Abstract
West Nile virus (WNV), a Flavivirus with an avian primary host, is already widespread in Europe and might also pose an infection risk to Germany, should competent mosquito vectors be present. Therefore, we analysed the ability of WNV to infect German Culex mosquitoes with special emphasis on field collected specimens of Culex torrentium and Culex pipiens biotype pipiens. We collected egg rafts of Culex mosquitoes over two subsequent seasons at two geographically distinct sampling areas in Germany and differentiated the samples by molecular methods. Adult females, reared from the various egg rafts, were challenged with WNV by feeding of artificial blood meals. WNV infection was confirmed by real-time RT-PCR and virus titration. The results showed that field collected C. pipiens biotype pipiens and C. torrentium mosquitoes native to Germany are susceptible to WNV infection at 25 °C as well as 18 °C incubation temperature. C. torrentium mosquitoes, which have not been established as WNV vector so far, were the most permissive species tested with maximum infection rates of 96% at 25 °C. Furthermore, a disseminating infection was found in up to 94% of tested C. pipiens biotype pipiens and 100% of C. torrentium. Considering geographical variation of susceptibility, C. pipiens biotype pipiens mosquitoes from Southern Germany were more susceptible to WNV infection than corresponding populations from Northern Germany. All in all, we observed high infection and dissemination rates even at a low average ambient temperature of 18 °C. The high susceptibility of German Culex populations for WNV indicates that an enzootic transmission cycle in Germany could be possible.
Keywords: Culex mosquitoes, West Nile virus, Infection assay
1. Introduction
West Nile virus [(WNV); family Flaviviridae, genus Flavivirus] infections are a growing concern to Europe as illustrated by repeated outbreaks of West Nile fever (WNF) and West Nile neuroinvasive disease (WNND) in south-eastern parts of Europe [1]. Further, the increase of imported human WNV infections into Germany [2], [3] raises concerns that the virus may also become established here. Since the emergence of arboviruses is closely linked to the presence of suitable vectors and susceptible hosts, the knowledge of principle vector species is essential for selection of adequate control measurements [4].
WNV is maintained in nature within an enzootic cycle involving ornithophilic mosquitoes and birds, but it can infect humans, equines and other vertebrates as illustrated by WNF and WNND in humans [5], [6], [7]. Since its first isolation in Uganda in 1937 [8], WNV has been isolated from mosquitoes in Eurasia [1] and Australia [9], [10]. Moreover, following a single introduction to New York City in 1999, WNV has also spread throughout the Americas [11], [12]. Members of the Culex (C.) pipiens complex (Linnaeus 1758), especially C. pipiens, Culex tarsalis and Culex quinquefasciatus, have been described as enzootic and bridge vectors for WNV in the United States of America and other WNV endemic regions [4], [13], [14]. The C. pipiens complex members C. pipiens biotypes pipiens and molestus as well as Culex torrentium are abundant in Central Europe [15], [16]. The C. pipiens biotype pipiens and C. torrentium preferentially take blood meals from birds, rendering them potential enzootic vectors for WNV in Central Europe [17]. Recently, two studies have demonstrated the potential of a Dutch laboratory colony of C. pipiens to serve as a vector for WNV [18], [19]. Up to now, there are no data available on the vector competence of C. torrentium for WNV. In contrast, C. torrentium is a proven enzootic vector of another arbovirus, the Sindbis virus, in Sweden [20], [21], [22].
Species identification within the Culex genus is difficult using classical morphological methods. Differentiation of C. pipiens and C. torrentium females relies on the occurrence of pre-alar scales, or measurement of the wing veins [23] but both methods are difficult to apply on large number of samples. The difficulties of correct assignment of the C. pipiens biotypes and C. torrentium might lead to misinterpretations of their vector potential, especially since virus isolation of field collected mosquitoes is a main marker for involvement in transmission.
Here we analyse the ability of Central European populations of C. pipiens biotype pipiens and C. torrentium to become infected with WNV and, in doing so, deliver a proxy for the vector competence estimations of these Culex populations for WNV. To avoid culturing effects, we used field collected samples from two geographically distinct regions in Germany and first separated the species and/or biotypes by multiplex qPCR [16]. Secondly, we analysed infection and dissemination rates after experimental feeding of WNV lineage 1 strain NY99 in C. pipiens biotype pipiens and C. torrentium females.
2. Material and methods
2.1. Mosquito strains and field collected mosquito samples
The C. quinquefasciatus (Malaysia) laboratory colony was obtained from Bayer (Bayer, Leverkusen, Germany). The C. pipiens biotype molestus colonies were established in our laboratory and originated from Heidelberg (Mol S), Wendland (Mol W) and Langenlehsten (Mol LL). The Mol S colony was maintained for 3 years in the laboratory prior to infection experiments. The Mol W colony was established from blood fed gravid females collected in 2012 in Wendland/Germany and the Mol LL colony was established from egg rafts collected in 2013 in Langenlehsten/Germany.
C. pipiens biotype pipiens and C. torrentium mosquitoes were obtained from egg raft collections carried out in Hamburg area/Germany [Altes Land 53°35′N 9°32′E, Langenlehsten 53°30′N 10°44′E and Hamburg City 53°32′N, 9°57′E; hereafter referred to as the North population (N)] and Lake Constance/Germany [Radolfzell-Böhringen 47°44′N, 8°58′E and Mettenau 47°43′N, 8°59′E; hereafter referred to as the South population (S)]. Specimens for infection experiments were collected from August to October in 2012 and 2013. The egg collection was carried out using gravid traps filled with hay infusion placed in proximity to natural breeding sites of Culex mosquitoes, i.e. water bodies to attract gravid females and stimulate egg deposition. Traps were checked twice a day for freshly deposited egg rafts, which were retrieved from water surface using a wooden spatula and placed in individual plastic cups for transportation to the laboratory.
