Abstract
Maintenance of the adipose tissue requires a proper balance between self-renewal and differentiation of adipose progenitors (AP). Any deregulation leads either to fat overexpansion and obesity or fat loss and consequent lipodystrophies. Depending on the fat pad location, APs and adipocytes are heterogeneous. However, information on the pharmacological sensitivity of distinct APs to drugs known to alter the function of adipose tissue, especially HIV protease inhibitors (PIs) is scant. Here we show that PIs decreased proliferation and clonal expansion of APs, modifying their self-renewal potential. Lopinavir was the most potent PI tested. Decrease in self-renewal was accompanied by a reduced expression of the immediate early response gene IER3, a gene associated with tissue expansion. It was more pronounced in chin-derived APs than in knee-derived APs. Furthermore, lopinavir lowered the activin A–induced ERK1/2 phosphorylation. Expressions of the transcription factor EGR1 and its targets, including INHBA were subsequently altered. Therefore, activin A secretion was reduced leading to a dramatic impairment of APs self-renewal sustained by the activin A autocrine loop. All together, these observations highlight the activin A autocrine loop as a crucial effector to maintain APs self-renewal. Targeting this pathway by HIV-PIs may participate in the induction of unwanted side effects.
Introduction
The adipose tissue (AT) represents the most adaptable tissue of an organism. It exists as functionally different depots that display opposite functions to fulfill the energy demand. In response to elevated calorie intake, white adipose tissue expansion allows energy storage as triglycerides. It represents the most abundant adipose tissue in adult humans. In contrast, brown adipose tissue is a key thermogenic organ able to produce heat from nutriments by uncoupling respiration from ATP synthesis. It surrounds the deepest organs1 and represents the lesser part of adipose tissue. White AT is present all over the human body and is composed of distinct depots that are heterogeneous in terms of cellular composition, proliferation and differentiation2, 3.
The adipose progenitor (AP) pool hosted within the adipose tissues is crucial for AT development and to form new fat cells upon appropriated stimulus that induce adipocyte differentiation. This process is essential because like most mature and specialized healthy cells, adipocytes are generated through differentiation of progenitor cells as they do not divide in vivo. Thus, the AP pool is directly correlated with adipose tissue development and linked to fat mass4. Our lab has recently shown that the AP pools are derived from distinct embryonic origins. In rodents, while the intra-abdominal depots originate from mesoderm, those from the face display a neuro-ectodermal origin5. This feature is also encountered in humans since APs from chin and knee do not express the same homeobox genes (HOX) code. Indeed, they produce adipocytes displaying different phenotypes: upon adipose differentiation, chin APs give rise preferentially to cells with brown/brite adipocytes features while knee APs are prone to generate white adipocytes6. All these observations suggest that the different fat depots are not equal either in terms of structures but also for physiological and metabolic functions. As AP cells need to perpetuate all life long, this pool is located in niches that maintain their intrinsic properties and control the self-renewal throughout adult life. Many factors present in the adipose tissue microenvironment are involved in a proper regulation of the balance between self-renewal and differentiation of these cells, including activin A which maintains the AP pool through autocrine and paracrine mechanisms7, 8. In addition, we recently identified the immediate early response 3 (IER3)9 as an important gene to control the activin A-induced expansion of this pool of cells7. IER3 is induced in response to distinct microenvironmental effectors that are susceptible to be modulated by therapeutic treatments. However, information linking the sensitivity of the distinct AP pools to drugs that may affect fat depot development is limited. Individual responses of APs to distinct medicines are not well defined so far.
Treatment of AIDS patients with antiretroviral therapy (ART) dramatically improved the life of patients, their immune functions and has reduced morbidity and mortality resulting from AIDS-related complications. Several classes of antiretroviral drugs are used to treat HIV-infected patients. Among them, proteases inhibitors (PIs) prevent the HIV protease to cleave precursor proteins that are essential to form infectious viral particles. Unfortunately, this therapeutic class of molecules displays unwanted side effects which are prejudicial for adhesion of patients to the treatment. In various regimens, PIs have been associated with abnormal fat distribution and selective loss of fat depots, dyslipidemia, hypertriglyceridemia, insulin resistance and an increased risk of cardiovascular diseases10, 11. ART therapy has been responsible for the development of acquired lipodystrophies that represents the most predominant type in the population12 as compared to genetically acquired disorders13. Despite the development of new and safer molecules14, these effects prevail as 57% of the 2–18 years-old HIV-positive population treated with ART displays lipodystrophy15.
ART therapy induces a loss of the subcutaneous fat, notably within the depots of the face, and an excess deposition in the neck and the abdomen, indicating that all the fat depots are not affected in a similar way16 and these differences in sensitivity were reported within the same person. The heterogeneity in these various responses may result from intrinsic differences within the precursor cells.
Several reports point out that PIs impair adipocyte differentiation reducing then the number of fat cells generated from APs17. Of note, the fat loss in AIDS patients worsens with ongoing ART therapy and discontinuation of the treatment neither inverted this situation nor its associated complications. This observation implies that not only the differentiation process is altered by ART therapy. Fewer reports describe the effects of PIs on AP cells issued from distinct fat depots and information on the process leading to a modification of the intrinsic properties of the AP pool in response to ART therapy is rather scant.
A better comprehension of the molecular alterations induced by HIV-ART molecules on APs represents a valuable approach to illustrate the specificity of the distinct depots and to identify the signaling pathways important for adipose tissue development. It also allows a better comprehension of PIs-induced lipodystrophy development that may be of interest to improve the panel of therapeutic options.
In this study, we used HIV-PIs to better understand how self-renewal and differentiation were regulated in APs of different origins. We show that, in addition to our reference model (hMADS cells18), AP cells isolated from distinct fat depots display heterogeneous sensitivities to HIV-PIs. Lopinavir (LPV) is a peptide mimetic HIV-protease inhibitor. It altered proliferation of APs, chin-derived APs being the most sensitive, and it diminished differentiation of some but not all APs. In contrast, darunavir (DRV), a nonpeptidic antiretroviral protease inhibitor very efficient to decrease the activity of the HIV protease, reduced APs differentiation at high doses and displayed more moderate effects on proliferation. The expression of IER3 was altered by LPV treatment. In addition, the activinA-induced ERK 1/2 phosphorylation was blunted thus promoting an alteration of the downstream signaling pathway. It especially lowered the expression of the early growth response 1 gene EGR1, a transcription factor also known as NGFI-A, Krox-24, ZIF268 and TIS8 which is highly dependent on ERK phosphorylation in self-renewing stem cells19, 20. EGR1 controls important cellular processes including survival, cell growth21, 22 and appears as a determinant gene targeted by LPV. This cascade of events decreased activin A production and secretion impairing then its ability to maintain self-renewal, either in a paracrine or in an autocrine manner.
All together our results show that alterations of self-renewal properties occurred predominantly in the AP pool issued from the adipose depots of the face in response to LPV and they point out that LPV targets the activin A autocrine loop which is crucial to maintain the AP pool.
