strait, where it can be prohibitively expensive and logistically difficult to conduct, due to its geographic isolation, being approximately 800 km from the nearest mainland city of Cairns.
2. Ae. albopictus invades Australia
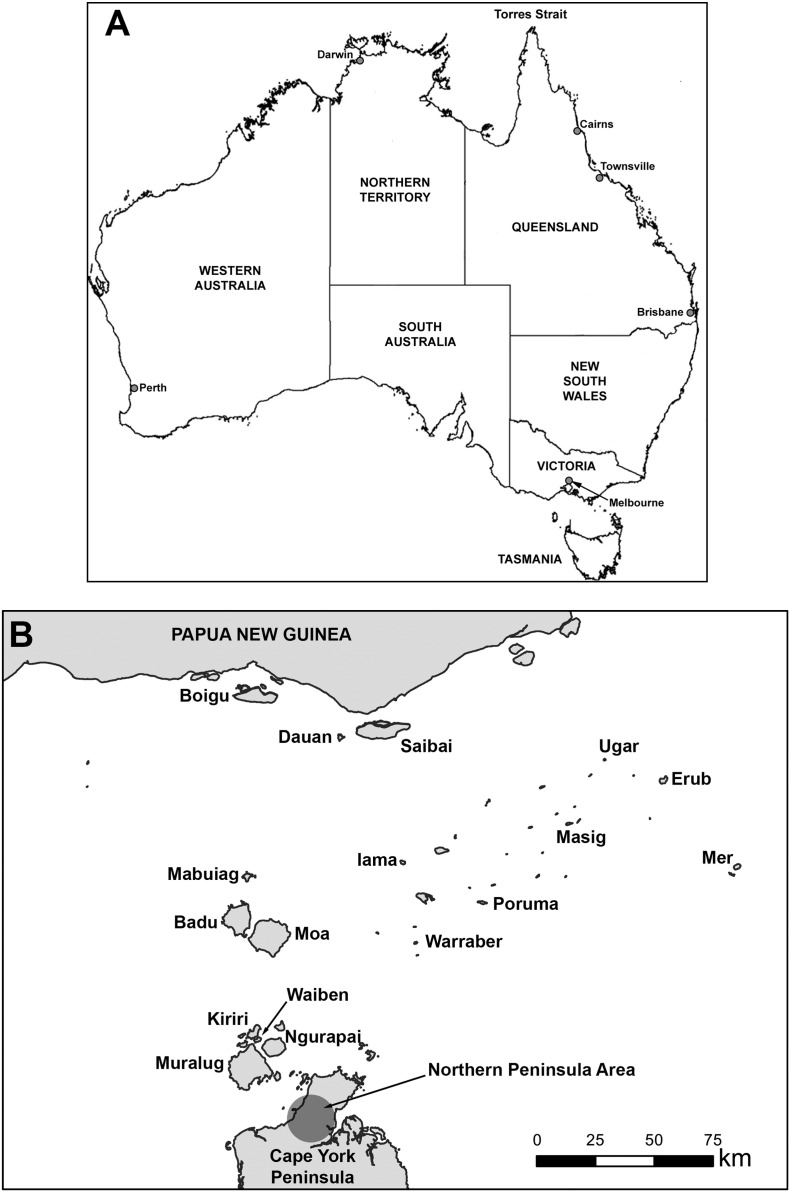
The risk of introduction and establishment of Ae. albopictus in Australia has been recognized for a number of years and has resulted in the implementation of quarantine procedures to detect incursions [9]. Between 1997 and 2005, there were at least 28 detections of Ae. albopictus by the then Australian Quarantine and Inspection Service at international seaports including Darwin, Cairns, Townsville, Brisbane, Sydney and Melbourne [10], but there was no evidence that the species had become established. In April 2005, ovitrap collections were undertaken on Masig Island in the Torres Strait (Fig. 1) to collect Aedes scutellaris for DENV type 2 transmission experiments [11]. During the collection trip, Biogents sentinel traps (BGS) were opportunistically deployed on the island. Unexpectedly, 42 out of 44 adult mosquitoes collected using BGS were identified as Ae. albopictus, with the remaining two being Ae. scutellaris [8]. Furthermore, when the ovitrap collections were hatched, 69% of adults were identified as Ae. albopictus. This discovery was surprising, as this species had never been detected during previous surveys conducted on the island, including during a dengue outbreak in 2004 [12], or on any other Torres Strait island [13]. This was despite the species being present in the villages of the Fly River region of southern PNG since at least the late 1980s [9], [14]. Retrospective analysis of the Masig Is specimens collected in 2004 using a newly developed molecular diagnostic assay [15] suggested that Ae. albopictus was present in low numbers [8]. A delimiting survey was subsequently conducted in 2005 across the 17 inhabited islands of the Torres Strait and communities of the Northern Peninsula Area (NPA), revealing that the incursion of Ae. albopictus was widespread, being present on 10 islands, although not in the mainland communities [8].
