Abstract
The pathogenesis of chronic inflammatory joint diseases such as adult and juvenile rheumatoid arthritis and Lyme arthritis is still poorly understood. Central to the various hypotheses in this respect is the notable involvement of T and B cells. Here we develop the premise that the nominal antigen-independent, polyclonal activation of preactivated T cells via Toll-like receptor (TLR)-2 has a pivotal role in the initiation and perpetuation of pathogen-induced chronic inflammatory joint disease. We support this with the following evidence. Both naive and effector T cells express TLR-2. A prototypic lipoprotein, Lip-OspA, from the etiological agent of Lyme disease, namely Borrelia burgdorferi, but not its delipidated form or lipopolysaccharide, was able to provide direct antigen-nonspecific co-stimulatory signals to both antigen-sensitized naive T cells and cytotoxic T lymphocyte (CTL) lines via TLR-2. Lip-OspA induced the proliferation and interferon (IFN)-γ secretion of purified, anti-CD3-sensitized, naive T cells from C57BL/6 mice but not from TLR-2-deficient mice. Induction of proliferation and IFN-γ secretion of CTL lines by Lip-OspA was independent of T cell receptor (TCR) engagement but was considerably enhanced after suboptimal TCR activation and was inhibitable by monoclonal antibodies against TLR-2.
Keywords: co-stimulation, lipoproteins, rheumatoid arthritis, T lymphocytes, Toll-like receptor
Introduction
Chronic inflammatory joint diseases (CIJDs) such as adult and juvenile rheumatoid arthritis and Lyme arthritis were first considered to be diseases caused and perpetuated by autoimmune processes, including the production of autoantibodies, immune complexes and/or autoreactive T cells [1,2]. Recently, T cells have attracted most attention, and their activities, together with an autonomous role for the synovial lining cells, are now thought to be responsible for initiating and sustaining the inflammation. The re-emergence of the notion that cells of the innate immune system are essential in generating and perpetuating an immune response has focused attention on the involvement of these cells in chronic inflammatory disorders too [3].
The question of how the immunopathological processes are set off remains controversial. One leading cause seems to be microbial infection [3,4]. Microbes are recognized not only by T and B cells of the adaptive immune system with their highly specific, monospecific receptors, but also by other cell types that use germline-encoded receptors to interact with microbes. For instance, conserved structural features of molecular determinants on pathogens, termed pathogen-associated molecular patterns, such as lipopolysaccharide (LPS), flagellin, peptidoglycans, microbial DNA and bacterial lipoproteins, are recognized by a set of germline-encoded receptors on host cells, the Toll-like receptor (TLR) family [5-8]. These TLRs are crucial in sensing infections, in the induction of antimicrobial genes and for the control of innate and adaptive immunity [7]. Recent observations have shown that TLRs are expressed not only by cells of the innate immune system but also by cells of the adaptive immune system, including B cells and T cells [9,10]. Ligands for TLRs are found in rheumatoid synovium [11] and are involved in the pathogenesis and severity of inflammatory arthritis [12,13].
T cells of multiple specificities, including self-specificities, are a frequent finding in inflammatory joint diseases such as Lyme arthritis and rheumatoid arthritis [14-17]. At present, two mechanisms by which individual microbes induce disease-promoting T cells are in vogue: one is antigen-specific, the other antigen-nonspecific [18].
Antigen-specific activation, termed epitope mimicry, predicts that during infection T cells are activated that recognize both a microbial antigen and a related self peptide, with the consequence that these T cells would eventually crossreact with host tissue and result in its destruction. The antigen-nonspecific theory predicts that during infection T cells with any specificity, including non-crossreactive autoreactive T cells, can develop into effector cells in inflammatory microenvironments, thereby contributing to tissue destruction. These normally quiescent T cells need to be activated (that is, made competent) by processes that are independent of particular classical (that is, MHC-I-defined) microbial antigenic determinants and that can be elicited via a multitude of mechanisms, termed bystander activation.
In the two-signal model of lymphocyte activation, optimal activation requires a specific interaction of the antigen (peptide–MHC complex for T cells, antigen as such for B cells) with the T cell receptor (TCR) and B cell receptor complex, respectively (signal 1) and additional co-stimulatory signals (signal 2) [19]. For T cells, signal 2 is normally delivered by a dedicated set of receptor–ligand interactions between the antigen-presenting cell (APC) and the T cell, but it can apparently also be delivered by other cell-surface receptor types such as cytokine receptors and extracellular matrix receptors [20,21] and by receptors that recognize microbial (cell wall) products [22-24]. Of particular relevance is co-stimulation in B cell physiology: LPS, a constituent of the outer cell wall of Gram-negative bacteria, has long been known as a polyclonal B cell stimulator and, in the presence of interleukin (IL)-4, as an inducer of differentiation. In this function, LPS can replace a CD40-derived signal and induce class switch recombination [25,26]. The receptor for LPS is TLR-4 [27].
Here we have investigated whether a prototype outer surface lipoprotein, namely OspA of Borrelia burgdorferi, the causative agent of Lyme arthritis, is able to directly activate antigen-sensitized naive and/or effector T cells from mice by binding to its nominal receptor, TLR-2. For this purpose we used mouse strains with deficiencies for either TLR-2 (TLR-2-/-) or TLR-4 (TLR-4def).
Materials and methods
Mouse strains
C57BL/6 (B6) mice and mouse strains deficient for TLR-2 (129Sv/C57BL/6.TLR-2-/- [28,29]) or TLR-4 (C57BL/10ScNCr, homozygous for a null mutation of TLR-4, TLR-4def [27,30]) were maintained under pathogen-free conditions in the animal facilities of the Max-Planck-Institut für Immunbiologie, Freiburg, Germany. Male and female mice between 7 and 9 weeks of age were used in all experiments, which were conducted in accordance with the ethical guidelines of the Federation of European Laboratory Animal Science Associations.
Enrichment/purification of cells
Purified T cells from spleen
Splenocytes from age- and sex-matched B6, TLR-2-/- and TLR-4def mice (two mice per group) were pooled and stained with fluorescein isothiocyanate (FITC)-labelled anti-B220 (RA3-6B2), anti-Mac-1 (M1/70), anti-Gr-1 (RB6-8C5), anti-CD11c (HL3) and anti-I-Ab (25-9-17) monoclonal antibodies (mAbs) (Pharmingen, Heidelberg, Germany) and anti-NK1.1 (PK136; Caltag, Hamburg, Germany). T cells from these populations were then negatively sorted by fluorescence-activated cell sorting (FACS) (MoFlo; Cytomation, Freiburg, Germany). Sorted T cells were re-analysed for purity by staining with allophycocyanin-labelled anti-B220, anti-NK1.1, anti-Mac-1 or anti-Gr-1, with anti-I-A/anti-I-E-PE (M5/114.15.2;), anti-CD11c-FITC or anti-Thy1.2-biotin (CD90.2, 53-2.1), all purchased from Pharmingen. Analysis was made with a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany) and CellQuest software.
