Abstract
IL-18, an immunoregulatory and proinflammatory cytokine, has been shown to play an important pathogenic role in Th1-driven autoimmune disorders. In this study, we evaluated the circulating levels and salivary-gland expression of IL-18 in patients with Sjögren's syndrome (SS), a mainly Th1-mediated disease. IL-18 serum levels were measured by ELISA in 37 patients with primary SS, 42 with rheumatoid arthritis, and 21 normal controls. We demonstrated high IL-18 serum levels in SS, similar to those in rheumatoid arthritis patients and significantly higher than in controls (P < 0.01). In addition, IL-18 serum concentrations were significantly higher in anti-SSA/Ro+ and anti-SSB/La+ than in anti-SSA/Ro- and anti-SSB/La- SS patients (respectively, P = 0.01, P < 0.01). Serum IL-18 correlated strongly with anti-SSA/Ro (P = 0.004) and anti-SSB/La (P = 0.01) titers. Salivary gland IL-18 expression was investigated by single/double immunohistochemistry in 13 patients with primary SS and in 10 with chronic sialoadenitis, used as controls. The expression of IL-18 was also examined in periductal inflammatory foci in relation to the acquisition of features of secondary lymphoid organs such as T–B compartmentalization, formation of follicular dendritic cell networks, and presence of germinal-center-like structures. IL-18 expression in SS salivary glands was detected in 28 of 32 periductal foci of mononuclear cells (87.5%), while no IL-18 production by infiltrating cells was detected in patients with chronic sialoadenitis. Within the inflammatory foci, IL-18 immunoreactivity co-localized almost exclusively with CD68+ macrophages. In addition, IL-18 was found in 15 of 19 foci (78.9%) with no evidence of T–B cell compartmentalization (nonsegregated) but in 100% of the segregated aggregates, both in T- and B-cell-rich areas. Strikingly, IL-18 was strongly expressed by CD68+ tingible body macrophages in germinal-centre-like structures both in SS salivary glands and in normal lymph nodes. IL-18 expression was observed in the ducts of all SS biopsies but in only 4 of 10 patients with nonspecific chronic sialoadenitis (P < 0.01). This study provides the first evidence of increased circulating levels and salivary gland expression of IL-18 in SS, suggesting an important contribution of this cytokine to the modulation of immune inflammatory pathways in this condition.
Keywords: chronic sialoadenitis, germinal centre, interleukin-18, Sjögren's syndrome, tingible body macrophages
Introduction
Sjögren's syndrome (SS) is an autoimmune disease affecting salivary and lacrimal glands, characterized by chronic periductal mononuclear-cell infiltration and local autoantibody production, which lead to architectural destruction of the glands, resulting in the classical clinical signs and symptoms of mouth and eye dryness. A large body of evidence from human studies suggests that the local immune response in SS is mainly Th1-mediated [1-6], although a Th2-mediated process may contribute at different stages of the disease [4,5]. The presence of Th1-related cytokines has been demonstrated in salivary glands from patients with SS both in terms of protein and mRNA expression. Increased levels of IL-1β, IL-6, tumor necrosis factor (TNF)-α, and IFN-γ have been reported in saliva from patients with SS in comparison with controls with histologically normal salivary glands, confirming the role of Th1-cell-mediated tissue damage [2]. However, little is known in SS regarding the molecules acting upstream of the immune-mediated events that lead to the amplification of the inflammatory cascade.
IL-18, although capable of inducing Th2 cytokines in an IL-4 independent manner [7], has been conclusively shown to be a critical regulator of Th1 responses [8]. IL-18 was initially identified as a major inducer of IFN-γ [9] and were shown to be instrumental in Th1 cell induction and activation in the presence of IL-12 [10]. Accordingly, functional IL-18R is expressed on mature Th1 but not Th2 lymphocytes [11]. Furthermore, IL-18 has more recently been shown to be capable of directly inducing expression of proinflammatory cytokines such as TNF-α and IL-1β in mature Th1 cells, macrophages, and natural killer cells [12-14], to up-regulate production of both CC and CXC chemokines [15], to enhance expression of costimulatory molecules such as CD40L and CD86 [16,17], and to induce tissue damage through the induction of cell-mediated cytotoxicity [18-21] and the release of matrix metalloproteinases [22,23].
This wide range of proinflammatory properties renders this cytokine a crucial candidate mediator of chronic inflammation, as demonstrated both in animal models of autoimmunity and human autoimmune diseases [13,24-32]. To date, however, the expression and function of IL-18 in the autoimmune sialoadenitis of SS has not been investigated, aside from a recently reported study [3] in which the mRNA expression of several cytokines (including IL-18) in minor salivary gland biopsies from patients with SS was evaluated. Thus, so far there are no definite reports regarding the expression of IL-18 at protein level in salivary glands of patients with SS. This is of particular relevance, because IL-18 is synthesized as 23-kDa pro-IL-18 and undergoes post-translational modifications, mainly upon cleavage by caspase 1, before it can function as a mature, active, 18-kDa glycoprotein [33]. In addition, there are no data addressing the relationship between IL-18 expression and local or systemic manifestation of SS.
The aims of the present study were, first, to evaluate IL-18 serum concentration in patients with primary SS and its relationship with autoantibody production and clinical parameters of this condition. Second, since SS is mainly a localized disorder, we examined the expression and distribution of IL-18 in salivary glands of SS. Third, we characterized the nature of IL-18-producing cells within the salivary glands. Fourth, we assessed the relationship between IL-18 expression and the histomorphological characteristics of the periductal immune/inflammatory infiltrates. The results provided in this study strongly support a prominent role for IL-18 in the local immune processes in salivary glands of patients with SS.
