Abstract
Activated platelets promote cancer progression and metastasis. Nevertheless, the prognostic value of platelet indices in melanoma had been rarely reported. The aim of this study was to investigate the predictive significance of platelet indices in melanoma. A total of 220 consecutive patients with melanoma were retrospectively enrolled between January 2009 and December 2009. The relationship between PDW and clinicopathological characteristics were analyzed. Kaplan-Meier method and Cox regression were used to evaluate the prognostic impact of PDW. Of the 220 patients, high platelet distribution width (PDW) levels were observed in 63 (28.6%) patients. Increased PDW was associated with tumor subtype (P < 0.001). Survival curves found that patients with increased PDW had significantly shorter survival time than those with normal PDW (P < 0.001). Cox regression analysis revealed that elevated PDW was an independent prognostic factor for overall survival (hazard ratio, 2.480; 95% confidence interval [CI], 1.386–4.436, P = 0.002). In conclusion, PDW is easily available in routine blood test. Our findings indicated that PDW is an independent predictor and that it may also be a potential parameter for targeted therapy in melanoma.
Introduction
Malignant melanoma is an aggressive form of cancer with an increasing incidence and mortality worldwide. Despite multiple and aggressive therapeutic interventions, some patients still recur after treatment. Therefore, it is of great importance to look for appropriate and effective prognostic markers in melanoma.
Platelets play an essential role in cancer development, progression and metastasis though their direct interaction with tumor cell1. Platelet actions trigger autocrine and paracrine activation processes that cause phenotypic changes in stromal cells which contribute to the development of cancer2. Increased platelets were associated with poor prognosis in patients with a wide spectrum of malignancies, such as pancreatic cancer, gastric cancer, colorectal cancer, endometrial cancer, and ovarian cancer3–7. However, platelet count is determined by the balance between the rate of production and consumption of platelets. A normal platelet count could conceal the presence of highly hypercoagulative and pro-inflammatory cancer phenotypes in the presence of efficient compensatory mechanisms8.
Mean platelet volume (MPV), the most commonly used measure of platelet size, is an index of platelet activation and is available in clinical practice9. Platelet distribution width (PDW), another platelet index, indicates variation in platelet size10. Altered MPV levels were reported in gastric cancer, ovarian cancer, lung cancer, colon cancer, and breast cancer. However, the clinical implications of PDW have not been well defined. In the current study, therefore, we aimed to evaluate the prognostic roles of MPV and PDW in patients with melanoma.
Results
Between Jan, 2009 and Dec, 2009, a total of 220 patients were enrolled in this study. Among the 220 patients, 116 (52.7%) were women and 104 (47.3%) were men, and the median age was 56.3 ± 12.4 years (range 21–86). In terms of the staging system, 36 cases were categorized as stage I and stage II, 129 as stage III and stage IV.
A ROC curve for OS prediction was plotted to verify the optimal cut-off value for PDW, which was 17.2 (Fig. 1). It demonstrated that PDW predicts cancer prognosis with a sensitivity of 51.1% and a specificity of 68.3% (AUC = 0.683, 95% CI: 0.618–0.744, p < 0.0001). Then, patients were divided into 2 groups: patients with PDW ≤ 17.2% and patients with PDW > 17.2%. There were 157 (71.4%) patients with PDW ≤ 17.2% and 63 (28.6%) patients with PDW > 17.2%.
Figure 1.
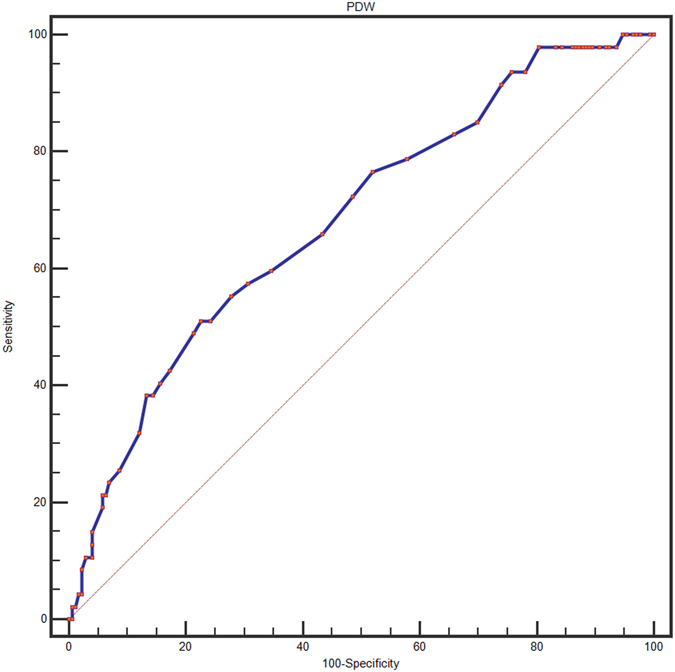
Optimized cut-off was determined for PDW using standard ROC curve analysis.
