Abstract
Cell-free extracts of twenty terrestrial cyanobacteria were evaluated for their antioxidant properties in terms of free-radical scavenging (DPPH and ABTS) and metal chelating activity and deoxyribose protection. Extract of Anabaena constricta was the most prominent antioxidant agent (IC50 for DPPH activity 0.91 mg ml−1, ABTS 0.23 mg ml−1, deoxyribose protection 0.63 mg ml−1 and Fe+2-ion chelating 0.9 mg ml−1). The extracts of cyanobacterial species contained high quantity of total phenol and total flavonoid that were supposed to impart prominent antioxidant properties. Cyanobacterial species also showed fairly high PAL activity. We reported varied quantities of polyphenolics gallic, chlorogenic, caffeic, vanillic and ferulic acids and flavonoids rutin, quercetin and kaempferol in cyanobacterial extracts. The presence of these polyphenolics was linked with the free radical scavenging, metal chelating and antioxidative damage protecting properties of the organisms. Cyanobacteria are the most feasible, promising and alternative candidates for searching out new chemical leads for industrial applications in pharmaceuticals, neutraceuticals and biomolecules of importance. Presence of biomolecules such as polyphenolics and their connection with the prominent biological functions (e.g., antioxidant properties) make these organisms a potential source of secondary metabolites with predominant biological activities. Additionally, dominant presence of polyphenols as antioxidant agents in cyanobacterial species may reflect their adaptation strategies against abiotic stresses for their ecological success in different habitats.
Keywords: Cyanobacteria, Flavonoids, Antioxidants, HPLC, Gallic acid, Polyphenolics
Introduction
Cyanobacteria are promising prokaryotic photosynthetic microorganisms that act as potential cell factories producing a wide array of secondary metabolites of different class and groups including venturamideA, dragomabin, dragonamide -A, -B, carmabin -A, -B, calothrixins -A, -B, dolastatins and nostocarboline (Rastogi and Sinha 2009a, b; Guedes et al. 2013; Xue and He 2015). Additionally, these organisms have the capabilities to express plant P450 proteins that have essential functions in the biosynthesis of natural product molecules (Xue and He 2015). Chemically diverse group of secondary metabolites of biotechnological and industrial significance (Rastogi and Sinha 2009a, b; Mandal and Rath 2015) potentiate cyanobacteria with promising biological activities (antibacterial, antialgal, antiviral, anticancer, immuno-suppressant, anti-inflammatory, antioxidant and biotoxins) (Singh et al. 2001; Aydas et al. 2013). Such compounds also impart intrinsic self-defense mechanisms to the cells that directly interact with the diverse environmental conditions (Camacho 2008). Many species exhibit wide range of chemical diversity with characteristic structures coupled with bioactivity that make these organisms a potential candidate for pharmaceuticals (Mandal and Rath 2015). Cyanobacterial species also exhibit potential adaptation strategies against oxidative stresses mainly by the activation of antioxidant defense system that comprises activation of various enzymes (superoxide dismutase, catalase, glutathione reductase and ascorbate peroxidase) (Bharanidharan et al. 2013; Guedes et al. 2013).
Phenylpropanoids and their derivatives are probably the largest group of secondary metabolites isolated from natural sources (plants, microorganisms and microalgal cultures) (Wojdylo et al. 2007; Vogt 2010). They are potential antioxidants that scavenge reactive oxygen species (ROS) (Perron and Brumaghim 2009). The detoxification of ROS by means of enzymatic and/or non-enzymatic antioxidants like phenylpropanoids protects cell damage and minimizes the loss due to oxidative responses (Reuter et al. 2010). Since these organisms are among the most primitive prokaryotes having faced several extreme environmental stresses during their evolution (Tomitani et al. 2006), it is imperative to understand that their capabilities to produce antioxidative enzymes and secondary metabolites might have offered them protective strategies against abiotic stresses.
Plant phenolics and flavonoids are the known antioxidants that reduce the oxidative damage inside the cells (Nordberg and Arnér 2001; Singh et al. 2009; Fraga et al. 2010). Because of the presence of unique phenylpropanoids, cyanobacteria could be a new source of these secondary metabolites (Xue and He 2015). We evaluated antioxidant property of the extracts of terrestrial cyanobacterial species from the genera Anabaena, Nostoc, Microcheate, Oscillatoria, Synechocystis, Hapalosiphon, Mastigocladus, Scytonema, Westiellopsis, Cylindrospermum, Aulosira, Chroococcus, Lyngbya, Calothrix, Dichothrix, Limnothrix and Phormidium. Targeted metabolomics using qualitative and quantitative determination of polyphenolics (phenolic acids and flavonoids) was performed in these species using High Performance Liquid Chromatography (HPLC) followed by liquid chromatography-mass spectrometry (LC–MS) to identify and quantify the compounds of interest.
Materials and methods
Reagents, organisms and culture conditions
Twenty different cyanobacterial species were obtained from National Agriculturally Important Microbial Culture Collection (NAIMCC) at ICAR-NBAIM, Maunath Bhanjan, India. These species were Anabaena doliolum (NAIMCC-1), Calothrix geitonos (NAIMCC-4), Dichothrix sp. (NAIMCC-7), Limnothrix obliqueacuminata (NAIMCC-8), Microcheate tenera (NAIMCC-12), Oscillatoria acuta (NAIMCC-17), Nostoc ellipsosporum (NAIMCC-28), Anabaena constricta (NAIMCC-29), Synechocystis sp. (NAIMCC-33), Hapalosiphon fontinalis (NAIMCC-36), Mastigocladus laminosus (NAIMCC-43), Scytonema simplex (NAIMCC-45), Calothrix brevissima (NAIMCC-48), Westiellopsis prolifica (NAIMCC-51), Cylindrospermum sp. (NAIMCC-70), Aulosira fertilissima (NAIMCC-94), Chroococcus sp. (NAIMCC-100), Limnothrix sp. (NAIMCC-107), Lyngbya sp. (NAIMCC-111), and Phormidium tenue (NAIMCC-113). Organisms were grown and maintained in BG11 medium (Stanier et al. 1971) in flasks (100 ml × 6) at 25 ± 1 °C with light/dark cycle of 12 h till exponentially growing phase is attained. Culture flasks were bubbled with air containing 1% (v/v) carbon dioxide and were kept under continuous illumination at 70 µ Em−2 s−2 from incandescent lamps. Exponentially growing cultures were harvested by centrifugation at 5000g at room temperature and cell pellets were finally used for extraction using appropriate solvents.
Biochemical assays
Chlorophyll and total carotenoid content in cyanobacterial cultures was quantified by extracting pigments from exponentially growing cyanobacterial cultures (0.5 g fresh wt, moisture content 15 ± 2%) in 80% acetone for 3–4 h at room temperature. After removal of the cell debris through centrifugation at 15,000g for 10 min, chlorophyll and carotenoids in the supernatant were quantified in terms of A665 and A450, respectively (Ferjani et al. 2003).
Total protein content was extracted and quantified as per the method described by Ferjani et al. 2003. Freshly harvested one gm cultures (moisture content 15 ± 2%) was macerated with 1% tricholoroacetic acid (5 ml) with intermittent sonication in cold conditions and the suspension was centrifuged (Sigma D-37520) at 15,000g for 10 min at 4 °C. The cell pellet was suspended in 1 N NaOH (4 ml), boiled for 30 min, cooled and again centrifuged at 15,000g for 5 min. The supernatant was quantified for total protein content by Lowry method (Lowry et al. 1951) taking bovine serum albumin (BSA) as standard.
Preparation of cell-free extracts
Two gm of fresh cyanobacterial culture was extracted with 10 ml of methanol for 4 h followed by re-extraction with 10 ml of water:methanol (1:1, v/v) for 3 h. Intermittent sonication for 4–5 min was given. The process was repeated thrice and the supernatant was pooled together and centrifuged at 10,000g for 15 min. Clear greenish supernatant was collected and lyophilized to obtain cell-free extracts. Dried extract was then dissolved in 2 ml of HPLC grade methanol by vortexing and was stored at 4 °C for further analysis.
Antioxidant assays
DPPH activity
Free radical scavenging activity (FRSA) was determined using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) solution (0.6 µM l−1 in ethanol) (Yen and Duh 1994) and was expressed as antiradical power (ARP). Briefly, 1 ml of methanolic cyanobacterial extract was mixed with 4 ml of DPPH solution and reaction mixture was kept in dark for 30 min. Discoloration in the reaction mixture was monitored at initial (0 min) and after 30 min at 517 nm taking methanol as blank. The results were recorded as % discoloration.