2.2. Rearing of larvae and adult mosquitoes
Field collected and laboratory bred mosquitoes were kept at 23 +/− 2 °C with a relative humidity of 80% and a 16 h:8 h light:dark photoperiod. Field collected egg rafts were floated separately in dechlorinated water and hatched larvae were fed on TetraMin flaked fish food (Tetra GmbH, Melle, Germany). From each individual egg raft, 4–5 larvae were used for molecular taxonomic identification as described previously [16]. Once identified, larvae were pooled according to species or biotype and emerging females (4–14 days of age) were distributed into plastic vials at 10–15 females each. Adult mosquitoes were fed on fructose pads (8% D(−)-Fructose, Carl Roth GmbH, Karlsruhe, Germany; 0.02% 4-Aminobenzoic acid, Sigma Aldrich, Seelze, Germany) for maintenance and starved overnight prior to infection. To facilitate egg production of C. quinquefasciatus and C. pipiens biotype molestus laboratory colonies, a blood meal consisting of human erythrocyte concentrate (Blood group 0, Blood bank, University Hospital Hamburg)/50% FCS (PAA/GE Healthcare Life Sciences, Germany)/0.5% fructose (Carl Roth GmbH, Karlsruhe, Germany) was provided weekly.
2.3. Experimental infection and dissemination assays
Mosquitoes used in experiments were kept in incubators at 25 °C or 18 °C/80% humidity. Infection was performed overnight via an artificial blood meal containing 1.0–1.6 × 107 PFU WNV lineage 1 strain NY99 [24]/mL blood meal presented on cotton sticks. This method has been shown to lead to efficient WNV infection in C. pipiens [25]. Fully engorged mosquitoes were either frozen at − 80 °C (day 0) or kept at 25 °C or 18 °C for 14 to 35 days. The two temperatures were chosen to mimic the climatic conditions in Germany with 25 °C representing the mean average temperature in Germany in July/August in the south of Germany and 18 °C representing the maximum average temperature during a minimum of 4 months/year in the north and south of Germany. For WNV RNA purification, mosquitoes were homogenised separately in 500 μl of medium (Schneider's Drosophila Medium, PAN Biotech, Aidenbach, Germany). To calibrate our infection assay, we used laboratory strains of C. pipiens biotype molestus and C. quinquefasciatus. C. pipiens biotype molestus has been described as a WNV vector in Israel [26], and C. quinquefasciatus was described by several studies from North America as a competent vector for WNV [14], [27], [28]. For the analysis of disseminating infection, frozen mosquitoes were beheaded under a dissection microscope and the body and head were separately homogenized in medium. The virus detection in heads as a method to measure the dissemination rate has been established previously by several other studies [29], [30], [31], [32].
2.4. Quantification of viral RNA and infectious particles
WNV RNA purification was performed with QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturers' instructions. The quantitative real-time PCR was performed using QuantiTec Probe RT-PCR MasterMix Kit (Qiagen, Hilden, Germany), with Light Cycler 480 II (Roche, Rotkreuz, Switzerland) and using 9 μl reaction mix containing 0.6 μM of the following primers and 0.2 μM of the following probes: OSM_145: GGCAATGGAGTCATAATG; OSM_146: GCATCTCAGGTTCGAATC; OSM_147: -FAM-CCAACGGCTCATACATAAGCG-BHQ1 and 2 μl RNA. For the analysis of virus titres, mosquito organ homogenate was filtered using 0.20 μm filters and inoculated on Vero cells (96-well format) with 10-fold dilutions and indirect immunofluorescent revelation after 3 days. Briefly, inoculated Vero cells were fixed in 4% formaldehyde for 30 min and immunostained using WNV recombinant E protein mouse monoclonal antibody (ABIN782271, antibodies-online GmbH, Germany) diluted 1:100 in PBT for 1–2 h and then with fluorescein (FITC)-conjugated AffiniPure goat anti-mouse IgG (115-095-003, Jackson ImmunoResearch Laboratories, Inc., USA) diluted 1:200 in PBT for 1 h. Infected wells were counted and viral titres were calculated using the Spearman and Kärber algorithm described by Hierholzer and Killington [33].
2.5. Statistics
Fisher's exact test was applied to assess differences of proportions between the species groups. A p value of less than 0.05 is deemed statistically significant. The statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, Inc., CA, USA).