CD4+/CD8+ T cells from spleen
Spleen cells from B6 mice were labelled with biotinylated antibodies against Thy1.2 (53-2.1; Pharmingen), followed by labelling with streptavidin-conjugated paramagnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Labelled cells were positively selected on magnetic cell separation (MACS) columns (Miltenyi Biotec) and subsequently labelled with antibodies against CD4 (GK1.5; Pharmingen) and CD8 (53-6.7; Southern Biotechnology Associates, Eching, Germany). CD4 and CD8 single-positive cells were then isolated by FACS (MoFlo; Cytomation). The purity of the cells was greater than 99%.
Macrophages from bone marrow
Bone marrow macrophages were cultivated as described elsewhere [31]. In brief, bone marrow cells were harvested from B6 mice and cultured for 7 days in Dulbecco's modified Eagle's medium (Gibco BRL, Karlsruhe, Germany) supplemented with 2 mM L-glutamine (Gibco), 50 μM 2-mercaptoethanol (Roth, Karlsruhe, Germany), 1 mM sodium pyruvate (Gibco), 1 × non-essential amino acids (Gibco), 5% heat-inactivated horse serum (Cell Concepts, Umkirch, Germany), 10% heat-inactivated fetal calf serum (PAA Laboratories, Cölbe, Germany) and 15–20% L-conditioned medium (sterile filtered supernatant of L929 cells, cultured for 7 days in Dulbecco's modified Eagle's medium and supplemented with 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1 × non-essential amino acids and 10% heat-inactivated fetal calf serum).
Isolation of mature B cells and marginal-zone B cells from spleen
Spleen cells from B6 mice were labelled with biotinylated antibodies against CD4 (GK1.5; Pharmingen) and CD8 (53-6.7; Pharmingen) followed by labelling with streptavidin-conjugated paramagnetic microbeads (Miltenyi Biotec). Labelled cells were negatively depleted on MACS columns (Miltenyi Biotec). Negative cells were labelled with antibodies against B220 (RA3-6B2; Pharmingen), IgM (Jackson Immuno Research, via Dianova, Hamburg, Germany), CD23 (B3B4; Pharmingen) and CD21 (7G6; Pharmingen). Mature B cells (CD23+, B220++ and IgM+) and marginal-zone B cells (CD23-, B220++, CD21++ and IgM++) were then isolated by FACS (MoFlo; Cytomation). The purity of the cells was greater than 99%.
Generation of cytotoxic T lymphocyte lines (mixed lymphocyte culture)
Generation of primary alloreactive cytotoxic T lymphocytes (CTLs) and restimulation of these cell lines was performed as described [32]. In brief, for the generation of primary alloreactive CTLs in vitro (primary mixed lymphocyte culture [MLC]), responder splenocytes (one spleen, isolated from B6, TLR-2-/- or TLR-4def mice) were co-cultured with irradiated (3000 rad) allogeneic stimulator splenocytes from BALB/c mice (H-2d, 3/4 spleen) in 40 ml of complete cell culture medium (minimal essential medium [Pan Biotech, Aidenbach, Germany] supplemented with 10% fetal calf serum [Sigma-Aldrich, Taufkirchen, Germany], 100 μg/ml kanamycin [Gibco], 10 μg/ml tylosin [ICN, Eschwege, Germany] and 50 μM 2-mercaptoethanol). CTLs were used on day 6 for cytotoxicity assays and restimulated on day 7. Restimulation for secondary MLC was performed by incubating CTLs derived in vitro (5 × 104/ml) with irradiated BALB/c stimulator cells (2.5 × 106/ml) in complete cell culture medium supplemented with IL-2 (10% of supernatant of rat splenocytes, stimulated with concanavalin A [ConA; Amersham Pharmacia Biotech, Freiburg, Germany] plus 20 mg/ml α-methyl-D-mannopyranoside [Roth]). Cells were used for experiments on day 4 or 5 and restimulated on day 7.
For analysis of the composition of these CTL lines, cells were stained with anti-CD4-FITC (H129.19), anti-CD8a-allophycocyanin (53.6.7), anti-B220-PE (RA3-6B2), anti-NK1.1-PE (PK136), anti-CD19-PE (1D3), anti-CD3ε-biotin (500A2), anti-Thy1.2-biotin (53-2.1) (all purchased from Pharmingen) and anti-F4/80-FITC (Cl:A3-1; Serotec, Eching, Germany).
Functional analysis and proliferation assay of purified T cells or CTL lines
Unselected and purified T cells or CTL lines from MLC were incubated in complete cell culture medium for 72 h (T cells) or 24–48 h (CTLs) in 96-well flat-bottomed plates (Nunc, via Multimed, Kirchheim/Teck, Germany; 4 × 104 cells; 200 μl per well) either coated with rabbit anti-hamster (ha) IgG (Dianova, Hamburg, Germany, 0.5 μg per well) and anti-CD3 (145-2C11; cell culture supernatant purified with Protein A–Sepharose; T cells, 3 ng per well; CTLs, 0.03 or 0.3 ng per well) or with rabbit anti-haIgG alone. The cultures were supplemented or not with recombinant Lip-OspA (strain ZS7, S&K, lot OPA152; GlaxoSmithKline, Rixensart, Belgium), recombinant Met-Asp-Pro (MDP)-OspA (delipidated form, ZS7, S&K, lot 46C33; GlaxoSmithKline; 10 μg/ml maximal concentration of each), human recombinant IL-2 (Sandoz, Basel, Switzerland; 50 U/ml), LPS (S. minnesota, R595; C Galanos, Max-Planck-Institut für Immunbiologie, Freiburg, Germany; 1 μg/ml) or ConA (Amersham Pharmacia Biotech; 5 μg/ml). Anti-TLR-2 mAb (clone mT2.5 [33], at 25, 2.5 or 0.25 μg/ml) or the respective isotype control (mouse IgG; Dianova) was added at various concentrations to cell cultures to analyse their inhibitory potential. For the last 20 h of incubation, 1 μCi of [3H]thymidine (Perkin Elmer, Boston, MA, USA) was added to each well. Incorporation of [3H]thymidine was determined by scintillation counting (cell harvester, Inotech [Dunn Labortechnik, Asbach, Germany]; counting system, TRACE 96 [Dunn Labortechnik]). Means ± SEM for three to six individual wells are given.
