Abstract
[Purpose] To improve walking efficiency could be useful for reducing fatigue and extending possible period of walking in children with cerebral palsy (CP). For this purpose, current study compared conventional parameters of gross motor performance, step length, and cadence in the evaluation of walking efficiency in children with CP. [Subjects and Methods] Thirty-one children with CP (21 boys, 10 girls; mean age, 12.3 ± 2.7 years) participated. Parameters of gross motor performance, including the maximum step length (MSL), maximum side step length, step number, lateral step up number, and single leg standing time, were measured in both dominant and non-dominant sides. Spatio-temporal parameters of walking, including speed, step length, and cadence, were calculated. Total heart beat index (THBI), a parameter of walking efficiency, was also calculated from heartbeats and walking distance in 10 minutes of walking. To analyze the relationships between these parameters and the THBI, the coefficients of determination were calculated using stepwise analysis. [Results] The MSL of the dominant side best accounted for the THBI (R2=0.759). [Conclusion] The MSL of the dominant side was the best explanatory parameter for walking efficiency in children with CP.
Keywords: Cerebral palsy, Maximum step length, Walking efficiency
INTRODUCTION
Walking efficiency is decreased in children with cerebral palsy (CP) compared to that in healthy children1), and may lead to fatigue in walking2). Kerr et al. found that walking efficiency in these children deteriorated over time and became most inefficient at around 12 years of age3). In adults with CP, approximately 75% of individuals stopped walking by 25 years of age4). Useful parameters to monitor and improve walking efficiency should be established in childhood.
For greater energy efficiency during walking, it is important to increase step length5). Abel et al. reported that the capacity for longer step length was lower in children with CP compared to that in healthy children6). Therefore, to monitor and to increase walking efficiency in children with CP, the focus should be on the assessment and the training methods of forward stepping.
Several exercise programs have been shown to increase walking efficiency and step length significantly7,8,9). In these programs, subjects performed various exercises, such as hip and knee flexion and forward or lateral step up7,8,9). Although task relevant training is a very important aspect of fulfilling functional improvement9, 10), it has not been clarified how type of exercise or direction of motion are related to walking efficiency and step length.
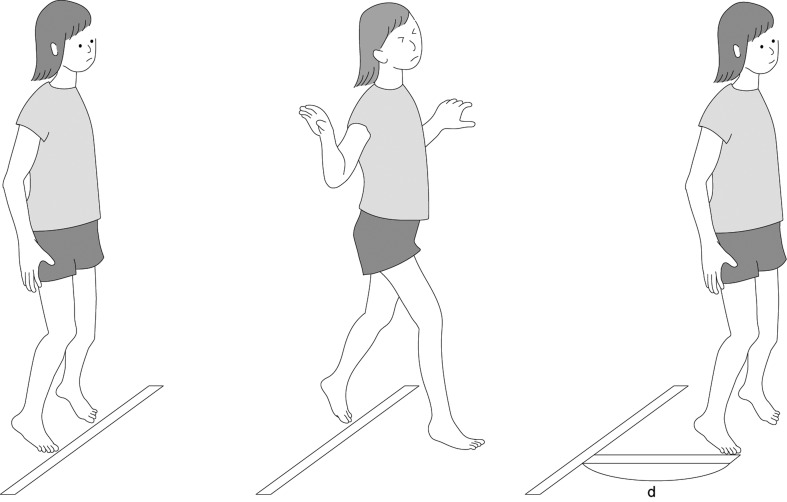
The maximum step length (MSL) has been used in Japan since 1997 to evaluate the risk of falls in elderly adults11, 12). The capacity to step out as far as possible in a forward direction decreases with age13, 14). A short step length is a common problem in both elderly adults and children with CP. The conventional method used to assess a patient’s step length was complicated, as it required walking with maximum possible speed. On the other hand, the MSL test involves taking a large forward step, and is therefore simple (Fig. 1). We have reported that some items in the Gross Motor Function Measure15) and the MSL were correlated with walking efficiency16). These common factors were mainly one leg movement under the support of the contralateral leg16). However, the previous report was examined in relatively small number of participants16). Furthermore, in the evaluation of contribution to the walking efficiency, few quantitative parameters representing a step out capacity beside the MSL were included.
