Abstract
[Purpose] The aim of this study was to evaluate the effects of a self-managed physical activity program using a pedometer and diary on physical function, ADL, and QOL in patients with chronic respiratory disease. [Subjects and Methods] 17 outpatients with chronic respiratory disease were assessed for dyspnea, muscle strength, exercise tolerance, ADL, and QOL at baseline, after 3-, and 6-months after the start of the program. Patients were randomly assigned to “Control” or “Diary” group. In the Diary group, the number of steps was counted with a pedometer and recorded in a diary together with self-evaluation of physical activity, while patients assigned to the Control group did not use a pedometer or keep a diary. [Results] The Diary group showed significant improvement in the daily step count over time. The Diary group showed significant improvement of the dyspnea, muscle strength, and exercise tolerance at 3 months, dyspnea and muscle strength at 6 months. Significant differences found between two groups with regard to the extent of change in the muscle strength, exercise tolerance, and QOL at 3 months. [Conclusion] This study suggests that a self-managed physical activity program using a pedometer and diary can increase the level of physical activity.
Keywords: Physical activity, Self-management, Pedometer
INTRODUCTION
Physical activity is increasingly considered important for patients with chronic respiratory disease (CRD) given the beneficial effect of regular physical activity on the prognosis of CRD1). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends physical activity for all patients with chronic obstructive lung disease (COPD)2), and improving the level of physical activity is a key goal in the management of COPD3). Importance of physical activity has also been reported for patients with interstitial pneumonia4, 5).
Guidelines generally recommend that rehabilitation programs be implemented two or three times per week6). However, it is common for elderly outpatients in Japan to undergo less frequent pulmonary rehabilitation7, 8) because they cannot attend hospital by themselves. Self-management is an important part of any formalized patient education program that aims to teach the skills needed to implement disease-specific regimens and guide health behavior changes so that patients can control their disease and improve well-being9, 10). Many self-management programs include specific education and encouragement of behavioral change10) so that patients can manage their diseases more effectively at home.
However, teaching self-management skills alone is not enough to change behavior and patients need to integrate these skills into daily life9). As these skills are successfully utilized in various situations, the patient develops a sense of self-efficacy, which is the confidence an individual gains in relation to specific actions and his or her ability to perform these actions11). Self-efficacy plays an important part in determining which activities or situations an individual will perform or avoid9), and an international statement has recommended enhancement of self-efficacy as part of pulmonary rehabilitation1).
COPD patients are typically advised to walk more for exercise, but advice alone has a limited impact on sedentary behavior12). Behavior change is most likely to occur when individuals are motivated to change13). Motivation has been classified into two main categories, which are intrinsic or extrinsic14). Previous studies have suggested that interventions should target enhancement of intrinsic motivation to change behavior and encourage patients to engage in self-management13,14,15), and self-monitoring is a method of enhancing intrinsic motivation. Physical activity can be assessed by various methods, including questionnaires, step counters (pedometers), activity monitors (accelerometers), and doubly-labelled water16). Programs based on use of pedometers or accelerometers for self-monitoring of physical activity are reported to be effective for increasing physical function and physical activity, as well as for enhancing the quality of life (QOL)12, 17,18,19,20). In previous studies, subjects were instructed to achieve a target number of steps or encouraged to increase their physical activity (extrinsic motivation), and the effects of self-management without external encouragement (intrinsic motivation) have not been clarified in patients with CRD. To clarify the effect of enhancing intrinsic motivation is beneficial for elderly CRD patients who cannot attend hospital by themselves, because they can maintain and increase physical activity without undergoing frequent rehabilitation at hospital.
Accordingly, this study was performed to evaluate the effects of a self-managed physical activity program using a pedometer and diary on physical function, activities of daily living (ADL), and QOL in CRD patients.
