Abstract
We first identified and isolated cellular subpopulations with characteristics of mesenchymal progenitor cells (MPCs) in osteoarthritic cartilage using fluorescence-activated cell sorting (FACS). Cells from osteoarthritic cartilage were enzymatically isolated and analyzed directly or after culture expansion over several passages by FACS using various combinations of surface markers that have been identified on human MPCs (CD9, CD44, CD54, CD90, CD166). Culture expanded cells combined and the subpopulation derived from initially sorted CD9+, CD90+, CD166+ cells were tested for their osteogenic, adipogenic and chondrogenic potential using established differentiation protocols. The differentiation was analyzed by immunohistochemistry and by RT-PCR for the expression of lineage related marker genes. Using FACS analysis we found that various triple combinations of CD9, CD44, CD54, CD90 and CD166 positive cells within osteoarthritic cartilage account for 2–12% of the total population. After adhesion and cultivation their relative amount was markedly higher, with levels between 24% and 48%. Culture expanded cells combined and the initially sorted CD9/CD90/CD166 triple positive subpopulation had multipotency for chondrogenic, osteogenic and adipogenic differentiation. In conclusion, human osteoarthritic cartilage contains cells with characteristics of MPCs. Their relative enrichment during in vitro cultivation and the ability of cell sorting to obtain more homogeneous populations offer interesting perspectives for future studies on the activation of regenerative processes within osteoarthritic joints.
Keywords: cartilage, mesenchymal progenitor cell, osteoarthritis
Introduction
Mesenchymal progenitor cells (MPCs) from bone marrow are able to differentiate in various types of connective tissue, including cartilage, bone and adipose tissue [1-3]. This led to more precise characterization of these cells by analysis of cell surface markers and differentiation related gene expression [4-9]. In parallel, it was recognized that MPCs not only reside in bone marrow but also in various other connective tissues, such as periost, and adipose and muscle tissue [5,6,10-14]. Cells within the joint that are capable of differentiating into chondrocytes, osteoblasts and adipocytes were recently described in synovia, patellar fat pad and articular cartilage [4,5,15-18].
In the present study we purified progenitor-like cells from the cartilage of human osteoarthritic joints and showed that these cells are capable of proliferation and osteogenic, adipogenic and chondrogenic lineage progression. Those cells could be distinguished from articular chondrocytes by simultaneous staining with several triple combinations of cell surface antigens [4-6]. We used these marker sets for quantification of MPCs by flow cytometric analysis in the original cell population and after in vitro cultivation. Finally, we sorted these cells according to the expression of triplicate surface markers and demonstrated that this subpopulation is capable of osteogenic, adipogenic and chondrogenic differentiation. These findings should provide a basis for identification of MPCs in articular cartilage and for studies of their roles in joint physiology and disease, as well as in induction of regenerative processes within osteoarthritic joints.
Methods
Patient characteristics
Human osteoarthritic cartilage (OC) was obtained during routine surgical procedures with informed consent from seven patients with end-stage osteoarthritis, in accordance with the terms of the Ethics Committee of the University of Ulm. The age of the donors ranged from 55 to 89 years (mean 74 years). The diagnosis was based on clinical and radiological criteria. None of the donors had received corticosteroids or cytostatic drugs during the previous few months. Patients with systemic inflammatory diseases such as rheumatoid arthritis or spondyloarthropathies were excluded.
Cell isolation, expansion and cryopreservation
For cell culture samples, pure cartilage from regions with macroscopically mild-to-moderate osteoarthritic changes was extracted and then subjected to the following: two rinses with phosphate-buffered saline (PBS; Invitrogen, Karlsruhe, Germany) supplemented with antibiotic solution (100 units/ml penicillin, 100 μg/ml streptomycin; Biochrom, Berlin, Germany); fine mincing and digestion with 0.2% pronase (Roche, Mannheim, Germany) for 45 min at 37°C; and two further washes followed by enzymatic digestion overnight at 37°C in 0.025% collagenase (Roche). After filtration through a 40 μm pore membrane, the cells were washed twice in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal calf serum (FCS; Biochrom) and antibiotic solution (100 units/ml penicillin, 100 μg/ml streptomycin), and counted and plated at low density (5 × 104 isolated cells/cm2). DMEM supplemented with 10% FCS was used as a medium during the proliferation phase. The cultures were incubated at 37°C in a humidified 5% carbon dioxide atmosphere, and media were changed three times a week. Cultures were split by trypsin treatment (0.05% trypsin, 0.02% EDTA; Biochrom) at 75% confluence.