Isolation of RNA and analysis by LightCycler®
Purified T cells from B6 mice (ex vivo, purified by cell sorting for Thy1.2-positive cells), whole splenocytes from TLR-2-/- mice or alloreactive CTLs from B6 or TLR-2-/- anti-BALB/c derived from in vitro MLC (purified by cell sorting for CD8+ cells) were stimulated for 24 h with phorbol 12-myristate 13-acetate (PMA; Calbiochem, Schwalbach, Germany; 2.5 ng/ml) and ionomycin (Calbiochem; 500 ng/ml) or frozen directly in TriReagent (Sigma, Taufkirchen, Germany) for RNA isolation. RNA was isolated with a modified guanidine thiocyanate/acid phenol method [34] with TriReagent in accordance with the manufacturer's instructions. After treatment with DNAse I (Ambion, Huntingdon, Cambridgeshire, UK), up to 2 μg of RNA was incubated with Random Hexamer primers (Promega, Mannheim, Germany; 1 μM) and Omniscript RT (Qiagen, Hilden, Germany; 4 U).
The cDNA obtained was used as a template for real-time quantitative polymerase chain reaction, which was performed with the LC FastStart DNA Master SYBR GreenI® (Roche Diagnostics, Mannheim, Germany) in a LightCycler® instrument (Roche). Cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C for 15 s, a primer-dependent temperature for 10 s and 72°C depending on the length of the polymerase chain reaction product (one second per 25 base pairs), all with a temperature transition rate of 20°C/s. Copy numbers were calculated on the basis of amplification of DNA in a 10-fold dilution series. The resulting calculation curves showed an error rate of less than 0.05. Moreover, fluorescence was measured at 2°C below the melting temperature of the amplified DNA, thereby excluding irrelevant amplification products. The numbers of copies of the mRNA under study were compared, assuming constancy in the number of 18S rRNA copies per cell (about 3 × 106 per cell [35]). The primers used are listed in Table 1.
Table 1.
Primers used
| Primer | Sequence |
| 18S rRNA upper | 5'-GCC CGA GCC GCC TGG ATA C-3' |
| 18S rRNA lower | 5'-CCG GCG GGT CAT GGG AAT AAC-3' |
| mTLR1 upper | 5'-GGC ATA CGC CAG TCA AAT A-3' |
| mTLR1 lower | 5'-ATG CAG AAA TGG GCT AAC TT-3' |
| mTLR2 upper | 5'-TCT GCT GTG CCC TTC TCC TGT TGA-3' |
| mTLR2 lower | 5'-GGC CGC GTC GTT GTT CTC GT-3' |
| mTLR4 upper | 5'-AGC CGG AAG GTT ATT GTG GTA GT-3' |
| mTLR4 lower | 5'-TGC CGT TTC TTG TTC TTC CTC T-3' |
| mTLR6 upper | 5'-ATA CCA CCG TTC TCC ATT T-3' |
| mTLR6 lower | 5'-GAC GTG CTC TAT CAT CAG TG-3' |
As a control for plausibility the copy number of mRNA for the low-abundance housekeeping gene TBP (TATA-box binding protein) was also determined and was expected to be between 20 and 40 copies per normal resting cell (data not shown).
Measurement of cytokine secretion
Purified T cells (ex vivo) or CTLs from MLC were cultured in 96-well plates as described above, and supernatants were harvested after 60 h (purified T cells) or 6 h (CTLs), pooled (from six wells per group) and frozen at -20°C until analysed. The concentrations of interferon (IFN)-γ, tumor necrosis factor-α, IL-4 and IL-6 in the supernatants were measured in duplicate with enzyme-linked immunosorbent assay (ELISA) kits from Pharmingen; measurements were performed in accordance with the manufacturer's instructions (IFN-γ, tumor necrosis factor-α and IL-6, cytokine sandwich ELISA; IL-4, OptEIA mouse IL-4 set).
Statistical analysis
Statistical significance was calculated with the two-tailed Student's t-test for comparison of means with unequal variances. P < 0.05 was considered statistically significant.
Results
Recombinant Lip-OspA provides co-stimulatory signals to T cells via TLR-2
To determine a direct co-stimulatory effect of bacterial lipoproteins on T cell proliferation, the preparation of T cell populations of high purity and free from B cells and APCs is critical. Accordingly, T cells were enriched from spleens of B6, TLR-2-/- and TLR-4def mice by negative selection via FACS sorting, by using a panel of mAbs against surface markers of B cells (B220), NK cells (NK1.1), dendritic cells (DCs) (CD11c/I-A) and macrophages (Mac-1). Sorted cell populations of B6 and TLR-2-/- mice contained more than 97% T cells and those of TLR-4def mice more than 93% T cells (Fig. 1c). The percentages of cells positive for the markers B220, Mac-1, NK1.1 and CD11c/I-A were variable between the three selected T cell populations and ranged between 0% and 0.7%.
Figure 1.
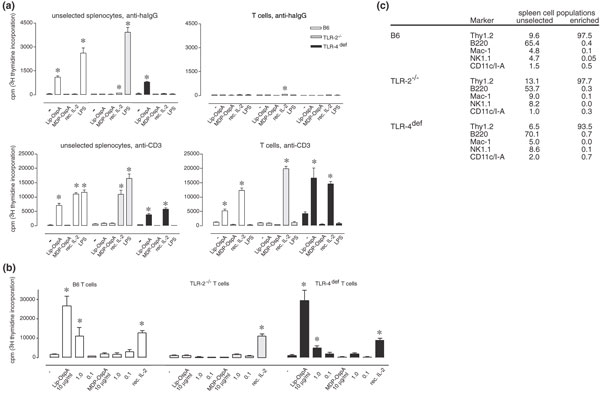
Direct co-stimulation of pre-sensitized T cells via Toll-like receptor (TLR)-2. (a) Unselected splenocytes or fluorescence-activated cell sorting (FACS)-sorted T cells were cultivated on anti-hamster (ha)IgG plus anti-CD3 (3 ng per well) or anti-haIgG coated plates (control) in the presence or absence of Lip-OspA, Met-Asp-Pro (MDP)-OspA (10 μg/ml each), recombinant interleukin-2 (rec. IL-2; 50 U/ml) or lipopolysaccharide (LPS; 1 μg/ml) for 72 h. Proliferation of cells was measured by [3H]thymidine incorporation. Means ± SEM for six different wells are given. Asterisk denotes significant difference (P < 0.05) from control (anti-haIgG or anti-CD3 without supplements). One representative experiment is shown. (b) FACS-sorted T cells were stimulated with anti-CD3 (3 ng per well) and different amounts of Lip-OspA or MDP-OspA (10, 1 or 0.1 μg/ml each) or with 50 U/ml recombinant IL-2. Proliferation of cells was measured by [3H]thymidine incorporation. Means ± SEM for six different wells are given. Asterisk denotes significant difference (P < 0.05) from control (anti-CD3 without supplements). (c) Analysis of splenocytes from C57BL/6 (B6; wild-type), TLR-2-/- and TLR-4def C57BL/10ScNCr mice for different cell populations before and after FACS sorting for T cells (re-analysis). CD11c+ and I-A+ are, in combination, characteristic markers for dendritic cells. Data are given in percentages.