Materials and methods
Serology
Thirty-seven consecutive patients with primary SS were enrolled in this study (35 females, 2 males; mean age [range] 54.1 years [28–77], mean disease duration [range] 71.2 months [2–360]). Patients were classified as having SS on the basis of the recently revised criteria of the American–European Consensus Group [34]. The presence of other, underlying autoimmune diseases or hepatitis C virus infection was carefully excluded. As negative control population, sera from 21 normal healthy subjects matched for sex and age were studied, while sera from 42 patients with rheumatoid arthritis (RA) classified according to American Rheumatism Association criteria were used as disease controls. From each patient and control a blood sample was taken and sera were stored at -20°C until they were tested. Patients with SS were also analyzed for the presence of extraglandular manifestations such as arthralgia/arthritis, cryoglobulinemia, Raynaud's phenomenon, and hepatic, pulmonary, or renal involvement. Twelve patients with SS had extraglandular manifestations (four arthralgia/arthritis, three Raynaud's phenomenon, two pleuritis, two cutaneous vasculitis, one renal involvement).
Antinuclear antibodies were evaluated by indirect immunofluorescence using Hep2 cells as substrate. Sera were diluted 1:40 before the immunofluorescence assay. Rheumatoid factor was detected using an immunonephelometry test (Behring, Marburg, Germany) as described elsewhere [35].
Anti-SSA/Ro and anti-SSB/La antibodies of IgG isotype were measured by commercial enzyme-linked immunosorbent assay (ELISA) (Diamedix, Miami, FL, USA). Results were expressed in IU in accordance with the manufacturer's instructions, and values above 20 IU were considered positive.
Anti-α-fodrin antibodies of both IgA and IgG isotypes were tested using a commercial ELISA (Aesku.lab Diagnostika, Wendelsheim, Germany). Results were expressed in U/ml, and values above 5 U/ml and 6 U/ml, respectively, were considered positive.
IL-18 serum levels were detected as previously reported [36]. Briefly, an anti-IL-18 monoclonal antibody (R&D Systems, Minneapolis, MN, USA) was used to coat (2 μg/ml in PBS) a polystyrene ELISA plate (Maxisorb), which was then incubated overnight at room temperature (RT). Plates were then blocked for 2 hours at RT with PBS/BSA (1%), sucrose (5%). After a washing with PBS–Tween 20 (0.05%), a solution made of TBS–BSA(0.1%)–Tween 20 (0.05%) was used to dilute standards (rhIL-18, R&D Systems) and sera. After 2 hours of incubation and further washing, a secondary biotinylated antibody (R&D Systems) was added (250 ng/ml) and incubated for 2 hours at RT. After further washing, peroxidase-conjugated streptavidin was added and incubated for 20 min at RT. The reaction was then developed with a solution of tetramethylbenzidine in the presence of H2O2, stopped with 4 N sulfuric acid, and read at 450 nm wavelength.
Immunohistochemistry (IHC)
Tissue samples
Formaldehyde-fixed, paraffin-embedded tissue samples were obtained from minor labial salivary gland biopsies of a smaller subset of 13 patients with histologically proven SS (i.e. with focus score ≥ 1) (11 females, 2 male; mean age [range] 52.1 years [36–67], mean disease duration [range] 70.8 months [24–180], 9 [69%] positive for anti-Ro and/or anti-La, 9 [69%] for rheumatoid factor, and 10 [77%] for antinuclear antibodies) and from 10 patients with nonspecific chronic sialoadenitis as controls. All samples were obtained, after informed consent, during routine diagnostic procedures. Minor salivary gland biopsies from patients with chronic sialoadenitis showed no infiltration or the presence of a diffuse mononuclear infiltration in the absence of focal organization. In most SS patients and controls, it was possible to analyze multiple biopsies taken at the same time. Two samples of parotid gland from SS patients were studied but not considered in the overall evaluation and statistical analysis. Histological evaluation of the salivary glands was performed according to the classification of Chisholm and Mason [37] and a periductal mononuclear-cell aggregate was defined as a focus when at least 50 periductal mononuclear cells with focal organization were counted. The histological evaluation of the number of foci, the presence of IL-18 in inflammatory foci and ducts, the degree of organization of the mononuclear aggregates, and the presence of germinal centers (GCs) were assessed blind by two observers (MB and FB). A periductal inflammatory focus was considered positive for IL-18 when at least three cells were stained within the focus.
Normal human lymph nodes were obtained from patients requiring vascular surgery. Procedures were performed after informed consent approved by the hospital Ethics Committee (LREC no. 99/03/19).
Primary antibodies
Mouse monoclonal anti-human IL-18 (IgG1 clone 2D3B6) was used to detect IL-18 [13]. Monoclonal antibodies directed against CD68 (macrophages) (clone PG-M1; DAKO A/S, Cambridge, UK), CD20 (B lymphocytes) (clone L26; DAKO), CD21 (follicular dendritic cells [FDCs]) (clone 1F8; DAKO), and rabbit polyclonal anti-CD3 (T lymphocytes) (Cod A0452; DAKO) were also used.
Detection of IL-18 expression, identification of IL-18-producing cells, and characterization of the periductal mononuclear-cell infiltrates in salivary glands of patients with SS and controls
For IL-18 detection, formalin-fixed, paraffin-embedded 3-μm sections were dewaxed in xylene, rehydrated through graded alcohol solutions, and washed in TBS, and antigen was retrieved after proteolytic digestion with a solution of 0.1% trypsin in phosphate-buffered saline (PBS), pH 7.3. After three washings in Tris-buffered saline (TBS), sections were incubated at RT for 60 min at RT with anti-IL-18 at the appropriate dilution in TBS containing 0.1% bovine serum albumin (BSA). Sections incubated with an isotype-matched control antibody were used as negative control. After exposure to the primary antibody, the sections were washed three times in TBS and incubated for 30 min at RT with a DAKO Envision alkaline-phosphatase-conjugated polymer. After three washings with TBS, colour reaction was developed using Vector Red (Vector Laboratories, Peterborough UK) and slides were slightly counterstained with Mayer's hematoxylin (Sigma, Poole, Dorset, UK), dehydrated through graded ethanol solutions and xylene, and mounted in DePex (BDH, Poole, Dorset, UK).