Flow cytometry analysis of cells
Either isolated cells from OC were directly used for flow cytometric analysis or cells were used after adherence and cultivation, as described above. Cells were washed twice with PBS containing 1% FCS and 0.02% sodium azide (Sigma, Taufkirchen, Germany). The cells were incubated with 1 μg/106 cells for each mouse anti-human monoclonal antibody that had been directly conjugated to a fluorochrome or biotinylated in the dark for 20 min on ice. The antibodies used are listed in Table 1. After a washing step, second staining for biotin-conjugated monoclonal antibodies was done with streptavidin peridinin chlorophyll protein conjugate in a working titre of 1:100. After 30 min in the dark on ice, cells were washed again twice with PBS buffer before flow cytometric analysis. MPCs were characterized by three-colour immunoflourescence and 2 × 104 cells per sample were analyzed on a Becton Dickinson FACScalibur system using CELLQuest software (Becton Dickinson, Heidelberg, Germany). Dead cells were excluded by propidium iodide (Sigma) staining. Cells were gated on forward and side scatter to exclude debris and cell aggregates. To calculate the percentages of cells staining positive for antigen-specific fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, allophycocyanine-conjugated, or biotin-conjugated monoclonal antibodies, a maximum of 2% positive cells by staining with isotype control antibody was allowed and therefore used to calibrate the channel display by setting the markers. CD133/1 (AC133)-biotin and CD133/2 (AC141)-biotin were obtained from Miltenyi Biotec (Bergisch-Gladbach, Germany). All other antibodies and the isotype controls FITC mouse IgG1κ, R-PE mouse IgG1κ, biotin mouse IgG1κ and biotin mouse IgG2b were provided by Becton Dickinson.
Table 1.
Cell surface markers used for fluorescence activated cell sorting analysis
| CD locus and label | Detection of MPCs | Common name |
| CD9 FITC | Positive | Tetraspan |
| CD44 FITC | Positive | HCAM |
| CD54 PE | Positive | ICAM-1 |
| CD90 biotin | Positive | Thy-1 |
| CD166 PE | Positive | ALCAM |
| CD133 biotin | Negative | AC133 |
| CD45 FITC | Negative | Leukocyte common antigen |
| IgG1 FITC, IgG1 biotin | - | IgG1 isotype control |
| IgG2b PE | - | IgG2b isotype control |
The classification mesenchymal progenitor cell specific surface marker was done in conformity with previous publications [6,30]. ALCAM, activated leukocyte cell adhesion molecule; FITC, fluorescein isothiocyanate; HCAM, homing cell adhesion molecule; ICAM, intercellular adhesion molecule; MPC, mesenchymal progenitor cell; PE, phycoerythrin.
Fluorescence-activated cell sorting
For cell sorting, native isolated cells from OC were stained with saturating concentrations of CD9-FITC, CD90-allophycocyanine and CD166-PE. Single cells were sorted into the flow cytometry tubes (Becton Dickinson) using a Becton-Dickinson FACStarplus cell sorter. OC cells were gated based on forward and side scatter, and the frequencies of CD90+ and CD166+ cells were determined following a second gate on CD9+ cells.
In vitro chondrogenesis assay
Pellet cultures were performed as described previously [15]. Briefly, expanded OC-derived cells and sorted OC cells were released by trypsin treatment, counted and resuspended in 15 ml polypropylene conical tubes at a density of 2 × 105–106, and short spun down at 500 g. The medium was changed to 500 μl DMEM with 10% FCS, 1% antibiotic mix (penicillin/streptomycin), 37.5 μg/ml (100 μmol/l) ascorbate-2 phosphate, and 10-7 mol/l dexamethasone. Pellet cultures were incubated with 10 ng/ml recombinant human transforming growth factor-β3 (Tebu, Offenbach, Germany) during chondrogenesis. All cultures were maintained at 37°C in 5% carbon dioxide, and the medium was changed every third day. After 3 weeks the samples were used for histological and immunohistological studies, and for RT-PCR gene expression analysis.
Histology and immunohistochemistry
The samples were fixed in 4% para-formaldehyde and embedded in paraffin. For histological evaluation, sections were deparaffinized and either stained with haematoxylin or Alcian blue at pH 2.5, with additional Kernechtrot counter-staining, according to standard protocols.
For immunohistochemical analysis of collagen types I and II, and cartilage oligomeric matrix protein (COMP) in chondrogenic differentiated pellet cultures, 3 μm sections were deparaffinized and treated with 1 mg/ml pepsin (Sigma) in 0.5 mol/l acetic acid for collagen type I, with 500 μg/ml proteinase K in Tris-buffered saline (Sigma) for collagen type II, and 1 mg/ml hyaluronidase (Sigma) and proteinase K (500 μg/ml in Tris-buffered saline) for COMP at room temperature for different times to facilitate antibody access. Endogenous peroxidase was blocked by 3% H2O2. The slides were incubated for 30 min in blocking reagent in order to prevent nonspecific binding. Sections were then incubated overnight at 4°C with primary antibodies. Rabbit anti-human polyclonal antibodies against collagen type I (DPC Biermann, Bad Nauheim, Germany), collagen type II (DPC Biermann), and COMP (kindly provided by Dr F Zaucke and Professor M Paulsson, Institute for Biochemistry II, University of Köln, Köln, Germany) were used. The antibody directed against collagen type I was diluted 1:1000, the antibody against collagen type II was diluted 1:400, and the antibody against COMP was used at a 1:300 dilution in 1% bovine serum albumin in PBS. Biotinylated anti-mouse, anti-rabbit secondary antibodies were used for 30 min incubation followed by streptavidin treatment (30 min). Finally, sections were stained using the AEC kit (DAKO, Hamburg, Germany), in accordance with the manufacturer's instructions. Nuclei were counterstained with haematoxylin.