Fig. 1.
The Maximum step length test. The participants take a forward step, as far as possible, with unilateral leg (anterior leg), and then move the contralateral leg (posterior leg) to the medial side of the anterior leg. The distance from the start line to the most distal surface of the foot of the anterior leg was measured. The higher value was chosen in twice trial.
In previous studies, the physiological cost index8) and energy expenditure index17) were used as measures of walking efficiency in children with CP. However, a low repeatability, due to the variability of the resting heart rate, has recently been reported for both of these indices in children with CP18, 19). IJzerman and Nene concluded that there was no benefit in using the difference between the walking heart rate and the resting heart rate compared to using the walking heart rate alone in children with CP18). In 2002, Hood et al. reported that THBI is advantageous because the formula for calculation of the THBI does not include the resting heart rate20); it is therefore considered to be a better parameter in the evaluation of walking efficiency in children with CP19, 20).
The purpose of this study was to determine the influence of step length and cadence on walking efficiency in children with CP. In addition, this study investigated the relationship between type of exercise or direction of motion and walking efficiency to develop a training program to improve walking efficiency in children with CP.
SUBJECTS AND METHODS
Thirty-one children (21 boys, 10 girls) with spastic diplegic CP, who were treated in the inpatient and outpatient clinics of the Akita Prefectural Center on Development and Disability, were included in the study. All participants were able to follow verbal instructions. To rule out the effect of equipment on walking efficiency, children who required devices for walking were excluded. Additionally, children who had undergone orthopedic surgery and/or serial casting within 6 months were also excluded from the study. The children were classified as level I or level II, based on the Gross Motor Function Classification System21). Approval for this study was obtained from the medical ethics committee at the Akita University Graduate School of Health Sciences. Participants and parents received an explanation of the purpose and methods of this study, and written informed consent was obtained prior to initiation of the study.
The tests described below were conducted and the results were recorded by one of the authors (MK). In testing the MSL, the maximum side-step length, the step number, the lateral step-up number, and single-leg standing time, the measurements were performed twice for each side with a 30-second interval, and the higher value for each side was adopted. Information on right and left lower leg selectivity in each patient was obtained from medical records. The high selectivity side was defined as the dominant side, and low selectivity side as the non-dominant side. The average value of the dominant and non-dominant sides was also calculated. All tests were performed barefoot without any support.
Walking efficiency was determined using the total heart beat index (THBI)19, 20). The participants walked at a comfortable speed on a rectangular walkway (10 m long, 7 m wide), and the distance covered in 10 minutes was measured as the walking distance. The heartbeats were monitored using the RS800 CX (Polar Electro Oy, Kempele, Finland). The number of heartbeats during 10 minutes of walking was totaled (walking heartbeats), and THBI was calculated using the following formula:
THBI (beats/m) = walking heartbeats/walking distance19)
To measure spatio-temporal parameters of walking, the participants walked a 10-meter straight walkway (with an additional 3 meters at each end for acceleration and deceleration) 3 times, at a comfortable speed22). The number of steps and the walking time were measured, and the values from each of the 3 trials were then averaged. The speed, the step length, and the cadence were calculated.
The MSL was measured according to a method reported in prior studies11, 12, 16). The participants stood at the start line with their feet together and took a step forward as far as possible with one leg (anterior leg), and then moved the contralateral leg (posterior leg) to the medial side of the anterior leg (Fig. 1). The distance from the start line to the most distal surface on the foot of the anterior leg was measured. If the anterior leg was the dominant side, this value was recorded as the MSL of the dominant side.
The maximum side-step length was as described in the literature23). A start line and a 7-meter guiding line perpendicular to the start line were marked on the floor. Participants stood and set the outer edge of the unilateral foot on the start line. Then, they took continuous 5 steps to the side as far as possible along the guiding line. The length from the start line to the outer edge of the foot after the 5 steps was measured and the length divided by the number of steps was calculated. If the leg of side stepping was the dominant side, this value was recorded as the maximum side-step length of the dominant side.