Subsequently, the enriched T cell populations were incubated in the presence of plate-bound anti-CD3 mAb, at concentrations known to be insufficient for the induction of proliferation [23], together with either Lip-OspA, its delipidated form MDP-OspA, LPS or recombinant IL-2; anti-haIgG served as negative control. Figure 1 shows one representative experiment (out of three with similar results). The successful depletion of APCs, including B cells and macrophages/DCs, was revealed by comparing proliferative responses of the enriched T cell populations to the various stimuli with those of unselected spleen cells. Unselected spleen cells responded as expected [23]: when incubated on plates coated with anti-haIgG, B6 spleen cells proliferated in the presence of both Lip-OspA and LPS, but not in the presence of MDP-OspA or recombinant IL-2, indicating that most responding cells are B cells (Fig. 1a, upper left panel). As expected, proliferation of unselected TLR-2-/- and TLR-4def spleen cells was seen only after incubation with either LPS or Lip-OspA, respectively, under similar conditions.
When unselected spleen cells were incubated in the presence of plate-bound anti-CD3 mAb, all three genotypes responded to recombinant IL-2, indicating the expansion of IL-2-responsive TCR-sensitized T cells, in addition to B cells (Fig. 1a, lower left panel) [23,24]. In contrast, proliferative responses were not observed in enriched T cell populations of all three genotypes when cells were incubated on plate-bound anti-haIgG, independently of the presence or absence of additional stimuli (Fig. 1a, upper right panel). This finding indicates that the enriched T cell populations were devoid of Lip-OspA and/or LPS-sensitive target cells, particularly B cells, macrophages and DCs. As expected from previous studies [23], all three anti-CD3-stimulated T cell populations proliferated in response to recombinant IL-2. However, after anti-CD3 stimulation only T cells from B6 and TLR-4def mice, but not those from TLR-2-/- mice, responded to Lip-OspA. Under these conditions the three cell populations did not proliferate in response to MDP-OspA (Fig. 1a, lower right panel). Most importantly, the three T-cell populations also did not respond to LPS in the presence of anti-CD3, indicating that the T cell populations were devoid of APCs, like macrophages and DCs [23].
Together with the fact that unselected spleen cells from B6 and TLR-2-/- mice that were sensitized with anti-CD3 were responsive to LPS under similar conditions (Fig. 1a, lower left panel), the data support the notion that co-stimulatory signals provided by LPS to T cells are mediated indirectly, most probably via APCs [23,24]. The co-stimulatory effect of Lip-OspA for anti-CD3-sensitized T cells from B6 and TLR-4def mice is dose dependent, whereas T cells from TLR-2-/- mice are again unresponsive to Lip-OspA at any concentration tested (Fig. 1b).
To determine the functional potential of T cells that were stimulated with plate-bound anti-CD3 mAb in the presence of Lip-OspA, supernatants of the respective cultures from B6 T cells were analysed for cytokine activities. From four cytokines analysed (IFN-γ, tumor necrosis factor, IL-4 and IL-6), only IFN-γ was detectable in the range 0.9–1.4 ng/ml in independent experiments. Levels of IFN-γ production were similar when T cell populations from B6 mice were co-stimulated with anti-CD3 in the presence of Lip-OspA or recombinant IL-2. Cytokine activity was not detectable at all when enriched B6 T cell populations were cultured solely on anti-CD3 mAb or incubated in the presence of either Lip-OspA, MDP-OspA or LPS alone (data not shown). Taken together, these data suggest that TLR-2 functions as a co-stimulatory signal for the maturation of TCR-sensitized T cells.
Recombinant Lip-OspA induces proliferation and IFN-γ secretion in CD8+ cytolytic T effector cell lines via TLR-2 in the absence of TCR engagement
To determine whether Lip-OspA is also stimulatory for T effector cells, alloreactive (anti-H-2d) CD8+ CTL lines derived in vitro from B6, TLR-2-/- and TLR-4def mice were analysed. Figure 2 shows one representative experiment (out of three with similar results). After the third restimulation in vitro, the three CTL lines consisted of more than 99% T cells (Thy-1.2+), including 93.1–97.4% CD8+ and 0.7–2.0% CD4+ T cells (Fig. 2 legend). When incubated on plate-bound control anti-haIgG, proliferative responses of CTL lines from B6 and TLR-4def mice, but not TLR-2-/- mice, were significantly increased in the presence of either Lip-OspA or recombinant IL-2 alone (Fig. 2, left panels). When the same CTL populations were seeded on anti-CD3 mAb-coated plates at a concentration insufficient to induce cell growth on its own, again only the addition of Lip-OspA and recombinant IL-2, but not of MDP-OspA or LPS, led to proliferative responses of CTL lines of B6 and TLR-4def mice but not TLR-2-/- mice (0.03 ng per well; Fig 2, middle panels). When the CTL lines were stimulated with 0.3 ng of anti-CD3 mAb per well (Fig. 2, right panels) the proliferative responses were increased about 5-10-fold compared with those plated on anti-haIgG or on 0.03 ng of anti-CD3 per well. The three CTL populations did not show proliferative responses to MDP-OspA, ConA or LPS above background, independently of whether they were cultivated on plate-bound anti-haIgG or anti-CD3 mAb. Note that the proliferative response of TLR-2-/- CTLs with anti-CD3 mAb alone was higher than that of B6 and TLR-4def CTLs, but that it was not altered by the addition of Lip-OspA.
Figure 2.

Direct co-stimulation of cytotoxic T lymphocyte (CTL) lines via Toll-like receptor (TLR)-2. CD8+ T cells from CTL lines (generated against BALB/c, sixth stimulation, day 4) were cultivated on anti-hamster (ha)IgG plus anti-CD3 (0.3 or 0.03 ng per well) or anti-haIgG coated plates (control) in the presence or absence of Lip-OspA, Met-Asp-Pro (MDP)-OspA (10, 1 or 0.1 μg/ml each), recombinant interleukin-2 (rec. IL-2; 50 U/ml), concanavalin A (ConA; 5 μg/ml) or lipopolysaccharide (LPS; 1 μg/ml) for 48 h. Proliferation of cells was measured by [3H]thymidine incorporation. Means ± SEM for six different wells are given. Asterisk denotes significant difference (P < 0.05) from control (anti-haIgG or anti-CD3 without supplements). One representative experiment is shown. Phenotypic analysis (fluorescence-activated cell sorting) of C57BL/6 (B6), TLR-2-/- and TLR-4def anti-BALB/c CTL lines (third stimulation, day 4): Thy1.2+, 99.0–99.5%; CD8+, 93–95%, CD4+, 0.7–2%; CD19+ (B cells), F4/80+ (macrophages), NK1.1+ (NK cells) ≤ 0.2%. SI, stimulation index (calculated based on results with anti-haIgG plus anti-CD3 or with anti-haIgG alone, without the addition of supplements).