3. Results
3.1. Susceptibility of German mosquitoes for WNV infection
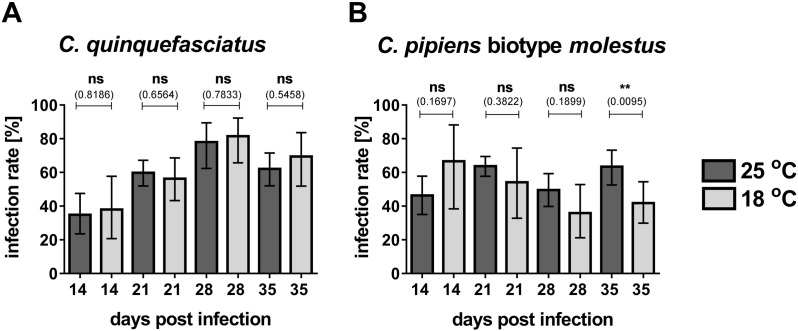
Susceptibility of German mosquitoes for WNV was analysed using 719 egg rafts of C. pipiens biotype pipiens and 373 egg rafts of C. torrentium collected in 2012 and 2013 (Table 1). Laboratory strains of C. quinquefasciatus and C. pipiens biotype molestus served as positive controls to calibrate the infection assay. Analysis of day 0 females of C. quinquefasciatus and C. pipiens biotype molestus for viral RNA content and viral particles revealed that 100% of tested females were positive for WNV viral RNA with viral titres of 1.4 × 101–7.76 × 103 PFU/ml in C. quinquefasciatus and 8 to 7.7 × 104 PFU/ml in C. pipiens biotype molestus. We further tested the correlation between viral RNA and infectious viral particles at different time points after infection to verify the use of virus RNA detection as a proxy for infection in the following experiments and to define the cut-off for qPCR detection. Selected samples from three independent infection experiments of C. quinquefasciatus and C. pipiens biotype molestus sampled at day 14 and day 21 post-infection were used. All samples tested positive in qRT-PCR with Ct-values below or equal 35 were also tested positive for infectious viral particles. Virus titres were 1.2 × 101–1.4 × 102 PFU/ml at day 14 and 4.0–7.7 × 103 PFU/ml at day 21 in C. quinquefasciatus and 7.8–2.5 × 101 PFU/ml at day 14 and 7.8–1.4 × 102 PFU/ml at day 21 for C. pipiens biotype molestus. Next, we analysed the WNV infection rates within the two positive controls. Infection rates were calculated from the percentage of females tested positive for WNV infection with respect to the total number of blood fed females. For C. quinquefasciatus at the maximum infection rate of 82% (n = 38) was found at 18 °C on day 28 post-infection (Fig. S1A). Furthermore, we did not find significant differences in C. quinquefasciatus between mosquitoes incubated at 25 °C or 18 °C temperature (Fig. S1A). In the case of C. pipiens biotype molestus mosquitoes the maximum infection rate of 67% (n = 15) was found at day 14 post-infection and 18 °C incubation temperature (Fig. S1A). Significant differences between infection rates at 25 °C and 18 °C were only observed at day 35 post-infection, where 63% (n = 90) and 42% (n = 67) were infected, respectively (p value = 0.0095, Fig. S1B).
Table 1.
Summary of mosquito samples used for WNV infection assays in 2013 and 2012.
The following lab strains were kept in captivity for 2 month to 2 years prior to the WNV infection assay: Culex quinquefasciatus (C. qui; Malaysia strain, Bayer Company), Culex pipiens biotype molestus Heidelberg (C. Mol S), Culex pipiens biotype molestus Wendland (C. Mol W) and Culex pipiens biotype molestus Langenlehsten (C. Mol LL).
The following species were reared from field-collected egg rafts: Culex pipiens biotype pipiens from Hamburg/Langenlehsten/Altes Land (C. pip North), Culex pipiens biotype pipiens from Lake Constance (C. pip South), Culex torrentium from Hamburg/Langenlehsten/Altes Land (C. tor North) and Culex torrentium from Lake Constance (C. tor South).
For each infection time point, the numbers of individual blood feeding assays performed (experiments) are listed. In addition, the combined number of mosquitoes presented with an infected blood meal during these blood meal assays (individuals) is also depicted.
| Species | Origin | Egg rafts | Temperature [°C] | Infection time (days) | Individuals [#] | Experiments [#] |
|---|---|---|---|---|---|---|
| C. qui | Malaysia | Lab strain | 25 | 14 | 66 (88) | 9 (4) |
| 25 | 21 | 169 (88) | 12 (4) | |||
| 25 | 28 | 41 | 5 | |||
| 25 | 35 | 103 | 7 | |||
| 18 | 14 | 29 | 6 | |||
| 18 | 21 | 64 | 6 | |||
| 18 | 28 | 38 | 5 | |||
| 18 | 35 | 36 | 5 | |||
| C. pip | North | 186 (222) | 25 | 14 | 22 (94) | 5 (3) |
| 25 | 21 | 65 (173) | 7 (3) | |||
| 25 | 28 | 53 | 6 | |||
| 25 | 35 | 69 | 5 | |||
| 18 | 14 | 23 | 5 | |||
| 18 | 21 | 41 | 5 | |||
| 18 | 28 | 46 | 5 | |||
| 18 | 35 | 72 | 5 | |||
| C. pip | South | 311 | 25 | 14 | 8 | 2 |
| 25 | 21 | 24 | 4 | |||
| 25 | 28 | 11 | 3 | |||
| 25 | 35 | 10 | 3 | |||
| 18 | 14 | 8 | 2 | |||
| 18 | 21 | 26 | 4 | |||
| 18 | 28 | 12 | 3 | |||
| 18 | 35 | 14 | 3 | |||
| C. mol | S, W, LL | Lab strains | 25 | 14 | 80 | 6 |
| 25 | 21 | 273 | 14 | |||
| 25 | 28 | 109 | 11 | |||
| 25 | 35 | 90 | 6 | |||
| 18 | 14 | 15 | 3 | |||
| 18 | 21 | 24 | 4 | |||
| 18 | 28 | 39 | 5 | |||
| 18 | 35 | 67 | 5 | |||
| C. tor | North | 225 (119) | 25 | 14 | 12 (130) | 3 (4) |
| 25 | 21 | 34 (138) | 6 (4) | |||
| 25 | 28 | 9 | 3 | |||
| 25 | 35 | 9 | 3 | |||
| 18 | 14 | 12 | 3 | |||
| 18 | 21 | 15 | 3 | |||
| 18 | 28 | 16 | 3 | |||
| 18 | 35 | 19 | 3 | |||
| C. tor | South | 29 | 25 | 14 | 0 | 0 |
| 25 | 21 | 12 | 3 | |||
| 25 | 28 | 3 | 1 | |||
| 25 | 35 | 0 | 0 | |||
| 18 | 14 | 0 | 0 | |||
| 18 | 21 | 7 | 2 | |||
| 18 | 28 | 10 | 2 | |||
| 18 | 35 | 0 | 0 |
The numbers written in parentheses represent the number of individuals/experiments/field-collected egg rafts used for experiments in 2012. All other values are data from 2013.