The step number was measured according to the method described by Hill et al24). The participants stood in front of a platform 20 cm in height. Stepping onto the platform and returning the foot to the floor with one leg, while supported by the contralateral leg, was repeated as many times as possible in 30 seconds. The step number was defined as the number of repetitions; if the stepping leg on the platform was the dominant side, this number was recorded as the step number of the dominant side.
The lateral step-up number was measured as described in the literatures7, 9, 25). The participants were positioned with one foot (step-up side) on a platform 20 cm in height and the contralateral foot on the floor beside the platform. Bringing the foot from the floor to the platform constituted 1 lateral step-up. This was repeated as many times as possible for 30 seconds. The number of repetitions equaled the lateral step-up number. If the step-up side was the dominant side, this number was recorded as the lateral step-up number of the dominant side.
The single-leg standing time was measured according to a method reported in prior studies12, 23). If the value was over 30 seconds, the single-leg standing time was recorded as 30 seconds. If the standing side was the dominant side, this value was recorded as the single-leg standing time of the dominant side.
The SPSS software package version 22 was used for all statistical analyses. The normality of each parameter was assessed using the Shapiro-Wilk test. Paired t-testing was used to analyze the difference between the dominant and the non-dominant sides on normally distributed parameters obtained for testing the MSL, the maximum side-step length, the step number, the lateral step-up number, and single-leg standing time. The Wilcoxon signed rank test was used for other parameters.
Pearson’s correlation coefficient was used to analyze the relationship between normally distributed parameters obtained for testing the MSL, the maximum side-step length, the step number, the lateral step-up number, and single-leg standing time, and spatio-temporal parameters of walking or walking efficiency parameters. Spearman rank-correlation coefficients were used for other parameters. The correlations were interpreted according to guidelines adopted from Altman26), with r<0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, very good.
Multiple regression analyses were performed using a stepwise procedure to determine the parameters of walking (step length and cadence) and gross motor performance that had the strongest influence on the THBI, walking heartbeats, and/or waking distance. The values of step length, cadence, and both dominant and non-dominant sides in gross motor performance were included as independent parameters. When the value of each gross motor performance was not significantly different, only the average value of the dominant and non-dominant sides was used. The participant’s age, height, weight, and leg length were included as independent parameters. The significance level was p<0.05.
RESULTS
The characteristics of the subjects are presented in Table 1. The values of each parameter on the dominant and non-dominant sides, and their mean values are shown in Table 2. There was a significant difference between the values of the MSL and the single-leg standing time for the 2 sides. The values of other parameters on the 2 sides were not significantly different.
Table 1. Characteristics of subjects (n=31).
| Mean ± SD | ||
|---|---|---|
| Anthropometrics | ||
| Age (years) | 12.3 ± 2.7 | |
| Body height (cm) | 143.0 ± 15.1 | |
| Body weight (kg) | 39.6 ± 13.7 | |
| Leg length (cm) | 72.8 ± 8.1 | |
| Body mass index (kg/m2) | 18.8 ± 3.6 | |
| Gender (number) | Boy, 21; Girl, 10 | |
| Treatment status | ||
| Botulinus toxin injection (yes/no) | 4/27 | |
| Gross motor function | ||
| GMFCS level (number) | I, 22; II, 9 | |
| GMFM dimension D score (%) | I, 96 ± 5; II, 75 ± 11 | |
| GMFM dimension E score (%) | I, 90 ± 22; II, 64 ± 17 | |
| GMFM total score (%) | I, 98 ± 2; II, 88 ± 7 | |
| Spatio-temporal parameters | ||
| Speed (m/min) | 72.4 ± 11.1 | |
| Step length (m) | 0.552 ± 0.080 | |
| Cadence (steps/min) | 132 ± 15 | |
| Walking efficiency parameters | ||
| THBI (beats/m) | 2.11 ± 0.44 | |
| Walking heartbeats (beats) | 1,246 ± 235 | |
| Walking distance (m) | 600 ± 71 | |
THBI: total heart beat index; GMFCS: gross motor function classification system; GMFM: gross motor function measure
Table 2. Comparison between dominant and non-dominant side (n=31).