Results
Differentiation of APs derived from different adipose depots in presence of PIs is not equivalent
Lopinavir but not darunavir, altered adipose progenitors differentiation
We measured the impact of DRV treatment on hMADS cells differentiation and compared it to LPV which is known to affect negatively the adipocyte maturation. We used concentrations in the range of those measured in the plasma of PI-treated patients. The therapeutic range for DRV was 10 µM onwards23 and it was comprised between 2–12 mg/ml, i.e. 3.2–19 µM, for LPV24. Indeed treatment of hMADS cells incubated with the differentiation cocktail in presence of 20 µM DRV allowed the apparition of lipid droplets as shown by Oil Red O staining (Fig. 1A and B) indicating that DRV is a weak inhibitor of adipose differentiation. In contrast differentiation was dramatically affected by a 10 µM LPV-treatment (Fig. 1C), indicating that the cells responded differently to these two drugs. Larger magnifications are presented in supplementary Fig. 1. These results were further confirmed by analysis of adipose differentiation markers. After 17 days of differentiation in hMADS cells, we observed that DRV significantly decreased the expression of genes important for adipocyte differentiation in a dose dependent manner, such as the transcription factors C/EBPα and PPARγ (Fig. 1D–E).In addition, LPV reduced the protein expression of the adipose marker FABP4 (Fig. 1F). Note that LPV treatment led to stronger effect while using lower concentrations as compared to DRV.
Figure 1.
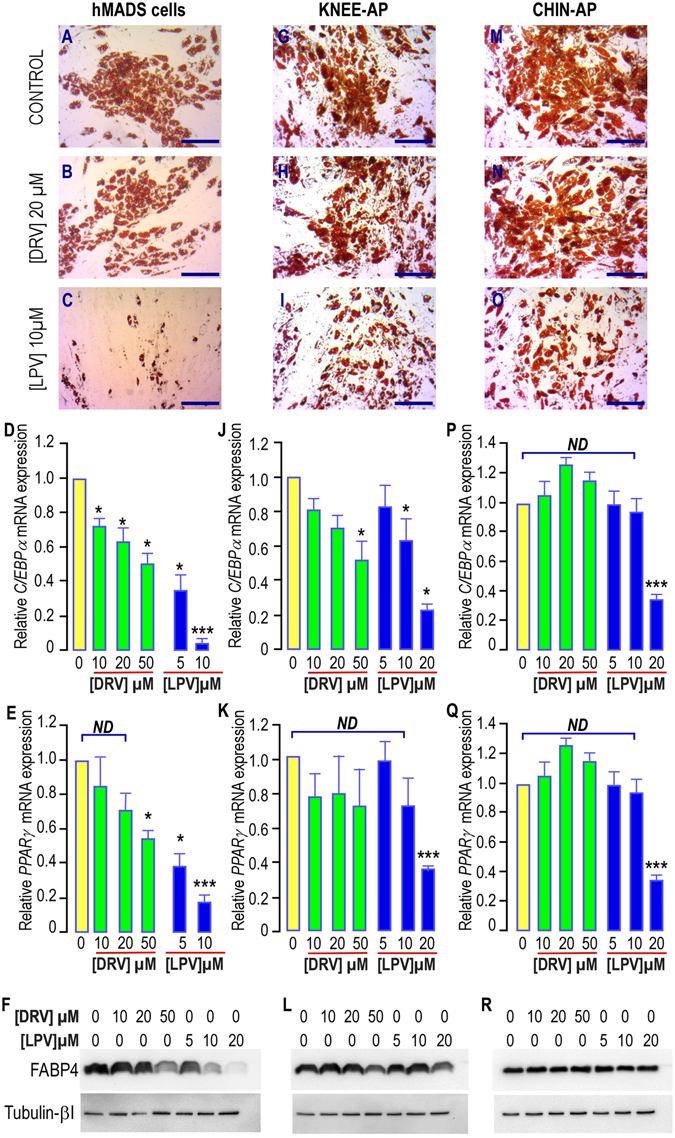
Effects of PIs treatment on differentiation of hMADS cells, chin and Knee APs. (A–C,G–I,M–O) Adipocyte differentiation in hMADS, knee or chin APs. After induction of differentiation for 17 days in absence or presence of PIs at the indicated concentrations, cells were fixed and further stained with Oil Red O. Differentiated cells displayed red staining within the lipid droplets. (D–E,J–K,P–Q) Expression of the adipogenic markers C/EBPα and PPARγ after 17 days of differentiation in absence or presence of increasing concentrations of PIs. Marker expression was assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Marker expression was measured in cells grown in the differentiation medium in absence of PIs (yellow bars) or in presence of DRV (green bars) or LPV (blue bars). The means ± SEM. were calculated from 3 (hMADS and knee) and 5 (chin) independent experiments, with determinations performed in duplicate (*p < 0.05, **p < 0.01, ***p < 0.001). (F,L,R) Expression of the adipogenic marker FABP4 after 17 days of differentiation in absence or presence of increasing concentrations of PIs. FABP4 expression was measured in cells grown in the differentiation medium in absence of PIs or in presence of increasing concentrations of DRV or LPV. Expression of Tubulin-βI used as a loading control (lower panel) and of FABP4 (upper panel) was analyzed by Western blot using specific antibodies. A typical autoradiograph is shown.
APs from distinct fat depots displayed different sensitivity to LPV’s effect
We determined the response of APs isolated from two distinct adipose depots from the same donor (chin and knee)6 to the inhibitory effect of PIs on the differentiation process.
We noted that adipose differentiation occurred in all APs cells treated or not with 20 µM DRV as shown by oil Red O staining (Fig. 1G,H,M,N) while LPV impacted this mechanism in a larger extend without abolishing it (Fig. 1I and O). In good correlation with our observations on hMADS cells, marker analysis revealed a dose-dependent impairment of differentiation when it was carried out in presence of DRV at concentrations higher than 20 µM in APs from knee (Fig. 1J). This result was further confirmed by protein analysis (Fig. 1l). In contrast, no effect was observed on APs derived from chin even at 50 µM DRV and at 10 µM LPV, a concentration that was detrimental for hMADS cells and knee-APs differentiation (Fig. 1D,P and Q). Here again, LPV did not prevent expression of FABP4 in chin-APs (Fig. 1R). Note that C/EBPα expression was more sensitive to PIs treatment in chin and knee APs than PPARγ (Fig. 1J, K and P, Q), while these two genes were affected in a similar manner in hMADS cells (Fig. 1D,E).
All together these results indicated that depending on their origins, the different APs exhibit distinct sensitivities to the inhibitory effect of DRV or LPV on differentiation that may reflect the discrepancies observed in the antiretroviral-induced lipodystrophies.
Lopinavir but not darunavir, altered adipose progenitors proliferation
As a decrease in differentiation alone is not sufficient to explain the unwanted side effects of PIs observed in the face of patients treated with PIs, we analyzed the effects of PIs on APs self-renewal.
We first treated hMADS cells with LPV or DRV for 4 days (MTT assay) or 5 days (cell counts) to assess their impact on proliferation. While increasing doses of DRV did not cause a huge decrease in the cell number, LPV treatment significantly reduced proliferation by 25 to 75% for concentrations starting from 10 µM (Fig. 2A and B).
Figure 2.