Fig. S1.
Infection rates for Cx. quinquefasciatus and Cx. pipiens biotype molestus laboratory strains.
(A-B) Adult females were sorted into small plastic containers 5–10 days after emergence and feed over night with human blood containing 1–1.5 × 107 PFU WNV lineage 1 strain NY99. Fully engorged females were separated and incubated at 25 °C or 18 °C and 80% humidity in a climate chamber for 14 to 35 days.
Graphs represent the infection rate (in percentage) of Cx. pipiens quinquefasciatus (panel A) and Cx. pipiens biotype molestus (panel B), at 25 °C (dark grey bars) and 18 °C (light grey bars) as determined via WNV-specific qRT-PCR (cut-off = 35 cycles). Detailed numbers of individuals and numbers of independent experiments used for analysis are listed in Table 1. Data presented in these graphs are pooled data from North and South populations. Statistical analyses were performed using the GraphPad Prism software and Fischer's exact test (p < 0.05).
C. pipiens biotype pipiens specimens from Germany showed infection rates similar to those of C. quinquefasciatus and C. pipiens biotype molestus with a maximum infection rate of 75% (n = 67) at 18 °C on day 21 (Fig. 1A). Viral titres in C. pipiens biotype pipiens at 25 °C incubation temperature were 4–1.4 × 105 PFU/ml at day 14 and 8–2.5 × 103 PFU/ml at day 21 post infection. The percentage of WNV RNA positive females increased over time at both incubation temperatures without significant differences between 25 °C and 18 °C incubation temperature. C. torrentium mosquitoes had the highest infection rates amongst all species or C. pipiens biotypes tested in this study, with a maximum infection rate of 96% (n = 46) at 25 °C on day 21 post infection (Fig. 1B). Interestingly, significant differences between infection rates between 18 °C and 25 °C at 14, 21 and 35 days post-infection were observed (p-values = 0.0033 (14 dpi), 0.0116 (21 dpi), 0.0346 (35 dpi)). At 25 °C incubation temperature infection rates of 83% (n = 12) were detected as early as 14 days post-infection, whereas only 16% (n = 12) of the tested females were tested positive for WNV RNA at 18 °C incubation temperature on the same day. Infection rates in C. torrentium at 18 °C increased over time and were higher compared to 25 °C at 35 days post-infection. Virus titres at 25 °C incubation temperature were 1.4 × 101–6.9 × 104 PFU/ml at 14 day post-infection and 8–1.4 × 103 at 21 days post-infection.
Fig. 1.
Temperature dependence of infection rates for field populations C. pipiens biotype pipiens and C. torrentium.
(A–B) Adult females were sorted into small plastic containers 5–10 days after emergence and feed over night with human blood containing 1–1.5 × 107 PFU WNV lineage 1 strain NY99. Fully engorged females were separated and incubated at 25 °C or 18 °C and 80% humidity in a climate chamber for 14 to 35 days.
Graphs represent the infection rates (in percentage) of C. pipiens biotype pipiens (panel A) and C. torrentium (panel B), at 25 °C (dark grey bars) and 18 °C (light grey bars) as determined via WNV-specific qRT-PCR (cut-off = 35 cycles). Detailed numbers of individuals and numbers of independent experiments used for analysis are listed in Table 1. Data presented in these graphs are pooled data from North and South populations collected in 2013. Statistical analyses were performed using the GraphPad Prism software and Fischer's exact test (p < 0.05).
Dissemination rates were measured by separate qPCR testing of heads and bodies and calculated as the number of WNV positive heads with respect to the number of WNV-positive females. We observed maximum dissemination rates of 94% (n = 34) in C. pipiens biotype pipiens at 25 °C and 28 days of infection (Fig. 2A) and 100% (n = 11) in C. torrentium at 25 °C on day 28 of infection (Fig. 2B). For both species no significant difference of dissemination rates between 18 °C and 25 °C were observed.
Fig. 2.
C. pipiens biotype pipiens and C. torrentium mosquitoes display high dissemination rates at 18 and 25 °C incubation temperature.
(A–B) Adult females were sorted into small plastic containers 5–10 days after emergence and feed over night with human blood containing 1–1.5 × 107 PFU WNV lineage 1 strain NY99. Fully engorged females were separated and incubated at 25 °C or 18 °C and 80% humidity in a climate chamber for 14 to 35 days.