In order to identify the cell type expressing IL-18 in the inflammatory infiltrates, double IHC was performed for CD68/IL-18 using the DAKO EnVision Doublestain System. Briefly, for double staining, after antigen unmasking with 0.1% trypsin in PBS, endogenous peroxidase was blocked for 5 min at RT, and primary anti-CD68 antibody appropriately diluted in TBS/0.1% BSA was added. After the sections had been incubated for 1 hour and washed, a horseradish-peroxidase-labelled polymer was added and sections were incubated for 30 min at RT. After further washing, a colour reaction was developed using 3,3'-diaminobenzidine (Sigma) substrate-chromogen until optimal staining was achieved, and the section was blocked with Doublestain Block for 3 min at RT. The sections were rinsed in TBS and stained for anti-IL-18 as described above. To verify the specificity of the staining, sections with omission of the first, the second, or both of the primary antibodies were used as negative controls (see Fig. 5).
Figure 5.
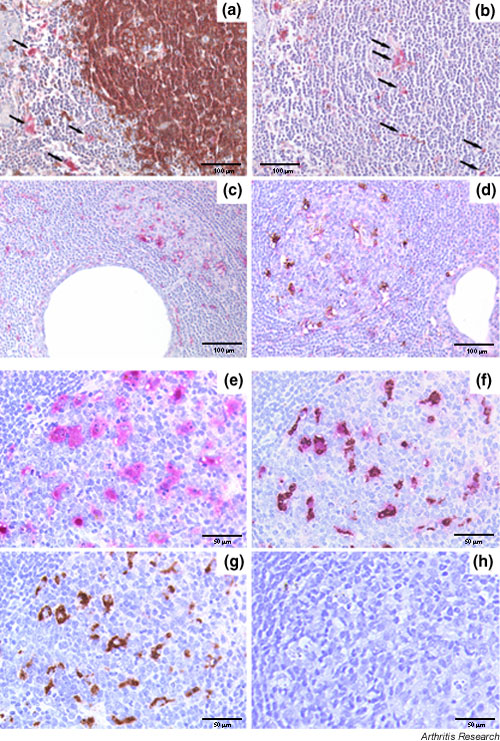
Relationship between IL-18 expression and B-/T-cell compartmentalization (a,b) and germinal-center-like (GC-like) structures (c,d) in the salivary glands of patient with Sjögren's syndrome (SS) and, for comparison, in normal lymph nodes (e–h). Representative section of a large segregated aggregate double-stained for CD20 (brown) and IL-18 (purple) (a), and (b) sequential section with an irrelevant antibody replacing the anti-CD20, demonstrating the presence of IL-18-producing cells both in the T-cell (a, arrows) and B-cell (b, arrows) areas. (c) Single staining for IL-18, demonstrating a large number of IL-18-producing cells within ectopic GC-like structures in salivary gland from SS. (d) Double immunohistochemical staining for CD68 (brown) and IL-18 (purple), demonstrating the exclusive co-localization of IL-18 with CD68 macrophages. (e–h) An identical pattern of distribution in terms of IL-18 expression and co-localization with CD68 macrophages was observed in GCs of secondary lymphoid organs. Histomorphological analysis of the IL-18 positive cells within the GC showed evidence of engulfed apoptotic bodies in the cytoplasm (e) that identifies these cells as tingible body macrophages (TBMs). (f) Double immunohistochemical staining for CD68/IL-18 confirmed the exclusive co-localization of IL-18 with TBMs within the GC. Sequential sections in which the anti-CD68 (e), anti-IL-18 (g), or both the primary antibodies (h) were replaced with an isotype-matched irrelevant antibody confirmed the specificity of the double staining (h, negative control). Original magnification (a–d) × 200, (e-h) × 400
In order to examine the relationship of IL-18 expression and the level of structural organization of the periductal inflammatory foci, samples were analyzed for T and B lymphocytes, the presence of FDCs, and the appearance of GC-like structures. Sections were double-stained for CD3 (T cells) and CD20 (B cells) using the DAKO EnVision Doublestain System and single-stained for FDC (CD21). For CD3/CD20 double staining, we adopted the same protocol described above for CD68–IL-18, with the exception of a different procedure for antigen unmasking, in that sections were heated for 45 min at 95°C in 0.02 Hcitrate buffer (pH 6) before primary antibodies were added. For CD21, we used a standardized protocol using proteolytic digestion as antigen-retrieving method and overnight incubation with the primary antibody at appropriate dilution. On the basis of the CD3, CD20, and CD21 staining, lymphocytic foci were classified either as nonsegregated (when no clear compartmentalization of T and B cells in discrete areas could be recognized) or as segregated (when inflammatory aggregates displayed a well defined organization in separated T- and B-cell-rich areas), with or without ectopic GC-like structures identified on the basis of the histologic appearance and confirmed by the presence of FDC networks, as previously described by Stott and co-workers [38].
Statistical analysis
A two-tailed Mann–Whitney U test was used to compare continuous variables in the different groups. Spearman's rank correlation was performed to correlate IL-18 serum concentration with the titer of serum antibodies and with clinical parameters. A χ2 test with Yates' correction when required or Fisher's exact test when appropriate was used to evaluate associations of qualitative variables in the different groups. P < 0.05 was considered statistically significant.
Results
IL-18 concentration in the serum of patients with SS in comparison with normal and diseased controls, and its relationship with autoantibody production
IL-18 is increased in the systemic circulation of autoimmune diseases such as RA and Crohn's disease, in which the principal site of production of this cytokine has been demonstrated to reside in the inflamed target tissue [13,29,39,40]. IL-18 serum concentrations (mean ± SEM) were significantly higher (379 ± 45 pg/ml) in patients with SS than in normal controls (196 ± 27 pg/ml; P < 0.01) and were comparable to those found in patients with RA (477 ± 86 pg/ml; PNS). As expected, IL-18 serum levels in RA patients were also significantly higher than in the control population (P < 0.05).