SUBJECTS AND METHODS
This study was approved by the Institutional Review Board of Jobu Hospital for Respiratory Diseases (Gunma, Japan) (approval no. 20130828), and written informed consent was obtained from all of the subjects. Patients with CRD were eligible for enrolment if they regularly attended Jobu Hospital for Respiratory Diseases, but were excluded if they had severe heart disease, other medical conditions, orthopedic diseases, or were judged to be inappropriate for this study by the treating physician. CRD in this study was defined as diseases with chronic exertional dyspnea and obstructive, restrictive, or combined (obstructive and restrictive) pulmonary dysfunction. Patients with acute diseases, malignancy, asthma, agraphia, or dementia were also excluded. All study visits were conducted at Jobu Hospital for Respiratory Diseases. Following baseline assessment at enrolment, patients were re-assessed after 3 and 6 months. Patients who consented to participate in the study were randomly assigned to one of two groups (“Control” group or “Diary” group) by a stratified randomization sequence that was generated using Excel before enrolment commenced. Patients assigned to the Diary group received an Omron HJ-205IT pedometer (Omron, Tokyo, Japan) and instructions on how to use it. They recorded the number of steps and performed self-evaluation of the level of physical activity in a diary provided for the study. The Omron HJ-205IT pedometer displays the cumulative daily step count and also stores the step counts for the previous 7 days. Daily physical activity was self-evaluated by 10 points scoring: 0 point (did not move) to 10 points (moved very much). Patients assigned to the Control group did not use a pedometer or keep a diary, but only were requested to participate in assessment after 3 months and 6 months. Patients in both groups were not given a target number of steps or a target level of physical activity.
At the baseline, after 3 months, and after 6 months, patients were assessed for dyspnea, muscle strength, exercise tolerance, ADL, and QOL. Dyspnea was assessed by using the modified Medical Research Council dyspnea scale21). To investigate muscle strength, isometric knee extension strength was measured with a hand-held dynamometer (µ-tas F-1; Anima, Tokyo, Japan) and leg strength was assessed by the 30-second chair stand test (CS-30)22). Exercise tolerance was measured by the 6-minute walking test23). ADL was assessed by the Nagasaki University Respiratory ADL questionnaire (NRADL)24), while QOL was investigated by the St. George’s Respiratory Questionnaire (SGRQ)25) and the COPD Assessment Test (CAT)26).
Results were expressed as the mean ± standard deviation (mean ± SD). Baseline values were compared between the two groups by using the independent t-test or the Mann-Whitney U-test for parametric and nonparametric data, respectively. Student’s paired t-test or the Wilcoxon signed rank test was respectively employed to compare differences of parametric or nonparametric data between baseline and after 3 or 6 months of intervention in each group. In addition, changes of variables after 3 and 6 months were compared between the two groups with the independent t-test or Mann-Whitney U-test for parametric and nonparametric data, respectively. Daily step counts were collected from the diaries and Dunnett’s test was used to compare step counts among 0–2 months (the initial 2 months of the study), 2–4 months (middle 2 months), and 4–6 months (final 2 months). All analyses were performed using IBM SPSS Statistics software (version 21) and a p value <0.05 was defined as indicating statistical significance.
There are no conflicts of interest for any of the authors.
RESULTS
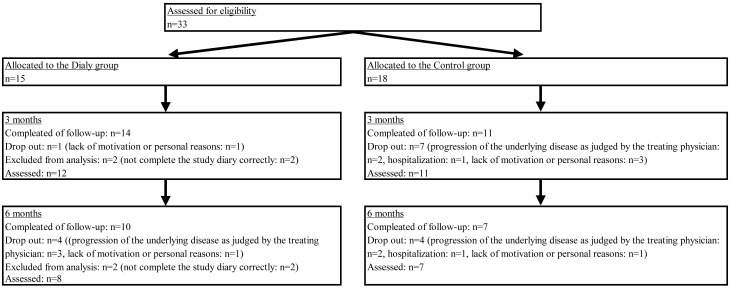
Thirty-three patients were recruited, among whom 25 completed 3 months of follow-up and 17 completed 6 months of follow-up. Sixteen patients dropped out due to progression of the underlying disease as judged by the treating physician (n=8), hospitalization (n=2), or lack of motivation/personal reasons (n=6). In addition, two patients in the Diary group did not complete the study diary correctly and were excluded from analysis (Fig. 1). The baseline characteristics of the Diary group and the Control group showed no significant differences (Table 1).
Fig. 1.