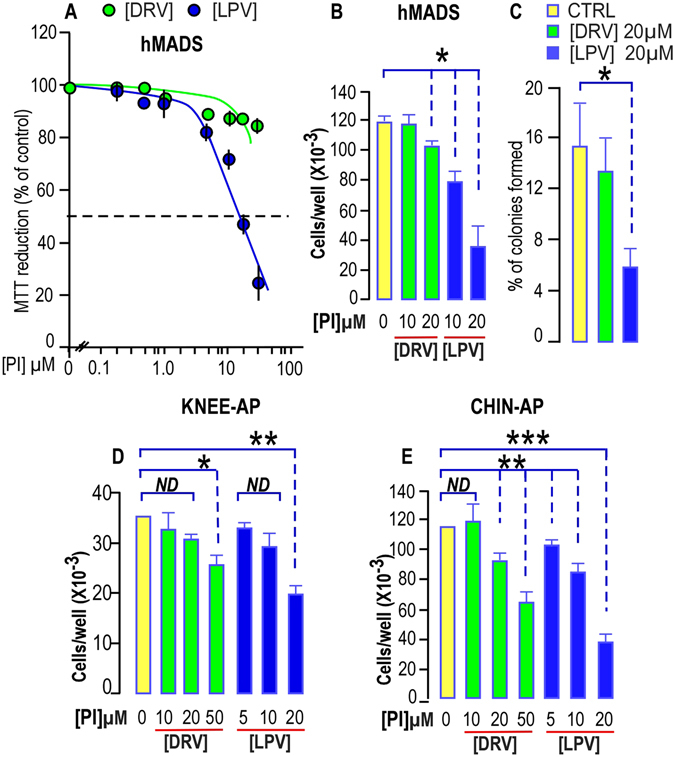
PIs impair proliferation and clonogenicity of hMADS cells, chin and Knee APs. (A) Dose response curve for DRV and LPV effect on hMADS cell proliferation. Proliferation was evaluated using the soluble tetrazolium salt MTT reduction assay after 4 days of proliferation in presence of DRV (green dots) or LPV (Blue dots). The results represent the mean ± SEM of three experiments carried out in triplicate. Error bars were omitted when the SEM was smaller than the size of the symbol. (B) Dose dependent effects of DRV and LPV on hMADS cell proliferation. Cells were seeded in 12-well plates and grown for 5 days in complete culture medium and counted using a BeckmanZ1 coulter. The results represent the mean ± SEM of three experiments carried out in triplicate (*p < 0.05). (C) Lopinavir but not Darunavir reduces clonogenicity of hMADS cells. After plating and growing 200 cells for 3 weeks in complete culture medium, the percentage of colonies obtained from hMADS cells was calculated. Mean ± SEM was representative of three independent experiments (*p < 0.05). (D,E) Dose dependent effects of DRV and LPV on knee and chin APs cell proliferation respectively. Cells were seeded in 12-well plates and grown for 5 days in complete culture medium and counted using a BeckmanZ1 coulter. Experiment was carried out using APs derived from the same person. The results represent the mean ± SEM of three experiments carried out in triplicate (*p < 0.05, **p < 0.01, ***p < 0.001). ND means “no significant difference with the control condition”.
In addition LPV dramatically reduced their ability to grow at a single cell level while DRV did not display any effect. All together these results indicate that LPV, but not DRV, alters self-renewal of hMADS cells (Fig. 2C).
These results prompted us to analyze self-renewal of APs isolated from different sites of the same donor upon PIs treatment. APs derived from chin were significantly impacted mainly by LPV while those derived from knee were less sensitive to PIs treatment (Fig. 2D and E). These results were confirmed after measuring the effects of PIs on proliferation of chin and knee APs both coming from another distinct donor (Supplementary Fig. 2).
We investigated the cytotoxic effects of PIs on APs, as we previously showed that low doses of LPV (1 µM) induced apoptosis in cancer stem cells expressing an embryonic signature25. After four days of culture in presence of LPV or DRV, cells were well attached to the tissue culture dish and more than 99.5% of cells were excluding trypan blue. We did not notice any induction of apoptosis by means of caspase 3 cleavage (Supplementary Fig. 3) indicating that the impairment of APs proliferation induced by HIV-PI did not result from cytotoxic effects.
Signaling pathways altered by PIs
HIV-PIs’ effect on IER3 mRNA expression
To gain mechanistic insights on PIs’ effect, we analyzed IER3 expression in cells submitted to PIs treatment. In good correlation with the absence of inhibition of proliferation, a 5-day treatment with low doses of DRV did not impact significantly IER3 expression either in hMADS cells or in the different APs used (Fig. 3A–C). In contrast LPV, which altered dramatically self-renewal, reduced IER3 by 80% in hMADS cells and in chin APs (Fig. 3A and B). Knee APs were less sensitive as IER3 expression was only reduced by 40% (Fig. 3C).
Figure 3.
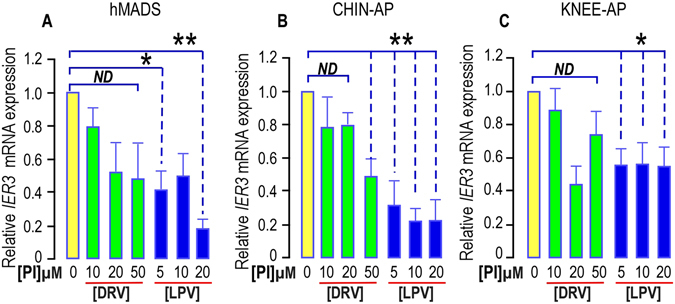
PIs impair IER3 expression in hMADS cells, chin and Knee APs. IER3 expression was determined in APs submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 5 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM. was representative of three independent experiments. (*p < 0.05 and **p < 0.01). ND means “no significant difference”.
We analyzed IER3 expression after a longer chronic treatment of APs with PIs, as short term outcomes are not always reflecting the changes occurring in life-long cures. Indeed this decrease in IER3 expression was still observed and was significant when a 35-day treatment was achieved in hMADS cells (Fig. 4A).
Figure 4.
Long term treatment with PIs impairs IER3 expression and ERK1/2 phosphorylation in hMADS cells. (A) Effect of PIs on IER3 expression. IER3 expression was determined in hMADS cells submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 35 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM was representative of three independent experiments. (*p < 0.05 and **p < 0.01). (B) PIs alter Activin A -induced ERK1/2 phosphorylation. Thirty µg of proteins prepared from hMADS cells treated with DRV or LPV for 35 days were loaded onto a 10% acrylamide gel. Sub-confluent cells were stimulated or not with 100 ng/ml activin A after 3 days of serum deprivation. Total or phosphorylated forms of ERK1/2 were assessed with specific antibodies. Activin A induced an increased phosphorylation of ERK1/2 that was blunted in cells treated with LPV. Expression of Tubulin-βI used as a loading control (lower panel). A representative Western blot is shown. (C) Quantification of the signals. The activin A-induced phosphorylation of ERK1/2 was measured using the Quantity one Program and compared to the expression of Tubulin-βI. Four and six determinations were analyzed when cells were treated respectively with DRV and LPV. Analysis was performed taking the unstimulated control as reference (black stars) or the activin A stimulated condition (green and blue stars) (*p < 0.05 and **p < 0.01). ND means “no significant difference”.