In general, T cells from TLR-2-/- mice were more reactive to appropriate stimuli (compare the stimulation indices with recombinant IL-2 in the left and middle panels of Fig. 2) but also seemed to function at an accelerated pace (compare absolute counts in the right panels of Fig. 2). When a mAb against mouse TLR-2 with inhibitory potential [33] was included in the cell culture, a significant and dose-dependent decrease in the proliferative response of anti-CD3 (both 0.3 and 0.03 ng per well) plus Lip-OspA-stimulated B6 CTL lines compared with control cultures (without anti-TLR-2 mAb or in the presence of the isotype control antibody) was observed (Fig. 3).
Figure 3.
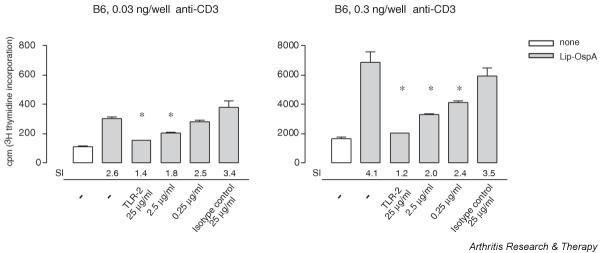
Direct co-stimulation of cytotoxic T lymphocyte (CTL) lines by Lip-OspA can be inhibited by anti-Toll-like receptor (TLR)-2 monoclonal antibody. CD8+ T cells from C57BL/6 (B6) CTL lines (generated against BALB/c, fifth stimulation, day 4) were cultivated on anti-hamster IgG plus anti-CD3 (0.3 or 0.03 ng per well) in the presence or absence of Lip-OspA (10 μg/ml) with or without varying concentrations of anti-TLR-2 monoclonal antibody (25, 2.5 or 0.25 μg/ml) or the respective isotype control antibody (25 μg/ml) for 24 hours. Proliferation of cells was measured by [3H]thymidine incorporation. Means ± SEM for three to six different wells are given. Asterisk denotes significant difference (P < 0.05) from control (plus Lip-OspA without the addition of anti-TLR-2 monoclonal antibody). One representative experiment (out of two with similar results) is shown. SI, stimulation index (calculated based on results with anti-CD3 without the addition of supplements; white bars).
In addition to proliferative responses, the production of IFN-γ by CTL lines was tested. The result of one representative ELISA (out of three with similar results) is shown in Table 2. When cultured on anti-haIgG, Lip-OspA, but not MDP-OspA or LPS, was able to induce IFN-γ production in B6-derived, but not in TLR-2-/- -derived, CTL lines (Table 2). IFN-γ release was similar to or even higher than that obtained with recombinant IL-2 and significantly (about 11-fold) exceeded those in the presence of MDP-OspA or in the absence of any stimulus (Table 2). When cultured on anti-CD3, Lip-OspA, but not MDP-OspA, further increased IFN-γ secretion in B6-derived, but not in TLR-2-/- -derived, CTL lines. These stimuli, including Lip-OspA, did not have any effect on the cytotoxic activities of the three CTL populations, as measured by specific target cell lysis or by the level of TCR-induced exocytosis of granzyme A (data not shown).
Table 2.
Interferon-γ production by cytotoxic T lymphocyte lines after incubation on anti-hamster IgG or anti-CD3 in the presence or absence of Lip-OspA, Met-Asp-Pro-OspA, recombinant interleukin-2 or lipopolysaccharide
| Addition | B6 anti-BALB/c, ng/ml (SI) | TLR-2-/- anti-BALB/c, ng/ml (SI) |
| Anti-haIgG | 0.2 | 0.3 |
| + Lip-OspA | 2.3a (11.5) | 0.2 (<1) |
| + MDP-OspA | 0.3 (1.5) | 0.2 (<1) |
| + rec. IL-2 | 0.5 (2.5) | 0.4 (1.3) |
| + LPS | 0.2 (1.0) | 0.4 (1.3) |
| 0.03 ng per well anti-CD3 | 0.2 | 0.3 |
| + anti-CD3 + Lip-OspA | 5.4a (27) | 0.2 (<1) |
| + anti-CD3 + MDP-OspA | 0.2 (1.0) | 0.3 (1.0) |
| + anti-CD3 + rec. IL-2 | 0.8a (4.0) | 0.6 (2.0) |
| + anti-CD3 + LPS | 0.3 (1.5) | 0.1 (<1) |
| 0.3 ng per well anti-CD3 | 4.9 | 7.2 |
| + anti-CD3 + Lip-OspA | 29.1a (5.9) | 6.8 (<1) |
| + anti-CD3 + MDP-OspA | 6.0 (1.2) | 9.5 (1.3) |
| + anti-CD3 + rec. IL-2 | 10.9a (2.2) | 10.2 (1.4) |
| + anti-CD3 + LPS | 2.7 (<1) | 7.2 (1.0) |
aSignificant difference (P < 0.05) from control (anti-haIgG or anti-CD3 without supplements). C57BL/6 (B6) and Toll-like receptor (TLR)-2-/- cytotoxic T lymphocyte lines (generated against BALB/c, fourth stimulation, day 4) were incubated for 6 h on anti-haIgG (control) with or without anti-CD3 (0.03 ng per well or 0.3 ng per well) in the presence or absence of Lip-OspA, Met-Asp-Pro (MDP)-OspA (10 μg/ml each), recombinant interleukin-2 (rec. Il-2; 50 U/ml) or lipopolysaccharide (LPS; 1 μg/ml). The amount of the secreted interferon-γ in the supernatant was then tested in duplicate using the enzyme-linked immunosorbent assay technique. One representative experiment is shown. The detection limit was 0.1 ng/ml. SI, stimulation index (calculated based on results with anti-hamster (ha)IgG plus anti-CD3 or anti-haIgG alone, without the addition of supplements).