In vitro adipogenesis assay
For adipogenic differentiation, 1 × 105 cells were washed and plated in six-well plates (Becton Dickinson). Adipogenic differentiation was induced with 1 μmol/l dexamethasone, 1 μg/ml insulin, 0.5 mmol/l isobutyl-methylxanthine and 100 μmol/l indomethacin. Stimulation was carried out for 2 weeks with the media changed every 3–4 days and supplements added fresh to each culture. Differentiation was confirmed by RT-PCR gene expression analysis.
In vitro osteogenesis assay
After trypsin treatment 2 × 104 cells were washed in DMEM with 10% FCS, and cultured in six-well plates (Becton Dickinson). Medium for osteogenic differentiation containing DMEM with 0.1 μmol/l dexamethasone, 10 mmol/l β-glycerophosphate and 50 μg/ml ascorbic acid was changed every third day, as described previously [19]. Osteogenic differentiation was confirmed by RT-PCR gene expression analysis.
Reverse transcription polymerase chain reaction and analysis of gene expression
Total RNA was isolated from fresh OC, which was cut in the operating room into small pieces, immediately frozen in liquid nitrogen and stored at -80°C. For RNA extraction of native tissue, OC samples were homogenized using a Dismembrator (Braun Biotech, Melsungen, Germany). Both OC and cultivated cells were lysed in 600 μl lysis buffer with 6 μl mercaptoethanol, by using the RNeasy® system and reverse transcription was done with Omniscript™ RT Kit (all Qiagen, Hilden, Germany), in accordance with the manufacturer's instructions.
PCR reactions were performed using a Robocycler® (Stratagene, Amsterdam, The Netherlands) using HotStarTaq™ Master Mix Kit (Qiagen). PCR was performed under linear conditions using the following cycle profile: initial incubation (15 min at 95°C); followed by 30 cycles of annealing (45 s at 60°C), extension (45 s at 72°C) and denaturation (60 s at 94°C); and terminating with 15 min at 72°C. PCR products were separated on a 1.5% agarose gel and stained with ethidium bromide, visualized and digitalized with an ImageMaster VDS system (Amersham Biosciences, Freiburg, Germany). The primer sequences are shown in Table 2.
Table 2.
Polymerase chain reaction primers
| Target | Primers |
| AP | 5'-ACC TCG TTG ACA CCT GGA AG-3' |
| 5'-CCA CCA TCT CGG AGA GTG AC-3' | |
| BSP | 5'-TGC ATT GGC TCC AGT GAC ACT-3' |
| 5'-TGC TCA GCA TTT TGG GAA T-3' | |
| Col1 | 5'-TAA CTT CTG GAC TAT TTG CGG ACT TTT GG-3' |
| 5'-CAA CCT CAG CCC ATT GGC GCT G-3' | |
| GAPDH | 5'-CGG AGT CAA CGG ATT TGG TCG TAT-3' |
| 5'-AGC CTT CTC CAT GGT TGG TGA AGA C-3' | |
| OCN | 5'-CTG GCC CTG ACT GCA TTC TGC-3' |
| 5'-AAC GGT GGT GCC ATA GAT GCG-3' |
For primer design, Primer3 was used (Rozen S, Skaletsky HJ [1998; available at http://www-genome.wi.mit.edu/genome_software/other/primer3.html]), with published DNA sequences from GenBank (NCBI). Used parameters: product size 180–600 base pairs, annealing temperature 60°C, and primer length 18–30 base-pairs. AP, alkaline phosphatase; BSP, bone sialoprotein; Col1, collagen type I; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OCN, osteocalcin.
Results
Flow cytometric analysis of mesenchymal progenitor cells from human osteoarthritic cartilage
No single surface marker protein has yet been found to characterize MPCs. From the accepted markers, we chose seven different cell surface markers and used them in triple combinations for fluorescence-activated cell sorting (FACS) analysis. Immediately after overnight isolation, chondrocytes from OC were directly stained with seven triple combinations of CD9, CD44, CD54, CD90 and CD166 as positive markers, and CD45, CD133/-1 and -2 as negative markers to eliminate haematopoietic and endothelial cells (Table 2). The expression of progenitor typical markers varied from nearly no detectable staining to relatively high levels of expression. As shown in Fig. 1, in the forward/side scatter the fresh isolated OC cells were a heterogeneous population. The majority of the cells stained negative for CD9, CD90 and CD166. The proportion of CD9+/CD90+/CD166+ triple positive cells was only about 5%.
Figure 1.