To examine the relationship between IL-18 serum levels and autoantibody production, we categorized patients on the basis of anti-SSA/Ro, anti-SSB/La, anti-α-fodrin antibodies, antinuclear antibodies, and rheumatoid factor. Anti-SSA/Ro and anti-SSB/La antibodies were found in 25 (68%) and 17 (46%) of 37 SS patients, respectively. Anti-α-fodrin antibodies of IgA isotype were found in 22 of 37 patients (59%), while 6 patients (16%) expressed anti-α-fodrin IgG. In addition, antinuclear antibodies were detected in 29 SS patients (78%), while rheumatoid factor was present in 27 (73%).
When patients with SS were grouped on the basis of the presence or absence of the various autoantibodies, serum IL-18 was found to be significantly increased in SS patients who were anti-SSA/Ro+ (443 ± 57 pg/ml) and anti-SSB/La+ (497 ± 78 pg/ml) in comparison with anti-SSA/Ro- (245 ± 58 pg/ml; P = 0.01) and anti-SSB/La- (278 ± 41 pg/ml, P < 0.01) patients (Fig. 1b,1c, respectively). Importantly, there was direct correlation between IL-18 serum levels and autoantibody production. Serum IL-18 concentration positively correlated with both anti-SSA/Ro (r = 0.466, P = 0.004) and anti-SSB/La serum titers (r = 0.414, P = 0.01). In contrast, no significant difference was observed in IL-18 serum levels comparing patients with SS with or without anti-α-fodrin IgG or IgA antibodies, antinuclear antibodies, and rheumatoid factor.
Figure 1.
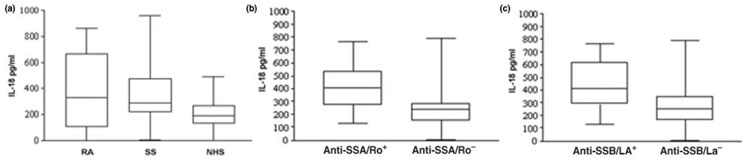
Serum IL-18 concentrations in patients with Sjögren's syndrome (SS) and relationship with the presence of anti-SSA/Ro and anti-SSB/La antibodies. Box–whisker plots showing serum IL-18 concentration in patients with SS compared with patients with rheumatoid arthritis (RA) and normal healthy subjects (NHS) (a), and in patients with SS who are positive or negative for anti-SSA/Ro (b) or anti-SSB/La antibodies (c). See text for statistical analysis.
Finally, we analyzed IL-18 serum concentrations and autoantibody production in relationship with the presence or absence of extraglandular involvement. No significant difference was found in IL-18 serum concentrations or autoantibody levels among these groups.
Tissue distribution of IL-18 expression and identification of IL-18-producing cells in salivary glands of patients with SS and chronic sialoadenitis
IL-18 expression in inflammatory foci
On the basis of the histological evaluation of the salivary glands performed according to the Chisholm and Mason classification [37], we could identify in patients with SS a total of 32 periductal inflammatory aggregates fulfilling focus definition, while no foci were observed in chronic sialoadenitis.
IL-18 expression was detected in periductal mononuclear cells in all 13 SS samples studied, with a total of 28 of 32 inflammatory foci (87.5%). A considerable amount of IL-18-producing cells (mean ± SEM number of positive cells/focus = 9.6 ± 1.4) was found to be distributed scattered within the focal infiltrates. Typical distribution of IL-18 within a periductal focus is shown in Fig. 2a,2b. Moreover, cells expressing IL-18 were frequently observed surrounding acinar structures, but only in close proximity to the inflammatory aggregates (Fig. 2c), with no IL-18 expression in areas devoid of infiltrating cells. No IL-18 production by infiltrating cells within the salivary glands was detected in any patient with chronic sialoadenitis (Fig. 2d,2e,2f).
Figure 2.
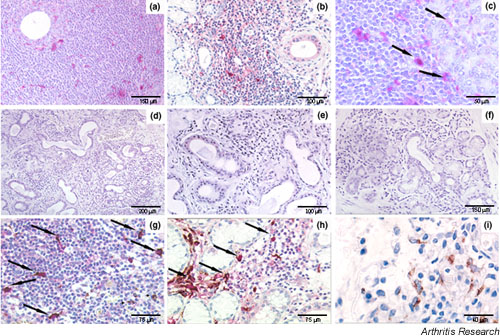
Immunohistochemical (IHC) detection of IL-18 in salivary glands of patients with Sjögren's syndrome (SS) (a–c,g–i) and in nonspecific chronic sialoadenitis (d–f). (a,b) Paraffin-embedded section of glands in SS, showing high amounts of IL-18-expressing cells distributed in a scattered fashion within the periductal mononuclear infiltrate. (c) IL-18-positive cells were also observed surrounding acini (arrows) in proximity with the inflammatory aggregate. (d–f) Paraffin-embedded sections of glands from patients with nonspecific chronic sialoadenitis, demonstrating the absence of IL-18 expression in mononuclear cells in nonfocal periductal infiltrates. (g) Paraffin-embedded sections of glands from patients with SS, double-stained for CD68 (brown) and IL-18 (purple), showed exclusive co-localization of IL-18 expression in most of the CD68+ macrophages (arrows) within the periductal inflammatory infiltrates. (h) Macrophages expressing a large amount of IL-18 (arrows) were also observed surrounding acini in contiguity with a focal lymphocytic aggregate. (i, same sample as g) Conversely, CD68+ macrophages adjacent to a nonfocal infiltrate remained single-stained. Original magnification (a,b,d) × 100, (c,e–i) × 200.
Identification of IL-18-producing cells in inflammatory foci
Morphological analysis demonstrated that infiltrating mononuclear cells expressing IL-18 had abundant cytoplasm and vesicular nuclei, compatible with a monocyte-derived cell lineage (Fig. 2a,2b,2c). Monocyte/macrophage cells have been shown to represent the major source of IL-18 in other chronic inflammatory conditions such as RA and Crohn's disease [13,29]. Using double IHC for IL-18 and CD68, we observed IL-18 exclusively in CD68+ cells adjacent to and within the foci (Fig. 2g,2h). IL-18+/CD68+ macrophages were detected only in the context of lymphocytic infiltration in the periductal foci, while CD68+ macrophages outside the focal infiltrates exhibited no detectable IL-18 (Fig. 2i). Thus, although macrophages are known to produce IL-18 constitutively, the discrete pattern of expression of IL-18 only in macrophages within the periductal infiltrate is suggestive of an inducible phenomenon associated with the microarchitectural organization of periductal aggregates.