The relationships between PDW and clinical characteristics were shown in Tables 1 and 2. Our study revealed that PDW was associated with tumor subtype (P < 0.001). However, no significant differences were observed between the groups with regard to age, gender, tumor location, ulceration, tumor size, lymph node metastasis, distant metastasis, and clinical stage.
Table 1.
Baseline characteristics of melanoma patients according to PDW levels.
| Variables | Total n (%) | PDW ≤ 17.2 n (%) | PDW > 17.2 n (%) | P value |
|---|---|---|---|---|
| Age (years) | 0.849 | |||
| <60 | 127 (57.7) | 90 (57.3) | 37 (58.7) | |
| ≥60 | 93 (42.3) | 67 (42.7) | 26 (41.3) | |
| Gender | 0.119 | |||
| Male | 104 (47.3) | 69 (43.9) | 35 (55.6) | |
| Female | 116 (52.7) | 88 (56.1) | 28 (44.4) | |
| Tumor location | 0.728 | |||
| Sun-exposed (head and neck) | 22 (10.0) | 15 (9.6) | 7 (11.1) | |
| Sun-protected (others) | 198 (90.0) | 142 (90.4) | 56 (88.9) | |
| Ulceration | 0.091 | |||
| Negative | 138 (62.7) | 93 (59.2) | 45 (71.4) | |
| Positive | 82 (37.3) | 64 (40.8) | 18 (28.6) | |
| Tumor Size | 0.187 | |||
| ≥2 mm | 127 (57.7) | 95 (60.5) | 32 (50.8) | |
| <2 mm | 93 (42.3) | 62 (39.5) | 31 (49.2) | |
| Tumor subtype | <0.001 | |||
| ALM | 89 (40.5) | 66 (42.0) | 23 (36.5) | |
| SSM | 65 (29.5) | 45 (28.7) | 20 (31.7) | |
| LMM | 26 (11.8) | 17 (10.8) | 9 (14.3) | |
| NM | 29 (13.2) | 24 (15.3) | 5 (7.9) | |
| Others | 11 (5.0) | 5 (3.2) | 6 (9.5) | |
| Lymph node metastasis | 0.116 | |||
| Negative | 166 (75.0) | 123 (78.3) | 43 (68.3) | |
| Positive | 54 (25.0) | 34 (21.7) | 20 (31.7) | |
| Distant metastasis | 0.366 | |||
| Absent | 192 (87.3) | 135 (86.0) | 57 (90.5) | |
| Present | 28 (12.7) | 22 (14.0) | 6 (9.5) | |
| Clinical stage | 0.192 | |||
| I/II | 157 (71.4) | 116 (73.9) | 44 (65.1) | |
| III/IV | 63 (28.6) | 41 (26.1) | 22 (34.9) |
SSM, superficial spreading melanoma; LMM, lentigo maligna melanoma; ALM, acrolentigous melanoma; NM, nodular melanoma; PDW, platelet distribution width.
Table 2.