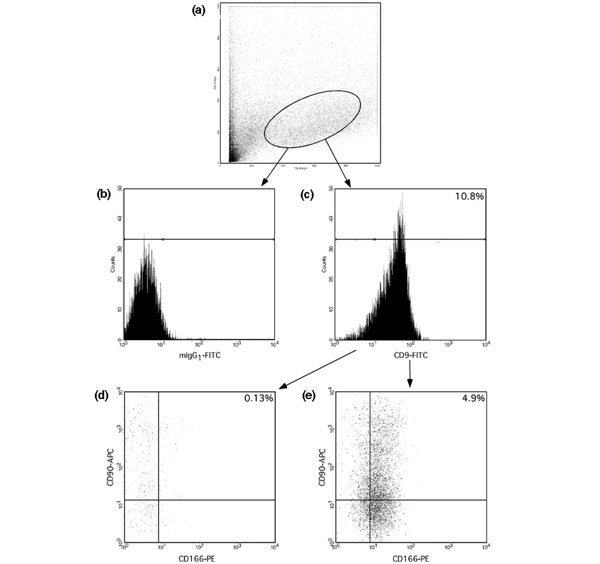
Fluorescence activated cell sorting analysis of fresh isolated chondrocytes from osteoarthritic cartilage. (a) Forward/side scatter. (b) Markers were set in the channel display with a maximum of 2% positive cells by staining with isotype control antibody fluorescein isothiocyanate (FITC)-conjugated mouse IgG1. (c-e) Triple staining experiments for CD9-FITC/CD90-allophycocyanine (APC)/CD166- phycoerythrin (PE). Panel c shows a histogram of FL1 CD9-FITC. Based on isotype and histogram, cells were divided into positive or negative: panel d, CD9-, double-stained CD90-APC/CD166-PE; and panel e, CD9+, double-stained CD90-APC/CD166-PE.
CD9+/CD166+ cells could be subdivided in two equivalent populations comprising about 8% of total cells that were either positive or negative for CD90. We analyzed CD9- but CD90+/CD166+, CD90-/CD166+ and CD90-/CD166- cells, and found that these groups comprised 23.0%, 29.7% and 33.7% of cells, respectively. No CD90+/CD9-/CD166- cells were detectable.
The isotype control antibody revealed no specific staining. The distribution of OC cells in forward/side scatter exhibited no difference between isotype and antibody staining. A maximum of 2% positive cells by staining with isotype antibody mouse IG1 or IG2 conjugated with FITC, PE or biotin was allowed and therefore was used to set the markers within the channel display. The distinction to negative assessed cells is presented in Fig. 1 by showing an exemplary FITC-isotype antibody mIgG1 staining.
Comparing total quantities of triple positive cells from OA cartilage (Fig. 2), CD9+/CD44+/CD166+ and CD9+/CD54+/CD90+ cells were detected in (mean ± standard deviation) 12.2 ± 10% and 13.3 ± 5.7%, respectively (n = 8). The frequencies of CD9+/CD90+/CD166+ and CD9+/CD44+/CD54+ cells were 8.2 ± 10.4% and 2.5 ± 1.8%, respectively.
Figure 2.
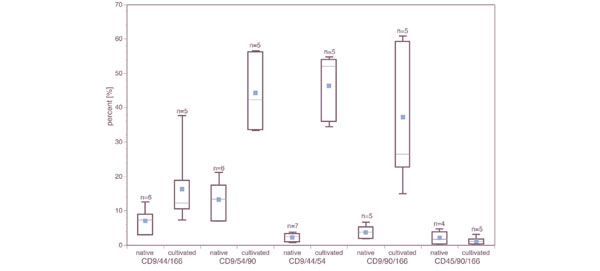
Flow cytometric analysis of combinations of progenitor markers on freshly isolated and culture expanded chondrocytes from osteoarthritic cartilage. The label 'native' represents the fluorescence-activated cell sorting analysis after tissue digestion, whereas 'cultivated' indicates the analysis after culture expansion. The number of patients used for every analysis is indicated above every box plot. The dot presents the arithmetic mean of all the data in the category.
The combinations CD45+/CD90+/CD166+ and CD9+/CD133(1 or 2)+/CD166+ exhibited less than 1% staining. MPC cultures isolated from bone marrow from different donors served as controls. In these samples 95–98% of all gated cells were triple positive for various combinations of the markers CD9/CD54/CD90/CD166 (data not shown).
Analysis of chondrocytes after adherence and cultivation
The change in cellular morphology and the acquisition of a fibroblastic shape became increasingly apparent as the cells were cultured on plastic. In the primary culture, nonadherent or few loosely adherent small round cells were also present, but these disappeared from the first to the second passage. After an initial lag time of 2–3 days, cells entered a proliferative phase, reaching confluence within 48 hours. An average of one doubling every 3 days was observed upon subsequent passages.
In order to determine the percentage of culture expanded progenitor cells that express antigens recognized by CD9/CD44/CD54/CD90/CD166 monoclonal antibodies, the number of immunoreactive cells was quantified by flow cytometry. Data from adherent, culture expanded cells were collected and the number of triple-positive events was expressed as a percentage of the total cell number. An example for the distribution of cultured OC cells is shown in Fig. 3. Compared with fresh isolated OC cells, as seen in Fig. 1, the cultured cells are a homogeneous population on forward/side scatter.
Figure 3.