This indicated that concomitantly with APs proliferation, LPV impaired IER3 mRNA expression. Its effects depended on the original location of the distinct APs.
ERK activation was blunted by LPV
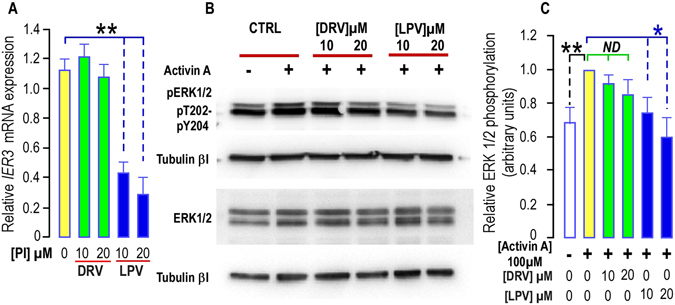
We next examined the effects of PIs on signaling pathways important for cell proliferation in hMADS cells grown for 35-days in presence or absence of PIs. We measured the ERK1/2 phosphorylation in response to activin A which is essential to maintain APs (hMADS) self-renewal7, 8.
While DRV did not significantly affect ERK 1/2 phoshorylation in response to activin A, LPV dose-dependently blunted the phosphorylation of ERK1/2 observed in response to this factor (Fig. 4B and C). In presence of LPV, we measured a phosphorylation level that was not significantly different from the non-stimulated control level (Fig. 4C).
Neither DRV, nor LPV affected the activin A-induced SMAD2 phosphorylation indicating that this pathway leading to inhibition of differentiation through a decrease in CEBPβ-LAP expression is not targeted by PIs (Supplementary Fig. 4).
HIV-PIs modified the immediate early gene EGR1 expression: consequences for INHBA gene expression
To assess the consequences of the LPV-induced limitation in ERK1/2 phosphorylation on the ERK cascade, we measured EGR1 expression in cells that received or not PIs treatment. We observed that neither a 5-day, nor a 35-day treatment with different concentrations of DRV significantly modified EGR1 expression (Fig. 5A–D). In contrast, LPV strongly and significantly impaired EGR1 expression irrespective of the length of the treatment (Fig. 5A–D). Inhibition was already reaching the maximum value at 5 µM LPV in chin-APs. A significant decrease was also observed at the protein level after 35 days of treatment with a 20 µM LPV concentration (Fig. 5E and F).
Figure 5.
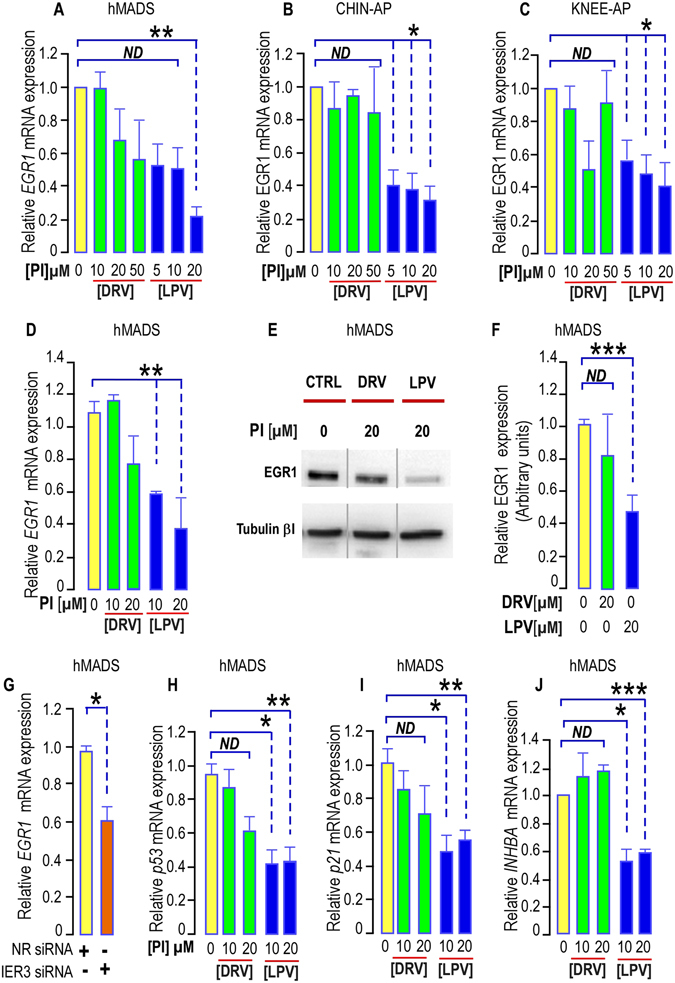
PIs impair EGR1 expression in hMADS cells, chin and Knee APs. (A–D) Effect of PIs on EGR1 expression. (A–C) EGR1 expression was determined in APs submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 5 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM. was representative of three independent experiments. (*p < 0.05 and **p < 0.01). ND means “no significant difference”. (D) EGR1 expression was determined in hMADS cells submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 35 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM. was representative of three independent experiments. (*p < 0.05 and **p < 0.01). (E) LPV decreases EGR1 expression. Thirty µg of proteins prepared from hMADS cells grown for 35 days with DRV or LPV were loaded onto a 10% acrylamide gel. A representative Western blot showing the expression of Tubulin-βI used as a loading control (lower panel) and of EGR1 (upper panel) is presented. All bands are issued from the same exposure of the blots. (F) Quantification of the signals. EGR1 expression was measured using the Quantity one Program and compared to the expression of Tubulin-βI. Four independent determinations were analyzed (***p < 0.001). ND means “no significant difference”. (G) EGR1 expression was determined in hMADS cells transfected with non-relevant siRNA or with siRNA to decrease IER3 expression for 3 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM. was representative of four independent experiments (p = 0.0107). (*p < 0.05). (H,I,J) Expression of EGR1 downstream genes. p53, p21 and INHBA expressions were determined in hMADS cells submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 35 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM. was representative of three independent experiments (ND = not significantly different, *p < 0.05, **p < 0.01, ***p < 0.001).
In addition, from RNA interference experiments with siRNAs to IER3 that were previously validated7, we observed that EGR1 expression was dependent on IER3 expression (Fig. 5G), indicating that LPV may alter IER3 prior to EGR1.
We measured the expression of p53 which is directly controlled by EGR1 at the transcriptional level26 and of its direct target p21. We found that the LPV-induced decrease in EGR1 expression after 35 days of treatment was accompanied by a significant reduction in the mRNA levels of both p53 and p21 (Fig. 5H and I). DRV which does not modify EGR1 expression did not affect either the expression of p53 or p21. This indicates that the decrease in EGR1 expression is followed by functional consequences on the gene expression pattern of LPV-treated cells. Although a decreased expression of p53 may favor cell growth, we did not observe such an event in LPV-treated cells.