Quantitative analysis of TLR expression on resting and activated T cell populations
To support these functional data, the expression of mRNA for TLRs on T cells was analysed. As shown in Table 3, enriched naive resting splenic B6 T cells do express TLR-2 and TLR-1 but not (or only at marginal levels) TLR-4. However, the latter transcripts were readily found in unselected spleen cells from TLR-2-/- mice, isolated mature resting B cells, marginal-zone B cells and, above all, macrophages. In addition, these data strongly argue against a contamination of the purified T cells with B cells or macrophages (Table 3). TLR-1, which is known to form heterodimers with TLR-2 and to modify its ligand-binding specificity [36-38], is expressed at considerable levels in naive and PMA/ionomycin-activated T cells. Expression of TLR-6, which is also able to modify the ligand-binding specificity of TLR-2 by heterodimerization [38-41], was detected at low levels in naive CD4+ and CD8+ T cell populations. CTLs expressed higher levels of TLR-2 and TLR-6 transcripts, but not of TLR-1 transcripts, than resting T cells. Activation of CTLs with PMA and ionomycin exhibited a dual effect in that TLR-2 expression increased but TLR-1 and TLR-6 expression decreased. In addition, CTLs from B6 and TLR-2-/- mice expressed low levels of TLR-4. For comparison, expression levels are given for two B cell subsets and for bone marrow-derived macrophages.
Table 3.
Expression of TLRs on resting and activated T cells in comparison with macrophages and B cells
| Cell population | Molecules per cell | |||
| TLR-1 | TLR-2 | TLR-4 | TLR-6 | |
| Spleen Thy1.2+ (ex vivo) | 611 | 46 | n.d. | n.d. |
| Spleen Thy1.2+ + PMA/ionomycin | 40 | 24 | 6 | n.d. |
| Spleen CD4+ T cells | 1874 | 30 | 7 | 47 |
| Spleen CD8+ T cells | 602 | 31 | 0 | 39 |
| CTLs | 248 | 219 | 43 | 56 |
| CTLs + PMA/ionomycin | 19 | 335 | 53 | 19 |
| CTLs (TLR-2-/-) | 0 | -a | 28 | 6 |
| Spleen (TLR-2-/-, ex vivo, unselected) | 101 | -a | 78 | 18 |
| Bone marrow macrophages (cultured) | -b | 6375 | 7056 | 113 |
| Spleen mature B cells | -b | 25 | 25 | 13 |
| Spleen marginal-zone B cells | -b | 49 | 33 | 11 |
aNot determined; sterile fusion transcripts of the mutated Toll-like receptor (TLR)-2 gene can be found with the indicated primer pairs; however, no protein product is detectable (CJ Kirschning, unpublished observations). bNot determined. Purified T cells (Thy1.2+, CD4+ or CD8+) or B cells (mature, marginal zone) from B6 mice, whole splenocytes from TLR-2-/- mice or purified cytotoxic T lymphocytes (CTLs) from C57BL/6 and TLR-2-/- anti-BALB/c mixed lymphocyte culture (purified by cell sorting for CD8-positive cells) were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 24 h or frozen directly in TriReagent for RNA isolation and real-time polymerase chain reaction, as described in Materials and methods. As a control, cultured bone marrow-derived macrophages were used. Experiments, except for the measurement of mRNA in CTL lines and spleen cells that were stimulated with PMA and ionomycin, were repeated twice and gave similar results, both in the sense of inter-experimental and intra-experimental reproducibilities. n.d., not detectable.
These data demonstrate that naive resting and effector T cells express TLRs appropriate for binding pathogen-associated molecular patterns of B. burgdorferi and are fully compatible with the results shown in Figs 1, 2 and 3.
Discussion
Our present findings show that a microbe-derived lipoprotein, Lip-OspA from B. burgdorferi, can function as co-stimulator for both antigen-sensitized naive T cells and effector T cells, namely CTLs, and that this co-stimulatory signal is directly mediated via TLR-2. These data stress the crucial role of TLRs not only as sensors of the innate immune responses against microbial pathogens [42] but also as co-stimulators of cells of the adaptive immune system. TLR-2 engagement therefore influences the differentiation of T cells not only by the activation of DCs (indirect pathway [23,43]) but also directly via co-stimulation. In the latter function TLRs can sustain ongoing specifically induced immune responses in a polyclonal manner. In this respect, the activity of Lip-OspA is comparable to the action of LPS, a polyclonal B cell activator that engages TLR-4 [27].
Specificity of the interaction
The present finding that Lip-OspA directly co-stimulates anti-CD3-sensitized T cells from B6 and TLR-4def mice, but not from TLR-2-/- mice, to proliferate and to develop into effector cells explains our previous findings that Lip-OspA augments proliferative and cytokine responses of mouse and human T cells [23,24]. Here we describe a direct involvement of TLR-2 expressed on T cells as the underlying molecular mechanism. This conclusion is supported by the fact that naive and presensitized T cells are shown to express the respective receptor, although at low levels, in line with previous reports on TLR expression in murine T cell lines [44] and in thymic and splenic T cells [9]. We found that after polyclonal activation with PMA and ionomycin, the expression of TLR-2 increased in CTLs but not in freshly isolated splenic T cells. Whereas the expression of TLR-4 transcripts was not seen in naive T cells, TLR-4 mRNA could be detected after stimulation and was even higher in CTLs.
These findings extend reported data [9] in which an increase in TLR-2 but not in TLR-4 transcripts was observed after stimulation of splenic and thymic T cells. The expression of TLRs that have been described as partners in a heterodimeric complex with TLR-2, namely TLR-1 and TLR-6 [36-38,41], are also regulated rather markedly: a more than 10-fold decrease in TLR-1 was found in PMA/ionomycin-treated freshly isolated B6 cells, as well as in CTL lines. Whether this TLR modulation would translate into a change of susceptibility for activation by these specific ligands has not been studied.
However, the differential effect of Lip-OspA on B6, TLR-4def and TLR-2-/- T cell populations suggests the surface expression of the respective lipoprotein receptor on presensitized T cells and CD8+ T effector cells. This assumption is furthered by the fact that co-stimulation of B6 CTL lines by Lip-OspA was inhibited by a mAb against TLR-2, known to interfere with ligand–receptor interaction [33]. It is not yet clear which level of (protein) expression of TLRs in general is necessary for efficient signaling of the target cell, but all evidence points to a low expression of most TLRs [45]; however, this does not seem to interfere with an efficient biological response to a stimulus. In this regard it is significant that TLR-4, which is the receptor for LPS, a polyclonal B-cell activator and inducer of differentiation, is expressed at comparable levels in B cells (Table 3) [25,26].
Neither naive splenic T cells nor CTL lines responded to LPS. This finding is remarkable for two reasons: first, in view of the fact that the co-stimulatory activity of LPS for T cells is strictly dependent on APCs [23] it verifies the successful enrichment of the responder populations; second, it conflicts with the (albeit low) expression of TLR-4 on CTLs and the reported responsiveness of TLR-4-positive regulatory T cells to LPS [46]. Optimal signaling by LPS requires, besides TLR-4, several accessory molecules such as LBP, MD-2 and CD14 [47,48]. We do not yet know whether the absence of a response to LPS by T lymphocytes that do express TLR-4 is due to qualitative or quantitative aspects of signal transduction by TLR-4 on T lymphocytes.