Fig. 1.
Map of Australia (A) and the Torres Strait (B) showing locations mentioned in the text. The island names listed on the map are the traditional Torres Strait Islander names. In the text, we have used the European names of Thursday and Horn islands, for Waiben Is and Ngurapai Is, respectively. The inner islands of the Torres Strait are Waiben, Ngurapai, Muralug and Kiriri, whilst the remainder are referred to as the outer islands.
In response to this alarming discovery, a strategy was initiated during the 2005–2006 wet season, with the primary objective of eliminating Ae. albopictus from the Torres Strait. This Ae. albopictus Eradication Programme has cost approximately $750,000 per annum with the funding provided by the Commonwealth Government Department of Health and Ageing [16]. This programme initially consisted of a) a surveillance component, which utilized container surveys and human bait sweep net collections to characterise the Ae. albopictus populations; and b) a control component which relied on elimination of larval containers, by removing or treating them with λ-cyhalothrin, a residual pyrethroid insecticide, or treatment of fixed containers, such as rainwater tanks or wells, with s-methoprene, an insect growth regulator. The λ-cyhalothrin (25 g/L active ingredient (a.i.); Demand® Insecticide, Syngenta Crop Protection, North Ryde, Australia) was diluted at label rate of 16 ml/L of water and sprayed almost to the point of runoff on targeted surfaces. The s-methoprene was applied to smaller containers as pellets (40 g/kg a.i.; ProLink® Pellets Mosquito Growth Regulator, Wellmark International, USA), at a rate of 1 pellet/L of estimated container volume. Larger containers, such as rainwater tanks and wells, were treated with ProLink® XR Briquets (18 g/kg a.i.) applied at 1 briquet/5000 L water.
Although some success was achieved in reducing populations on some islands, a combination of logistical issues with operating in remote locations, and a demonstrated risk of re-invasion (see below), suggested that a change in strategy was required. Consequently, in 2008, the programme changed from a focus on eradication to one of containment, whereby a cordon sanitaire approach was enabled on the inner islands of Thursday (Waiben) Is and Horn (Ngurapai) Is. These islands represent the primary administrative centers and the transport hubs to both the outer islands and onto mainland Australia, which had up until that point, remained free of Ae. albopictus.
Intensive control activities mainly targeting larval habitats were unable to keep Ae. albopictus off these inner islands, as it was discovered on Horn and Thursday Islands in 2010. Consequently, the surveillance and control methodologies utilized on Thursday and Horn Islands were augmented with the deployment of lethal tyre piles and harbourage spraying. The lethal tyre pile consists of 7–9 water-filled tyres treated with λ-cyhalothrin and s-methoprene pellets at the rates described above, to which female Ae. albopictus are attracted and come into contact with the insecticide. Harbourage spraying involves treating foliage and leaf litter in shaded areas with λ-cyhalothrin as described above with the objective of killing Ae. albopictus adults where they preferentially rest [17]. This strategy appears to have been extremely successful, with the number of positive human bait collection sites reduced from > 20 sites on each island per visit in the first year of discovery to < 5 sites in later visits.
In addition to the programme on the inner islands of the Torres Strait, a comprehensive surveillance system was implemented on the Australian mainland. This involved regular surveys in the communities of the NPA (Fig. 1) and a network of adult surveillance traps focussing on high risk areas, such as the seaport and airport, in Cairns, the main destination of sea and air traffic from the Torres Strait. In March 2009, Ae. albopictus was detected in one larval site on the NPA, triggering intensive vector control operations. Subsequent surveys provided no evidence that it had become established. Furthermore, Ae. albopictus originating from the Torres Strait have not been identified as having become established in Cairns.