In this regard, we focused on pathways important to sustain self-renewal in APs. In silico analysis revealed the presence of EGR1 binding elements in the INHBA promoter. We further measured the effect of LPV on INHBA expression. After 35 days of treatment with PIs, we observed that LPV decreased significantly INHBA expression in hMADS cells while DRV had no effect (Fig. 5J).
This observation prompted us to quantify INHBA expression in cells treated with PIs for a shorter period- i. e. 5 days. We observed that a short term treatment with LPV was sufficient to reduce INHBA expression in hMADS cells and in chin AP (Fig. 6A and B). To confirm these observations, we measured activin A secretion in PI-treated cells. Indeed we noticed that a four-day treatment with LPV reduced activin A secretion (Fig. 6C). All together, our results indicated that LPV altered self-renewal by both decreasing the production of activin A and impairing its signaling pathway, hindering then the autocrine loop sustaining the AP pool.
Figure 6.
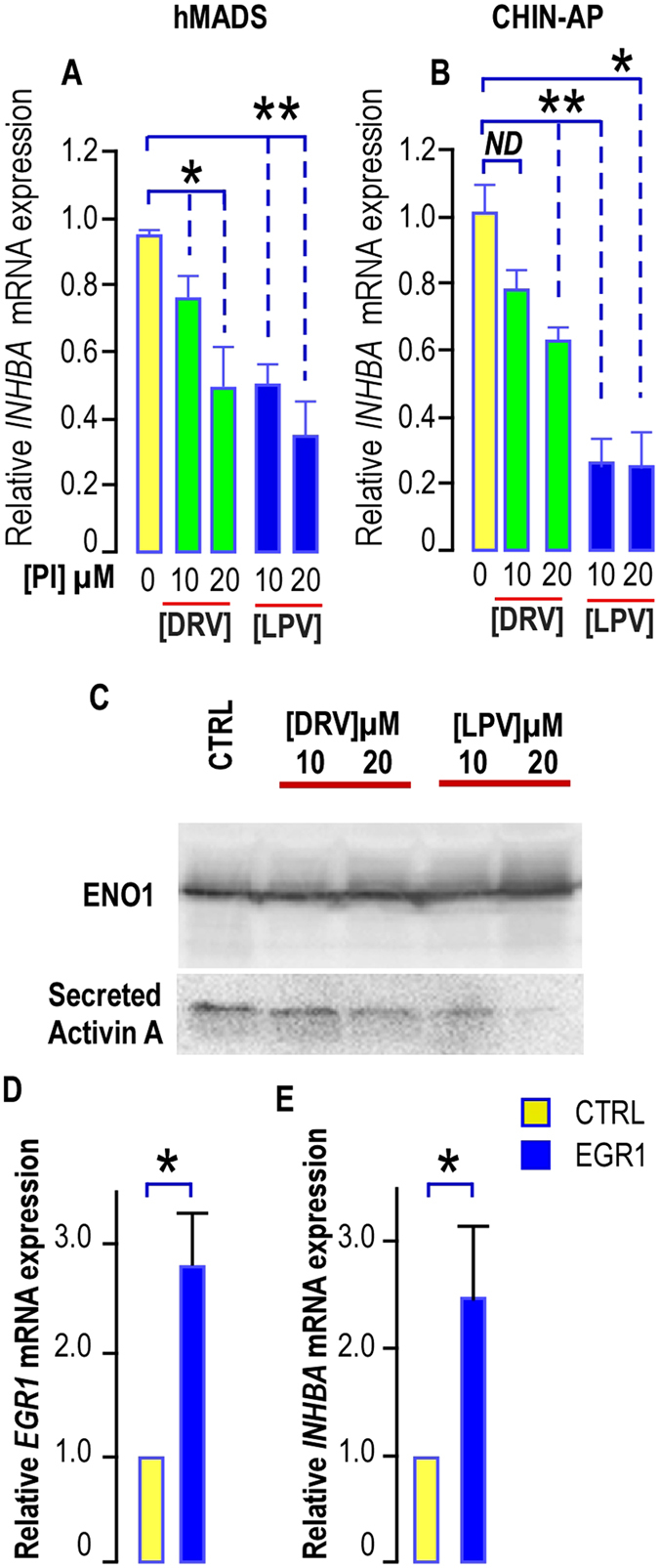
LPV impairs INHBA expression and activin A secretion. (A,B) Expression of INHBA in hMADS cells and in chin-AP treated with PIs. INHBA expression was determined in hMADS cells and chin APs submitted or not to increasing doses of DRV (green bars) or LPV (blue bars) for 5 days. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM was representative of four (hMADS) or three (chin-AP) independent experiments (ND = not significantly different, *p < 0.05, **p < 0.01, ***p < 0.001). (C) Effects of HIV-PIs on activin A secretion. Culture media were collected after a 4-day treatment of hMADS cells with or without PIs, filtered on 0.45 µM membranes, and concentrated with Amicon ultra-15 columns (NMWL, three KDa; Millipore). Activin A secretion was analyzed by Western blot performed under non reducing conditions because anti–activin A antibody selectively binds to the dimeric form of activin A. A representative Western blot showing the expression of Enolase 1 (ENO1) used as a loading control (upper panel) and of activin A (Lower panel) is presented. (D,E) Effect of EGR1 overexpression on INHBA. EGR1 and INHBA expressions were determined in hMADS cells transduced with a lentivirus allowing EGR1 expression. The analysis findings were assessed by real-time RT-PCR and normalized for the expression of 36B4 mRNA. Mean ± SEM was representative of four independent experiments. (*p < 0.05)
To ensure that EGR1 was involved in this regulation, INHBA expression was measured in cells overexpressing EGR1 after lentiviral transduction. Indeed, we observed that a 3-time increase in EGR1 expression led to an enhanced expression of INHBA (Fig. 6D and E) indicating that expression of INHBA was dependent on the presence of EGR1.
The summary of the results and the pathway altered by HIV-PIs is presented in Fig. 7.
Figure 7.
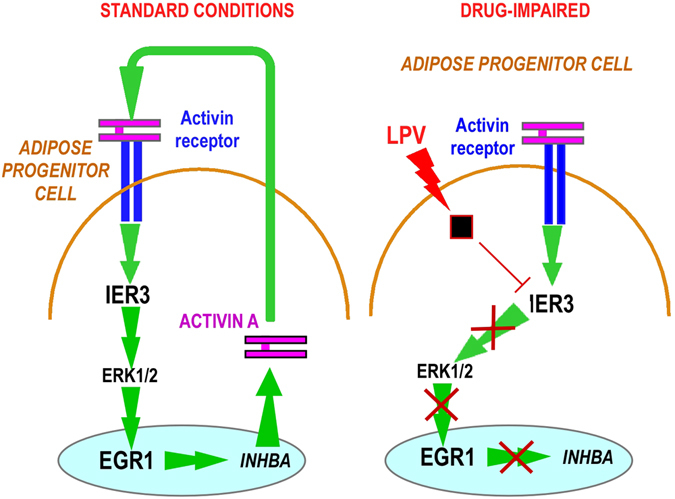
: Summary of the results found. Activin A sustains self-renewal through activation of IER3 expression. LPV impairs IER3 expression that impacts ERK1/2 phosphorylation and EGR1 expression. EGR1 downstream pathway is then affected. This implies a decrease in Activin A production and an impairment of the activin A autocrine signaling that alters proliferation and clonal expansion of APs.