ABTS activity
The assay was based on the ability of antioxidant compounds/extracts to scavenge 2,2′-azino-bis(ethylbenzthiazoline-6-sulfonic acid (ABTS·+) radical cation (Giao et al. 2007). The radical cation was prepared by mixing 7 mM ABTS solution with potassium persulfate (1/1; v/v) while leaving the reaction mixture for 6 h. Scavenging activity of cell free extracts of cyanobacteria was evaluated in a reaction mixture containing 0.9 ml of ABTS+ free radical and 0.1 ml of cyanobacterial methanolic extracts. The absorbance of the reaction mixture was recorded at 734 nm after 15 min and expressed as % ABTS activity.
Deoxyribose protection assay
The efficacy of methanolic extract of cyanobacteria on prevention of Fe2+/H2O2-induced decomposition of deoxyribose was determined (Halliwell and Gutteridge, 1984). In brief, a reaction mixture contains 450 µl of 0.2 M phosphate buffer (pH = 0.7), 150 µl of 10 mM deoxyribose, 150 µl of 10 mM FeSO4, 150 µl of 10 mM EDTA, 500 µl of distilled water and 100 µl of methanolic extracts. The reaction was started by the addition of 150 µl of 10 mM H2O2 and after incubation at 37 °C, reaction was stopped by adding 750 µl of 2.8% TCA and 750 µl of 1% TBA in 50 mM NaOH. The absorbance was recorded at 532 nm in spectrophotometer (Thermo UV10) taking ascorbic acid as reference.
Fe+2-ion chelating activity
The ferrous (Fe+2)-ion chelating activity of methanolic extracts of cyanobacteria was determined (Dinis et al. 1994). Methanolic extracts (0.5 ml) were mixed with 100 μl of 2 mM ferrous chloride solution and the reaction was initiated by adding 200 μl of 5 mM ferrozine. The mixture was shaken vigorously and incubated at room temperature for 10 min. The absorbance of the solution was measured at 562 nm. The percentage of inhibition of ferrozine-Fe+2 complex formation was calculated using the following formula.
Ferrous ion chelating ability (%) = [(A 0 − A)/A 0] × 100 where, A 0 is the absorbance of the control solution (containing all reagents except the extract); A is absorbance in the presence of the extract sample. The experiment was carried out in triplicate and ethylenediaminetetraacetate (EDTA) was used as standard.
Enzyme assays
Enzyme extract of all the twenty cyanobacterial strains was prepared by extracting 1 g fresh culture (moisture content 15 ± 2%) with 3 ml of 0.05 M phosphate buffer (pH 7.8) containing 1 mM EDTA and 2% (w/v) polyvinylpyrolidone. The suspension was sonicated and the supernatant from the homogenate was obtained by centrifugation at 13,000g for 15 min at 4 °C. The supernatant was used for the estimation of phenylalanineammonialyase (PAL) activity.
PAL activity
PAL (EC 4.3.1.24) activity in enzyme extracts of cyanobacterial strains was determined as per the method reported by McCallum and Walker (1990). The assay mixture consisted of 500 µl of phosphate buffer (100 mM, pH 7.0), 200 μl enzyme extract and 1.3 ml distilled water. The reaction was initiated by the addition of 1 ml l-phenylalanine (100 mM). Tubes were incubated at 30 °C for 30 min (or one h when the activity was low) and the reaction was stopped by the addition of 500 µl of 1 M trichloroacetic acid (TCA). Tubes were then centrifuged for 5 min at 5000 g and the absorbance was recorded at A290. Cinnamic acid was used as standard to calculate PAL activity. Triplicate assays were performed for each cyanobacterial strain.
Estimation of total phenolics and flavonoids
Total phenolic content (TPC) in the cyanobacterial strains was assessed using Folin-Ciocalteu’s reagent (Machu et al. 2015). Gallic acid was used as standard compound and total phenol content was expressed in terms of mg gallic acid equivalents (GAE) g−1 fresh wt.
Total flavonoid content was measured by aluminum chloride colorimetric method (Kim et al. 2003). Cyanobacterial extract (0.5 ml) was mixed with 0.1 ml of 10% aluminum chloride hexahydrate, potassium acetate (0.1 ml of 1 M) and 2.8 ml of deionized water. After incubation for 30 min, the absorbance of the reaction mixture was recorded at 415 nm and quantified taking quercetin as standard. Total flavonoid content (TFC) was expressed in terms of µg quercetin equivalents (QE) g−1 fresh cell wt.
HPLC analysis of extracts
Estimation of polyphenolics (phenolic acids and flavonoids) in the cyanobacterial cell-free extracts was performed using HPLC system (515, Waters, USA) equipped with binary Waters 515 reciprocating pumps, a variable photodiode array (PDA) detector (Waters 2996) and system controller empowered with EmpowerTM software for data acquisition, integration and analysis. Reverse phase liquid chromatographic separation of the phenolic extracts was carried out at room temperature with the following conditions; injection volume 10 µl/sample, separation mode- isocratic, column C-18 (250 × 4.6 mm i.d., particle size 5 µm), mobile phase- methanol:0.4% acetic acid in water (55:45%, v/v), flow rate- 1 ml/min and detection at 254 and 280 nm (Singh et al. 2011). Phenolic acids (gallic, chlorogenic, caffeic, vanillic and ferulic acids) and flavonoids (rutin, quercetin, and kaempferol) (Sigma-Aldrich) were used as standard compounds. Samples were subjected to membrane filtration (0.45 µm) prior to injection in the HPLC column. Characterization of the compounds in the sample was done by comparing retention time with the standard compounds and by co-injection while quantification was done by comparing peak areas of identified compounds in the samples with those of the standard compounds.
Qualitative LC–MS
Phenolic constituents were used as standards. Their presence in each cyanobacterial strains was validated by mass spectrometric analysis (Singh et al. 2009). Compounds were detected by their respective m/z values of parent and product ions.
Results and discussion
Plants have always remained potential source of phenylpropanoids and other natural chemicals. Biosynthetic mechanisms for the synthesis of phenolic acids, flavonoids, flavones and their derivatives have been well established (Vogt 2010). Microalgae, especially cyanobacteria being non-conventional and less explored source of phenylpropanoids, pose immense scope for searching out unique secondary metabolites with potential bioactivities because of the ease of mass-scale cultivation in limited time frame, small space and low cost. Various other qualities of cyanobacteria like oxygenic photosynthesis, ability to survive in extreme habitats and amenability to genetic engineering make them prominent platform as cell factories for the targeted biosynthesis of secondary metabolites (Xue and He 2015).
We evaluated twenty strains of cyanobacteria belonging to different genera including Anabaena, Nostoc, Microcheate, Oscillatoria, Synechocystis, Hapalosiphon, Mastigocladus, Scytonema, Westiellopsis, Cylindrospermum, Aulosira, Chroococcus, Lyngbya, Calothrix, Dichothrix, Limnothrix and Phormidium that were isolated from different habitats. Growth of these organisms was recorded in terms of chlorophyll, carotenoids and protein content at the time of harvesting in their exponential phase (Table 1). There has been marked variation in the content of total chlorophyll, protein and carotenoids of cyanobacterial strains. Maximum content of chlorophyll (3.35 mg g−1 fresh wt), total protein (7.62 mg g−1 fresh wt) and total carotenoid (605.67 µg g−1 fresh wt) was found in Lyngbya sp. while minimum content of chlorophyll (0.53 mg g−1 fresh wt) was recorded in H. fontinalis, total protein (1.32 mg g−1 fresh wt) in L. obliqueacuminata and total carotenoids (77.73 µg g−1 fresh wt) in S. simplex.
Table 1.