Whilst the Torres Strait population does not appear to have expanded onto the Australian mainland, there is a risk that Ae. albopictus may be introduced via another mainland port from other international ports. For instance, since 2012, this species has been intercepted at international seaports in Melbourne, Townsville, Darwin and Cairns, and Perth airport. A Melbourne incursion in 2012 was particularly concerning, as the importation occurred via a shipment of Dracaena spp. (lucky bamboo) to a nursery. Fortunately, the infestation was confined to a quarantine-approved premise and there was no evidence that it had breached this containment. Multiple incursions occurred in 2012 into Townsville, with all linked to tyre imports from Japan via PNG. Despite instances such as these, as of September 2015, there is no evidence to suggest Ae. albopictus has become established on the Australian mainland.
3. Using population genetic tools to track the source of the Torres Strait Ae. albopictus population
As soon as the population was discovered in the Torres Strait, there was speculation as to the origins of the incursions. Given that Ae. albopictus had historically been detected in the villages of the lower Fly River region of the Western Province of PNG [9], [14], this appeared to be the most likely source. It was hypothesized that the establishment of water storage infrastructure in response to the 1997 drought in PNG had provided the ideal environment for Ae. albopictus populations to proliferate and eventually spill over into the Torres Strait. Once it was introduced, it was then assumed that local boat traffic ferried the mosquito between islands.
Using a combination of opportunistic collections from PNG, Indonesia and the Torres Strait, and population genetics methodologies, the movement of Ae. albopictus from PNG villages and between islands of the Torres Strait was examined [18]. Surprisingly, analysis of the mitochondrial DNA and microsatellites revealed that the Torres Strait and Southern Fly region Ae. albopictus were distinct from the extant PNG populations. Instead, affinity between the introduced population and Indonesian material strongly suggested that it was this region that was the source of the invasion. This expansion into the Torres Strait and southern Fly River region was linked to mosquitoes inhabiting water supplies on foreign fishing vessels that were fishing illegally in Australian waters. Furthermore, it was revealed that there were multiple mitochondrial DNA haplotypes moving between islands, indicating that if the mosquito was eradicated from an island, there was a high risk of re-introduction. This was one of the primary reasons that the control program was changed from one of elimination to a cordon sanitaire of the inner islands. Additional work is currently being undertaken to further genetically characterize the populations of Ae. albopictus in the Indonesian, Australasian and western Pacific regions, and to provide a repository to potentially infer the source of incursions into mainland ports.
4. Ae. albopictus as an arbovirus vector in Australia
There is concern that should Ae. albopictus become established on the Australian mainland where Ae. aegypti is absent, such as the densely populated cities of the eastern seaboard, then it will render these regions receptive to local transmission of DENVs, CHIKV and Zika virus. In addition to exotic viruses, Ae. albopictus could play a role in the transmission of endemic viruses. However, as none of the key Australian arboviruses has been isolated from field populations of Ae. albopictus, laboratory vector competence experiments have been used to incriminate this species in virus transmission. The only previously published experiments with endemic viruses demonstrated that overseas populations of Ae. albopictus were highly efficient laboratory vectors of a Ross River virus (RRV) strain isolated from the Cook Islands [19], [20]. The vector competence of a Masig Island population of Ae. albopictus for a number of endemic and exotic viruses has recently been examined [11], [21], [22]. This population was a relatively competent vector of the alphaviruses, RRV, Barmah Forest virus and CHIKV, and DENV type 3, but less so for DENV type 2, Murray Valley encephalitis, West Nile (Kunjin subtype) and Japanese encephalitis viruses. In addition, Ae. albopictus was able to transmit RRV and CHIKV after 4 and 2 days, respectively [21], which concurs with previous studies on the extrinsic incubation period of these viruses [23], [24].
Whilst the intrinsic ability of Ae. albopictus to transmit arboviruses has been established in the vector competence experiments, it is important to consider these results in an ecological context. A number of other factors can influence the ability of a species to serve as a vector, including daily survival rate, population density, and host feeding behaviour. Jansen et al. [25] used a simple model which integrates these key biological parameters with vector competence to provide a relative measure of the vectorial capacity of several key Australian mosquito species implicated as CHIKV vectors. This analysis demonstrated that the anthropophilic behavior of Ae. aegypti facilitated it being the key potential vector of CHIKV in Australia. The study also showed that Ae. albopictus could be an important vector, particularly at high population densities and when the majority of blood meals were obtained from humans. Given that Ae. albopictus is an opportunistic blood feeder [26], obtaining blood meals from both humans and other vertebrates, it has the ability to also be involved in transmission of RRV, a zoonotic virus which uses species, such as marsupials, as amplifying hosts.