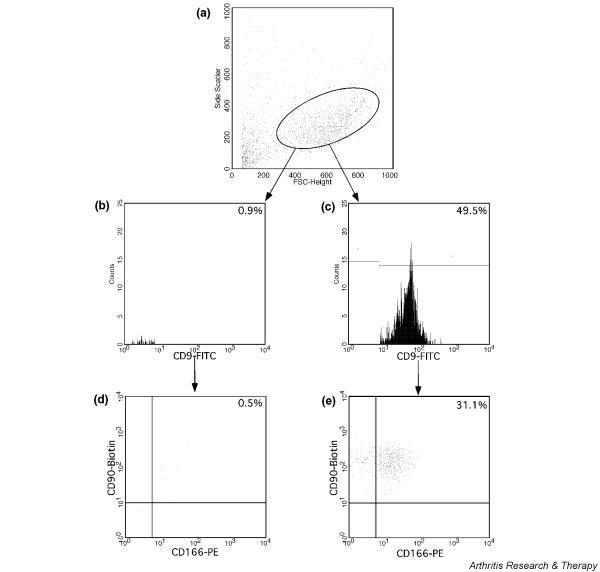
Fluorescence-activated cell sorting analysis of culture expanded chondrocytes. (a) Forward and side scatter (FCS/SSC) of cultured cells. Histograms of CD9-fluorescein isothiocyanate (FITC) (b) negative and (c) positive stained cells. Dot plots show the expression of triple stained cells: CD9-FITC gated (d) negative and (e) positive double-stained CD90-Biotin/CD166-phycoerythrin (PE).
In these experiments, the mean frequency of every triple positive staining of cultured OC cells increased markedly compared with native OC cells (Fig. 2). The total frequency of the CD9+/CD90+/CD166+ population rose 4.1-fold compared with freshly isolated OC chondrocytes. After cultivation in monolayer, we found 18.8-fold more CD9+/CD44+/CD54+ cells than among native, uncultured OC cells. Analysis of the CD9+/CD44+/CD166+ subpopulation revealed only 1.6-fold expansion, whereas CD9+/CD54+/CD90+ cells expanded 3.4-fold. Within the in vitro expanded cells, CD45+ was still not detectable, whereas the total frequency of CD45-/CD90+/CD166+ population was around 29–45%. These results indicate that cultivation enriches a subpopulation of OC cells that express cell surface markers for MPCs.
Sorting and cultivation of progenitor marker positive, fresh isolated osteoarthritic cartilage cells
Findings in fresh isolated chondrocytes suggested that there is a subpopulation in OC that expresses progenitor-associated markers and is capable of osteogenic and chondrogenic differentiation. If a common progenitor cell exists, then it should be found among cells with a CD9+, CD90+ and CD166+ phenotype. Based on phenotypic analysis by FACS, we therefore isolated CD9+/CD90+/CD166+ OC cells from five patients and analyzed the kinetics of cultivation and the potential for differentiation. The data analysis after sorting is exemplarily shown for one patient in Fig. 4. The gates used for cell sorting are shown in Fig. 4.
Figure 4.
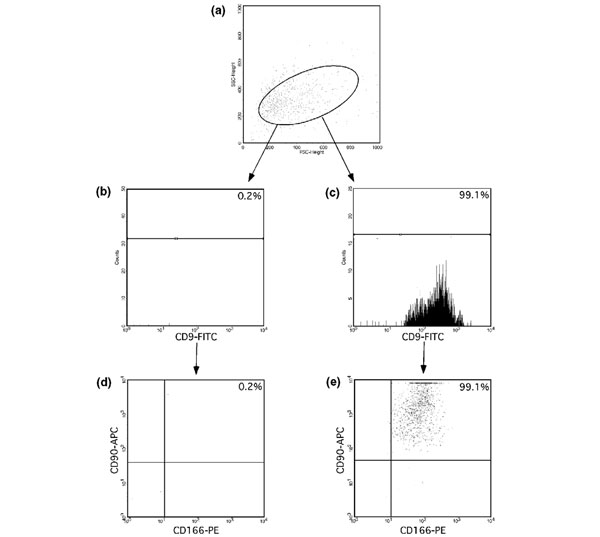
Reanalysis of triple positive sorted cells. (a) Forward and side scatter characteristics of sorted osteoarthritic cartilage cells. (b-d) CD9-fluorescein isothiocyanate (FITC)/CD166-phycoerythrin (PE), CD9-FITC/CD90-allophycocyanine (APC) or CD90-APC/CD166-PE double positive cells and the fluorescence gate used for sorting. Triple staining: (e) CD9+/CD90+/CD166+ and (d) CD9-/CD90+/CD166+.
In these experiments, the mean frequency of the native CD9+/CD90+/CD166+ population was 32%. Figure 4a shows the forward and side scatter characteristics of sorted OC cells. To confirm the quality of sorting, reanalysis of triple positive sorted cells was performed, and it was found that 99.1% were again triple positive (Fig. 4e). Serial observations of each culture well that contained triple positive cells were performed after 3, 7, 14, 21 and 28 days. The earliest point at which the growth of triple positive sorted could be detected was after 3 days. Between 7 and 14 days of culture, adherent, fibroblast-like cells scattered in a random pattern across the surface of the culture well. For 21 days of culture, continuous growth of the adherent, fibroblastic cells was observed.