Graphs represent the dissemination rate (in percentage) of C. pipiens biotype pipiens (panel A) and C. torrentium (panel B), at 25 °C (dark grey bars) and 18 °C (light grey bars) as determined via WNV-specific qRT-PCR (cut-off = 35 cycles). Detailed numbers of individuals and numbers of independent experiments used for analysis are listed in Table 1. Data presented in these graphs are pooled data from North and South populations collected in 2013. Statistical analyses were performed using the GraphPad Prism software and Fischer's exact test (p < 0.05).
3.2. Temporal and spatial variation of infection rates
Comparison of WNV infection rates in C. pipiens biotype pipiens collected in 2012 and 2013 showed significant differences in infection rates at day 14 post-infection (p value = 0.0208), whereas no significant difference was observed at day 21 post-infection (Fig. 3A). For C. torrentium we observed higher infection rates in 2013 for both time points, which were only significant at day 21 post-infection (p value = 0.0019). The infection rates were higher in C. torrentium compared to C. pipiens biotype pipiens in both subsequent years (Fig. 3A).
Fig. 3.
Spatial and temporal variation in vector competence of wild C. pipiens biotype pipiens and C. torrentium populations.
(A–C) For comparison of infection rates between two years and two geographically distinct mosquito populations of the same species, the data obtained through infection experiments described in Table 1 and Fig. 1 of 2013 were analysed together with data obtained in 2012.
(A) Infection rates for 2012 are indicated by dark grey bars and infection rates for 2013 are represented by light grey bars. Statistical analysis was performed using GraphPad Prism and Fisher's exact test (p < 0.05). Detailed numbers of individuals and numbers of independent experiments used for analysis are listed in Table 1.
(B and C) Infection rates are determined via WNV-specific qRT-PCR (cut-off = 35 cycles). Statistical analysis was performed using GraphPad Prism and Fisher's exact test (p < 0.05). Detailed numbers of individuals and numbers of independent experiments used for analysis are listed in Table 1.
(B) Infection rates for C. pipiens biotype pipiens at 21 days post-infection and two temperatures, 18 °C and 25 °C, for the North population (N) and South population (S) respectively.
(C) Infection rates for C. torrentium at 21 days post-infection and two temperatures, 18 °C and 25 °C, for the North population (N) and South population (S) respectively.
To determine spatial variations of susceptibility to WNV infection, we stratified the infection data obtained with the 2013 C. pipiens biotype pipiens and C. torrentium specimens according to population origin (North (N) and South (S)) and focused on the infection rates at 21 days post-infection. WNV infection rates of C. pipiens biotype pipiens revealed significant differences between the N and S populations (Fig. 3B). Infection rates of the S population were 22 percentage points higher than N population at 18 °C (p value = 0.0469, N population n = 41; S population n = 26) and 37 percentage points higher at 25 °C (p value = 0.0011, N population n = 65; S population n = 24). The analysis of WNV infection data for C. torrentium showed a 44 percentage point difference between S (43%; n = 7) and N (87%; n = 15) at 18 °C, which was not, however, statistically significant (p value = 0.0536, Fig. 3C). At 25 °C comparable proportions were observed.
4. Discussion
We demonstrated that Culex mosquitoes native to Germany are susceptible to WNV infection. The infection rates measured for C. pipiens biotype pipiens within our study largely match infection rates measured in field populations of C. pipiens in the US [25]. Furthermore, the high dissemination rates suggest an efficient amplification of the virus in those mosquitoes and a successful escape of the midgut barrier. Although this does not necessarily translate into high transmission rates, dissemination is a prerequisite for successful transmission. Thus, our data can provide evidence for potential vector competence of Central European mosquitoes. However, further transmission studies with larger sample sizes in the respective study strata, are needed to clarify their vector competence. Recent studies with Dutch laboratory colonies of C. pipiens and WNV lineage 1 NY99 and WNV lineage 2 also demonstrated the potential of Central European mosquitoes to serve as vectors for WNV [18]. In contrast to the strong correlation of infection rates with incubation temperature in this study, showing significantly decreased infection rates with WNV lineage 2 virus at lower temperature (18 and 23 °C) [18], we did not find significant differences in C. pipiens biotype pipiens at 18 °C and 25 °C incubation temperature. It is, however, difficult to compare these results and make assumption as to whether the differential temperature dependence is due to the origin of mosquitoes (field collected versus laboratory colony) or due to the virus lineage (lineage 1 versus lineage 2). In this context it is also important to note, that laboratory strains have a different genetic makeup and genetic variability compared to field collected populations due to selection processes during colony establishment. These differences translate into differential susceptibility of laboratory strains and field population for arboviruses as exemplified by experimental infection in C. tarsalis with Western equine encephalomyelitis virus (WEEV) and Aedes albopictus with dengue virus [34], [35], [36]. Thus more comparative transmission experiments using field collected mosquitoes with WNV of both lineages would be needed to answer this question. Nevertheless, our infection data suggest that susceptibility to WNV infection as well as efficient dissemination of the virus within the mosquitoes are not affected by lower ambient temperatures in field population of C. pipiens biotype pipiens.