5. Ecological factors that could impact the invasion of Australia by Ae. albopictus
There are a number of biotic and abiotic factors that could influence the ability for Ae. albopictus to become established on the Australian mainland. Ae. albopictus is relatively unique amongst the Aedes (Stegomyia) spp., in that it has colonised many temperate regions of the world, including northeast China, the Korean peninsula, Japan, North America and Europe. The key biological trait that allows Ae. albopictus to survive unfavourable winters in these regions is the ability to undergo diapause, whereby the fully-developed first instar larva suppresses hatching until conditions are favourable for larval development [27]. Predictive models have been developed in an attempt to determine the potential distribution of Ae. albopictus on the Australian mainland [10], [28]. These models predicted that a tropical strain of Ae. albopictus could establish itself down the entire eastern seaboard as far south as the state of Victoria. When diapausing populations from temperate regions were considered, the distribution extended even further, south to Tasmania and extended into inland areas [28].
To supplement these theoretical predictions of its potential geographical range, Nicholson et al. [29] examined the ability for a Torres Strait Ae. albopictus population to survive in temperate areas of Australia. These laboratory-based experiments demonstrated that although this Ae. albopictus population would increase in size during temperate summers, it was not able to complete immature development at average temperate winter climates. However, 17% of eggs were able to survive 3 months under these winter conditions, providing a mechanism for Ae. albopictus to survive before proliferating in the summer. As the Torres Strait Ae. albopictus population is of tropical origin, it may not readily undergo diapause. Introduction of a diapausing population or evolution of diapause in the Torres Strait population [30], could increase the survivorship and population performance of Ae. albopictus, thus enhancing their ability to become established at the temperature limits of its predicted distribution on the Australian mainland. Conversely, the egg survival experiments also demonstrated that conditions of low humidity, irrespective of temperature, were detrimental to survival, suggesting that the species would struggle to colonise the inland arid regions of Australia.
Whenever a species invades a new territory, there is an interaction between the invasive species and the resident species, which can be detrimental to either or both species. For instance, when Ae. albopictus invaded the southeastern USA, it rapidly supplanted the established populations of Ae. aegypti in some areas, whilst in others Ae. aegypti predominated, or the species co-existed [31], [32]. These observations were attributed to larval competition for limited resources in container habitats, asymmetric interspecific mating, and introduction of a parasite by Ae. albopictus, or a combination of these factors [3]. The establishment of Ae. albopictus in the northern region of Australia where Ae. aegypti currently exists, could lead to a reduction in the resident species, particularly in the coastal regions. Paradoxically, Ritchie et al. [8] suggests that the reduction in Ae. aegypti populations could lead to a reduced DENV transmission risk, because Ae. albopictus is not as efficient a vector of these viruses as Ae. aegypti [33]. However, a recent paper using blood feeding on viraemic patients, suggests that the difference in vector competence between the two species for DENVs may not be as great as has been previously thought [34]. Combined with the major role that Ae. albopictus has played in the re-emergence of CHIKV, this indicates that the displacement of Ae. aegypti by Ae. albopictus may not have as pronounced effect on transmission of these viruses.
There is always the possibility that endemic container inhabiting species could adversely impact the ability of Ae. albopictus to become established on the Australian mainland. One such species is Aedes notoscriptus, which has been observed inhabiting the same containers as Ae. albopictus in the Torres Strait and is the most common container-inhabiting Aedes spp. in Australia. Consequently, the effects of cohabitation with Ae. notoscriptus on Ae. albopictus were assessed under different climatic conditions, nutrient levels, nutrient source and species densities [35]. The experiments revealed that although increasing densities of Ae. notoscriptus reduced the survivorship and population performance of Ae. albopictus, it was not sufficient to prevent the population increasing in size through successive generations. It was concluded that the presence of Ae. notoscriptus should not prevent the establishment of Ae. albopictus in Australia.