Differentiation of the cultured sorted cells was determined by RT-PCR, histochemistry and immunohistochemistry. The findings confirmed that the CD9/CD90/CD166 triple positive cell population derived from OC was capable of multipotent mesenchymal differentiation.
Osteogenesis, adipogenesis and chondrogenesis of culture expanded osteoarthritic cartilage cells
To study the possible multilineage capacity of some OC derived cells, we differentiated these cell cultures toward the osteogenic, adipogenic and chondrogenic lineages.
Pellet cultures of OC derived cells resulted in the formation of dense nodules consistent with chondrogenic differentiation. These nodules were associated with an Alcian Blue-positive extracellular matrix, which indicates the presence of sulphated proteoglycans within the matrix (Fig. 5).
Figure 5.
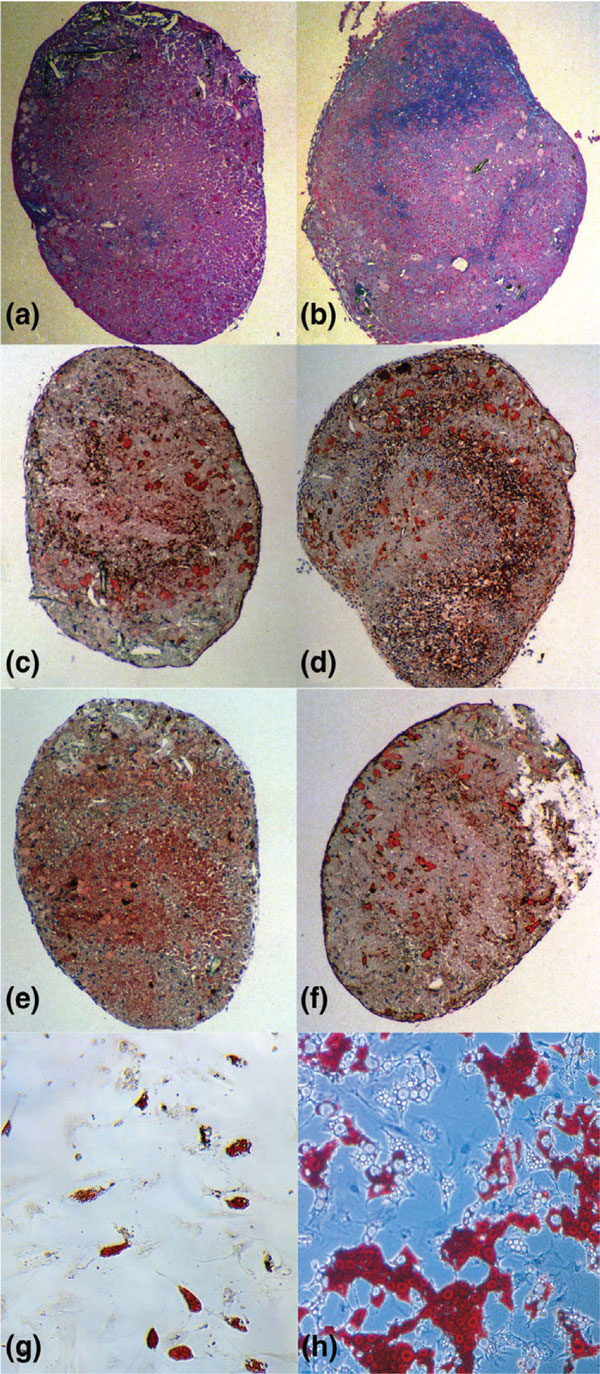
Chondrogenesis and adipogenesis of culture expanded and progenitor marker sorted osteoarthritic cartilage derived cells. Culture expanded cells were stained as follows: (a) Alzian blue, (c) collagen type II, (e) cartilage oligomeric matrix protein (COMP) and (g) oil-red. The marker sorted cells are shown in (b) Alzian blue, (d) collagen type II, (f) COMP and (h) oil-red.
Cartilaginous nodules were also observed upon pellet cultures of bone marrow derived MPCs. In addition to the presence of sulphated proteoglycans within the extracellular matrix, transforming growth factor-β3 supplemented OC-derived cells expressed collagen type II and COMP in pellet culture (Fig. 5a,5b,5c,5d,5e,5f). Overall, these results indicate that a subpopulation of OC-derived cells has the capacity to differentiate toward the chondrogenic lineage. To determine whether OC cells undergo adipogenesis, cells were cultured in medium containing dexamethasone, isobutyl-methylxanthine and indomethacin. About 10–30% of the OC cells were reproducibly induced toward the adipogenic lineage as early as 2 weeks after induction (Fig. 5g,5h). Using PCR, the expression of peroxisome proliferator-activated receptor-γ demonstrated adipogenic differentiation by progenitor marker sorted and culture-derived OC cells (data not shown).
Differentiation of OC derived cells into osteoblasts was induced in vitro by treating the cells with ascorbic acid, β-glycerophosphate and dexamethasone [2,20,21]. OC derived cells and bone marrow MPCs formed an extensive network of dense, multilayered nodules that stained positive for alkaline phosphatase.