Source of organisms and total content of chlorophyll, carotenoids and protein in cyanobacteria
| Organismsa | Accession and Origin | AChlorophyll content (mg g−1 fresh w) | BTotal protein content (mg g−1 fresh w) | CTotal carotenoid (µg g−1 fresh w) |
|---|---|---|---|---|
| Anabaena doliolum | NAIMCC-1; Porompat, Manipur | 2.14 ± 0.07b | 5.57 ± 0.11d | 356.60 ± 9.4f |
| Calothrix geitonos | NAIMCC-4; Singda, Manipur | 0.70 ± 0.07f–i | 1.74 ± 0.07k | 178.47 ± 7.9j |
| Dichothrix sp. | NAIMCC-7; Ukhrul, Manipur | 0.71 ± 0.07fgh | 1.64 ± 0.08k | 102.9 ± 5.6n |
| Limnothrix obliqueacuminata | NAIMCC-8; Wangkhei, Manipur | 0.75 ± 0.08fg | 1.32 ± 0.03l | 155.60 ± 11.7k |
| Microcheate tenera | NAIMCC-12; Kumbi, Manipur | 0.67 ± 0.06f–i | 2.19 ± 0.09i | 146.80 ± 5.3kl |
| Oscillatoria acuta | NAIMCC-17; Tamu, Burma | 1.69 ± 0.16c | 7.53 ± 0.087a | 526.70 ± 8.8b |
| Nostoc ellipsosporum | NAIMCC-28; Varanasi, U.P. | 0.77 ± 0.06fg | 3.17 ± 0.075g | 243.97 ± 8.5h |
| Anabaena constricta | NAIMCC-29; Varanasi, U.P. | 1.01 ± 0.14e | 3.27 ± 0.095g | 361.60 ± 5.3f |
| Synechocystis sp. | NAIMCC-33; Jodhpur, Rajasthan | 1.99 ± 0.12b | 6.21 ± 0.11b | 508.17 ± 4.7c |
| Hapalosiphon fontinalis | NAIMCC-36; Nagpur, Maharastra | 0.53 ± 0.06i | 1.96 ± 0.09j | 94.43 ± 1.8n |
| Mastigocladus laminosus | NAIMCC-43; Nagpur, Maharastra | 1.82 ± 0.11c | 5.83 ± 0.08c | 480.40 ± 7.3d |
| Scytonema simplex | NAIMCC-45; Baharaich, U.P. | 0.68 ± 0.05f–i | 1.66 ± 0.07k | 77.73 ± 5.9o |
| Calothrix brevissima | NAIMCC-48; Baharaich, U.P. | 0.84 ± 0.09f | 3.53 ± 0.09f | 206.43 ± 4.9i |
| Westiellopsis prolifica | NAIMCC-51; Baharaich, U.P. | 1.36 ± 0.08d | 5.57 ± 0.14d | 449.53 ± 9.4e |
| Cylindrospermum sp. | NAIMCC-70; South, Goa | 1.03 ± 0.1e | 4.40 ± 0.17e | 237.2 ± 6.1h |
| Aulosira fertilissima | NAIMCC-94; Tamil Nadu | 0.68 ± 0.08f–i | 2.68 ± 0.19h | 130.0 ± 3.6m |
| Chroococcus sp. | NAIMCC-100; IARI, New Delhi | 0.56 ± 0.07hi | 3.60 ± 0.17f | 154.5 ± 5.2k |
| Limnothrix sp. | NAIMCC-107; Takyelpat, Manipur | 1.12 ± 0.06e | 3.55 ± 0.18f | 268.5 ± 8.9g |
| Lyngbya sp. | NAIMCC-111; Takyelpat, Manipur | 3.35 ± 0.12a | 7.62 ± 0.11a | 605.67 ± 10.7a |
| Phormidium tenue | NAIMCC-113; West Agartala, Tripura | 0.60g ± 0.07hi | 2.64 ± 0.09h | 137.03 ± 5.4lm |
| SEM± | 0.05 | 0.07 | 4.01 | |
| CD (p < 0.05) | 0.15 | 0.190 | 11.46 | |
| CV (%) | 7.9 | 3 | 2.6 | |
aObtained from ICAR-National Bureau of Agriculturally Important Microorganisms (ICAR-NBAIM), Mau, India Culture Collection (NAIMCC)
Values represent average ± standard deviation for three replications; values in the same column with different superscripts are significantly different at p < 0.05
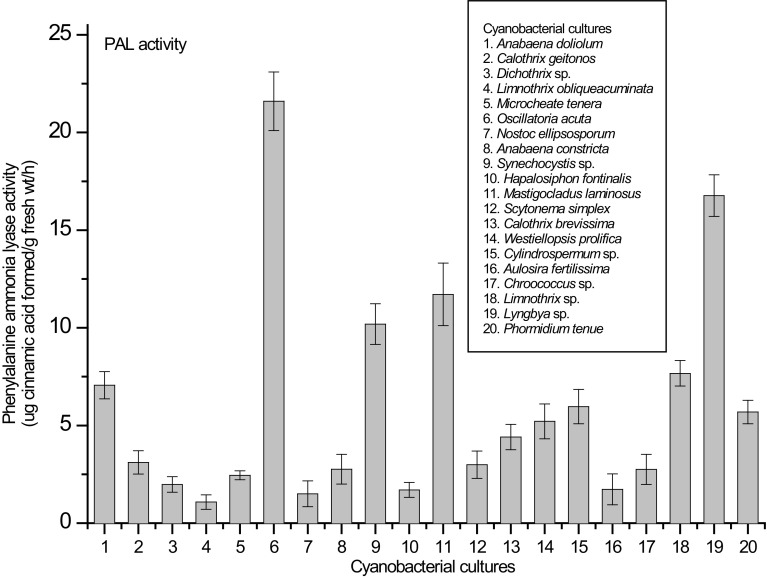
Since we determined antioxidant properties and polyphenolics content of cyanobacterial extracts, it was pertinent to assess the activity of PAL enzyme which catalyzes the initial reaction of phenylpropanoid biosynthesis in plants (Hanson and Havir 1981). The activity of PAL is known to undergo dramatic changes in the cells under the conditions of biotic and abiotic stresses (Ronald and Soderhall 1985; Aydas et al. 2013). PAL activity leading to the production of cinnamic acid may further lead to the biosynthesis of a number of polyphenolics within the cells. We reported maximum PAL activity in O. acuta (21.6 µg cinnamic acid g−1 fresh wt h−1) followed by Lyngbya sp. (16.8 µg cinnamic acid g−1 fresh wt h−1 and M. laminosus (11.7 µg cinnamic acid g−1 fresh wt h−1) (Fig. 1). Minimum PAL activity (1.08 µg cinnamic acid g−1 fresh wt h−1) was recorded in L. obliqueacuminata. After PAL estimation, total phenol content (TPC) and total flavonoid content (TFC) was determined in the cyanobacterial extracts (Table 2). High quantity of TPC (290.23, 107.07 and 92.03 mg GAE g−1 fresh wt in O. acuta, M. laminosus and Lyngbya sp., respectively) and TFC (634.0, 370.47 and 275.47 µg QE g−1 fresh wt in O. acuta, M. laminosus and Synechocystis sp., respectively) was recorded (Table 2). Other organisms also possess high quantity of TPC and TFC. High content of TPC in three strains namely O. acuta, M. laminosus and Lyngbya sp. is linked with the high activity of PAL enzyme in these organisms. Such observations reflect that a high PAL activity can result in increased biosynthesis of polyphenolics in the cells and due to this, high accumulation of total phenolics and total flavonoids was observed in these organisms.
Fig. 1.
Phenylalanine ammonia lyase activity of cyanobacterial cultures
Table 2.