6. Surveillance and control of Ae. albopictus
A number of strategies have been used in Australia for assessing the presence, distribution and population densities of Ae. albopictus. Ovitraps have been used successfully by quarantine and public health personnel to detect incursions of this mosquito at ports on the Australian mainland [36]. Five minute human bait sweep net collections have been used for rapid assessment of Ae. albopictus populations in the Torres Strait [8]. These human bait collections have been supplemented with container surveys, which can be labour intensive and key productive containers can often be missed. Furthermore, it is difficult to morphologically distinguish larvae of Ae. albopictus from Ae. scutellaris, although this issue has been overcome with the development and implementation of molecular diagnostic assays [15], [37]. Several traps have recently been developed to specifically collect container inhabiting Aedes spp. The BGS (http://www.biogents.com/cms/website.php?id=/en/traps/mosquito_traps/bg_sentinel.htm) has been employed for Ae. albopictus surveillance overseas [38] and it was this trap that yielded the first collections of this species in Australia [8]. A network of BGS is used by local public health officers in Cairns for surveillance of Ae. albopictus and they are now being implemented at a number of first ports around Australia to supplement existing ovitrap and light trap collections. Although effective, the BGS do suffer from several drawbacks, including the relatively high cost, requirement for a source of electricity (mains power or battery) and documented issues with trap reliability [39]. Another adult trap that shows promise is the Gravid Aedes Trap (GAT), which was recently developed for collecting Ae. aegypti and is commercially available (http://www.biogents.com/cms/website.php?id=/en/traps/biogents-trap-systems/bg_gat.htm) [40], [41]. The GAT has also been shown to be effective for collecting female Ae. albopictus in recent trials in the Torres Strait and the Solomon Is (S. A. Ritchie and C. Butafa, unpublished data). Furthermore, Johnson and Ritchie [42] found that addition of a sound lure playing the conspecific female wing beat frequency enabled the GAT to capture male Ae. aegypti. A similar approach could be used with Ae. albopictus, providing an inexpensive passive trap to capture male and female Aedes.
Control of Ae. albopictus is extremely challenging, due to a high level of heterogeneity in its distribution, cryptic larval habitats, opportunistic feeding patterns and resting behaviour. Initial control strategies implemented as part of the Ae. albopictus eradication program, including source reduction and insecticide treatment of containers proved to be unsustainable on the outer islands of the Torres Strait and there was also the risk of re-introduction. However, there appears to have been success on the inner islands when these methods were supplemented with harbourage spraying and strategic placement of lethal tyre piles. The accessibility of the inner islands means that application of insecticides can occur on regular basis to ensure continued efficacy of treatment. Indeed, there has been a sustained reduction in Ae. albopictus populations on these islands when compared to their widespread distribution during the wet season when they were first discovered. Despite this success, there are major issues with the sustainability of the current control programme. First and foremost, it requires a guarantee of ongoing funding, based on periodic resubmissions to Commonwealth and State governments. Another issue is the reliance on application of residual pyrethroids, which could lead to resistance in the local Ae. albopictus populations. Nonetheless, the successful suppression on the inner islands provides a model template for rapid control of Ae. albopictus, should established populations be discovered on the Australian mainland. Whatever surveillance and control strategies are deployed, authorities must be able to quickly locate and eliminate mainland incursions before this rapidly invasive mosquito spreads beyond our capacity to control it. It is imperative that sound contingency plans for rapid, effective response to Ae. albopictus are formulated. A key component of these contingency plans is formal agreements between key stakeholders as to their role in any incursion response.
There are several emerging technologies primarily developed for Ae. aegypti and which may be harnessed for the control of Ae. albopictus populations or for rendering them incapable of transmitting arboviruses [43]. The first of these is the mass release of mosquitoes transinfected with the endosymbiotic bacterium Wolbachia [44]. Wolbachia induces a number of phenotypes in the infected host, including cytoplasmic incompatibility which drives the infection into the wild population, life shortening and inhibition of arbovirus replication. The wMel strain of Wolbachia was recently transinfected into Ae. albopictus producing complete bidirectional cytoplasmic incompatibility and inhibition of DENV and CHIKV transmission [45], [46]. The methodology used for release of Wolbachia-infected Ae. aegypti could also be applied to releases of Ae. albopictus [47]. Another strategy involves the release of male mosquitoes carrying a dominant lethal gene (RIDL), which induces a conditional female-specific late-acting flightless phenotype and is expected to result in a reduction of the wild population [48]. A final strategy that may be utilised for Ae. albopictus suppression in the future is RNA interference mediated gene knockdown, recently demonstrated in Ae. aegypti, whereby mosquitoes treated with double stranded RNA exhibit reduced fertility or a highly male-biased population of mosquitoes [49]. Regardless of the technology, a number of regulatory approvals must be obtained before any releases, and may include gene technology and quarantine approvals. In addition, there should be a high level of community consultation and rigorous biosafety assessment, which is something the Wolbachia-based Eliminate Dengue program for Ae. aegypti has effectively achieved [50], [51].