Baseline characteristics of melanoma patients according to PDW levels.
| Variables | PDW ≤ 17.2 | PDW > 17.2 | P value |
|---|---|---|---|
| Age (years) | 56.2 (12.6) | 56.5 (12.2) | 0.834 |
| Smoker (n, %) | 17 (10.8) | 13 (20.6) | 0.570 |
| Drinking (n, %) | 14 (8.9) | 16 (25.4) | 0.525 |
| BMI (kg/m2) | 24.8(3.4) | 23.6 (2.9) | 0.007 |
| FPG (mmol/L) | 5.10 (4.80–5.62) | 5.00 (4.70–5.50) | 0.444 |
| Albumin (g/L) | 43.3 (4.3) | 44.6 (4.5) | 0.047 |
| WBC (×109/L) | 6.44 (2.05) | 5.94 (1.87) | 0.095 |
| Neutrophils (×109/L) | 3.84 (1.74) | 3.49 (1.65) | 0.173 |
| Lymphocytes (×109/L) | 2.07 (1.36) | 1.85 (0.58) | 0.239 |
| Hemoglobin (g/dl) | 138.8 (29.8) | 139.3 (17.4) | 0.903 |
| Platelet count (×109/L) | 242.2 (67.7) | 217.3 (60.5) | 0.011 |
| MPV (fL) | 8.6 (1.2) | 9.2 (1.5) | 0.001 |
| NLR | 2.12(1.32) | 2.15(2.30) | 0.904 |
| PLR | 133.8 (58.9) | 125.4 (46.2) | 0.265 |
Data are expressed as means (SD) or median (IQR). FPG, fasting plasma glucose; WBC, white blood cell; BMI, body mass index; MPV, mean platelet volume; PDW, platelet distribution width; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
With a median follow up of 60 months, 47 (21.4%) patients had death events. Patients with PDW ≤ 17.2% had a significantly better 5-year OS than patients with PDW > 17.2% (85.4% vs. 61.9%, P < 0.001). The Kaplan-Meier OS curves of the normal versus elevated PDW showed a significant separation (Fig. 2).
Figure 2.

Kaplan–Meier analysis of overall survival in melanoma patients.
In univariate analysis, lymph node metastasis, PDW (categorical variable), albumin, and clinical stage were significant predictors of OS (Table 3). Age (categorical variable) (p = 0.093), lymphocytes (p = 0.071), and tumor subtype (p = 0.075) showed weak associations. Other parameters were not found to be in correlation with OS. Next, all the factors with a P value less than 0.05 in univariate analysis were included in multivariate analysis (Table 4). In multivariate analyses, we demonstrated that PDW was an independent prognostic factor in patients with melanoma. Patients with PDW > 17.2% had a hazard ratio (HR) of 2.480 [95% confidence interval (CI): 1.386–4.436, P = 0.002] for OS.
Table 3.
Univariate analysis of overall survival in melanoma patients.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (years) (≥60 versus <60) | 0.591 | 0.320–1.092 | 0.093 |
| Gender (male versus female) | 0.769 | 0.433–1.364 | 0.368 |
| Smoker (yes versus no) | 1.060 | 0.257–4.368 | 0.936 |
| Drinking (yes versus no) | 0.423 | 0.152–1.180 | 1.000 |
| BMI (kg/m2) | 0.945 | 0.865–1.033 | 0.215 |
| FPG (mmol/L) | 1.360 | 0.897–2.063 | 0.148 |
| Albumin (g/L) | 1.075 | 1.009–1.145 | 0.026 |
| WBC (×109/L) | 0.894 | 0.756–1.057 | 0.189 |
| Neutrophils (×109/L) | 0.931 | 0.766–1.133 | 0.477 |
| Lymphocytes (×109/L) | 0.612 | 0.359–1.042 | 0.071 |
| NLR | 1.105 | 0.941–1.298 | 0.224 |
| PLR | 1.004 | 0.978–1.013 | 0.613 |
| Hemoglobin (g/dl) | 0.996 | 0.983–1.009 | 0.562 |
| Platelet count (×109/L) | 1.001 | 0.997–1.005 | 0.681 |
| MPV (fL) | 0.918 | 0.737–1.143 | 0.444 |
| PDW (%) (>17.2 versus ≤17.2) | 2.857 | 1.612–5.063 | <0.001 |
| Tumor location (Sun-exposed versus Sun-protected) | 1.721 | 0.771–3.843 | 0.185 |
| Ulceration (Yes versus No) | 0.675 | 0.361–1.261 | 0.218 |
| Tumor subtype (SSM + NM versus ALM + LMM + others) | 0.592 | 0.332–1.055 | 0.075 |
| Tumor Size (mm) (≥2.0 versus<2.0) | 1.102 | 0.616–1.974 | 0.743 |
| Lymph node metastasis (Yes versus No) | 2.889 | 1.625–5.136 | <0.001 |
| Distant metastasis (Yes versus No) | 1.219 | 0.546–2.721 | 0.629 |
| Clinical stage (III/IV versus I/II) | 3.386 | 1.908–6.009 | <0.001 |
Table 4.