Total phenol and flavonoids content and antioxidant potential of cyanobacterial cell-free extracts in terms of DPPH, ABTS, Deoxyribose protection and Fe+2-ion chelating activity
| Organisms | Phenolics content | Antioxidant power (IC50) (mg ml−1 cell-free extract) | ||||
|---|---|---|---|---|---|---|
| Total phenol content (mg GAE g−1 fresh w) | Total flavonoid content (µg QE g−1 fresh w) | DPPH activity | ABTS activity | Deoxyribose protection activity | Fe+2-ion chelation activity | |
| Anabaena doliolum | 47.77 ± 3.4fg | 106.93 ± 4.25j | 2.64 ± 0.19b | 0.39 ± 0.04efg | 5.26 ± 0.11b | 1.7 ± 0.36gh |
| Calothrix geitonos | 22.80 ± 2.3j | 91.0 ± 1.8kl | 1.06 ± 0.18b | 0.52 ± 0.06cd | 3.94 ± 0.05c | 3.5 ± 0.4e |
| Dichothrix sp. | 35.23 ± 3.3hi | 53.37 ± 3.81o | 1.72 ± 0.24b | 0.47 ± 0.06cde | 2.96 ± 0.09e | 3.2 ± 0.35e |
| Limnothrix obliqueacuminata | 44.80 ± 3.9g | 106.9 ± 7.3j | 2.95 ± 0.2b | 0.45 ± 0.07def | 7.95 ± 0.09a | 3.7 ± 0.45e |
| Microcheate tenera | 45.57 ± 5.9g | 88.37 ± 3.8l | 4.28 ± 0.5b | 0.37 ± 0.05efg | 1.84 ± 0.08h | 8.7 ± 0.36a |
| Oscillatoria acuta | 290.23 ± 2.2a | 634.0 ± 5.1a | 2.63 ± 0.36b | 0.97 ± 0.09a | 3.64 ± 0.05d | 1.4 ± 0.2hi |
| Nostoc ellipsosporum | 39.03 ± 1.8h | 115.03 ± 5.2i | 8.91 ± 0.73a | 0.45 ± 0.07def | 2.59 ± 0.07f | 4.7 ± 0.8d |
| Anabaena constricta | 59.37 ± 4.0d | 201.97 ± 3.3d | 0.91 ± 0.04b | 0.23 ± 0.07h | 0.63 ± 0.04n | 0.9 ± 0.22i |
| Synechocystis sp. | 54.93 ± 2.65de | 275.47 ± 2.8c | 1.52 ± 0.06b | 0.38 ± 0.08efg | 0.86 ± 0.05lm | 1.3 ± 0.2hi |
| Hapalosiphon fontinalis | 33.43 ± 3.65hi | 79.43 ± 2.9m | 3.0 ± 0.19b | 0.44 ± 0.04d–g | 2.44 ± 0.12g | 2.46 ± 0.36f |
| Mastigocladus laminosus | 107.07 ± 4.04b | 370.47 ± 2.7b | 1.67 ± 0.11b | 0.32 ± 0.04gh | 1.35 ± 0.06j | 2.42 ± 0.45fgh |
| Scytonema simplex | 32.77 ± 2.65i | 71.1 ± 2.8n | 1.42 ± 0.19b | 0.68 ± 0.09b | 0.75 ± 0.13mn | 4.5 ± 0.38d |
| Calothrix brevissima | 31.47 ± 2.01i | 95.13 ± 2.7k | 2.24 ± 0.18b | 0.34 ± 0.07fgh | 0.92 ± 0.05l | 2.4 ± 0.45fg |
| Westiellopsis prolifica | 58.07 ± 4.51d | 199.2 ± 3.13d | 3.52 ± 0.26b | 0.67 ± 0.04b | 0.66 ± 0.05n | 3.8 ± 0.8e |
| Cylindrospermum sp. | 34.07 ± 4.27hi | 157.9 ± 2.8g | 1.27 ± 0.06b | 0.32 ± 0.03gh | 0.44 ± 0.08o | 1.3 ± 0.26hi |
| Aulosira fertilissima | 22.17 ± 1.81j | 82.27 ± 2.05m | 2.27 ± 0.19b | 0.58 ± 0.04bc | 1.78 ± 0.11h | 7.8 ± 0.45b |
| Chroococcus sp. | 52.07 ± 1.32ef | 166.67 ± 1.96f | 1.56 ± 0.09b | 0.32 ± 0.06gh | 0.32 ± 0.04o | 2.4 ± 0.28fg |
| Limnothrix sp. | 24.93 ± 2.4j | 130.77 ± 1.7h | 1.82 ± 0.09b | 0.57 ± 0.05bc | 0.81 ± 0.04lm | 4.6 ± 0.49d |
| Lyngbya sp. | 92.03 ± 1.59c | 192.37 ± 2.0e | 2.61 ± 0.13b | 0.53 ± 0.07cd | 1.15 ± 0.05k | 2.6 ± 0.43c |
| Phormidium tenue | 30.63 ± 1.04i | 95.20 ± 2.05k | 2.69 ± 0.21b | 0.33 ± 0.05fgh | 1.54 ± 0.05i | 1.5 ± 0.31hi |
| SEM± | 1.895 | 2.035 | 1.449 | 0.036 | 0.044 | 0.24 |
| CD (p < 0.05) | 5.415 | 5.817 | 4.142 | 0.104 | 0.126 | 0.70 |
| CV (%) | 5.7 | 2.1 | 99 | 13.5 | 3.6 | 12.60 |
Values represent average ± standard deviation for three replications; values in the same column with different superscripts are significantly different at p < 0.05
ND Not Detected, GAE gallic acid equivalents, QE Quercetin equivalents
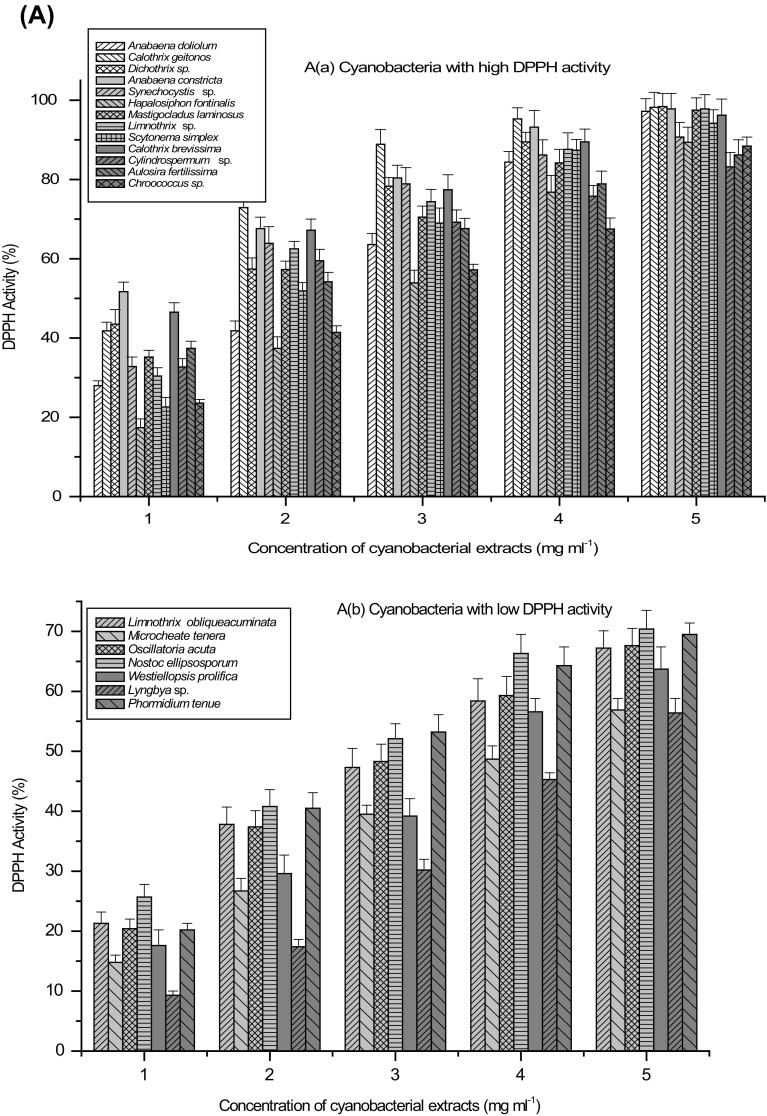
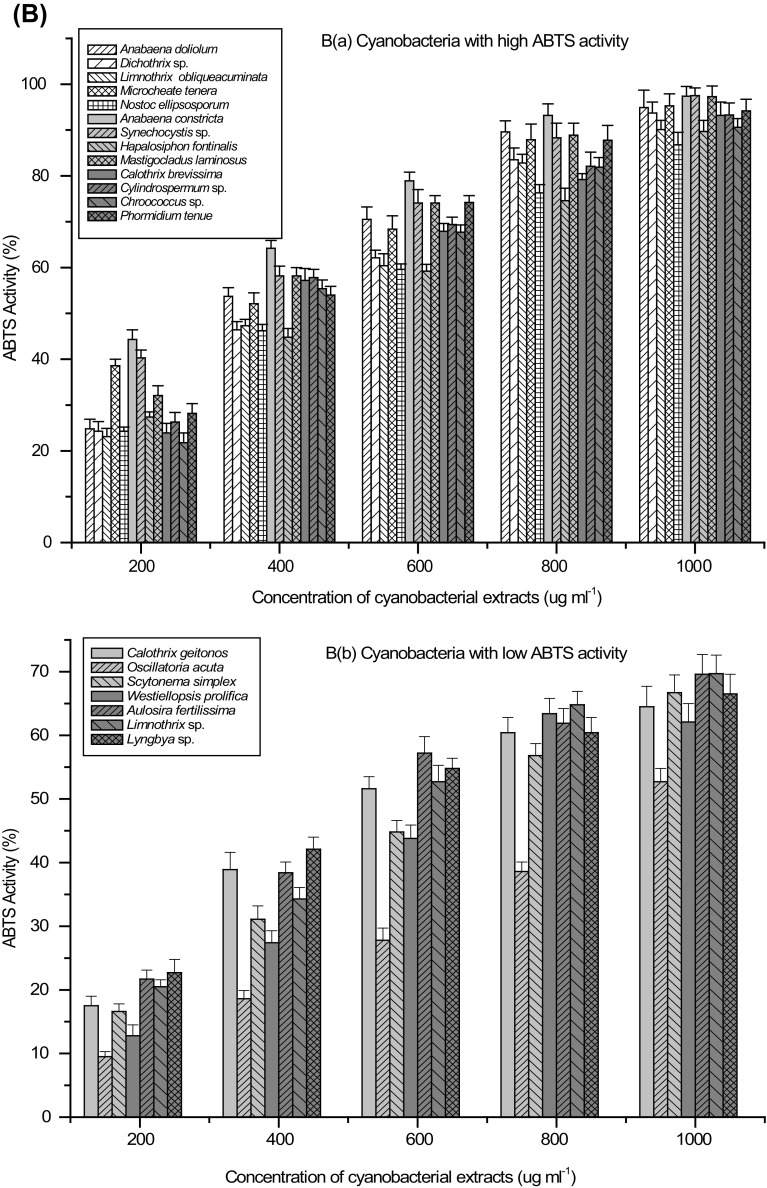