7. Conclusions
Based on global experience, it seems to be only a matter of time until Ae. albopictus becomes established on the Australian mainland. The results of the predictive modelling, climatic tolerance and cohabitation experiments suggest that much of the eastern Australia is receptive to establishment of this species. Populous regions in southern Australia would then become susceptible to local transmission of DENVs and CHIKV, as well as hosting another competent urban vector of RRV. Such a scenario would add another priority for mosquito control, necessitating an integration of container-based control methodologies into saltmarsh and groundwater mosquito control programs. The successful strategy of suppression which has so far contained the Torres Strait infestation could be implemented in these southern regions. Adoption of emerging control technologies will require significant investment into assessing their efficacy in the Australian environment, obtaining the necessary regulatory approvals and undertaking community consultation. It is essential that the formulation of control strategies, as well as further risk analyses and predictive modelling, be underpinned by a thorough understanding of the biology of Australian Ae. albopictus populations. Some of the key areas of research required include the analysis of host feeding patterns, influence of co-habitation with endemic Ae. aegypti, interspecific mating with endemic and exotic Aedes spp., dispersal characteristics, and temporal and geographical distribution.
Contributor Information
Andrew F. van den Hurk, Email: andrew.vandenhurk@health.qld.gov.au.
Jay Nicholson, Email: Jay.Nicholson@health.wa.gov.au.
Nigel W. Beebe, Email: n.beebe@uq.edu.au.
Joe Davis, Email: Joe.Davis@health.qld.gov.au.
Odwell M. Muzari, Email: Odwell.Muzari@health.qld.gov.au.
Richard C. Russell, Email: richard.russell@sydney.edu.au.
Gregor J. Devine, Email: Greg.Devine@qimrberghofer.edu.au.
Scott A. Ritchie, Email: scott.ritchie@jcu.edu.au.
References
- 1.Gratz N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsetsarkin K.A., Vanlandingham D.L., McGee C.E., Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lounibos L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 4.Reiter P. Aedes albopictus and the world trade in used tires, 1988–1995: the shape of things to come? J. Am. Mosq. Control Assoc. 1998;14:83–94. [PubMed] [Google Scholar]
- 5.Moore C.G., Mitchell C.J. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg. Infect. Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- 7.Bonizzoni M., Gasperi G., Chen X., James A.A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29:460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie S.A., Moore P., Carruthers M., Williams C., Montgomery B., Foley P. Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 2006;22:358–365. doi: 10.2987/8756-971X(2006)22[358:DOAWIO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Kay B.H., Ives W.A., Whelan P.I., Barker-Hudson P., Fanning I.D., Marks E.N. Is Aedes albopictus in Australia? Med. J. Aust. 1990;153:31–34. doi: 10.5694/j.1326-5377.1990.tb125460.x. [DOI] [PubMed] [Google Scholar]
- 10.Russell R.C., Williams C.R., Sutherst R.W., Ritchie S.A. Aedes (Stegomyia) albopictus — a dengue threat for southern Australia. Commun. Dis. Intell. 2005;29:296–298. doi: 10.33321/cdi.2005.29.31. [DOI] [PubMed] [Google Scholar]
- 11.Moore P.R., Johnson P.H., Smith G.A., Ritchie S.A., van den Hurk A.F. Infection and dissemination of dengue virus type 2 in Aedes aegypti, Aedes albopictus, and Aedes scutellaris from the Torres Strait, Australia. J. Am. Mosq. Control Assoc. 2007;23:383–388. doi: 10.2987/5598.1. [DOI] [PubMed] [Google Scholar]
- 12.Hanna J.N., Ritchie S.A. Outbreaks of dengue in north Queensland, 1990–2008. Commun. Dis. Intell. 2009;33:32–33. doi: 10.33321/cdi.2009.33.5. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie S., Montgomery B., Walsh I. Production of mosquitoes in rainwater tanks and wells on Yorke Island, Torres Strait: preliminary study. Environ. Heal. 2002;2:13–18. [Google Scholar]