Multivariate analysis of overall survival in melanoma patients.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Albumin (g/L) | 1.051 | 0.985–1.122 | 0.131 |
| PDW (%) (>17.2 versus ≤17.2) | 2.480 | 1.386–4.436 | 0.002 |
| Lymph node metastasis (Yes versus No) | 0.660 | 0.223–1.953 | 0.453 |
| Clinical stage (III/IV versus I/II) | 4.311 | 1.479–12.568 | 0.007 |
Discussion
This study showed that PDW is associated with patient’s survival and is an independent risk factor for prognosis in melanoma.
Platelets facilitate cancer progression and metastasis by inducing tumor growth, epithelial-mesenchymal transition, and invasion11. An increasingly body of evidence have identified the involvement of activated platelets in melanoma. Platelet-derived growth factor (PDGF) secreted by melanoma cell could stimulate the development of tumor stroma and new blood vessels12. Moreover, Boukerche H et al. showed platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex13. Kolber DL et al. confirmed that recombinant platelet factor 4, a known angiogenesis inhibitor, could effectively suppress tumor-induced neovascularization in mice14. In accordance with the studies above, the current study indirectly confirmed the findings using a simple platelet index. These data are also consistent with the current knowledge that anti-platelet is considered to be a part of cancer adjuvant therapy1. In addition, our study can form the basis for further mechanistic studies and ultimately aid in patient-tailored selection of therapeutic strategies.
The mechanisms to explain the association between PDW and survival are poorly understood. Bone marrow cells (including megakaryocytes) dys-function may contribute to altered PDW. PDW is a measure of platelet heterogeneity caused by heterogeneous demarcation of megakarocytes15. Recent reports demonstrated several cytokines, such as interleukin-6 (IL-6), granulocytes colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF), regulate megakaryocytic maturation, platelet production and platelet size16. IL-6 promotes tumor angiogenesis, metastasis and metabolism17. Furthermore, the cytokines G-CSF and M-CSF that be secreted by tumor cells could stimulate megakaryopoiesis and subsequent thrombopoiesis in cancer18. However, the clinical value of PDW has not been studied in melanoma. Another possible mechanism is that platelets promote the hypercoagulable state in cancer19. Activated platelets create a procoagulant micro-environment that enables the tumor cells to cover themselves with platelets and evade the host immune system20.
The present study has several limitations. First, this was a single-center retrospective study and additional larger validation studies with multiethnic groups are needed to confirm our results. Second, the mechanisms underlying the involvement of PDW in melanoma remains unclear, to which further investigation should be addressed. Third, the patients were composed of Chinese. The application to other ethnic groups still needs further investigation.
In conclusion, PDW is easily available with routine blood counts. Increased PDW may serve as a marker of adverse prognosis in melanoma. Further studies are warranted to clarify the exact role of PDW in melanoma.
Patients and Methods
Study population
This study consisted of 220 consecutive melanoma cases (mean age 56.3 ± 17.4 years, range 18–86 years). Cases were admitted to the Third Affiliated Hospital, Harbin Medical University between January 2009 and December 2009. All patients undergone complete surgical resection. The pathologic diagnoses of melanoma were evaluated by pathologists from biopsy reports. None of the patients received preoperative chemotherapy or radiation therapy. Patients were excluded if they had hematological disorders, coronary artery disease, hypertension, diabetes mellitus, and medical treatment with anticoagulant, statins, and acetylic salicylic acid.
Standard demographic and clinicopathological data were collected from the patients’ records in hospital. For all the study participants, venous peripheral blood samples were collected at admission. Survival data were obtained through follow-up. Overall survival (OS) was defined as the interval from the date of diagnosis to death or last follow-up. The median follow-up time was 60 months. The platelet-to-lymphocyte ratio (PLR) was calculated as the absolute platelet count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the absolute neutrophil count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L.