Study design
Table 1. Comparison of dyspnea, physical function, ADL and QOL over time in the each group.
| Diary group | Control group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (n=15) |
3 months (n=12) |
6 months (n=8) |
Baseline (n=18) |
3 months (n=11) |
6 months (n=7) |
||
| Age (years) | 73.0 ± 6.6 | 71.9 ± 5.8 | 73.4 ± 6.0 | 71.8 ± 7.5 | 72.6 ± 6.9 | 72.1 ± 6.6 | |
| Diagnosis (COPD/IPF) | 12/3 | 9/3 | 6/2 | 14/4 | 8/3 | 5/2 | |
| Gender (Male/Female) | 11/4 | 9/3 | 6/2 | 13/5 | 8/3 | 6/1 | |
| Height (cm) | 158.3 ± 10.3 | 158.3 ± 9.5 | 156.4 ± 11.2 | 161.2 ± 9.6 | 164.0 ± 10.7 | 166.5 ± 6.8 | |
| Weight (kg) | 59.5 ± 10.3 | 58.7 ± 8.2 | 55.5 ± 7.2* | 57.8 ± 8.6 | 60.5 ± 7.3 | 62.4 ± 6.7 | |
| Body Mass Index (kg/m²) | 23.6 ± 2.5 | 23.4 ± 2.0 | 22.6 ± 1.1* | 22.3 ± 3.2 | 22.6 ± 2.6 | 22.7 ± 3.5 | |
| mMRC | 1.9 ± 0.7 | 1.4 ± 1.0* | 0.6 ± 0.5* | 1.3 ± 1.0 | 1.5 ± 0.8 | 0.9 ± 0.7 | |
| Knee extension strength (WBI) | 0.601 ± 0.136 | 0.638 ± 0.107 | 0.701 ± 0.152 | 0.536 ± 0.204 | 0.530 ± 0.167 | 0.548 ± 0.268 | |
| 6MWD (m) | 451.1 ± 83.1 | 503.5 ± 79.4** | 510.8 ± 88.3 | 448.1 ± 118.3 | 460.7 ± 137.1 | 469.4 ± 177.4 | |
| CS-30 (time) | 14.3 ± 3.9 | 18.4 ± 3.4* | 19.5 ± 4.4* | 16.1 ± 4.8 | 15.4 ± 7.0 | 15.9 ± 8.6 | |
| NRADL | 96.8 ± 3.8 | 97.4 ± 5.4 | 98.4 ± 3.8 | 95.5 ± 8.6 | 96.9 ± 6.2 | 98.3 ± 1.9 | |
| SGRQ | 28.2 ± 18.5 | 23.0 ± 20.4 | 14.8 ± 17.0 | 21.2 ± 19.8 | 23.5 ± 20.4 | 20.0 ± 22.1 | |
| CAT | 10.9 ± 7.1 | 10.3 ± 9.5 | 6.0 ± 7.8 | 10.0 ± 8.6 | 9.6 ± 7.3 | 8.4 ± 10.3 | |
Mean ± standard deviation is presented. *p<0.05 and **p<0.01 compared to baseline in each groups. IPF: idiopathic pulmonary fibrosis; mMRC: modified Medical Research Council dyspnea scale; WBI: weight bearing index; 6MWD: 6-minute walking distance; CS-30: 30-second chair stand test; NRADL: The Nagasaki University Respiratory ADL questionnaire; SGRQ: The St. George’s Respiratory Questionnaire; CAT: The COPD Assessment Test
Compared with baseline, the Diary group showed significant improvement of the mMRC, 6MWT, and number of standings in the CS-30 at 3 months. After 6 months, there were also significant changes of the body weight, BMI, mMRC, and CS-30 in the Diary group. In contrast, the Control group showed no significant improvement after 3 or 6 months. Moreover, there were significant differences between the Diary and Control groups with regard to the changes of the 6MWT, CS-30, and SGRQ after 3 months, as well as significant differences in the changes of body weight and BMI after 6 months (Table 2).
Table 2. Comparison of changes between the Diary and Control group.