- 14.Cooper R.D., Waterson D.G.E., Kupo M., Sweeney A.W. Aedes albopictus (Skuse) (Diptera: Culicidae) in the Western Province of Papua New Guinea and the threat of its introduction to Australia. J. Aust. Entomol. Soc. 1994;33:115–116. [Google Scholar]
- 15.Beebe N.W., Whelan P.I., van den Hurk A.F., Ritchie S.A., Corcoran S., Cooper R.D. A polymerase chain reaction-based diagnostic to identify larvae and eggs of container mosquito species from the Australian region. J. Med. Entomol. 2007;44:376–380. doi: 10.1603/0022-2585(2007)44[376:apcrdt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Devine G., Muzari O. Tropical Public Health Services; Cairns, Australia: 2013. Report to DoHA: Aedes albopictus prevention and control program in the Torres Strait. [Google Scholar]
- 17.Muzari O.M., Adamczyk R., Davis J., Ritchie S., Devine G. Residual effectiveness of lambda-cyhalothrin harbourage sprays against foliage-resting mosquitoes in north Queensland. J. Med. Entomol. 2014;51:444–449. doi: 10.1603/me13141. [DOI] [PubMed] [Google Scholar]
- 18.Beebe N.W., Ambrose L., Hill L.A., Davis J.B., Hapgood G., Cooper R.D. Tracing the tiger: population genetics provides valuable insights into the Aedes (Stegomyia) albopictus invasion of the Australasian Region. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell C.J., Gubler D.J. Vector competence of geographic strains of Aedes albopictus and Aedes polynesiensis and certain other Aedes (Stegomyia) mosquitoes for Ross River virus. J. Am. Mosq. Control Assoc. 1987;3:142–147. [PubMed] [Google Scholar]
- 20.Mitchell C.J., Miller B.R., Gubler D.J. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J. Am. Mosq. Control Assoc. 1987;3:460–465. [PubMed] [Google Scholar]
- 21.Nicholson J.N., Ritchie S.A., van den Hurk A.F. Aedes albopictus (Diptera: Culicidae) as a potential vector of endemic and exotic arboviruses in Australia. J. Med. Entomol. 2014;51:661–669. doi: 10.1603/me13204. [DOI] [PubMed] [Google Scholar]
- 22.van den Hurk A.F., Hall-Mendelin S., Pyke A.T., Smith G.A., Mackenzie J.S. Vector competence of Australian mosquitoes for chikungunya virus. Vector Borne Zoonotic Dis. 2010;10:489–495. doi: 10.1089/vbz.2009.0106. [DOI] [PubMed] [Google Scholar]
- 23.Dubrulle M., Mousson L., Moutailler S., Vazeille M., Failloux A.B. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay B.H., Jennings C.D. Enhancement or modulation of the vector competence of Ochlerotatus vigilax (Diptera: Culicidae) for Ross River virus by temperature. J. Med. Entomol. 2002;39:99–105. doi: 10.1603/0022-2585-39.1.99. [DOI] [PubMed] [Google Scholar]
- 25.Jansen C.C., Williams C.R., van den Hurk A.F. The usual suspects: comparison of the relative roles of potential urban chikungunya virus vectors in Australia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richards S.L., Ponnusamy L., Unnasch T.R., Hassan H.K., Apperson C.S. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J. Med. Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estrada-Franco J.G., Craig G.B., Jr. PAHO Technical Paper. Vol. 42. 1995. Biology, relationships and control of Aedes albopictus; pp. 1–51. [Google Scholar]
- 28.Hill M.P., Axford J.K., Hoffman A.A. Predicting the spread of Aedes albopictus in Australia under current and future climates: multiple approaches and datasets to incorporate potential evolutionary divergence. Austral Ecol. 2014;39:469–478. [Google Scholar]
- 29.Nicholson J., Ritchie S.A., Russell R.C., Zalucki M.P., van den Hurk A.F. Ability for Aedes albopictus (Diptera: Culicidae) to survive at the climatic limits of its potential range in eastern Australia. J. Med. Entomol. 2014;51:948–957. doi: 10.1603/me14079. [DOI] [PubMed] [Google Scholar]
- 30.Lounibos L.P., Escher R.L., Lourenço-De-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2003;96:512–518. [Google Scholar]
- 31.Juliano S.A. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition. Ecology. 1998;79:255–268. [Google Scholar]