Biological effects
The biological effects of TLR engagement on target cells are poorly understood, which is partly due to insufficient knowledge about the signal transduction pathways. Recent evidence indicates that subgroups of TLRs use specific signaling pathways [49-51] aside from common pathways employed by all TLRs, IL-1 receptors and other surface receptors. Evidently CD28, the prototype co-stimulator of T lymphocytes, shares certain signaling pathways with TLRs [52]. Thus, the distinct outcome of a T cell response, such as differential cytokine production, as seen with human T cells co-stimulated by either Lip-OspA or CD28 [24], can be understood by implicating non-overlapping parts of distinct signaling pathways. Additional levels of sophistication seem to derive from a differential expression of TLRs on different T effector cells ([46], and this study) and the dependence of recognition on the physical state of the pathogen-associated molecular patterns. For example, the recognition of OspA by TLR-2/TLR-6 or TLR-2/TLR-1 heterodimers depends on the acylation state of the lipoprotein [36-41,53,54]. In addition, little is known about feedback regulation after engagement of TLRs and the consequences of the absence of particular TLRs on effector cells. The increased excitability of TLR-2-/- CTL lines should be considered in this context.
Current concepts of T cell activation imply that co-stimulatory molecules are necessary to initiate antigen-specific responses from naive T cells but are dispensable for triggering functions in effector T cells, including exocytosis and cytokine release [55]. The present finding that bacterial lipoproteins directly stimulate alloreactive CTLs to proliferate and to secrete IFN-γ via TLR-2 adds another facet to the functional potential of T effector cells. The fact that the same stimulatory signal leads neither to the release of cytotoxic effector molecules from CTLs, such as perforin and granzymes, nor to an enhancement of their cytotoxic potential in the presence of appropriate target cells or anti-CD3 mAb indicates that TCR-induced granula exocytosis is independent of TLR-2 signaling. These findings not only emphasize the differential signal requirements for the induction effector function in T cells [56], including CTLs, such as granula exocytosis and cytokine release [57]; they will certainly also contribute to a better understanding of T cell-driven pathological processes in inflamed tissues, even in situations where causal agents are elusive.
Relevance of the findings
The question of whether our findings are of any significance for the understanding of CIJD is justified and needs answering.
1. Involving microbial infections as a leading cause for CIJD would reconcile years of research in this area and numerous hypotheses on its pathogenesis [4,14,58,59].
2. Recent research implicates synovial lining cells, B cells and T cells in the pathogenesis of CIJD (for a recent review on this, see [2]).
3. The receptor system, implied by our findings, is present on synovial lining cells, B cells and T cells as is shown by our own data and published results [8,10].
4. TLR ligands have long been known as polyclonal activators of lymphocytes, in particular of B cells [27,60,61].
5. TLR ligands have been implicated by other groups as a cause of CIJD or as enhancing factors in the disease, for example hypomethylated bacterial DNA [12], LPS [13] and heat shock protein 60 [62].
6. TLR ligands are found in the synovia of patients with CIJD [11].
7. The cytokine profile in the serum of patients with inflammatory joint disease or produced by T cells isolated from synovia is congruent with that produced by the T cells in our experiments [63-66].
8. In our hypothesis a specific antigen is not required, leaving room for a multicausal hypothesis on the pathogenesis, including T cells of any specificity.
9. The pathogenesis and (histo)pathological findings in B. burgdorferi infection are compatible with those of CIJD [14,67-70].
These data suggest that the described inflammatory processes are elicited and maintained by direct interaction of intact spirochetes and/or extracellular membrane-bound vesicles [71] and molecules thereof with cells that either home to the affected tissue or infiltrate the diseased area. The observation that susceptibility to the development of chronic arthritis in patients with B.burgdorferi infection is linked, at least partly, to HLA-DRB1*0401 or related alleles [72,73], just as the predisposition of normal mouse strains with certain H-2 haplotypes to develop chronic joint inflammation [69,74] indicated the critical involvement of T cells in the pathogenesis of Lyme disease. The fact that human T cells with specificity for a particular OspA epitope in the context of HLA-DR4 protein co-recognize an epitope on a host adhesion molecule, LFA-1, led to the hypothesis that Lyme arthritis could be a consequence of a specific pathogen-induced autoimmunity [75]. However, at present, there is no convincing experimental evidence whatsoever for such a causal relationship [18,76-78]. In addition, no correlation was found between responses of T cells to LFA-1 peptide in patients with Lyme disease and their clinical status [79].
The findings that synovial T cells from patients with Lyme arthritis are polyclonal [14,15] and that pre-activated T cells, irrespective of their antigen specificity, effectively infiltrate inflammatory foci [80,81] suggest that T cells specific both for spirochetal and for third-party antigen can expand and secrete pro-inflammatory cytokines in infected tissue, thereby contributing to the disease progress. Engagement of TLR-2 and other TLRs with resident spirochetes or their products would give any pre-activated T cell a nonspecific stimulus that ensures the ongoing inflammation in a seemingly specific way (Fig. 4). This might also hold true for other pathogen-induced or non-pathogen-induced CIJD.
Figure 4.
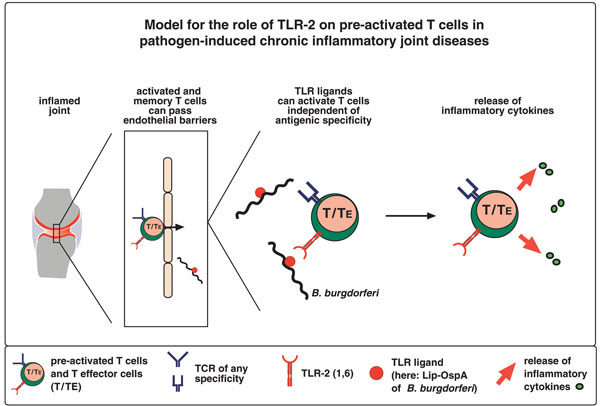
Explanation for the involvement of Toll-like receptor (TLR)-2 on pre-activated T cells in pathogen-induced chronic inflammatory joint diseases. Any inflammation will cause the induction of chemokine and cytokine production in several tissue-associated cells in the joint, including fibroblasts, macrophages and dendritic cells. Activated T cells and T effector cells of any specificity (also auto-specificities) can respond to these signals, migrate to the joint, breach endothelial barriers, infiltrate the inflamed foci and sustain inflammatory processes by secreting cytokines in response to direct co-stimulation via TLR-2, without the necessity of engagement of the T cell receptor.