Detection of IL-18 in ductal epithelial cells
IL-18 immune staining was detected in epithelial cells of ducts in all SS samples, while in patients with nonspecific chronic sialoadenitis, ductal IL-18 staining was observed only in 4 of 10 patients (P < 0.01). Notably, IL-18 expression in SS ducts showed considerable variability (mean percentage of positive ducts 46.3, range 18–82). Moreover, variable levels of IL-18 expression were detected in different ducts within the same gland (Fig. 3a), as well as among different samples studied. In addition, ductal IL-18 expression was observed both in the presence and in the absence of IL-18-producing cells in the periductal inflammatory focal infiltrate (Figs 2b,3b, respectively). Finally, ductal IL-18 expression was also found in the absence of a focal infiltrate (Fig. 3c) as well as in ducts surrounded by extensive fibrosis (Fig. 3d). Of relevance, in contrast to ductal epithelial cells, no staining for IL-18 was found in acinar cells (Fig. 3a,3d). In a minority of patients with chronic sialoadenitis, we observed similar ductal staining patterns (Fig. 3e,3f), with the exception of a consistently negative periductal infiltration for IL-18.
Figure 3.
IL-18 expression in salivary gland ducts of patients with Sjögren's syndrome (SS) (a–d) and nonspecific chronic sialoadenitis (e,f). IL-18-positive ducts were detected in all the SS samples but in only a minority of those from chronic sialoadenitis. A considerable range of variability of IL-18 expression was observed in ducts among different samples. Within the same glandular lobule, positive and negative (arrowheads) adjacent ducts were observed (a). Ductal IL-18 expression was found in ducts surrounded (b) and not surrounded (c) by focal infiltrate, as well as in ducts characterized by periductal fibrosis (d). In contrast to ductal epithelial cells, no staining for IL-18 was found in acinar cells (a,d, stars). In a minority of patients with chronic sialoadenitis, we observed similar ductal staining patterns. Representative examples of positive (e) and negative (f) ductal IL-18 staining in different patients with chronic sialoadenitis are shown. Original magnification × 200.
Relationship between IL-18 expression and lymphoid organization of inflammatory foci in salivary glands of patients with SS in comparison with secondary lymphoid organs
Expression of IL-18 by CD68+ macrophages only within periductal inflammatory foci rather than diffuse cellular infiltrate suggested that a 'critical mass' might be required for IL-18 expression. Moreover, anti-SSA/Ro and SSB/La autoantibodies are known to be principally produced in the salivary glands with lymphoid-like features [41]. For these reasons, IL-18 expression was analyzed in relationship with the level of lymphoid organization of the foci in terms of T–B cell segregation and the appearance of GC-like structures identified as described in Materials and methods, and foci were classified as nonsegregated and segregated with or without GC-like structures.
Of the 32 periductal inflammatory foci identified in the samples studied, 19 (59.4%) showed predominance of CD3+ lymphocytes mixed with CD20+ cells without clear compartmentalization of the two cell subsets (Fig. 4a). The remaining 13 inflammatory foci (40.6%) showed an increased number of B cells with a variable degree of B-T-cell compartmentalization into discrete areas (Fig. 4b). Furthermore, on the basis of the morphological analysis, confirmed by the presence of FDC network, GCs were detected in 6 of 32 (18.7%) periductal foci (Fig. 4c,4d). In the nonsegregated foci, IL-18 was expressed in 15 of 19 (78.9%), while IL-18-producing cells were detected in 100% of the segregated aggregates, both in T- and B-cell-rich areas (Fig. 5a,5b). IL-18 was also found to be highly expressed in all ectopic GC-like structures (Fig. 5c) in SS salivary glands. Double IHC confirmed that IL-18 was produced by CD68+ tingible body macrophages within the GCs (Fig. 5d). In order to assess whether the production of IL-18 in lymphoid-like structures in SS salivary glands is peculiar to this condition or is a common feature of secondary lymphoid-organ follicles, we performed a comparative IHC analysis in normal lymph nodes. This analysis demonstrated a very similar pattern, with high IL-18 expression in large mononuclear cells within GC with engulfed apoptotic bodies (Fig. 5e). Consistent with this, IL-18 immune reactivity appeared to overlap with CD68+ tingible body macrophages (Fig. 5g). Co-localization of IL-18 and CD68 staining was clearly confirmed by double IHC (Fig. 5f).
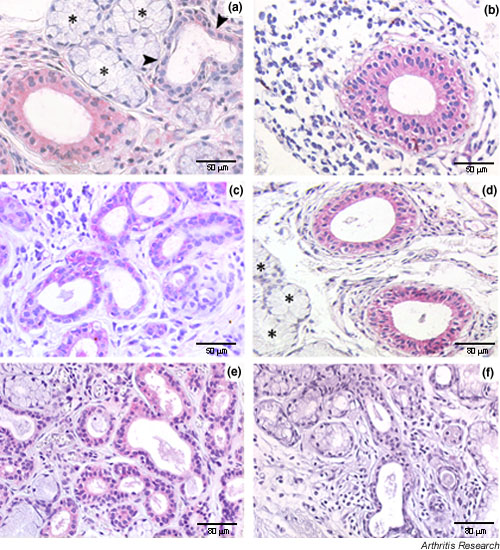
Figure 4.