Antioxidant activity of biological samples and/or natural compounds was evaluated by various methods. Two free radicals commonly used to evaluate antiradical and antioxidant power of biological extracts or natural compounds in vitro are 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and 2, 2-azinobis (3-ethylbenzothiazoline- 6-sulfonic acid) (ABTS). Free radical scavenging activity of the cyanobacterial extracts was evaluated against three important radicals, DPPH, ABTS+ and OH. DPPH method was widely used to determine antiradical and/or antioxidant power of biological extracts and/or purified compounds. It is relatively stable free radical that, upon reduction by an antioxidant loses its absorption (515 nm). Concentration-dependent assays for the determination of free radical scavenging activity using DPPH resulted in two categories of cyanobacterial strains, first with high activity while second with low activity. Extracts of A. doliolum, C. geitonos, Dichothrix sp., A. constricta, Synechocystis sp., H. fontanalis, M. laminosus, S. symplex, C. brevissima, Cylindrospermum sp., A. fertilissima and Chroococcus sp. exhibited high free radical scavenging activity in terms of DPPH activity in which more than 80% DPPH activity was recorded at the concentration of 5 mg ml−1 (Fig. 2a). Among cyanobacteria exhibiting low DPPH activity were L. obliqueaccuminata, M. tenera, O. acuta, N. ellipsoporum, W. prolifica, Lyngbya sp. and P. tenue in which less than 70% activity was achieved at 5 mg ml−1 and even at higher concentrations, the activity remained similar. Maximum DPPH activity (IC50 0.91 mg ml−1 cell-free extract) was recorded in A. constricta while minimum activity (IC50 4.28 mg ml−1 cell-free extract) was found in M. tenera (Table 2). Similarly, high ABTS activity (above 90% at 1 mg ml−1) was observed in A. doliolum, Dichothrix sp., L. obliqueaccuminata, M. tenera, N. ellipsosporum, A. constricta, Synechocystis sp., H. fontanalis, M. laminosus, C. brevissima, Cylindrospermum sp., Chroococcus sp. and P. tenue and low activity group included C. geitonos, O. acuta, S. simplex, W. prolifica, A. fertilissima, Limnothrix sp., and Lyngbya sp. (Fig. 2b).
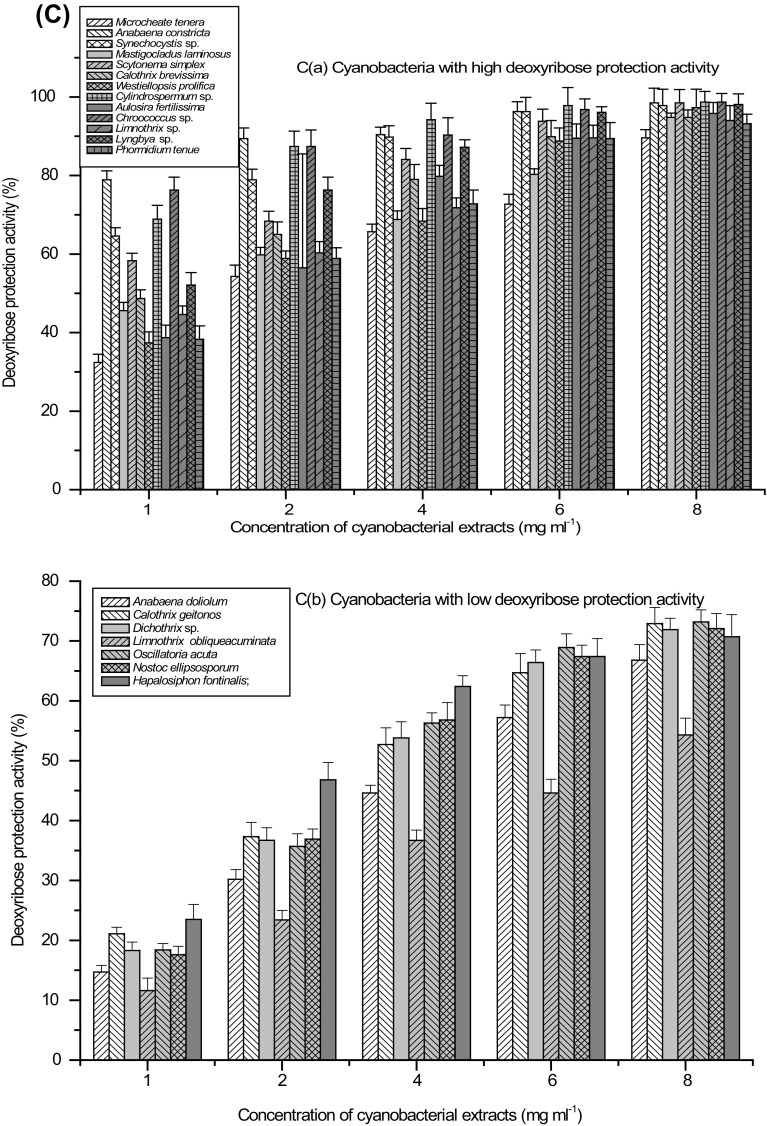
Fig. 2.
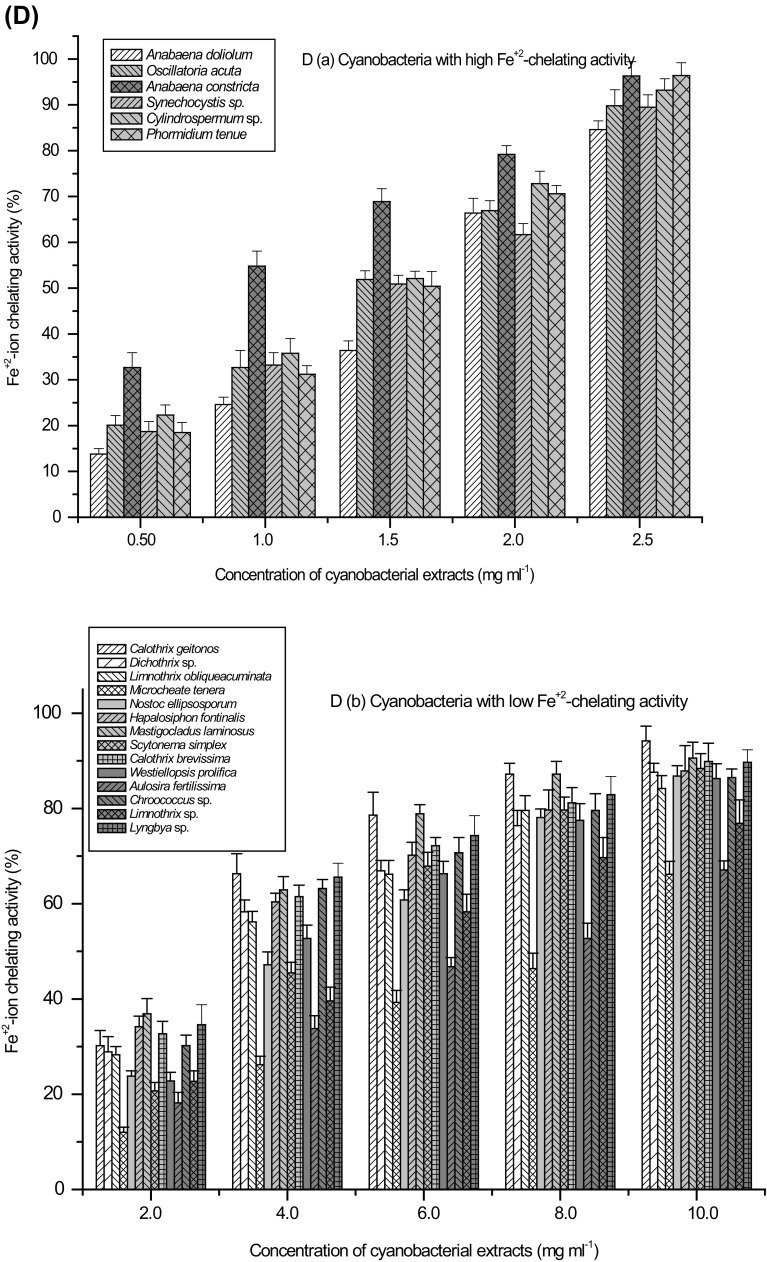
Concentration-dependent antioxidant activity (%) of cyanobacterial strains a DPPH activity, b ABTS activity, c Deoxyribose protection activity and d Fe+2-ion chelating activity; Extracts with high (a) and low (b) activity
ABTS activity of the cyanobacterial extracts ranged from 0.23 mg ml−1 (IC50) in A. constricta to 0.97 mg ml−1 (IC50) in O. acuta. ABTS assay determines relative ability of the extract or compound (antioxidant) to scavenge ABTS which is generated by reacting strong oxidizing agent such as potassium persulfate. Reduced blue-green ABTS radical by hydrogen-donating antioxidant in solution reflects antiradical power of the antioxidant extract or compounds. While comparing the results on DPPH and ABTS free radical scavenging activity of the extracts, it is evident that in contrast to DPPH method, ABTS method was more effective and precise. It has been shown that most phenolic antioxidants react slowly with DPPH, reaching a steady state in longer time (Bondet et al. 1997). The method also has limitations with the interference of the presence of anthocyanins that could lead to underestimation of the antioxidant activity of biological extract (Shalaby and Shanab 2013). The requirement of high concentrations of polyphenolics-rich cyanobacterial extracts for obtaining IC50 value of antioxidant activity using DPPH in comparison to ABTS may be explained on the basis of slow reaction of phenolics with DPPH.