It is of particular interest that we found the highest infection rates in C. torrentium mosquitoes, a species that has not yet been described in the context of WNV transmission. The high prevalence of C. torrentium in northern and Central Europe has just come to general attention due to the recent revision of the Culex complex distribution in Europe by several groups using molecular taxonomy methods [15], [16]. Thus, the contribution of C. torrentium to WNV circulation might be underestimated due to poor identification rate of the species among trapped mosquito females in WNV surveillance studies. It is to note, that the distribution of C. torrentium [15] seems to be restricted to the North and Central Europe, while most WNV cases have been reported from the South-eastern part of Europe. Consequently an overlap of WNV endemic area with the C. torrentium distribution is currently restricted to northern Italy and Austria. Nevertheless, C. torrentium has already been identified as a major vector for other relevant viruses, for example, Sindbis virus [20], [21], [22]. Our infection data indicate that C. torrentium should be taken into consideration as a potential WNV vector, especially in northern and Central Europe.
Analysing the spatial variation in susceptibility to WNV, we found population-based differences between C. pipiens biotype pipiens mosquitoes from northern and southern Germany at both incubation temperatures, which indicates that intrinsic factors in different C. pipiens populations contribute to a differential susceptibility to WNV infection. This hypothesis is supported by reports of spatial variation in vector competence of North American C. pipiens mosquitoes for WNV [25]. Furthermore, experimental infection studies with C. tarsalis and WEEV and Ae. albopictus and dengue virus highlight the significance of (i) a population based analyses of vector competence and (ii) the use of field collected populations in addition to laboratory colonies to estimate vector competence of resident mosquito species. However, experimental infection with Italian laboratory strains and field collected populations of C. pipiens did not show differences between these distinct populations [37]. There are several possible explanations for these contradicting observations. Firstly, the laboratory colonies used in the Italian study were only cultivated for approximately 1 year (F7-F11) so the discrepancy between laboratory colony and field collected samples might not be very high [38]. Secondly, it might be possible that Italian C. pipiens populations are more genetically uniform than German populations, which would account for the differing observations. However, recent studies of genetic diversity of C. pipiens in Italy and Germany, although not conducted at the sites used for sample acquisition in the infection experiments, hint to a high genetic diversity of population in Italy and low genetic diversity in Germany [39]. A third possible hypothesis is that different Wolbachia strains within distinct populations may change the genetic diversity of these populations [40] and also influence their vector competence [41].
Interestingly, WNV infection rates in C. torrentium mosquitoes were not significantly different between North and South populations at 25 °C, whereas a visible but not statistically different difference was found at 18 °C. Additionally, the pooled infection rates from northern and southern populations showed a significantly reduced infection rate at 18 °C in C. torrentium and delayed infection compared to 25 °C resulting in significant differences in infection rates at early and late time points after infection. Furthermore, similar dissemination rates at 18 and 25 °C 35 days post-infection, despite the significant higher infection rates at 18 °C at this time point, hint to delayed virus replication at lower temperatures in this species These results might point to a temperature dependent difference in susceptibility to WNV of C. torrentium populations from Germany. But the lack of experimental WNV infection data in C. torrentium makes it difficult to draw conclusions on spatial variation and temperature-based variation in this species at the moment. In contrast to our WNV infection data, infection studies with Sindbis virus in this species showed temperature independent high infection rates in C. torrentium [21]. Thus, there might be also virus specific variation in different mosquito populations.
We found temporal variation in infection rates for both species with generally higher infection rates in 2013 compared to 2012, which might be due to variation in population in these two years. For example, experimental studies with C. quinquefasciatus colonies have shown the influence of colony age, specific age on the female included into the experiment and temperature [27], and experimental infection using US field collected samples of C. pipiens have revealed similar variations [25].
Taken together, our experimental infection data with field populations of C. pipiens biotype pipiens and C. torrentium mosquitoes show that both are permissive to WNV infection and that there are spatial, temporal and species-based variation in susceptibility to this virus. These variations are of interest for further studies of the mechanisms of virus–mosquito interactions as well as for the development of targeted control programmes.
The following is the supplementary data related to this article.
Competing interest
The authors declare that they have no competing interest.
Author's contribution
MB collected the mosquito egg rafts for vector competence assays. ML, MB, SJ and KH carried out the vector competence assay experiments and participated in data analysis. MR, JB and AK did the taxonomic classification of mosquito specimens. ML and RK did the statistical analysis. JSC designed the WNV real-time PCR. ET, ML and JSC participated in study design and manuscript writing. SCB directed and conceived the study, analysed data and wrote the first draught of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by the Leibniz Association, grant number SAW-2011-BNI-3-29 and SAW-2014-SGN-3.
We thank Norbert Schwarz (Bernhard-Nocht-Institute for Tropical Medicine, Infectious Disease Epidemiology) for the help with the statistical analysis of the data.
Contributor Information
Mayke Leggewie, Email: leggewie@bnitm.de.
Marlis Badusche, Email: badusche@bnitm.de.
Martin Rudolf, Email: rudolf@bnitm.de.
Stephanie Jansen, Email: jansen@bnitm.de.
Jessica Börstler, Email: boestler@bnitm.de.
Ralf Krumkamp, Email: krumkamp@bnitm.de.
Katrin Huber, Email: katrinhuber1@gmx.de.
Andreas Krüger, Email: Krueger@bni-hamburg.de.
Jonas Schmidt-Chanasit, Email: jonassi@gmx.de.
Egbert Tannich, Email: tannich@bnitm.de.
Stefanie C. Becker, Email: stefanie.becker@tiho-hannover.de.