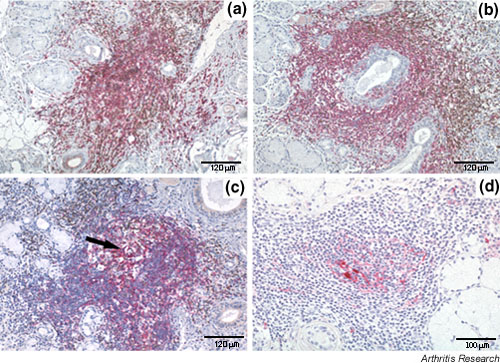
Degree of lymphoid organization of the periductal lymphocytic infiltrates in salivary gland of patients with Sjögren's syndrome (SS). Paraffin-embedded sections were double-stained for CD3 (brown) and CD20 (purple) (a–c) and single-stained with CD21 (d). Inflammatory foci were classified as nonsegregated when T and B lymphocytes were not compartmentalized in distinct areas (a), as segregated in the presence of evident compartmentalization of T and B cells (b), and as segregated with germinal-centre-like structures (arrow) when a clear histological appearance (c) and networks of CD21+ follicular dendritic cells (d) were observed. Original magnification × 200.
Discussion
In this study, we report the first demonstration of increased systemic and local expression of IL-18 in patients with SS. SS is an autoimmune disease characterized by the destruction of epithelial cells in salivary and lacrimal glands, leading to exocrine dysfunction. The histological hallmark of the disease is the presence of a periductal mononuclear-cell infiltrate that can become organized in follicle-like structures. Chronic inflammation within the salivary glands leads to the local production of autoantibodies and cell-mediated mechanisms of tissue damage. Although the presence of Th2 cytokines in salivary glands of patients with SS has been demonstrated [4,5], several lines of evidence suggest that the immune cellular infiltrate in SS is mainly represented by CD4 lymphocytes expressing a Th1 profile. Accordingly, enhanced production of Th1-related cytokines such as IFN-γ, TNF-α, IL-2, and IL-1β has been demonstrated, both by mRNA and protein expression analysis [1-5,42,43]. In particular, it has been observed that CD4+ T cells infiltrating salivary glands from patients with SS produce over 40-fold more IL-2 and IFN-γ mRNA than peripheral blood CD4 T cells isolated from the same patients as well as from salivary glands of normal controls [42]. In addition, IFN-γ mRNA expression from cultured lymphocytes isolated from salivary glands of patients with SS correlates with the degree of lymphocytic infiltration in salivary gland, a finding that indirectly suggests that the increase in lymphocytic infiltration is accompanied by the up-regulation of Th1 cytokines [4]. Despite these observations, factors regulating this Th1 response in SS have not been well characterized.
The crucial role for IL-18 in the development of Th1 immune responses has been established since its identification as a major IFN-γ-inducing factor [9] in cooperation with IL-12 [44]. Furthermore, IL-18 has been demonstrated to exert additional proinflammatory properties such as the ability to directly stimulate the production of TNF-α in macrophages, CD3+/CD4+ cells, and natural killer cells, with subsequent release of IL-1β and IL-8 [13,15]; to up-regulate the expression of both CC and CXC chemokines [12]; and to stimulate adhesion molecule expression in different cell types [45]. The broad range of proinflammatory activities and the demonstrated pathogenic role of IL-18 in other chronic Th1-mediated autoimmune diseases such as RA and Crohn's disease [13,28,29,39] make this cytokine a prime candidate also in the pathogenesis of SS.
Increased serum levels and salivary gland expression of IL-18 in patients with SS is in keeping with an active role for this cytokine in the tissue pathogenesis. IL-18 serum concentrations in patients with primary SS were significantly higher than in normal controls and comparable with those observed in patients with RA, a disease in which elevated IL-18 serum levels have been reported by our group and others [28,36]. Serum IL-18 levels were significantly increased in SS patients with anti-SSA/Ro+ and anti-SSB/La+ antibodies. The strength of this association was further emphasized by the positive correlation of IL-18 serum levels with both anti-SSA/Ro and anti-SSB/La serum titers. The observation that anti-SSA/Ro and anti-SSB/La serum levels have been shown to correlate with the presence of anti-SSA/Ro- and anti-SSB/La-producing cells in the salivary glands [46] induced us to investigate IL-18 expression at this site.
IL-18 protein was strongly expressed in periductal mononuclear cells infiltrating the salivary glands of all SS patients but not in patients with chronic sialoadenitis. Phenotypic analysis demonstrated that in SS samples, IL-18 production within the periductal inflammatory infiltrate was exclusively confined to CD68+ macrophages. Interestingly, the expression of IL-18 by CD68+ macrophages was observed only within periductal inflammatory foci and in periacinar macrophages adjacent to focal infiltrates, and not in isolated macrophages, an observation that suggests an activation state of macrophages. These observations reinforce an otherwise underestimated role of this cell type in the pathogenesis of SS, as also recently suggested [47].
In addition to macrophages, ductal epithelial cells appeared to represent a major source of IL-18 in SS salivary glands. Although the range of ductal IL-18 expression was wide (18–82% positive ducts), expression at some level was detected in all SS patients studied, but interestingly only in a minority of patients with chronic sialoadenitis. No staining for IL-18 was found in acinar cells, indicating that IL-18 production is confined exclusively to ductal epithelial cells. The detection of IL-18 in this cell type is in accordance with the notion that, although classical antigen-presenting cells such as monocytes/macrophages and dendritic cells are regarded as the pivotal source of IL-18 in the regulation of Th1-mediated immune responses [7], nonimmune cell types can also produce IL-18 [13,29,48]. Notably, in salivary gland of SS, ductal IL-18 expression was observed both in the presence and in the absence of IL-18-producing cells in the periductal inflammatory foci and was also found in the absence of a focal infiltrate as well as in ducts surrounded by extensive fibrosis. The lack of association between ductal IL-18 expression and the presence of periductal inflammation in all cases raises the intriguing possibility that the dysregulation of ductal IL-18 expression may become uncoupled from or independent of the level of cellular infiltration. In addition, the demonstration of increased ductal expression of IL-18 in SS would be in keeping with other autoimmune conditions such as Crohn's disease and psoriasis, in which a dysregulation of IL-18 expression in intestinal epithelial cells and skin keratinocytes, respectively, is considered an important component in the development of local chronic inflammation [29,48]. However, whether up-regulation of IL-18 expression in epithelial cells in SS, as well as in other autoimmune conditions, is an initiating event or is acquired later in the course of the inflammatory process is still unknown.