- 32.O'Meara G.F., Evans L.F., Jr., Gettman A.D., Cuda J.P. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 33.Lambrechts L., Scott T.W., Gubler D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehorn J., Kien D.T., Nguyen N.M., Nguyen H.L., Kyrylos P.P., Carrington L.B. comparative susceptibility of Aedes albopictus and Aedes aegypti to dengue virus infection after feeding on blood of viremic humans: implications for public health. J. Infect. Dis. 2015;212:1182–1190. doi: 10.1093/infdis/jiv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson J., Ritchie S.A., Russell R.C., Webb C.E., Cook A., Zalucki M.P. Effects of cohabitation on the population performance and survivorship of the invasive mosquito Aedes albopictus and the resident mosquito Aedes notoscriptus (Diptera: Culicidae) in Australia. J. Med. Entomol. 2015;52:375–385. doi: 10.1093/jme/tjv004. [DOI] [PubMed] [Google Scholar]
- 36.Whelan P., Russell R.C., Hayes G., Tucker G., Goodwin G. Exotic Aedes mosquitoes: onshore detection and elimination in Darwin, Northern Territory. Commun. Dis. Intell. 2001;25:283–285. doi: 10.33321/cdi.2001.25.52. [DOI] [PubMed] [Google Scholar]
- 37.Hill L.A., Davis J.B., Hapgood G., Whelan P.I., Smith G.A., Ritchie S.A. Rapid identification of Aedes albopictus, Aedes scutellaris, and Aedes aegypti life stages using real-time polymerase chain reaction assays. Am. J. Trop. Med. Hyg. 2008;79:866–875. [PubMed] [Google Scholar]
- 38.Fonseca D.M., Unlu I., Crepeau T., Farajollahi A., Healy S.P., Bartlett-Healy K. Area-wide management of Aedes albopictus. Part 2: gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag. Sci. 2013;69:1351–1361. doi: 10.1002/ps.3511. [DOI] [PubMed] [Google Scholar]
- 39.Crepeau T.N., Unlu I., Healy S.P., Farajollahi A., Fonseca D.M. Experiences with the large-scale operation of the Biogents Sentinel trap. J. Am. Mosq. Control Assoc. 2013;29:177–180. doi: 10.2987/12-6277r.1. [DOI] [PubMed] [Google Scholar]
- 40.Ritchie S.A., Buhagiar T.S., Townsend M., Hoffmann A., van den Hurk A.F., McMahon J.L. Field validation of the gravid Aedes trap (GAT) for collection of Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2014;51:210–219. doi: 10.1603/me13105. [DOI] [PubMed] [Google Scholar]
- 41.Eiras A.E., Buhagiar T.S., Ritchie S.A. Development of the gravid Aedes trap for the capture of adult female container-exploiting mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2014;51:200–209. doi: 10.1603/me13104. [DOI] [PubMed] [Google Scholar]
- 42.Johnson B.J., Ritchie S.A. The Siren’s song: exploitation of female flight tones to passively capture male Aedes aegypti mosquitoes. J. Med. Entomol. 2016;53:245–248. doi: 10.1093/jme/tjv165. [DOI] [PubMed] [Google Scholar]
- 43.McGraw E.A., O'Neill S.L. Beyond insecticides: new thinking on an ancient problem. Nat. Rev. Microbiol. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 44.Iturbe-Ormaetxe I., Walker T., O'Neill S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12:508–518. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blagrove M.S., Arias-Goeta C., Di Genua C., Failloux A.B., Sinkins S.P. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits chikungunya virus. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blagrove M.S., Arias-Goeta C., Failloux A.B., Sinkins S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. U. S. A. 2012;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann A.A., Montgomery B.L., Popovici J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 48.Labbe G.M., Scaife S., Morgan S.A., Curtis Z.H., Alphey L. Female-specific flightless (fsRIDL) phenotype for control of Aedes albopictus. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whyard S., Erdelyan C.N., Partridge A.L., Singh A.D., Beebe N.W., Capina R. Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit. Vectors. 2015;8:96. doi: 10.1186/s13071-015-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy B.J., Jansen C.C., Murray J., De Barro P. CSIRO; Indooroopilly: 2010. Risk Analysis on the Australian Release of Aedes aegypti (L.) (Diptera: Culicidae) Containing Wolbachia. [Google Scholar]
- 51.Kolopack P.A., Parsons J.A., Lavery J.V. What makes community engagement effective?: lessons from the Eliminate Dengue Program in Queensland, Australia. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003713. [DOI] [PMC free article] [PubMed] [Google Scholar]