| Dominant side Mean ± SD |
Non-dominant side Mean ± SD |
|
|---|---|---|
| Gross motor performance | ||
| Maximum step length (m) | 0.877 ± 0.225* | 0.844 ± 0.205 |
| Maximum side-step length (m) | 0.716 ± 0.217 | 0.717 ± 0.208 |
| Step number | 21 ± 10 | 21 ± 10 |
| Lateral step-up number | 14 ± 11 | 12 ± 12 |
| Single leg standing time (s) | 16.5 ± 12.8† | 9.1 ± 10.8 |
*p<0.05, Paired t-test; †p<0.05, Wilcoxon signed rank test
Correlations among spatio-temporal parameters, walking efficiency parameters, and gross motor performance are shown in Table 3. The THBI and walking distance showed significant correlations with speed and step length. The walking heartbeats showed significant correlation with step length, but cadence did not show any correlation with walking efficiency parameters. The step length, THBI, and walking heartbeats showed significant correlations with all gross motor performance tests. However, cadence was not related to any gross motor performance tests.
Table 3. Correlation coefficients among THBI and spatio-temporal parameters (n=31).
| Spatio-temporal parameters | Walking efficiency | ||||||
|---|---|---|---|---|---|---|---|
| Speed | Step length | Cadence | THBI | Walking heartbeats | Waking distance | ||
| Spatio-temporal paremeters | |||||||
| Speed | 0.76a | 0.47b | –0.52a | - | 0.73a | ||
| Step length | 0.76a | - | –0.71a | –0.41a | 0.69a | ||
| Cadence | 0.47b | - | - | - | - | ||
| Walking efficiency parameters | |||||||
| THBI | –0.52a | –0.71a | - | 0.85a | 0.49a | ||
| Walking heartbeats | - | –0.41a | - | 0.85a | - | ||
| Walking distance | 0.73a | –0.69a | - | –0.49a | - | ||
| Gross motor performance | |||||||
| Maximum step length (D) | 0.41a | 0.66a | - | –0.87a | –0.78a | 0.37a | |
| Maximum step length (N) | 0.44a | 0.71a | - | –0.86a | –0.73a | 0.43a | |
| Maximum side-step length (A) | - | 0.61a | - | –0.82a | –0.71a | 0.40a | |
| Step number (A) | 0.36a | 0.46a | - | –0.46a | –0.73a | - | |
| Lateral step-up number (A) | 0.37b | 0.46b | - | –0.77b | –0.60b | 0.42 | |
| Single-leg standing time (D) | 0.42b | 0.61b | - | –0.84b | –0.66b | 0.43b | |
| Single-leg standing time (N) | - | 0.40b | - | –0.53b | 0.42b | - | |
ap<0.05, Pearson’s product moment correlation coefficient; bp<0.05, Spearman’s rank correlation coefficient. Correlation values: 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80 (bold), good; 0.81–1.00 (underlined bold), very good. THBI: total heart beat index; D: dominant side; N: non-dominant side; A: average
The results of the stepwise multiple regression analyses are presented in Table 4. The MSL of the dominant side best accounted for variance in THBI (75.9%) and walking heartbeats (61.6%), and step length and cadence accounted for the variance in walking distance (57.1%).
Table 4. Stepwise multiple regression analysis (n=31).
| Dependent variable | THBI | Walking heartbeats | Walking distance |
|---|---|---|---|
| Maximum step length (D) | 0.759 | 0.616 | |
| Step length | 0.538 | ||
| Cadence | 0.033 | ||
| Total explained variance (R2) | 0.759* | 0.616* | 0.571* |
The coefficient of determination R2 for the variance of each dependent variable (standardized partial regression coefficient × correlation coefficient) and the total explanatory variance for each dependent variable. THBI: total heart beat index; D: Dominant side. *p<0.05
DISCUSSION
The THBI and walking distance showed significant correlations with speed and step length. The MSL of the dominant side best accounted for variance in the THBI. The walking heartbeats and walking distance were best explained by the MSL of the dominant side and step length, respectively.