Because of the strong evidence that IL-18 expression was present only within the inflammatory infiltrates showing focal organization, we analyzed the anatomical relationship between the presence of IL-18 within the focus, the main lymphocytic subsets, and the degree of structural organization of the periductal inflammatory aggregates. While we found expression of IL-18 by infiltrating cells in the majority but not all of the nonsegregated foci, a large number of IL-18-producing cells was detected in 100% of the aggregates with well demarcated T–B-cell compartmentalization both in T- and B-cell areas. Thus, the increasing expression of IL-18 in larger and more structured infiltrates would suggest that IL-18 is involved in the amplification of the chronic inflammatory processes leading to the acquisition of a more complex organization of the periductal foci. This possibility is further supported by the observation of prominent IL-18 expression in all the ectopic GC-like structures in salivary glands of patients with SS. IL-18 production within these structures was exclusively co-localized with CD68+ tingible body macrophages. An identical pattern of IL-18 expression was also observed in secondary lymphoid organs of normal individuals. In this regard, a recent study demonstrated that IL-18R is expressed and functional on GC B cells isolated from human tonsil and is up-regulated by IL-12 [49]. To our knowledge, this is the first report of IL-18 production within the GC by tingible body macrophages and suggests an active involvement of tingible body macrophages producing high levels of IL-18 in the regulation of GC reaction.
The relevance of IL-18 expression in B-cell-rich areas and GC-like structures in salivary glands of patients with SS relates to the demonstration that, as mentioned above, serum levels of IL-18 in our SS population were increased in patients positive for anti-SSA/Ro and anti-SSB/La in comparison with patients who were negative for these antibodies, and were closely correlated with the titers of these autoantibodies. Although our study did not address the direct relationship between IL-18 expression in GC-like structures, their functionality, and local production of autoantibodies, it has been reported that anti-SSA/Ro and anti-SSB/La are produced in SS salivary glands [46], and their serum levels correlate with the presence of ectopic GC-like structures [41]. Finally, although a conclusive demonstration of the functionality of ectopic GC-like structures in SS is required, Ig V gene rearrangement analysis has provided evidence of an antigen-driven B-cell response within microdissected GC-like structures in salivary glands of SS patients, suggesting their functionality in generating a local (auto) antibody response [38]. In this regard, further studies will be required to assess the functional role of IL-18 in participating in physiologic and ectopic GC formation and function.
Conclusion
In this study, we provide for the first time evidence of increased serum levels of IL-18 in patients with SS, which correlate with the production of autoantibodies. We also demonstrated that IL-18 is expressed at high levels within the inflammatory foci in salivary glands of patients with SS but not in chronic sialoadenitis and is exclusively produced by CD68+ macrophages within the periductal aggregates. In addition, IL-18 expression in SS salivary glands was particularly associated with inflammatory foci that acquired features of secondary lymphoid organs, with large amounts of IL-18 expressed within ectopic GC-like structures by tingible body macrophages. Similar findings were reproduced in normal lymph node GCs. Finally, we demonstrated that IL-18 was expressed in the salivary gland ducts of all SS patients but in only a minority of patients with nonspecific chronic sialoadenitis. Although further studies are needed to ascertain the direct functional relevance of IL-18 in regulating immune responses in SS, our data suggest an important contribution of this cytokine to the modulation of immune inflammatory pathways in SS.
Competing interest
None declared.
Abbreviations
BSA = bovine serum albumin; ELISA = enzyme-linked immunosorbent assay; FDC = follicular dendritic cell; GC = germinal center; IFN = interferon; IHC = immunohistochemistry/immunohistochemical; IL = interleukin; PBS = phosphate-buffered saline; RA = rheumatoid arthritis; RT = room temperature; SS = Sjögren's syndrome; TBS = Tris-buffered saline; Th1/Th2 = T helper cell type 1/2; TNF = tumor necrosis factor.
Acknowledgments
Acknowledgements
We would like to acknowledge the support of The Arthritis Research Campaign and The Wellcome Trust.
Contributor Information
Michele Bombardieri, Email: pikler@tin.it.
Francesca Barone, Email: frabarone@hotmail.com.
Valerio Pittoni, Email: v.pittoni@libero.it.
Cristiano Alessandri, Email: cristianoalessandri@hotmail.com.
Paola Conigliaro, Email: paolaconigliaro@yahoo.it.
Mark C Blades, Email: mark.blades@kcl.ac.uk.
Roberta Priori, Email: rob.pri@libero.it.
Iain B McInnes, Email: ibmi1w@clinmed.gla.ac.uk.
Guido Valesini, Email: guido.valesini@uniroma1.it.
Costantino Pitzalis, Email: costantino.pitzalis@kcl.ac.uk.