References
- 1.Calistri P., Giovannini A., Hubalek Z., Ionescu A., Monaco F., Savini G., Lelli R. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol J. 2010;4:29–37. doi: 10.2174/1874357901004010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel M., Emmerich P., Frank C., Fiedler M., Rashidi-Alavijeh J., Jochum C., Günther S., Auerhammer K., Rupprecht H.J., Blank R.T., Sacher N., Pertzborn L., Stark K., Schrauzer T., Schmidt-Chanasit J. Increase in West Nile virus infections imported to Germany in 2012. J. Clin. Virol. 2013;58:587–589. doi: 10.1016/j.jcv.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Schultze-Amberger J., Emmerich P., Günther S., Schmidt-Chanasit J. West Nile virus meningoencephalitis imported into Germany. Emerg. Infect. Dis. 2012;18:1698–1700. doi: 10.3201/eid1810.120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farajollahi A., Fonseca D.M., Kramer L.D., Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai T.F., Popovici F., Cernescu C., Campbell G.L., Nedelcu N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Bocanegra I., Jaen-Tellez J.A., Napp S., Arenas-Montes A., Fernandez-Morente M., Fernandez-Molera V., Arenas A. West Nile fever outbreak in horses and humans, Spain, 2010. Emerg. Infect. Dis. 2011;17:2397–2399. doi: 10.3201/eid1712.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platonov A.E., Shipulin G.A., Shipulina O.Y., Tyutyunnik E.N., Frolochkina T.I., Lanciotti R.S., Yazyshina S., Platonova O.V., Obukhov I.L., Zhukov A.N., Vengerov Y.Y., Pokrovskii V.I. Outbreak of West Nile virus infection, Volgograd region, Russia, 1999. Emerg. Infect. Dis. 2001;7:128–132. doi: 10.3201/eid0701.010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am.J.Trop. Med. Hyg. 1940;20:471–472. [Google Scholar]
- 9.Frost M.J., Zhang J., Edmonds J.H., Prow N.A., Gu X., Davis R., Hornitzky C., Arzey K.E., Finlaison D., Hick P., Read A., Hobson-Peters J., May F.J., Doggett S.L., Haniotis J., Russell R.C., Hall R.A., Khromykh A.A., Kirkland P.D. Characterization of virulent West Nile virus Kunjin strain, Australia, 2011. Emerg. Infect. Dis. 2012;18:792–800. doi: 10.3201/eid1805.111720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell R.C., Dwyer D.E. Arboviruses associated with human disease in Australia. Microbes Infect. 2000;2:1693–1704. doi: 10.1016/s1286-4579(00)01324-1. [DOI] [PubMed] [Google Scholar]
- 11.Nash D., Mostashari F., Fine A., Miller J., O'Leary D., Murray K., Huang A., Rosenberg A., Greenberg A., Sherman M., Wong S., Layton M. The outbreak of West Nile virus infection in the New York City area in 1999. The N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 12.Petersen L.R., Hayes E.B. West Nile virus in the Americas. The Medical Clinics of North America. 2008;92:1307–1322. doi: 10.1016/j.mcna.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Andreadis T.G., Anderson J.F., Vossbrinck C.R., Main A.J. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- 14.Andreadis T.G. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile virus in North America. J. Am. Mosq. Control Assoc. 2012;28:137–151. doi: 10.2987/8756-971X-28.4s.137. [DOI] [PubMed] [Google Scholar]
- 15.Hesson J.C., Rettich F., Merdic E., Vignjevic G., Ostman O., Schäfer M., Schaffner F., Foussadier R., Besnard G., Medlock J., Scholte E.J., Lundström J.O. The arbovirus vector Culex torrentium is more prevalent than Culex pipiens in northern and central Europe. Med. Vet. Entomol. 2014;28:179–186. doi: 10.1111/mve.12024. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf M., Czajka C., Börstler J., Melaun C., Jöst H., von Thien H., Badusche M., Becker N., Schmidt-Chanasit J., Krüger A., Tannich E., Becker S. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundström J.O. Mosquito-borne viruses in western Europe: a review. J Vector Ecol. 1999;24:1–39. [PubMed] [Google Scholar]
- 18.Fros J.J., Geertsema C., Vogels C.B., Roosjen P.P., Failloux A.B., Vlak J.M., Koenraadt C.J., Takken W., Pijlman G.P. West Nile virus: high transmission rate in north-western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fros J.J., Miesen P., Vogels C.B., Gaibani P., Sambri V., Martina B.E., Koenraadt C.J., van Rij R.P., Vlak J.M., Takken W., Pijlman G.P. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health. 2015;1:31–36. doi: 10.1016/j.onehlt.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundström J.O., Niklasson B., Francy D.B. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J. Med. Entomol. 1990;27:561–563. doi: 10.1093/jmedent/27.4.561. [DOI] [PubMed] [Google Scholar]
- 21.Lundström J.O., Turell M.J., Niklasson B. Effect of environmental temperature on the vector competence of Culex pipiens and Cx. torrentium for Ockelbo virus. Am. J. Trop. Med. Hyg. 1990;43:534–542. doi: 10.4269/ajtmh.1990.43.534. [DOI] [PubMed] [Google Scholar]
- 22.Hesson J.C., Verner-Carlsson J., Larsson A., Ahmed R., Lundkvist A., Lundström J.O. Culex torrentium mosquito role as major enzootic vector defined by rate of Sindbis virus infection, Sweden, 2009. Emerg. Infect. Dis. 2015;21:875–878. doi: 10.3201/eid2105.141577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Börstler J., Lühken R., Rudolf M., Steinke S., Melaun C., Becker S., Garms R., Krüger A. The use of morphometric wing characters to discriminate female Culex pipiens and Culex torrentium. J Vector Ecol. 2014;39:204–212. doi: 10.1111/j.1948-7134.2014.12088.x. [DOI] [PubMed] [Google Scholar]