After 14 days of differentiation, the gene expression profile of osteoblast markers was investigated. For OC cells and bone marrow MPCs we detected a strong signal for expression of genes for all tested osteogenic markers (Fig. 6): alkaline phosphatase, bone sialoprotein and osteocalcin.
Figure 6.
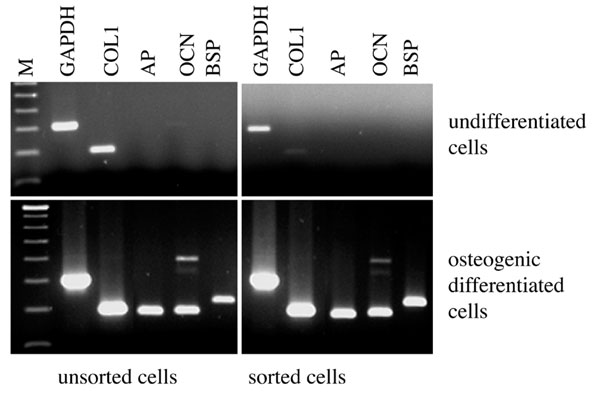
Polymerase chain reaction analysis of osteogenesis of culture expanded and progenitor marker sorted osteoarthritic cartilage derived chondrocytes. AP, alkaline phosphatase; BSP, bone sialoprotein; COL1, collagen type I; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; M, 100 base-pair size marker; OCN, osteocalcin.
Discussion
We could show that a defined population of MPCs resides within OC of knee joints. Although these were present only at a low percentage in native tissue, their relative amount increased markedly during cell cultivation, indicating that this subpopulation possibly could be targeted in vivo for novel tissue regeneration strategies.
In parallel to our findings in joints of osteoarthritic patients with mean age 74 years, Barbero and coworkers [18] described the plasticity of clonal populations of dedifferentiated human articular chondrocytes from much younger probands (mean age 30 years) without degenerative joint disease. This argues against the suggestion that development of a plastic phenotype is solely dependent on the presence or duration of disease. Using a clonal assay, Barbero and coworkers found that about 10% of freshly isolated cells had the capacity to differentiate toward chondrogenic, osteogenic and adipogenic lineages. This is in good agreement with our findings based on the quantification by FACS analysis using triple staining of MPC related cell surface markers. In addition, Jones and coworkers [22] recently identified cells with characteristics of MPCs in synovial fluid of osteoarthritic patients using FACS analysis and proved their potential to differentiate.
Apart from their functionality, there are several reports on phenotypic characterization of human bone marrow MPCs by expression of surface markers [4,8,23,24]. MPCs express a large number of adhesion molecules such as activated leukocyte cell adhesion molecule (CD166), the hyaluronate receptor (CD44) and intercellular adhesion molecule-1 (CD54), growth factor and cytokine receptors, integrins and additional markers such as tetraspan (CD9) and Thy-1 (CD90). In contrast, CD34 and the leukocyte common antigen CD45, which represent the haematopoietic lineage, are not expressed by ex vivo culture expanded MPCs. The early haematopoietic lineage is also characterized by the expression of CD133 [4,25-27]. Despite expanding knowledge on the biology of MPCs, until now it was not possible to characterize these cells using a single marker. Based on the expression of surface markers, the progenitor nature of a certain percentage of OC cells was suggested in our experiments by positive reactivity to a combination of established markers. For FACS analysis and cell sorting, we used various triple combinations of the markers CD9, CD44, CD54, CD90 and CD166. The amount of total triple positive cells varied to some extent between the selected combinations, indicating that the subpopulations – although exhibiting considerable overlap – are not absolutely identical. The expression of triple combinations of MPC typical surface markers, combined with the plasticity of differentiation, as was shown for the CD9+/CD90+/CD166+ subpopulation, indicates that the progenitor cells identified in OA cartilage have marked similarities to bone marrow MPCs. However, as a total population, unlike bone marrow MPCs, cartilage derived cells could not form bone in an in vivo osteochondrogenic assay [17]. This may be related, at least in part, to cellular heterogeneity and a lower percentage of pluripotent cells. The potency of MPC marker sorted, cartilage derived cells in such in vivo assays clearly deserves further investigation.
The observed increase in the relative percentage of triple positive cells after cultivation indicates that the subpopulation of progenitors from OC retained high proliferative capacity. It has been reported that bone marrow MPCs from patients with osteoarthritis have reduced adipogenic and chondrogenic differentiation potential [28]. Our findings concerning the differentiation of CD9+/CD90+/CD166+ sorted cells indicate that corresponding progenitor cells derived directly from the affected tissue at least have retained the potential to enter these lineages. Therefore, a certain cellular 'regenerative potential' is still present in OC. Future studies will have to address the important question of whether this capacity is not adequately used during early stages of degenerative joint disease or simply not sufficiently to cover demands.