References
- Ajjan RA, McIntosh RS, Waterman EA, Watson PF, Franklin CD, Yeoman CM, Weetman AP. Analysis of the T-cell receptor Valpha repertoire and cytokine gene expression in Sjogren's syndrome. Br J Rheumatol. 1998;37:179–185. doi: 10.1093/rheumatology/37.2.179. [DOI] [PubMed] [Google Scholar]
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- Kolkowski EC, Reth P, Pelusa F, Bosch J, Pujol-Borrell R, Coll J, Jaraquemada D. Th1 predominance and perforin expression in minor salivary glands from patients with primary Sjogren's syndrome. J Autoimmun. 1999;13:155–162. doi: 10.1006/jaut.1999.0289. [DOI] [PubMed] [Google Scholar]
- Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, Fujimura T, Yamada A, Itoh K, Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 1996;39:1376–1384. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- Sun D, Emmert-Buck MR, Fox PC. Differential cytokine mRNA expression in human labial minor salivary glands in primary Sjogren's syndrome. Autoimmunity. 1998;28:125–137. doi: 10.3109/08916939808996281. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/S1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita H, Torigoe K, Okura T, Fukuda S. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/S0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- Xu D, Chan WL, Leung BP, Hunter D, Schulz K, Carter RW, McInnes IB, Robinson JH, Liew FY. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, Joon DY, Reznikov LL, Kim SH, Rubinstein M. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–664. [PubMed] [Google Scholar]
- Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Gracie JA, Leung BP, Wei XQ, Liew FY. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–315. doi: 10.1016/S0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Yagita H, Ortaldo JR, Wiltrout RH, Young HA. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Iwagaki H, Yoshino T, Mori S, Morichika T, Itoh H, Yokoyama M, Kubo S, Kondo E, Akagi T, Tanaka N, Nishibori M. Prostaglandin E(2) inhibits IL-18-induced ICAM-1 and B7.2 expression through EP2/EP4 receptors in human peripheral blood mononuclear cells. J Immunol. 2002;168:4446–4454. doi: 10.4049/jimmunol.168.9.4446. [DOI] [PubMed] [Google Scholar]
- Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell Immunol. 1996;173:230–235. doi: 10.1006/cimm.1996.0272. [DOI] [PubMed] [Google Scholar]
- Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- Dao T, Mehal WZ, Crispe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J Immunol. 1998;161:2217–2222. [PubMed] [Google Scholar]
- Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- Moller B, Kessler U, Rehart S, Kalina U, Ottmann OG, Kaltwasser JP, Hoelzer D, Kukoc-Zivojnov N. Expression of interleukin-18 receptor in fibroblast-like synoviocytes. Arthritis Res. 2002;4:139–144. doi: 10.1186/ar390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171:1009–1015. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000;164:6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, Komai-Koma M, Wei X, Liew FY. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–5347. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- Rothe H, Hibino T, Itoh Y, Kolb H, Martin S. Systemic production of interferon-gamma inducing factor (IGIF) versus local IFN-gamma expression involved in the development of Th1 insulitis in NOD mice. J Autoimmun. 1997;10:251–256. doi: 10.1006/jaut.1997.0135. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Kawashima M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y, Makino H. Interferon-gamma-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum. 2001;44:275–285. doi: 10.1002/1529-0131(200102)44:2<275::AID-ANR44>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- Ishikura T, Kanai T, Uraushihara K, Iiyama R, Makita S, Totsuka T, Yamazaki M, Sawada T, Nakamura T, Miyata T, Kitahora T, Hibi T, Hoshino T, Watanabe M. Interleukin-18 overproduction exacerbates the development of colitis with markedly infiltrated macrophages in interleukin-18 transgenic mice. J Gastroenterol Hepatol. 2003;18:960–969. doi: 10.1046/j.1440-1746.2003.03097.x. [DOI] [PubMed] [Google Scholar]
- Wong CK, Li EK, Ho CY, Lam CW. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2000;39:1078–1081. doi: 10.1093/rheumatology/39.10.1078. [DOI] [PubMed] [Google Scholar]
- Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–3095. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardieri M, Alessandri C, Labbadia G, Iannuccelli C, Carlucci F, Riccieri V, Paoletti V, Valesini G. Role of anti-cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection-associated polyarticular involvement. Arthritis Res Ther. 2004;6:R137–R141. doi: 10.1186/ar1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittoni V, Bombardieri M, Spinelli FR, Scrivo R, Alessandri C, Conti F, Spadaro A, Valesini G. Anti-tumour necrosis factor (TNF) alpha treatment of rheumatoid arthritis (infliximab) selectively down regulates the production of interleukin (IL) 18 but not of IL12 and IL13. Ann Rheum Dis. 2002;61:723–725. doi: 10.1136/ard.61.8.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren's disease. J Clin Pathol. 1968;21:656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller B, Kukoc-Zivojnov N, Kessler U, Rehart S, Kaltwasser JP, Hoelzer D, Kalina U, Ottmann OG. Expression of interleukin-18 and its monokine-directed function in rheumatoid arthritis. Rheumatology (Oxford) 2001;40:302–309. doi: 10.1093/rheumatology/40.3.302. [DOI] [PubMed] [Google Scholar]
- Kanai T, Watanabe M, Okazawa A, Nakamaru K, Okamoto M, Naganuma M, Ishii H, Ikeda M, Kurimoto M, Hibi T. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn's disease. Gastroenterology. 2000;119:1514–1523. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmstrom P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- Fox PC, Grisius MM, Bermudez DK, Sun D. Cytokine mRNA expression in labial salivary glands and cytokine secretion in parotid saliva in Sjogren's syndrome. Adv Exp Med Biol. 1998;438:909–915. doi: 10.1007/978-1-4615-5359-5_129. [DOI] [PubMed] [Google Scholar]
- Koski H, Janin A, Humphreys-Beher MG, Sorsa T, Malmstrom M, Konttinen YT. Tumor necrosis factor-alpha and receptors for it in labial salivary glands in Sjogren's syndrome. Clin Exp Rheumatol. 2001;19:131–137. [PubMed] [Google Scholar]
- Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- Morel JC, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001;276:37069–37075. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren's syndrome. Arthritis Rheum. 1998;41:2238–2248. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bredberg A, Henriksson G, Larsson A, Sallmyr A, Manthorpe R. A role of the macrophage in Sjogren's syndrome? Scand J Rheumatol. 2003;32:255. doi: 10.1080/03009740310003785. [DOI] [PubMed] [Google Scholar]
- Naik SM, Cannon G, Burbach GJ, Singh SR, Swerlick RA, Wilcox JN, Ansel JC, Caughman SW. Human keratinocytes constitutively express interleukin-18 and secrete biologically active interleukin-18 after treatment with pro-inflammatory mediators and dinitrochlorobenzene. J Invest Dermatol. 1999;113:766–772. doi: 10.1046/j.1523-1747.1999.00750.x. [DOI] [PubMed] [Google Scholar]
- Airoldi I, Gri G, Marshall JD, Corcione A, Facchetti P, Guglielmino R, Trinchieri G, Pistoia V. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2000;165:6880–6888. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]