Discussion
APs isolated from different depots display distinct molecular signatures which have been related to their embryonic origin5, 6. In this paper we aimed to point out the heterogeneity of APs derived from human distinct fat depots by means of their respective sensitivities to pharmaceutical treatments. We focused on drugs known to affect adipose tissue homeostasis: the PIs which are part of HIV–ART therapy. Among its deleterious side effects, this treatment influences adipose tissues repartition. It promotes lipodystrophy development, leading then to a paucity of fat that impairs energy homeostasis. This represents a relevant approach to pinpoint the specific features (self-renewal and differentiation) of distinct APs and to identify the pathways playing a crucial role in their perpetuation.
We used APs from different origins6 in addition to abundantly described models of APs17, 18. Ten years ago, the inhibition of adipose differentiation induced by PIs and NRTI was reported in murine as well as human models17. A lack of differentiation was the only designed culprit responsible for lipodystrophies -even lipoatrophies- and this mechanism appeared as sufficient to explain the evolution of this unwanted side effect. In good agreement with these results, we found here that adipose differentiation of hMADS and APs from knee was drastically impacted by LPV treatment in a dose-dependent manner. However, the chin-derived APs were only impacted significantly when 20 µM LPV was used, thus pointing out important differences in sensitivity to LPV treatment among the distinct AP pools. This indicated that inhibition of differentiation may not be the major cause of lipoatrophy in the chin fat pad induced by HIV-ART. Indeed we found that proliferation of chin APs was dramatically reduced both by LPV and DRV, LPV being the most efficient molecule for doses as low as 5 µM significantly impaired cell growth. Note that these molecules did not display cytotoxic effects in APs while low doses of LPV induced apoptosis in cancer stem cells expressing an embryonic signature25, reflecting again huge differences in sensitivity between distinct cell types. In this regard, APs from different depots displayed proper sensitivities to DRV and LPV. We measured this effect in paired samples issued from different donors indicating that it was an unrestricted observation. Proliferation was also reduced in the other APs analyzed here, and this effect has to be considered in addition to the inhibition of differentiation, indicating that several processes may contribute all together to alteration of the fat pads expansion. Absence of differentiation efficiently reduces production of adipocytes when APs are present. In addition any reduction in the AP pool is susceptible to cause adipose tissue remodeling and to decrease fat depot development.
Our in vitro observations are in lane with clinical reports revealing that different manifestations of the HIV-ART were observed in AIDS patients. As an example, lipoatrophy of the face and neck adipose tissues can be observed concomitantly with hypertrophy of the visceral adipose tissue. These two antagonistic situations could even be observed in the same patient indicating that all adipose depots do not display identical responses to HIV-PI in vivo 16. Of note, PIs were reported as lipoatrophy-inducers in the face27. Thus, any shrinkage of the AP pool may account for the irreversible HIV-ART-induced lipoatrophies that were observed.
We then aimed to decipher the molecular mechanisms altered by HIV-ART that underlie the adverse effects on APs self-renewal.
Several genes and signaling pathways control the balance between self-renewal and differentiation in stem cells. Recently, we focused on activin A and its crucial role to maintain self-renewal in adipose progenitors7, 8. We pointed out that activin A was involved in the preservation of AP pools by coordinating the balance between self-renewal and differentiation in an autocrine manner7, 8. Similarly, activin A also sustains self-renewal of human ES cells28, 29 and its upregulation is required to maintain mesenchymal features of cancer stem-like cells in non-small cell lung cancer30 indicating that its role is not restricted to APs.
The immediate early response gene IER3 is expressed in a wide range of tissues31 and we recently showed that activin A controls its expression7. It has been reported to participate to several processes including cell survival, proliferation or apoptosis9, 32. Its pro-survival properties are relevant in a context of stem cells that need to perpetuate all life-long but also in a context of a tissue that needs to adapt to metabolic constraints33–35. Indeed, expression of IER3 was crucial to maintain and expand the adipose progenitor pool in response to effectors present in the microenvironment, including activin A7. Although, an elevation of IER3 expression was measured in the APs isolated from obese patients, information concerning its expression in lipodystrophic individuals is scant and difficult to reach due to ethical considerations. We noted that LPV treatment which altered self-renewal of APs lowered IER3 expression in these cells. This indicated that this gene is closely linked to the self-renewal status of the AP pools. Interestingly, not all the APs responded in a similar manner. We observed a consequent LPV-induced diminution in IER3 expression mainly in chin-derived APs, which is in lane with the LPV-induced alteration of proliferation measured in these cells.
From RNA interference experiments, we previously showed that IER3 supports activin A-induced self-renewal of APs through phosphorylation of ERK1/2 and activation of this regulatory network7. In good agreement, we observed that LPV, which reduces IER3 expression, significantly blunted the activin A–induced phosphorylation of ERK1/2 in APs. The alteration of this key pathway involved in cell proliferation may account for the negative impact of LPV on proliferation. In addition, these results and those previously reported7 show that IER3 expression is correlated with the expandability of the adipose tissue and reflects its adaptation to metabolic constraints in physiopathological conditions.
To further identify the cascade leading to a decrease in proliferation, we concentrated on the multifunctional transcription factor EGR1 which is highly dependent on ERK1/2 phosphorylation in self-renewing stem cells36, 37. Furthermore, EGR1 has been identified as one of the positively regulated target genes by activin A in the murine gonadotrope-derived LBT2 cell line38. EGR1 is a DNA binding protein able to regulate the expression of a large number of genes, then controlling important cellular processes such as survival, cell growth, differentiation, senescence, transformation or apoptosis. In addition to the three zinc finger motifs and two nuclear localization signals, the structural analysis reveal the presence of a strong activation followed by a repression domain while a weak activation domain is located at the C terminus, indicating that EGR1 can function as a positive or negative regulator of transcription39. For instance, EGR1 promotes cell proliferation in some cancers cells40, 41 and has also been associated with enhanced cell survival and tumor progression20, 42, while inhibition of EGR1 contributes to anti mitogenic effects in vascular smooth muscle cells43 or cancer cells44. EGR1 was shown to play an essential role in many differentiation processes leading to the production of functional cell types including B cells, macrophages, neuroblastoma cells and adipocytes45. Its anti-adipogenic action is important to maintain appropriate levels of adipogenesis and/or a pool of resting APs able to undergo specialization upon appropriate stimulus in their microenvironment. In agreement with this finding, RNA interference experiments revealed that EGR1 expression was depending on IER3, an important target in the activin A self-renewal signaling pathway in our model. Our results indicated that LPV negatively targeted EGR1 expression as well as the expression of its downstream genes including INHBA, while EGR1 overexpression promoted INHBA expression. All together, these observations are in lane with a LPV-induced alteration of self-renewal of APs and they bring further evidences in the identification of the actors involved in the activin A autocrine loop that sustains APs self-renewal.