Hydroxyl radical OH· is an extremely reactive free radical capable of reacting with various molecules like sugars, amino acids, lipids and nucleotides in the cells (Yasuda et al. 2000). Incubation of deoxyribose with H2O2 and Fe+2-EDTA as chelating agent reduces degradation of deoxyribose by the addition of scavenger of OH· radical. The process depends on the concentration of scavenger relative to deoxyribose (Gutteridge 1984). Extracts of the plants such as Moringa oleifera (Chumark et al. 2008) and Allium cepa (Singh et al. 2009) rich in polyphenolics have shown excellent antioxidant properties and were proven good scavengers of reactive species such as OH, O2 and ROO. We evaluated different concentration of cyanobacterial extracts rich in polyphenols to assess shielding effect against the degenerative reaction against deoxyribose caused by the OH· radical in the reaction mixture. Prominent deoxyribose protection activity was shown from 0.32 mg ml−1 cell free extract (IC50) in Chroococcus sp. to 7.95 mg ml−1 (IC50) in L. obliqueacuminata (Table 2). Extracts of M. tenera, A. constricta, Synechocystis sp., M. laminosus, S. simplex, C. brevissima, W. prolifica, Cylindrospermum sp., A. fertilissima, Chroococcus sp., Limnothrix sp., Lyngbya sp. and P. tenue showed high level (>90% activity at 6 mg ml−1 cell-free extract) of OH radical scavenging activity and extracts of A. doliolum, C. geitonos, Dichothrix sp., L. obliqueacuminata, O. acuta, N. ellipsosporum and H. fontinalis showed low deoxyribose protection (<75% activity at 8 mg ml−1 cell free extract) (Fig. 2c).
Iron (Fe+2) chelation power of cyanobacterial extracts was analysed. Methanolic extract of cyanobacteria exhibited significant capacity to chelate ferrous ions. These ions are among the most effective pro-oxidants and their reaction with H2O2 in the biological system leads to the formation of OH radicals. In the experiments, ferrozine quantitatively forms stable Fe+2-complex, the intensity of which was decreased in the presence of a chelating agent due to the interference with Fe+2-ferozine complex. The measurement of chelating activity therefore, reflects the ability of the chelating agent or the antioxidant to disrupt Fe+2-complex formation. All cyanobacterial extracts interfered with the Fe+2-complex formation in various capacities. Prominent Fe+2 chelating activity was recorded in the extracts of A. constricta, Synechocystis sp., Cylindrospermum sp., O. acuta¸ P. tenue and A. doliolum (IC50 0.9, 1.3, 1.3, 1.4, 1.5 and 1.7 mg ml−1 cell-free extract) (Table 2). Fe+2-ion chelating activity was found to be concentration-dependent and increased with the increasing concentration of cell free extracts. Evaluation of chelating activity showed that in the extracts of six strains namely A. constricta, Synechocystis sp., Cylindrospermum sp., O. acuta¸ P. tenue and A. doliolum, >90% activity was attained at the extract concentration of <2.5 mg ml−1 (Fig. 2D(a)). However, cyanobacteria with low Fe+2-ion chelating activity showed a maximum of 70–80% activity at the extract concentration of 8–10 mg ml−1 (Fig. 2D(b)). Excess of metal ions can lead to various anomalies in the cellular system. The iron (II) chelating activity of cyanobacterial extracts is of great significance and the transition metal ions contribute to the oxidative damage. Such activities of cyanobacterial cellular constituents might be thought to become protective to these organisms under the conditions of habitat stresses.
Specific biochemical fingerprints in terms of small-molecule metabolites in real time can be obtained with the help of LC–MS/MS studies (Daviss 2005). Targeted metabolic profiling of cyanobacterial polyphenolic-rich extracts using HPLC has resulted in the characterization of five phenolic acids namely gallic, chlorogenic, caffeic, ferulic and vanillic acids and three flavonoids namely rutin, quercetin and kaempferol in various quantities (Table 3). Persistent presence of gallic acid was observed in all the strains and the maximum content was 99.03 µg g−1 fresh wt in L. obliqueacuminata followed by 83.7 µg g−1 fresh wt in Dichothrix sp. and 77.73 µg g−1 fresh wt in P. tenue (Table 3). Minimum content of gallic acid was as low as 16.47 µg g−1 fresh wt in N. ellisposporum. Among other phenolic acids, presence of chlorogenic acid was detected in various quantities in 13 strains, caffeic acid in 12, ferulic acid in 10, vanillic acid in 14, rutin in 16, quercetin in 15 and kaempferol in 15 strains. Chlorogenic acid content was maximum (77.9 µg g−1 fresh wt) in M. tenera while minimum (1.9 µg g−1 fresh wt) in Lyngbya sp. Maximum content of caffeic acid (95.9 µg g−1 fresh wt) was found in A. fertilissima, ferulic acid (37.4 µg g−1 fresh wt) in O acuta, vanillic acid (27.4 µg g−1 fresh wt) in M. laminosus, rutin (29.4 µg g−1 fresh wt) in M. tenera, quecretin (23.8 µg g−1 fresh wt) in N. ellipsosporum and kaempferol (7.8 µg g−1 fresh wt) in M. tenera (Table 3). These metabolites were further verified by LC–MS/MS studies using [M–H]− ion for gallic m/z 169, chlorogenic m/z 353, caffeic m/z 179, vanillic m/z 167 and ferulic acid m/z 193 and rutin m/z 609, quercetin m/z 301 and kaempferol m/z 185.
Table 3.