The evolving concept that cartilage may have an intrinsic capacity for regeneration challenges a long-lasting paradigm. However, it has already been mentioned that chondrocytes may have several options in responding to injury, including recapitulation of development such as expression of procollagen type IIA [29]. Possibly, the activation of MPCs may contribute to the observed expression of this alternative splice variant. A misguiding of repair attempts may also lead to either enhanced terminal chondrogenic (collagen type X expression) or incomplete osteogenic differentiation, as is observed in OC.
Our observations of an enrichment of subpopulations with characteristics of MPCs during in vitro cultivation and proliferation also shed new light on the cell biological basis of chondrocyte transplantation. It may be assumed that the cell population derived from intact cartilage, which also contains a certain amount of progenitor cells [18], also increases in their relative percentage. This could have a profound influence on differentiation potential. Therefore, further studies on the proliferation and (re)differentiation potential of distinct subpopulations are necessary to improve further the functional quality of the cell populations used for transplantation. The methods of FACS analysis and cell sorting offer important approaches for quality control and application of cell populations with greater purity.
Conclusion
In conclusion, there is increasing evidence for cellular heterogeneity of cartilage derived cells in health and disease. The spectrum of cellular phenotypes contains a low percentage of progenitor cells that are bipotential or tripotential for mesenchymal lineage progression. The techniques of fluorescence automated flow cytometry and cell sorting using typical combinations of cell surface markers for MPCs offer powerful tools with which to study the biological properties of defined subpopulations and their involvement in disease or repair processes. Of course, additional studies are necessary to identify the most suitable combinations of cell surface markers for distinct subpopulations from early to late developmental stages. This knowledge may provide a basis to improve current approaches of cell based therapies for cartilage defects and to develop new strategies for guided tissue regeneration.
Competing interests
None declared.
Abbreviations
COMP = cartilage oligomeric matrix protein; DMEM = Dulbecco's modified Eagle's medium; FACS = fluorescence-activated cell sorting; FCS = fetal calf serum; FITC = fluorescein isothiocyanate; MPC = mesenchymal progenitor cell; OC = osteoarthritic cartilage; PBS = phosphate-buffered saline; PE = phycoerythrin; RT-PCR = reverse transcription polymerase chain reaction.
Acknowledgments
Acknowledgement
This study was founded by a grant of the IZKF, Ulm, Projekt B9.
Contributor Information
Jörg Fiedler, Email: joerg.fiedler@medizin.uni-ulm.de.
Rolf E Brenner, Email: rolf.brenner@medizin.uni-ulm.de.
References
- Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/S0301-472X(00)00482-3. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Shur I, Marom R, Lokiec F, Socher R, Benayahu D. Identification of cultured progenitor cells from human marrow stroma. J Cell Biochem. 2002;87:51–57. doi: 10.1002/jcb.10267. [DOI] [PubMed] [Google Scholar]
- Guo XM, Wang CY, Wang YH, Duan CM, Zhao Q, Sun DM. Experimental study of the isolation, culture and in chondrogenic differentiation of human bone mesenchymal stem cell [in Chinese] Zhonghua Kou Qiang Yi Xue Za Zhi. 2003;38:63–66. [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC., Jr Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang QY, Ma L, Ma GJ, Jiang XY, Zhao CH. Identification and isolation of mesenchymal stem cells from human fetal pancreas [in Chinese] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002;24:45–49. [PubMed] [Google Scholar]
- Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, Noda M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287:289–300. doi: 10.1016/S0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Fickert S, Fiedler J, Brenner RE. Identification, quantification and isolation of mesenchymal progenitor cells from osteoarthritic synovium by fluorescence automated cell sorting. Osteoarthritis Cartilage. 2003;11:790–800. doi: 10.1016/S1063-4584(03)00167-5. [DOI] [PubMed] [Google Scholar]
- Imabayashi H, Mori T, Gojo S, Kiyono T, Sugiyama T, Irie R, Isogai T, Hata J, Toyama Y, Umezawa A. Redifferentiation of dedifferentiated chondrocytes and chondrogenesis of human bone marrow stromal cells via chondrosphere formation with expression profiling by large-scale cDNA analysis. Exp Cell Res. 2003;288:35–50. doi: 10.1016/S0014-4827(03)00130-7. [DOI] [PubMed] [Google Scholar]
- Tallheden T, Dennis JE, Lennon DP, Sjogren-Jansson E, Caplan AI, Lindahl A. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;Suppl 2:93–100. doi: 10.2106/00004623-200300002-00012. [DOI] [PubMed] [Google Scholar]
- Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/en.134.1.277. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1159/000068710. [DOI] [PubMed] [Google Scholar]
- Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–233. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- Kuci S, Wessels JT, Buhring HJ, Schilbach K, Schumm M, Seitz G, Loffler J, Bader P, Schlegel PG, Niethammer D, Handgretinger R. Identification of a novel class of human adherent CD34- stem cells that give rise to SCID-repopulating cells. Blood. 2003;101:869–876. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- Handgretinger R, Gordon PR, Leimig T, Chen X, Buhring HJ, Niethammer D, Kuci S. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci. 2003;996:141–151. doi: 10.1111/j.1749-6632.2003.tb03242.x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]