| 3 months | 6 months | |||
|---|---|---|---|---|
| Diary (n=12) |
Control (n=11) |
Diary (n=8) |
Control (n=7) |
|
| ΔHeight (cm) | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.0 ± 0.2 | 0.0 ± 0.0 |
| ΔWeight (kg) | –0.7 ± 2.2 | 0.8 ± 1.4 | –3.1 ± 3.3 | 1.6 ± 1.9** |
| ΔBody Mass Index (kg/m²) | –0.3 ± 0.9 | 0.3 ± 0.5 | –1.3 ± 1.5 | 0.6 ± 0.7** |
| ΔmMRC | –0.4 ± 0.5 | –0.1 ± 0.5 | –1.0 ± 0.8 | –0.6 ± 1.0 |
| ΔKnee extension strength (WBI) | 0.030 ± 0.109 | 0.012 ± 0.076 | 0.079 ± 0.131 | –0.006 ± 0.148 |
| Δ6MWD (m) | 43.5 ± 46.1 | 1.1 ± 28.7* | 37.0 ± 63.4 | –0.3 ± 26.7 |
| ΔCS-30 (time) | 3.7 ± 2.8 | –0.6 ± 2.9** | 4.0 ± 3.7 | –1.1 ± 3.7 |
| ΔNRADL | 0.9 ± 2.8 | 1.6 ± 4.4 | 1.0 ± 3.1 | 0.1 ± 2.5 |
| ΔSGRQ | –4.7 ± 11.3 | 4.6 ± 9.2* | –3.9 ± 9.7 | –0.7 ± 5.6 |
| ΔCAT | –1.3 ± 4.5 | 1.7 ± 4.7 | –3.1 ± 4.3 | –0.1 ± 4.2 |
Mean ± standard deviation is presented. *p<0.05 and **p<0.01. mMRC: modified Medical Research Council dyspnea scale; WBI: weight bearing index; 6MWD: 6 minute walking distance; CS-30: 30-second chair stand test; NRADL: The Nagasaki University Respiratory ADL questionnaire; SGRQ: The St. George’s Respiratory Questionnaire; CAT: The COPD Assessment Test
In the diary group, there was significant improvement of the daily step count over time compared with the initial 2 months according to Dunnett’s test (Table 3). Patients in this group walked 8,220.0 ± 4,155.1 steps per day during the initial 2 months of the study versus 8,870.7 ± 4,271.6 steps per day in the middle 2 months (p<0.01) and 9,151.8 ± 5,179.8 steps per day in the final 2 months (p<0.01).
Table 3. Daily number of steps in the Diary group.
| 0–2 months (Initial 2 months) |
3–4 months (Middle 2 months) |
5–6 months (Final 2 months) |
|
|---|---|---|---|
| Daily step counts (steps/day) | 8,222.0 ± 4,155.1 | 8,870.7 ± 4,271.6** | 9,151.8 ±5,179.8** |
Mean ± standard deviation is presented. **p<0.01 compared to the initial 2 months.
DISCUSSION
The findings of the present study suggest that a self-managed physical activity program using a pedometer and diary can increase physical activity, at least with regard to the daily walking steps. To effect changes of behavior in daily life, patients need feedback on their behavioral performance9). In the present study, a diary was used for self-evaluation of physical activity and this feedback may have enhanced the self-efficacy and intrinsic motivation of patients in the Diary group.
The Diary group demonstrated significant improvement of the mMRC, 6MWT, and CS-30 test after 3 months of intervention, as well as significant improvement of mMRC and CS-30 after 6 months. The 6MWT improved by 52.4 m, which corresponded to the minimum clinically important difference27,28,29,30,31,32). These findings suggest that increasing the level of physical activity by walking further on a daily basis improved exercise tolerance, as indicated by better performance in the 6MWT. The CS-30 test measures leg strength at a submaximal number of repetitions and is considered to reflect performance during daily physical activity which is also not performed at maximum strength. It seems likely that a higher level of physical activity, including more repetitions of standing up to walk, may have improved leg muscle strength (as shown by the CS-30) in the present study. The decrease of the mMRC score showed improvement of dyspnea on exertion in the Diary group. Exertional dyspnea is related to poor exercise tolerance and low muscle strength, so improvement of these factors may have led to improvement of mMRC in the Diary group.
We found significant differences between the Diary and Control groups with regard to the changes of the 6MWT, CS-30, and SGRQ after 3 months. Improvement of the 6MWT and CS-30 were related to improvement of physical activity, as mentioned above, while the significant change of the SGRQ score showed that our pedometer program was also effective for improving QOL. While we did not find any significant differences of dyspnea, muscle strength, exercise tolerance, ADL, or QOL between the two groups after 6 months, this may well have been related to the small number of patients that remained in the study at that time. The subjects were elderly, and 10 patients (30.3%) dropped out due to hospitalization or progression of their underlying disease. Our findings with regard to physical activity and physical function differ somewhat from the results of several previous studies. Tödt et al.33) found no relationship between the level of physical activity and depression, anxiety, body composition, exacerbation, or systemic inflammation. Eagan et al.34) reported that 7 weeks of pulmonary rehabilitation led to significant reduction of total energy expenditure and breathlessness, along with improvement of exercise capacity, PiMax, and QOL, but no significant change in the daily average number of steps, duration of sedentary activity, METs consumed, or daily level of physical activity. Benzo et al.35) stated that hospitalization was predicted by the weekly level of physical activity, although there were only weak correlations between the 6MWT, maximum workload test using a bicycle ergometer, and physical activity, while neither the 6MWT nor maximum workload test predicted hospitalization. Our self-managed physical activity program did not lead to changes of physical function parameters at 6 months, but the increase of physical activity may still have been sufficient to prevent hospitalization and improve the prognosis of the subjects.