Quantitative content of phenolic acids and flavonoids in cyanobacterial cell-free extracts
| Cyanobacteria | Phenolic acids (µg g−1 fresh wt) | Flavonoids (µg g−1 fresh wt) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gallic acid (2.73A) | Chlorogenic acid (3.04A) | Caffeic acid (3.58A) | Vanillic acid (3.89A) | Ferulic acid (4.76A) | Rutin (4.06A) | Quercetin (9.24A) | Kaempferol (15.5A) | |
| Anabaena doliolum | 45.73g | 27.4 ± 6.1de | 7.6 ± 0.65de | 12.7 ± 1.07c | NDk | 12.8 ± 0.41b | 9.7 ± 0.66e | NDj |
| Calothrix geitonos | 34.40h | NDg | NDg | 3.8 ± 0.3f | 1.7 ± 0.09j | 12.0 ± 0.4c | 8.9 ± 0.5e | 3.0 ± 0.4efg |
| Dichothrix sp. | 83.70b | 72.0 ± 8.3b | NDg | 2.0 ± 0.31fgh | 3.7 ± 0.25i | 2.5 ± 0.4i | 7.5 ± 0.4f | 3.6 ± 0.56cde |
| Limnothrix obliqueacuminata | 99.03a | 28.1 ± 1.7de | NDg | NDh | 17.9 ± 0.65c | 3.0 ± 0.55hi | 12.4 ± 0.43c | 1.9 ± 0.45hi |
| Microcheate tenera | 47.03g | 77.9 ± 2.2a | NDg | 24.4 ± 3.15b | NDk | 29.4 ± 0.72a | 18.4 ± 0.85b | 7.8 ± 0.7a |
| Oscillatoria acuta | 25.67ij | NDg | 25.3 ± 4.03c | 7.6 ± 0.32e | 37.4 ± 1.2a | 3.4 ± 0.37gh | NDh | 1.8 ± 0.4hi |
| Nostoc ellipsosporum | 16.47k | 4.2 ± 0.22fg | 8.2 ± 0.45de | 1.4 ± 0.15gh | 8.9 ± 0.43e | NDk | 23.8 ± 1.03a | 4.3 ± 0.6bc |
| Anabaena constricta | 22.60j | NDg | 5.6 ± 0.5ef | 1.2 ± 0.36gh | 4.9 ± 0.35h | 1.5 ± 0.35j | NDh | NDj |
| Synechocystis sp. | 24.53ij | 8.2 ± .45f | NDg | NDh | NDk | 8.8 ± 0.62d | 6.7 ± 0.45f | NDj |
| Hapalosiphon fontinalis | 35.63h | 41.2 ± 4.2c | 45.1 ± 1.98b | NDh | 16.96 ± 1.06d | 3.7 ± 0.45gh | 11.7 ± 0.66cd | 4.8 ± 0.6b |
| Mastigocladus laminosus | 25.93ij | NDg | 8.8 ± 0.7d | 27.4 ± 3.15a | NDk | 13.4 ± 0.46b | NDh | NDj |
| Scytonema simplex | 27.60i | NDg | 5.5 ± 0.65ef | 2.6 ± 0.5fg | 5.8 ± 0.65g | 3.9 ± 0.8fg | 11.4 ± 0.65d | 2.8 ± 0.31fg |
| Calothrix brevissima | 62.03e | 31.8 ± 2.7d | NDg | NDh | NDk | 4.6 ± 0.56ef | 11.2 ± 0.62d | NDj |
| Westiellopsis prolifica | 43.13g | 9.3 ± 0.4f | 3.7 ± 0.5f | 2.8 ± 0.25fg | NDk | NDk | 3.7 ± 0.55g | 7.8 ± 0.46a |
| Cylindrospermum sp. | 36.97h | NDg | 6.6 ± 0.64de | 2.7 ± 0.4fg | NDk | NDk | 8.9 ± 0.8e | 3.9 ± 0.36cd |
| Aulosira fertilissima | 24.80ij | NDg | 95.9 ± 4.94a | NDh | NDk | NDk | 4.3 ± 0.55g | 3.3 ± 0.56def |
| Chroococcus sp. | 67.97d | 37.8 ± 2.9c | NDg | 10.5 ± 0.97d | 7.8 ± 0.7f | 1.4 ± 0.35j | NDh | 3.0 ± 0.4efg |
| Limnothrix sp. | 55.23f | 27.1 ± 3.3de | NDg | 3.8 ± 0.65f | NDk | 8.6 ± 0.57d | 11.3 ± 0.7d | 2.7 ± 0.47fg |
| Lyngbya sp. | 35.43h | 1.9 ± 0.2g | 3.7 ± 0.55f | 2.8 ± 0.7fg | NDk | 8.8 ± 0.6d | NDh | 2.5 ± 0.4gh |
| Phormidium tenue | 77.73c | 23.5 ± 3.8e | 5.5 ± 0.51ef | NDh | 27.3 ± 1.12b | 5.2 ± 0.46e | 11.3 ± 0.56d | 1.6 ± 0.25i |
| SEM± | 1.51 | 1.70 | 0.89 | 0.63 | 0.30 | 0.28 | 0.32 | 0.24 |
| CD (p < 0.05) | 4.32 | 4.86 | 2.55 | 1.80 | 0.87 | 0.79 | 0.92 | 0.68 |
| CV (%) | 5.90 | 15.10 | 14.00 | 20.70 | 8.00 | 7.80 | 6.90 | 15.00 |
Retention time in min; ND- not detectable; Values represent average ± standard deviation for three replications; values in the same column with different superscripts are significantly different at p < 0.05
Prominent DPPH, ABTS and deoxyribose protection activity was observed in the polyphenolics compounds that were identified in different quantities in cyanobacterial extracts (Table 4). Gallic (IC50 3.53 µg ml−1), chlorogenic (IC50 6.41 µg ml−1), caffeic (IC50 6.34 µg ml−1) were found more prominent in DPPH activity along with other compounds (except vanillic acid) than the standard compounds BHT (IC50 12.41 µg ml−1) and α-tocoferol (IC50 10.87 µg ml−1). Similarly, ABTS activity of kaempferol and gallic acid was (IC50 8.54 and 8.85 µg ml−1, respectively) very prominent in comparison to BHT and α-tocoferol and most of the compounds except vanillic acid identified in the cyanobacterial extracts were even more prominent than α-tocoferol in ABTS activity. Ferulic acid was found to be very effective in deoxyribose protection (IC50 4.51 µg ml−1) while other compounds also proven comparatively good in comparison to BHT and α-tocoferol. Such results reflect a clear correlation in between the presence of different polyphenolics biomolecules in the cyanobacterial extracts and prominent antioxidant activity shown by the extracts. This is probably a direct evidence to support the assumption that polyphenolics in cyanobacterial extracts are responsible for the antioxidant properties offered by the organisms. Phytochemicals of natural origin are becoming a prominent source of antioxidant molecules. Cyanobacteria could be one of the most potential source for the production of natural antioxidants. Phenolic acids and flavonoids from plant sources have been well-documented for their antioxidant, free-radical quenching and redox metal ion-chelating affects (Singh et al. 2009, 2014; Tutour 1990; Apati et al. 2003; Hossain et al. 2016) but cyanobacteria and microalgae are relatively new source for such compounds in recent years due to their potential benefits and ease of production (Guedes et al. 2013; Machu et al. 2015). Gallic, chlorogenic, caffeic and ferulic acids identified from plant sources have been known as strong antioxidants and free radical scavengers (Piazzon et al. 2012; Babić et al. 2015) but their presence in cyanobacteria is scarcely reported and this make these organisms more potential. Being most primitive, these organisms are considered to evolve with various levels of environmental adaptations to overcome these stresses for their survival over the time (Tandeau-de-Marsac and Houmard 1993). This study implicates that the antioxidant properties of the cyanobacterial extracts can be thought of a reflection of intrinsic strategies of these organisms to reduce the damage caused due to ROS generated during environmental stresses. The presence of polyphenolics (phenolic acids and flavonoids) and high PAL activity in cyanobacteria may also be considered as a part of the key functions responsible for the protection of these organisms from environmental stresses during the course of their evolution (Aydas et al. 2013; Singh et al. 2014). This further may add functional values such as free-radical quenching, metal chelation and ROS-scavenging activity to the properties of these organisms.
Table 4.
Antioxidant activity of phenolics acids and flavonoids identified in the cyanobacterial extracts
| Polyphenolics | Antioxidant activity | ||
|---|---|---|---|
| DPPH activity (IC50) (µg ml−1) | ABTS activity (IC50) (µg ml−1) | Deoxyribose protection activity (IC50) (µg ml−1) | |
| Gallic acid | 3.53 ± 0.24 | 8.85 ± 0.74 | 7.84 ± 0.72 |
| Chlorogenic acid | 6.41 ± 0.61 | 13.15 ± 0.71 | 8.53 ± 0.62 |
| Caeffic acid | 6.34 ± 0.37 | 18.04 ± 0.68 | 4.76 ± 0.67 |
| Vanillic acid | 416.7 ± 6.2 | 132.1 ± 3.4 | 91.1 ± 5.2 |
| Ferulic acid | 11.75 ± 0.75 | 9.47 ± 0.69 | 4.51 ± 0.37 |
| Rutin | 6.55 ± 0.54 | 13.78 ± 0.93 | 7.58 ± 0.66 |
| Quercetin | 4.71 ± 0.49 | 10.63 ± 0.83 | 20.1 ± 0.84 |
| Kaempferol | 17.30 ± 1.3 | 8.54 ± 0.6 | 7.17 ± 0.46 |
| BHT | 12.41 ± 1.6 | 16.12 ± 1.3 | 6.1 ± 0.45 |
| α-Tocoferol | 10.87 ± 1.2 | 32.41 ± 1.7 | 8.37 ± 0.72 |
Values represent average ± standard deviation for three replications
Conclusion
Despite of possessing unique secondary metabolites with potential biological activities, cyanobacteria are a non-conventional and less worked-out source of phenylpropanoids. Evaluation of cell-free extracts of twenty cyanobacterial strains from different genera i.e., Anabaena, Nostoc, Microcheate, Oscillatoria, Synechocystis, Hapalosiphon, Mastigocladus, Scytonema, Westiellopsis, Cylindrospermum, Aulosira, Chroococcus, Lyngbya, Calothrix, Dichothrix, Limnothrix and Phormidium indicated prominent biological potential based on DPPH, ABTS, deoxyribose protection and Fe+2 ion chelating activities. Presence of significant amount of PAL activity, total phenolics and flavonoids and phenylpropanoid metabolites is linked with the indicated biological effects. A direct correlation between phenylpropanoids in the extracts and the antioxidant properties was found indicating that the polyphenolics in the cyanobacterial extracts may be accountable for their antioxidant properties. Presence of phenylpropanoids gallic, chlorogenic, caffeic, vanillic and ferulic acids and flavonoids rutin, quercetin and kaempferol in cyanobacterial extracts and their correlation with antioxidant properties make these organisms potentially viable source of biomolecules. In addition, dominant presence of polyphenols as antioxidants in cyanobacterial species may be presumed as the adaptation strategies of these organisms against abiotic stresses due to their specific habitats.