- 24.Jia X.Y., Briese T., Jordan I., Rambaut A., Chi H.C., Mackenzie J.S., Hall R.A., Scherret J., Lipkin W.I. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet. 1999;354:1971–1972. doi: 10.1016/s0140-6736(99)05384-2. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick A.M., Fonseca D.M., Ebel G.D., Reddy M.R., Kramer L.D. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg. 2010;83:607–613. doi: 10.4269/ajtmh.2010.10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahori A.S., Sterk V.V., Goldblum N. Studies on the dynamics of experimental transmission of West Nile virus by Culex molestus. Am.J.Trop. Med. Hyg. 1955;4:1015–1027. doi: 10.4269/ajtmh.1955.4.1015. [DOI] [PubMed] [Google Scholar]
- 27.Richards S.L., Lord C.C., Pesko K.N., Tabachnick W.J. Environmental and biological factors influencing Culex pipiens quinquefasciatus (Diptera: Culicidae) vector competence for West Nile virus. Am.J.Trop. Med. Hyg. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fall G., Diallo M., Loucoubar C., Faye O., Sall A.A. Vector competence of Culex neavei and Culex quinquefasciatus (Diptera: Culicidae) from Senegal for lineages 1, 2, Koutango and a putative new lineage of West Nile virus. Am.J.Trop. Med. Hyg. 2014;90:747–754. doi: 10.4269/ajtmh.13-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vazeille M., Moutailler S., Coudrier D., Rousseaux C., Khun H., Huerre M., Thiria J., Dehecq J.S., Fontenille D., Schuffenecker I., Despres P., Failloux A.B. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2 doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amraoui F., Krida G., Bouattour A., Rhim A., Daaboub J., Harrat Z., Boubidi S.C., Tijane M., Sarih M., Failloux A.B. Culex pipiens, an experimental efficient vector of West Nile and Rift valley fever viruses in the Maghreb region. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0036757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolling B.G., Olea-Popelka F.J., Eisen L., Moore C.G., Blair C.D. Transmission dynamics of an insect-specific Flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology. 2012;427:90–97. doi: 10.1016/j.virol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanlandingham D.L., McGee C.E., Klinger K.A., Vessey N., Fredregillo C., Higgs S. Relative susceptibilties of south Texas mosquitoes to infection with West Nile virus. Am.J.Trop. Med. Hyg. 2007;77:925–928. [PubMed] [Google Scholar]
- 33.Hierholzer J.C., Killington R.A. Virus isolation and quantitation. In: Mahy B., Kangro H., editors. Virology Methods Manual. Academic Press; London: 1996. pp. 25–46. [Google Scholar]
- 34.Hardy J.L., Reeves W.C., Sjogren R.D. Variations in the susceptiblility of field and laboratory populations of Culex tarsalis to experimental infection with Western equine encephalomyelitis virus. Am. J. Epidemiol. 1976;103:498–505. doi: 10.1093/oxfordjournals.aje.a112251. [DOI] [PubMed] [Google Scholar]
- 35.Vazeille M., Mousson L., Rakatoarivony I., Villeret R., Rodhain F., Duchemin J.B., Failloux A.B. Population genetic structure and competence as a vector for dengue type 2 virus of Aedes aegypti and Aedes albopictus from Madagascar. Am.J.Trop. Med. Hyg. 2001;65:491–497. doi: 10.4269/ajtmh.2001.65.491. [DOI] [PubMed] [Google Scholar]
- 36.Vazeille M., Rosen L., Mousson L., Failloux A.B. Low oral receptivity for dengue type 2 viruses of Aedes albopictus from southeast Asia compared with that of Aedes aegypti. Am.J.Trop. Med. Hyg. 2003;68:203–208. [PubMed] [Google Scholar]
- 37.Fortuna C., Remoli M.E., Di Luca M., Severini F., Toma L., Benedetti E., Bucci P., Montarsi F., Minelli G., Boccolini D., Romi R., Ciufolini M.G. Experimental studies on comparison of the vector competence of four Italian Culex pipiens populations for West Nile virus. Parasit Vectors. 2015;8:463. doi: 10.1186/s13071-015-1067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson S.L., Richards S.L., Tabachnick W.J., Smartt C.T. Effects of West Nile virus dose and extrinsic incubation temperature on temporal progression of vector competence in Culex pipiens quinquefasciatus. J. Am. Mosq. Control Assoc. 2010;26:103–107. doi: 10.2987/09-5926.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonato M., Martinez-Sanudo I., Cavaletto G., Santoiemma G., Saltarin A., Mazzon L. High genetic diversity in the Culex pipiens complex from a West Nile virus epidemic area in southern Europe. Parasit Vectors. 2016;9:150. doi: 10.1186/s13071-016-1429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duron O., Raymond M., Weill M. Many compatible Wolbachia strains coexist within natural populations of Culex pipiens mosquito. Heredity (Edinb) 2011;106:986–993. doi: 10.1038/hdy.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborne S.E., Leong Y.S., O'Neill S.L., Johnson K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009;5(11) doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]