A variety of devices are available to measure physical activity. While the subjects of this study were elderly, they could use a pedometer correctly after receiving instructions. Activity monitors are more technologically advanced than pedometers and provide comprehensive data, but are more expensive36), while smartphones also have a higher cost and are unfamiliar technology for most elderly persons37). Therefore, we selected the “Omron HJ-205IT” pedometer because it is simple to use and the data are easy to view. Our present findings suggest that a pedometer can be an effective device for self-monitoring of activity levels by patients.
In a previous long-term study (5–8 years of follow-up)38), COPD patients with lower levels of physical activity had a shorter survival. In a 4 year cohort study of COPD patients, physical activity was the best predictor of mortality among a broad range of variables related to lung function and functional exercise capacity39). These reports suggest that increasing physical activity through self-management of exercise using a pedometer and diary may be able to improve the prognosis of COPD patients. Although the Diary group only received a single intervention at the beginning of our study, their level of physical activity increased, suggesting that self-management programs can be effective for improving the prognosis of elderly COPD patients who cannot attend hospital frequently.
Our study had some limitations. First, the sample size was small, which is a potential source of bias. Second, the Control group did not record the daily number of steps, so we could not compare physical activity between the two groups. Another limitation was the relatively short intervention period. Previous studies have monitored the prognosis over 4 years or more38, 39), so a longer follow-up period may have been needed to demonstrate improvement of the prognosis with our intervention.
In elderly CRD patients, a self-managed physical activity program using a pedometer and diary increased the level of physical activity and improved QOL. In conclusion, a pedometer is a simple device that patients can use for effective self-monitoring of their level of activity. This study suggesting that self-management program focused on internal motivation can be effective for improving elderly CRD patients who cannot attend rehabilitation at hospital frequently.
REFERENCES
- 1.Spruit MA, Singh SJ, Garvey C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation: An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med, 2013, 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 2.Global initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease updated 2015. http://www.goldcopd.org/ (Accessed Sep. 8, 2015)
- 3.Troosters T, van der Molen T, Polkey M, et al. : Improving physical activity in COPD: towards a new paradigm. Respir Res, 2013, 14: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama M, Bando M, Araki K, et al. : Physical activity in patients with idiopathic pulmonary fibrosis. Respirology, 2015, 20: 640–646. [DOI] [PubMed] [Google Scholar]
- 5.Wallaert B, Monge E, Le Rouzic O, et al. : Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest, 2013, 144: 1652–1658. [DOI] [PubMed] [Google Scholar]
- 6.Ries AL, Bauldoff GS, Carlin BW, et al. : Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest, 2007, 131: 4S–42S. [DOI] [PubMed] [Google Scholar]
- 7.Chigira Y, Takai T, Oda T, et al. : Difference in the effect of outpatient pulmonary rehabilitation due to variation in the intervention frequency: intervention centering on home-based exercise. J Phys Ther Sci, 2014, 26: 1041–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo Y, Dobashi K, Uga D, et al. : Effect of 12-month rehabilitation with low loading program on chronic respiratory disease. J Phys Ther Sci, 2016, 28: 1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourbeau J, Nault D, Dang-Tan T: Self-management and behaviour modification in COPD. Patient Educ Couns, 2004, 52: 271–277. [DOI] [PubMed] [Google Scholar]
- 10.Zwerink M, Brusse-Keizer M, van der Valk PD, et al. : Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev, 2014, 3: CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandura A: Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev, 1977, 84: 191–215. [DOI] [PubMed] [Google Scholar]
- 12.Bravata DM, Smith-Spangler C, Sundaram V, et al. : Using pedometers to increase physical activity and improve health: a systematic review. JAMA, 2007, 298: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 13.Bourbeau J, Lavoie KL, Sedeno M: Comprehensive self-management strategies. Semin Respir Crit Care Med, 2015, 36: 630–638. [DOI] [PubMed] [Google Scholar]
- 14.Dacey M, Baltzell A, Zaichkowsky L: Older adults’ intrinsic and extrinsic motivation toward physical activity. Am J Health Behav, 2008, 32: 570–582. [DOI] [PubMed] [Google Scholar]