Because THBI is calculated as the ratio of walking heartbeats to walking distance20), increase of cadence and/or step length should improve the walking efficiency. However, it was clarified that an increase in cadence had a negligible effect on walking efficiency in this condition17). Similarly, in the current study, there was no significant correlation between cadence and THBI. Therefore, to obtain greater walking efficiency in children with CP, the focus should be on the assessment of the step length. In fact, among the gross motor performance measures, the MSL of the dominant side was the best explanatory parameter for THBI.
Considering the definition of THBI, walking heartbeats and walking distance should be important factors in walking efficiency. The step length was the best explanatory parameter for walking distance. This study found that walking speed during prolonged walking increased primarily with the increase in step length. In addition, the current study showed that the MSL of the dominant side was the best explanatory parameter for walking heartbeats and efficiency. When the anterior leg was dominant, the MSL of the dominant side was longer than the non-dominant side. The cardiovascular load during walking probably decreased with the increase in a participant’s ability to lengthen a step. It was reported that the mechanical power that spent by the muscle in accelerating the limbs increase with the step frequency at a given walking speed27). The energy expenditure during a walk with small steps was higher than that during a walk with free step length in healthy adults28). It is likely that the MSL of the dominant side has considerable influence on the walking heartbeats and/or efficiency in children with CP. Increasing the MSL should decrease walking heartbeats and thus improve walking efficiency in children with CP.
Other parameters of gross motor performance, including maximum side-step length, step number, lateral step-up number, bilateral single-leg standing time, and the MSL of the non-dominant side, were not significant predictors of the THBI in multiple regression analyses. In the maximum side-step length test, the direction of motion is sideways23). Both the step number and the lateral step-up number assess the speed of leg movement with the support of the contralateral leg7, 9, 24). In the single-leg standing time test, the static balance on one leg is assessed12, 23). None of these parameters translated to the capability of a long step in the forward direction. Because movements during forward steps are similar to those of walking, the assessment of step length in a forward direction, as in the MSL test, is important.
The present findings should prove useful in developing programs of the specific training and in analyzing outcomes for interventions aimed at improving walking efficiency in children with CP. Compared with step length, cadence, and gross motor performance tests, the MSL of the dominant side better represents step length in a forward direction, and is the best predictor for the THBI. In addition, the MSL of the dominant side is the best predictor of walking heartbeats. The MSL test is a simple and easy procedure and is strongly associated with walking efficiency in children with CP. We conclude that increasing MSL will decrease walking heartbeats and improve walking efficiency in children with CP. Further studies are needed to clarify whether the specific training contained the MSL movement is able to improve walking efficiency in children with CP.
REFERENCES
- 1.Rose J, Gamble JG, Burgos A, et al. : Energy expenditure index of walking for normal children and for children with cerebral palsy. Dev Med Child Neurol, 1990, 32: 333–340. [DOI] [PubMed] [Google Scholar]
- 2.Rose J, Haskell WL, Gamble JG: A comparison of oxygen pulse and respiratory exchange ratio in cerebral palsied and nondisabled children. Arch Phys Med Rehabil, 1993, 74: 702–705. [DOI] [PubMed] [Google Scholar]
- 3.Kerr C, McDowell BC, Parkes J, et al. : Age-related changes in energy efficiency of gait, activity, and participation in children with cerebral palsy. Dev Med Child Neurol, 2011, 53: 61–67. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KP, Molnar GE, Lankasky K: Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol, 1995, 37: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 5.Rose J, Ralston HJ, Gamble JG: Energetics of walking. In: Human walking, 2nd ed. Baltimore: Williams & Wilkins, 1994, pp 77–102. [Google Scholar]