The Institutional Ethics Review Board of the 3rd Affiliated Hospital of Harbin Medical University approved this study prior to commencement of data collection and waived the informed consent requirement because it was a retrospective study.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). The descriptive statistics are presented as means ± SD or medians (interquartile range) for continuous variables and percentages of the number for categorical variables. Inter-group differences in categorical variables were assessed for significance using the Chi-square test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. The optimal cutoff value of PDW was determined by receiver operating characteristic (ROC) curve. We used univariate analysis to narrow down the list of possible prognostic factors. Variables with P value < 0.05 in univariate analysis were brought into multivariate Cox proportional hazard model to determine their independency. Kaplan-Meier curves and log-rank test were used to compare survival differences among groups. All reported p-values are two-sided and statistical significance was assumed as p < 0.05.
Acknowledgements
This work was supported financially by grants from the Harbin special fund for scientific and technological innovation talents (RC2016XK004068).
Author Contributions
N.L. and Z.Y.D. participated in manuscript preparation, data analysis and editing. XY.H., and Y.N. participated in data collection and data analysis. T.M.L. and Z.P.L. participated in study design, and manuscript revision. R.T.W. and K.J.Y. participated in study design, data analysis, and manuscript preparation. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Na Li and Zhiyong Diao contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui-tao Wang, Email: ruitaowang@126.com.
Kai-jiang Yu, Email: kaijiang_yu@yeah.net.
References
- 1.Mezouar S, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb. Res. 2016;139:65–76. doi: 10.1016/j.thromres.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin. Pharmacol. Toxicol. 2014;114(1):118–127. doi: 10.1111/bcpt.12156. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, et al. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology. 2004;51(57):847–853. [PubMed] [Google Scholar]
- 4.Long, Y, Wang, T, Gao, Q, Zhou, C. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget (2016). [DOI] [PMC free article] [PubMed]
- 5.Pietrzyk L, et al. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac. J. Cancer Prev. 2016;17(9):4433–4437. [PubMed] [Google Scholar]
- 6.Ekici H, et al. Do Leukocyte and Platelet Counts Have Benefit for \Preoperative Evaluation of Endometrial Cancer. Asian Pac. J. Cancer Prev. 2015;16(13):5305–5310. doi: 10.7314/APJCP.2015.16.13.5305. [DOI] [PubMed] [Google Scholar]
- 7.Qiu J, et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2012;38(4):651–657. doi: 10.1111/j.1447-0756.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 8.Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us. Platelets. 2015;26(2):114–118. doi: 10.3109/09537104.2014.894969. [DOI] [PubMed] [Google Scholar]
- 9.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation. Curr. Pharm. Des. 2011;17(1):47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 10.Kaito K, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 2005;128(5):698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 11.Meikle CK, et al. Cancer and Thrombosis: The Platelet Perspective. Front Cell Dev Biol. 2016;4:147. doi: 10.3389/fcell.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnhill RL, Xiao M, Graves D, Antoniades HN. Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-alpha receptor, but not the PDGF-beta receptor, in human malignant melanoma in vivo. Br. J. Dermatol. 1996;135(6):898–904. doi: 10.1046/j.1365-2133.1996.d01-1092.x. [DOI] [PubMed] [Google Scholar]
- 13.Boukerche H, et al. Platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex. Blood. 1989;74(2):658–663. [PubMed] [Google Scholar]
- 14.Kolber DL, Knisely TL, Maione TE. Inhibition of development of murine melanoma lung metastases by systemic administration of recombinant platelet factor 4. J. Natl. Cancer Inst. 1995;87(4):304–309. doi: 10.1093/jnci/87.4.304. [DOI] [PubMed] [Google Scholar]
- 15.Paulus JM. Recent advances in the story of megakaryocyte physiology. Pathol. Biol. 1981;29(3):133–135. [PubMed] [Google Scholar]
- 16.Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92(2):345–344. [PubMed] [Google Scholar]
- 17.Kumari, N., Dwarakanath, B. S., Das, A., Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. (2016). [DOI] [PubMed]
- 18.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer. 2016;138(9):2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 20.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]