So far, the intracellular receptor or target for LPV remains unknown. Our results indicate that LPV, but not DRV, blunts the proliferation/self-renewal pathway activated by activin A. Note that LPV reduces also its production by lowering the transcription of INHBA gene and the secretion of activin A in APs. This contributes to turn down one of the autocrine loops critical to maintain the stemness properties of the AP pool. Note that any deterioration of this pathway is sufficient to dramatically alter self-renewal.
In conclusion, our work underlines the heterogeneity in the responses of distinct APs to HIV-PIs. While proliferation and self-renewal were preferentially impaired in APs derived from chin, differentiation was mostly impacted in APs derived from knee, indicating that all features of APs may account for lipodystrophy development. We identified a cascade of events that were important to sustain the self-renewal ability of APs. Cell autonomous regulation through activin A production appeared as an important, and somehow underestimated so far, pathway to perpetuate the AP pools. In this regard, the identification of IER3 and EGR1 as crucial genes to control self-renewal in physio-pathological conditions may give new insights to target and control the adipose tissue development and indeed for successful de novo adipogenesis in restoring facial defects.
Material and Methods
Reagents
Unless specified otherwise, all reagents were obtained from Sigma (Saint-Quentin Fallavier, France).
Tissue culture media were obtained from LONZA (Levallois-Perret, France) and foetal calf serum (FCS) from Dutscher S.A. (Brumath, France). Protease Inhibitors were obtained by extraction from commercially available tablets and capsules and purified by silica gel column chromatography. Their purity was assessed by 1H and 13C nuclear magnetic resonance and mass spectroscopy. Pure molecules were dissolved in DMSO. The key intermediates used for structure-activity relationship studies were synthesized according to a previously reported procedure46.
Cell culture
Human APs cells used for in vitro studies derived from distinct sources.
hMADS cells6 were isolated more than fifteen years ago from adipose tissue, as surgical scraps from surgical specimen of various surgeries of young donors, with the informed consent of the parents. All methods were approved and performed in accordance with the guidelines and regulations of the Centre Hospitalier Universitaire de Nice Review Board.
Chin and knee paired APs were derived from the stroma vascular fraction of Caucasians women who underwent elective liposuction procedures. Informed consent was obtained from all patients44, 45. APs were grown, maintained and differentiated according to experimental conditions previously described for each cell type6, 45, 47.
For proliferation experiments
cells were seeded at 3 000 cells/cm2. They were incubated in complete culture medium. The cells were counted using a Beckman Coulter Z1.
Proliferation based on MTT assay
Proliferation of human APs was evaluated using the soluble tetrazolium salt MTT reduction assay48. Briefly, cells were seeded at 1500 cells/well in quadruplicate in 96-well plates and incubated at 37 °C for the indicated time. MTT (0.5 mg/ml) was added to each well and cells were further incubated for 4 h. Then the insoluble formazan was dissolved in isopropanol and DMSO (v/v) and the OD was determined spectrophometrically at 562 nm and 630 nm for background correction.
A clonogenic assay
was carried out by plating 200 cells in a 100 mm-dia. dish in complete growth medium (containing 10% SVF) for 21 days. Colonies were scored after staining with crystal violet (1%). Each experiment was carried out in triplicate.
Adipocytic differentiation
was carried out as previously described and was assessed by Oil Red O staining6, 47.
Viral infection
Lentiviral particles were produced transfecting the 293 T cell line with the 2K7BSD-EGR1 constructs along with the packaging vectors. After lentiviral infection, cell lines stably expressing EGR1 were selected in appropriate blasticidin containing- medium (1 μg/mL) for at least 15 days. Two stable cell lines were developed from independent viral productions/infections and exhibited identical behaviors. Cells stably expressing the TOMATO-Fluorescent Protein (TOMATO) were obtained after 2K7BSD-Tomato lentiviral particles infection and were used as control.
Gene expression analysis
Total RNA was extracted using the TRI-Reagent kit (Euromedex, Soufflweyersheim, France) and reverse transcription (RT) was performed using MMLV reverse transcriptase (Promega, Charbonnieres, France), as recommended by the manufacturers.
All primer sequences are described in supplementary section. Real-time PCR assays were run on an ABI Prism One step real-time PCR machine (Applied Biosystems, Courtaboeuf, France). Normalization was performed using 36B4 as a reference gene. Quantification was performed using the comparative Ct method.
Protein expression
Cells were rinsed in ice-cold PBS and whole cell extracts were prepared as described7. Briefly, cells were lysed in the cell lysis buffer49, sonicated for 10 s and centrifuged at 12000 g for 10 min.
Thirty micro grams of proteins were resolved by SDS–PAGE under reducing conditions and transferred to Immobilon–P membranes (Millipore, Molshiem, France). The detection antibodies listed in supplementary section were used according to the manufacturer’s instructions.
The bound primary antibody was detected by horseradish peroxidase-conjugated secondary antibody and visualized using an ECL detection kit (Millipore, Molsheim, France).
Chemiluminescence was observed using a molecular imager ChemiDoc XRS system (Bio-Rad, Marne la Coquette, France). The band intensity was quantified using Bio-Rad Quantity One software.
Activin A secretion
Activin A secretion was assessed as previously described8. Briefly, hMADS cells were maintained in 5 µg/ml insulin and 10 µg/ml transferrin medium in the absence or presence of LPV or DRV as indicated. Culture media were collected 4 days later, filtered on 0.45 µm membranes, and concentrated with Amicon ultra-15 columns (NMWL, three KDa; Millipore). Levels of activin A were analyzed by Western blot performed under nonreducing conditions because anti–activin A antibody selectively binds to the dimeric form of activin A. Secreted enolase one (ENO1) was used as a loading control50.
Statistical analysis
The results are shown as mean ± standard error of the mean (SEM), with the number of experiments indicated. Statistical significance was determined by t-tests using Micrococal Origin 6.0 (Micrococal Software, Northampton MA). Probability values <0.05 were considered statistically significant and are marked with a single asterisk, <0.01 with double asterisks and <0.001 with triple asterisks.
Electronic supplementary material
Acknowledgements
We thank our colleagues for helpful suggestions and discussions. We are indebted to Drs. Pascal Peraldi for a careful reading of this manuscript and Thierry Virolle for a generous gift of the lentiviral constructs used in this study and for helpful discussions. This work was supported by ANRS (French National Agency for Research on AIDS and viral hepatitis) (grant number #AO 2013-1 CSS3) and the French Government (National Research Agency, ANR) through the “Investments for the Future” LABEX SIGNALIFE: program reference #ANR-11-LABX-0028-01. CR is recipient of a pre-doctoral fellowship from ANRS and then from SIDACTION, M.P. is a pre-doctoral fellow from LABEX SIGNALIFE.