- 6.Abel MF, Damiano DL: Strategies for increasing walking speed in diplegic cerebral palsy. J Pediatr Orthop, 1996, 16: 753–758. [DOI] [PubMed] [Google Scholar]
- 7.Blundell SW, Shepherd RB, Dean CM, et al. : Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4–8 years. Clin Rehabil, 2003, 17: 48–57. [DOI] [PubMed] [Google Scholar]
- 8.Liao HF, Liu YC, Liu WY, et al. : Effectiveness of loaded sit-to-stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial. Arch Phys Med Rehabil, 2007, 88: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Sung IY, Yoo JY: Therapeutic effects of strengthening exercise on gait function of cerebral palsy. Disabil Rehabil, 2008, 30: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford OM: Muscular coordination and strength training. Implications for injury rehabilitation. Sports Med, 1988, 5: 196–202. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu T, Kim KJ, Kaminai T, et al. : Clinical factors as predictors of the risk of falls and subsequent bone fractures due to osteoporosis in postmenopausal women. J Bone Miner Metab, 2006, 24: 419–424. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Kim KJ, Komatsu T, et al. : Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J Bone Miner Metab, 2008, 26: 254–259. [DOI] [PubMed] [Google Scholar]
- 13.Medell JL, Alexander NB: A clinical measure of maximal and rapid stepping in older women. J Gerontol A Biol Sci Med Sci, 2000, 55: M429–M433. [DOI] [PubMed] [Google Scholar]
- 14.Schulz BW, Ashton-Miller JA, Alexander NB: Maximum step length: relationships to age and knee and hip extensor capacities. Clin Biomech (Bristol, Avon), 2007, 22: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell DJ, Rosenbaum PL, Cadman DT, et al. : The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol, 1989, 31: 341–352. [DOI] [PubMed] [Google Scholar]
- 16.Kimoto M, Noro Y, Katou C, et al. : Relation between walking efficiency and motor function in children with spastic diplegic cerebral palsy: examined gross motor function, side steps and maximum one step length. Jpn J Phys Ther, 2011, 45: 179–183. [Google Scholar]
- 17.Damiano DL, Abel MF: Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil, 1998, 79: 119–125. [DOI] [PubMed] [Google Scholar]
- 18.Ijzerman MJ, Nene AV: Feasibility of the physiological cost index as an outcome measure for the assessment of energy expenditure during walking. Arch Phys Med Rehabil, 2002, 83: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 19.Kimoto M, Noro Y, Katou C, et al. : Reproducibility of the physiological cost index and the total heart beat index for children with spastic diplegic cerebral palsy. Rigakuryoho Kagaku, 2009, 24: 653–658. [Google Scholar]
- 20.Hood VL, Granat MH, Maxwell DJ, et al. : A new method of using heart rate to represent energy expenditure: the Total Heart Beat Index. Arch Phys Med Rehabil, 2002, 83: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 21.Palisano R, Rosenbaum P, Walter S, et al. : Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol, 1997, 39: 214–223. [DOI] [PubMed] [Google Scholar]
- 22.Wade DT: Measurement in neurological rehabilitation. New York: Oxford University Press, 1992, pp 78–79. [Google Scholar]
- 23.Fujisawa H, Takeda R: A new clinical test of dynamic standing balance in the frontal plane: the side-step test. Clin Rehabil, 2006, 20: 340–346. [DOI] [PubMed] [Google Scholar]
- 24.Hill KB, McGann A, Maltese D, et al. : A new test of dynamic standing balance for stroke patients: reliability, validity and comparison with healthy elderly. Physiother Can, 1996, 48: 257–262. [Google Scholar]
- 25.Verschuren O, Ketelaar M, Takken T, et al. : Reliability of hand-held dynamometry and functional strength tests for the lower extremity in children with cerebral Palsy. Disabil Rehabil, 2008, 30: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 26.Altman DG: Practical statistics for medical research. London: Chapman and Hall, 1991, p 404. [Google Scholar]
- 27.Cavagna GA, Franzetti P: The determinants of the step frequency in walking in humans. J Physiol, 1986, 373: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarrugh MY, Todd FN, Ralston HJ: Optimization of energy expenditure during level walking. Eur J Appl Physiol Occup Physiol, 1974, 33: 293–306. [DOI] [PubMed] [Google Scholar]