- 15.McClure JB, Divine G, Alexander G, et al. : A comparison of smokers’ and nonsmokers’ fruit and vegetable intake and relevant psychosocial factors. Behav Med, 2009, 35: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watz H, Pitta F, Rochester CL, et al. : An official European Respiratory Society statement on physical activity in COPD. Eur Respir J, 2014, 44: 1521–1537. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza L, Horta P, Espinoza J, et al. : Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J, 2015, 45: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hospes G, Bossenbroek L, Ten Hacken NH, et al. : Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns, 2009, 75: 274–278. [DOI] [PubMed] [Google Scholar]
- 19.Tabak M, Vollenbroek-Hutten MM, van der Valk PD, et al. : A telerehabilitation intervention for patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot trial. Clin Rehabil, 2014, 28: 582–591. [DOI] [PubMed] [Google Scholar]
- 20.Vaes AW, Cheung A, Atakhorrami M, et al. : Effect of ‘activity monitor-based’ counseling on physical activity and health-related outcomes in patients with chronic diseases: a systematic review and meta-analysis. Ann Med, 2013, 45: 397–412. [DOI] [PubMed] [Google Scholar]
- 21.Bestall JC, Paul EA, Garrod R, et al. : Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax, 1999, 54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazono T, Kamide N, Ando M: The reference values for the chair stand test in healthy Japanese older people: determination by meta-analysis. J Phys Ther Sci, 2014, 26: 1729–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories: ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med, 2002, 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 24.Yoza Y, Ariyoshi K, Honda S, et al. : Development of an activity of daily living scale for patients with COPD: the Activity of Daily Living Dyspnoea scale. Respirology, 2009, 14: 429–435. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, et al. : A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis, 1992, 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 26.Jones PW, Harding G, Berry P, et al. : Development and first validation of the COPD Assessment Test. Eur Respir J, 2009, 34: 648–654. [DOI] [PubMed] [Google Scholar]
- 27.Holland AE, Hill CJ, Rasekaba T, et al. : Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil, 2010, 91: 221–225. [DOI] [PubMed] [Google Scholar]
- 28.Puhan MA, Mador MJ, Held U, et al. : Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J, 2008, 32: 637–643. [DOI] [PubMed] [Google Scholar]
- 29.Redelmeier DA, Bayoumi AM, Goldstein RS, et al. : Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med, 1997, 155: 1278–1282. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Townsend M, Pugsley SO, et al. : Bronchodilators in chronic air-flow limitation. Effects on airway function, exercise capacity, and quality of life. Am Rev Respir Dis, 1987, 135: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 31.Holland AE, Hill CJ, Conron M, et al. : Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir Med, 2009, 103: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 32.du Bois RM, Weycker D, Albera C, et al. : Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med, 2011, 183: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 33.Tödt K, Skargren E, Jakobsson P, et al. : Factors associated with low physical activity in patients with chronic obstructive pulmonary disease: a cross-sectional study. Scand J Caring Sci, 2015, 29: 697–707. [DOI] [PubMed] [Google Scholar]
- 34.Egan C, Deering BM, Blake C, et al. : Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med, 2012, 106: 1671–1679. [DOI] [PubMed] [Google Scholar]
- 35.Benzo RP, Chang CC, Farrell MH, et al. NETT Research Group: Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration, 2010, 80: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner LJ, Houchen L, Williams J, et al. : Reliability of pedometers to measure step counts in patients with chronic respiratory disease. J Cardiopulm Rehabil Prev, 2012, 32: 284–291. [DOI] [PubMed] [Google Scholar]
- 37.Fong SS, Ng SS, Cheng YT, et al. : Comparison between smartphone pedometer applications and traditional pedometers for improving physical activity and body mass index in community-dwelling older adults. J Phys Ther Sci, 2016, 28: 1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Rio F, Rojo B, Casitas R, et al. : Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest, 2012, 142: 338–346. [DOI] [PubMed] [Google Scholar]
- 39.Waschki B, Kirsten A, Holz O, et al. : Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest, 2011, 140: 331–342. [DOI] [PubMed] [Google Scholar]