Acknowledgements
The work was carried out under the Institute project of ICAR-NBAIM, India undertaken by DPS.
Compliance with ethical standards
Conflict of interest
Authors declared no conflict of interest.
References
- Apati P, Szentmihalyi K, KristoSz T, Papp I, Vinkler P, Szoke E, Kery A. Herbal remedies of Solidago, correlation of phytochemical characteristics and antioxidative properties. J Pharm Biomed Anal. 2003;32:1045–1053. doi: 10.1016/S0731-7085(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Aydas SB, Ozturk S, Aslim B. Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem. 2013;136:164–169. doi: 10.1016/j.foodchem.2012.07.119. [DOI] [PubMed] [Google Scholar]
- Babić O, Kovač D, Rašeta M, Šibul F, Svirčev Z, Simeunović J. Evaluation of antioxidant activity and phenolic profile of filamentous terrestrial cyanobacterial strains isolated from forest ecosystem. J Appl Phycol. 2015;28:2333–2342. [Google Scholar]
- Bharanidharan M, Sivasubramanian V, Raja SR, Nayagam V. Evaluation of antioxidant and antimicrobial potential of cyanobacteria, Chroococcus turgidus (Kützing) Nägeli. Int J Curr Microbiol App Sci. 2013;2:300–305. [Google Scholar]
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH.free radical method. Lebensm Wiss Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- Camacho FA. Macroalgal and cyanobacterial chemical defenses in freshwater communities. In: Amsler FA, editor. Algal chemical ecology. Berlin: Springer-Verlag; 2008. pp. 105–109. [Google Scholar]
- Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, Ratanachamnong P, Srisawat S, Pongrapeeporn KU. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam leaves. J Ethnopharmacol. 2008;116:439–446. doi: 10.1016/j.jep.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Daviss B. Growing pains for metabolomics. The Scientist. 2005;19:25–28. [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida MLM. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Ferjani A, Mustardy L, Sulpice R, Marin K, Suzuki I, Hagemann M, Murata N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003;131:1628–1637. doi: 10.1104/pp.102.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga CG, Galleano M, Verstraeten SV, Oteiza PI. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol Aspects Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Giao MS, Gonzalez-Sanjose ML, Perez MDR, Pereira CI, Pintado ME, Malcata FX. Infusion of Portuguese medicinal plants: dependence of final antioxidant capacity and phenolic content on extraction features. J Sci Food Agri. 2007;87:2638–2647. doi: 10.1002/jsfa.3023. [DOI] [PubMed] [Google Scholar]
- Guedes AC, Gião MS, Seabra R, Ferreira AC, Tamagnini P, Moradas-Ferreira P, Malcata FX. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar Drugs. 2013;11:1256–1270. doi: 10.3390/md11041256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JMC. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid-reactive material from deoxysugars, nucleotides and benzoate. J Biochem. 1984;224:761–767. doi: 10.1042/bj2240761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KR, Havir EA. Phenylalanine ammonia-lyase. In: Stumpf PK, Conn EE, editors. The biochemistry of plants. New York: Academic Press; 1981. pp. 577–625. [Google Scholar]
- Hossain MF, Ratnayake RR, Meerajini K, Kumara KLW. Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci Nutr. 2016 doi: 10.1002/fsn3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem. 2003;51:6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Machu L, Misurcova L, Ambrozova JV, Orsavova J, Mlcek J, Sochor J, Jurikova T. Phenolic acids and antioxidants in algal food products. Molecules. 2015;20:1118–1133. doi: 10.3390/molecules20011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Rath J. Secondary metabolites of cyanobacteria and drug development. In: Mandal S, Rath J, editors. Extremophilic cyanobacteria for novel drug development. Switzerland: Springer; 2015. pp. 23–43. [Google Scholar]
- McCallum JA, Walker JRL. Phenolic biosynthesis during grain development in wheat: changes in phenylalanine ammonia-lyase activity and soluble phenolic content. J Cereal Sci. 1990;11:35–49. doi: 10.1016/S0733-5210(09)80179-3. [DOI] [Google Scholar]
- Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Piazzon A, Vrhovsek U, Masuero D, Mattivi F, Mandoj F, Nardini M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J Agri Food Chem. 2012;60:12312–12323. doi: 10.1021/jf304076z. [DOI] [PubMed] [Google Scholar]
- Rastogi RP, Sinha RP. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv. 2009;27:521–539. doi: 10.1016/j.biotechadv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Rastogi RP, Sinha RP. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv. 2009;27(4):521–539. doi: 10.1016/j.biotechadv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation and cancer: how are they linked? Free Radical Bio Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald P, Soderhall K. Phenylalanine ammonia lyase and peroxidase activity in mycorrhizal and nonmycorrhizal short roots of scots pine, Pinus sylvestris L. New Phytol. 1985;101:487–494. doi: 10.1111/j.1469-8137.1985.tb02854.x. [DOI] [PubMed] [Google Scholar]
- Shalaby EA, Shanab SMM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanolic extracts of Spirulina platensis. Ind J Geo-Mar Sci. 2013;42:556–564. [Google Scholar]
- Singh DP, Tyagi MB, Kumar A, Thakur JK, Kumar A. Antialgal activity of a hepatotoxin-producing cyanobacterium, Microcystis aeruginosa. World J Microbiol Biotechnol. 2001;17:15–22. doi: 10.1023/A:1016622414140. [DOI] [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Singh DP, Sarma BK, Upadhyay G, Singh HB. Polyphenolics from various extracts/fraction of red onion (Allium cepa) peel with potential antioxidant and antimutagenic activities. Food Chem Toxicol. 2009;47:1161–1167. doi: 10.1016/j.fct.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Singh DP, Prabha R, Yandigeri MS, Arora DK. Cyanobacteria-mediated phenylpropanoids and phytohormones in rice (Oryza sativa) enhance plant growth and stress tolerance. Antonie Van Leeuwenhoek. 2011;100:557–568. doi: 10.1007/s10482-011-9611-0. [DOI] [PubMed] [Google Scholar]
- Singh DP, Prabha R, Meena KK, Sharma L, Sharma AK. Induced accumulation of polyphenolics and flavonoids in cyanobacteria under salt stress protects organisms through enhanced antioxidant activity. American J Plant Sciences. 2014;5:726–735. doi: 10.4236/ajps.2014.55087. [DOI] [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order-Chroococcales) Bacteriol Rev. 1971;35:171–305. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau-de-Marsac N, Houmard J. Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiol Rev. 1993;104:119–190. doi: 10.1111/j.1574-6968.1993.tb05866.x. [DOI] [Google Scholar]
- Tomitani A, Knoll AH, Cavanaugh CM, Ohno T. The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc Natl Acad Sci USA. 2006;103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutour BL. Antioxidative activities of algal extracts, synergistic effect with vitamin E. Phytochem. 1990;29:3759–3765. doi: 10.1016/0031-9422(90)85327-C. [DOI] [Google Scholar]
- Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Xue Y, He Q. Cyanobacteria as cell factories to produce plant secondary metabolites. Front Bioeng Biotechnol. 2015;3:57. doi: 10.3389/fbioe.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Inaba A, Ohmori M, Endo T, Kubo S, Ohsawa K. Urinary metabolites of gallic acid in rats and their radical scavenging effect on DPPH. J Nat Prod. 2000;63:1444–1446. doi: 10.1021/np0000421. [DOI] [PubMed] [Google Scholar]
- Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free radical and anti oxygen. Agric Food Chem. 1994;42:629–632. doi: 10.1021/jf00039a005. [DOI] [Google Scholar]