Author Contributions
C.R. design of experiments, data research and contributed to discussion. S.A. Chemical synthesis and purification and contributed to discussion. M.P. data research and contributed to discussion. C.D. design of experiments and contributed to discussion. A.L. design of experiments, data research, contributed to discussion and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Christian Dani and Annie Ladoux contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02807-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Tchkonia T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 3.Tchkonia T, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 4.Maumus M, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 5.Billon N, et al. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouidhi, M. et al. Characterization of human knee and chin adipose-derived stromal cells. Stem Cells Int2015, Epub 2015 Feb 2013 (2015). [DOI] [PMC free article] [PubMed]
- 7.Ravaud C, et al. IER3 is a crucial effector promoting expansion of adipose progenitor cells in response to distinct micro-environmental effectors. Stem cells. 2015;33:2564–2573. doi: 10.1002/stem.2016. [DOI] [PubMed] [Google Scholar]
- 8.Zaragosi LE, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arlt A, Schafer H. Role of the immediate early response 3 (IER3) gene in cellular stress response, inflammation and tumorigenesis. Eur J Cell Biol. 2011;90:545–552. doi: 10.1016/j.ejcb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Caron-Debarle M, Lagathu C, Boccara F, Vigouroux C, Capeau J. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol Med. 2010;16:218–229. doi: 10.1016/j.molmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 12.Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59:16–23. doi: 10.1038/jhg.2013.107. [DOI] [PubMed] [Google Scholar]
- 13.Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207:245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 14.Singhania R, Kotler DP. Lipodystrophy in HIV patients: its challenges and management approaches. HIV AIDS (Auckl) 2011;3:135–143. doi: 10.2147/HIV.S14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam N, et al. Body fat abnormality in HIV-infected children and adolescents living in Europe: prevalence and risk factors Fat abnormality in children. J Acquir Immune Defic Syndr. 2012;59:314–324. doi: 10.1097/QAI.0b013e31824330cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25:487–499. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Vernochet C, et al. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J Biol Chem. 2005;280:2238–2243. doi: 10.1074/jbc.M408687200. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez AM, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min IM, et al. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell. 2008;2:380–391. doi: 10.1016/j.stem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Virolle T, et al. Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J Biol Chem. 2003;278:11802–11810. doi: 10.1074/jbc.M210279200. [DOI] [PubMed] [Google Scholar]
- 21.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 22.Zwang Y, et al. Two phases of mitogenic signaling unveil roles for p53 and EGR1 in elimination of inconsistent growth signals. Mol Cell. 2011;42:524–535. doi: 10.1016/j.molcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boffito M, et al. Pharmacokinetics and antiretroviral response to darunavir/ritonavir and etravirine combination in patients with high-level viral resistance. Aids. 2007;21:1449–1455. doi: 10.1097/QAD.0b013e3282170ab1. [DOI] [PubMed] [Google Scholar]
- 24.Jackson A, et al. Pharmacokinetics of plasma lopinavir/ritonavir following the administration of 400/100 mg, 200/150 mg and 200/50 mg twice daily in HIV-negative volunteers. J Antimicrob Chemother. 2011;66:635–640. doi: 10.1093/jac/dkq468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darini CY, et al. Targeting cancer stem cells expressing an embryonic signature with anti-proteases to decrease their tumor potential. Cell Death Dis. 2013;4:e706. doi: 10.1038/cddis.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair P, et al. Early growth response-1-dependent apoptosis is mediated by p53. J Biol Chem. 1997;272:20131–20138. doi: 10.1074/jbc.272.32.20131. [DOI] [PubMed] [Google Scholar]
- 27.Carr A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 28.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 30.Wamsley JJ, et al. Activin upregulation by NF-kappaB is required to maintain mesenchymal features of cancer stem-like cells in non-small cell lung cancer. Cancer Res. 2015;75:426–435. doi: 10.1158/0008-5472.CAN-13-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldmann KA, Pittelkow MR, Roche PC, Kumar R, Grande JP. Expression of an immediate early gene, IEX-1, in human tissues. Histochem Cell Biol. 2001;115:489–497. doi: 10.1007/s004180100284. [DOI] [PubMed] [Google Scholar]
- 32.Shen L, Guo J, Santos-Berrios C, Wu MX. Distinct domains for anti- and pro-apoptotic activities of IEX-1. J Biol Chem. 2006;281:15304–15311. doi: 10.1074/jbc.M600054200. [DOI] [PubMed] [Google Scholar]
- 33.Pawlikowska P, et al. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ. 2009;17:1739–1750. doi: 10.1038/cdd.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steensma DP, et al. Rearrangements and amplification of IER3 (IEX-1) represent a novel and recurrent molecular abnormality in myelodysplastic syndromes. Cancer Res. 2009;69:7518–7523. doi: 10.1158/0008-5472.CAN-09-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trouillas M, et al. Three LIF-dependent signatures and gene clusters with atypical expression profiles, identified by transcriptome studies in mouse ES cells and early derivatives. BMC Genomics. 2009;10:73. doi: 10.1186/1471-2164-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook PJ, et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379–1389. doi: 10.1093/neuonc/now049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakakini N, et al. A Positive Feed-forward Loop Associating EGR1 and PDGFA Promotes Proliferation and Self-renewal in Glioblastoma Stem Cells. J Biol Chem. 2016;291:10684–10699. doi: 10.1074/jbc.M116.720698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazhawidza W, Winters SJ, Kaiser UB, Kakar SS. Identification of gene networks modulated by activin in LbetaT2 cells using DNA microarray analysis. Histol Histopathol. 2006;21:167–178. doi: 10.14670/HH-21.167. [DOI] [PubMed] [Google Scholar]
- 39.Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol. 1993;13:4556–4571. doi: 10.1128/MCB.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun T, Tian H, Feng YG, Zhu YQ, Zhang WQ. Egr-1 promotes cell proliferation and invasion by increasing beta-catenin expression in gastric cancer. Dig Dis Sci. 2013;58:423–430. doi: 10.1007/s10620-012-2356-4. [DOI] [PubMed] [Google Scholar]
- 42.Baron V, et al. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22:4194–4204. doi: 10.1038/sj.onc.1206560. [DOI] [PubMed] [Google Scholar]
- 43.Kimura TE, et al. Inhibition of Egr1 expression underlies the anti-mitogenic effects of cAMP in vascular smooth muscle cells. J Mol Cell Cardiol. 2014;72:9–19. doi: 10.1016/j.yjmcc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLigio JT, Zorio DA. Early growth response 1 (EGR1): a gene with as many names as biological functions. Cancer Biol Ther. 2009;8:1889–1892. doi: 10.4161/cbt.8.20.9804. [DOI] [PubMed] [Google Scholar]
- 45.Boyle KB, et al. The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death Differ. 2009;16:782–789. doi: 10.1038/cdd.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoll V, et al. X-ray crystallographic structure of ABT-378 (lopinavir) bound to HIV-1 protease. Bioorg Med Chem. 2002;10:2803–2806. doi: 10.1016/S0968-0896(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez AM, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315:255–263. doi: 10.1016/j.bbrc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 48.Velasco-Velazquez MA, et al. 4-Hydroxycoumarin disorganizes the actin cytoskeleton in B16-F10 melanoma cells but not in B82 fibroblasts, decreasing their adhesion to extracellular matrix proteins and motility. Cancer Lett. 2003;198:179–186. doi: 10.1016/S0304-3835(03)00333-1. [DOI] [PubMed] [Google Scholar]
- 49.Peraldi P, Xu M, Spiegelman BM. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Invest. 1997;100:1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiellini C, et al. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol. 2008;9:26. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