Conclusion
The present data reveal a new method of co-stimulation of T cells via TLR-2 that might have a critical role in pathogen-induced immunopathology. The important finding that bacterial lipoproteins can trigger the release of a proinflammatory cytokine also from T effector cells, even in the absence of TCR engagement, might help to elucidate causative signals of inflammatory diseases for which the original microbe has not been identified, such as rheumatoid arthritis. It has not escaped our attention that the novel mechanism of T cell activation described here might also open new avenues for the understanding and treatment of diseases other than chronic inflammatory disorders, for example cancer.
Competing interests
None declared.
Abbreviations
APC = antigen-presenting cell; B6 = C57BL/6; CIJD = chronic inflammatory joint disease; ConA = concanavalin A; CTL = cytotoxic T lymphocyte; DC = dendritic cell; ELISA = enzyme-linked immunosorbent assay; FACS = fluorescence-activated cell sorting; FITC = fluorescein isothiocyanate; ha = hamster; IFN = interferon; IL = interleukin; LPS = lipopolysaccharide; MACS = magnetic cell separation; MDP = Met-Asp-Pro; MLC = mixed lymphocyte culture; Osp = outer surface protein; PE = phycoerythrin; PMA = phorbol 12-myristate 13-acetate; TCR = T cell receptor; TLR = Toll-like receptor; TNF = tumor necrosis factor.
Acknowledgments
Acknowledgements
We thank Reina Brehm for expert technical assistance and Marina Freudenberg for providing TLR-2-/- and TLR-4def mice. We also thank Thomas Boehm for valuable suggestions and for reading the manuscript critically. This work was supported in part by the Deutsche Forschungsgemeinschaft (Si 214/9-2-1) and the Boehringer Ingelheim Fonds (VS).
Contributor Information
Vera Sobek, Email: sobek@immunbio.mpg.de.
Nico Birkner, Email: birkner@immunbio.mpg.de.
Ingrid Falk, Email: falk@immunbio.mpg.de.
Andreas Würch, Email: wuerch@immunbio.mpg.de.
Carsten J Kirschning, Email: carsten.kirschning@lrz.tu-muenchen.de.
Hermann Wagner, Email: h.wagner@lrz.tum.de.
Reinhard Wallich, Email: reinhard.wallich@urz.uni-heidelberg.de.
Marinus C Lamers, Email: lamers@immunbio.mpg.de.
Markus M Simon, Email: simon@immunbio.mpg.de.
References
- Steere AC, Gross D, Meyer AL, Huber BT. Autoimmune mechanisms in antibiotic treatment-resistant lyme arthritis. J Autoimmun. 2001;16:263–268. doi: 10.1006/jaut.2000.0495. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Klinman D. Does activation of the innate immune system contribute to the development of rheumatoid arthritis? Arthritis Rheum. 2003;48:590–593. doi: 10.1002/art.10852. [DOI] [PubMed] [Google Scholar]
- Silman AJ, Hochberg MC. Rheumatoid Arthritis. In: Silman AJ, Hochberg MC, editor. In Epidemiology of the Rheumatic Diseases. Oxford: Oxford University Press; 2001. pp. 31–71. [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin Immunol. 2000;12:185–188. doi: 10.1006/smim.2000.0230. discussion 257–344. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95:1378–1385. [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, Breedveld FC, Tak PP. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ronaghy A, Prakken BJ, Takabayashi K, Firestein GS, Boyle D, Zvailfler NJ, Roord ST, Albani S, Carson DA, Raz E. Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J Immunol. 2002;168:51–56. doi: 10.4049/jimmunol.168.1.51. [DOI] [PubMed] [Google Scholar]
- Terato K, Harper DS, Griffiths MM, Hasty DL, Ye XJ, Cremer MA, Seyer JM. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme Disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Yssel H, Shanafelt MC, Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NL, Zhang DQ, Zhou KY, Cartman A, Leroux JY, Poole AR, Zhang YP. Isolation and characteristics of autoreactive T cells specific to aggrecan G1 domain from rheumatoid arthritis patients. Cell Res. 2000;10:39–49. doi: 10.1038/sj.cr.7290034. [DOI] [PubMed] [Google Scholar]
- Verheijden GF, Rijnders AW, Bos E, Coenen-de Roo CJ, van Staveren CJ, Miltenburg AM, Meijerink JH, Elewaut D, de Keyser F, Veys E, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40:1115–1125. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- Benoist C, Mathis D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol. 2001;2:797–801. doi: 10.1038/ni0901-797. [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Yamada A, Kay J, Yamada KM, Akiyama SK, Schlossman SF, Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989;170:1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990;145:59–67. [PubMed] [Google Scholar]
- Brett SJ, Mazurov AV, Charles IG, Tite JP. The invasin protein of Yersinia spp. provides co-stimulatory activity to human T cells through interaction with beta 1 integrins. Eur J Immunol. 1993;23:1608–1614. doi: 10.1002/eji.1830230732. [DOI] [PubMed] [Google Scholar]
- Simon MM, Nerz G, Kramer MD, Hurtenbach U, Schaible UE, Wallich R. The outer surface lipoprotein A of Borrelia burgdorferi provides direct and indirect augmenting/co-stimulatory signals for the activation of CD4+ and CD8+ T cells. Immunol Lett. 1995;45:137–142. doi: 10.1016/0165-2478(94)00243-K. [DOI] [PubMed] [Google Scholar]
- Knigge H, Simon MM, Meuer SC, Kramer MD, Wallich R. The outer surface lipoprotein OspA of Borrelia burgdorferi provides co- stimulatory signals to normal human peripheral CD4+ and CD8+ T lymphocytes. Eur J Immunol. 1996;26:2299–2303. doi: 10.1002/eji.1830261005. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986;136:4538–4541. [PubMed] [Google Scholar]
- Karnowski A, Yu P, Achatz G, Lamers MC. The road to the production of IgE is long and winding. Am J Respir Crit Care Med. 2000;162:S71–S75. doi: 10.1164/ajrccm.162.supplement_2.ras-3. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts C, Tapping RI, Mathison JC, Chuang TH, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- Poltorak A, Merlin T, Nielsen PJ, Sandra O, Smirnova I, Schupp I, Boehm T, Galanos C, Freudenberg MA. A point mutation in the IL-12R beta 2 gene underlies the IL-12 unresponsiveness of Lps-defective C57BL/10ScCr mice. J Immunol. 2001;167:2106–2111. doi: 10.4049/jimmunol.167.4.2106. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kramer MD, Eichmann K, Modolell M, Museteanu C, Simon MM. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci USA. 1990;87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Hausmann M, Tran T, Ebnet K, Tschopp J, Tha Hla R, Mullbacher A. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, et al. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest. 2004;113:1473–1481. doi: 10.1172/JCI200420762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Science; 2002. [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/S0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- Morr M, Takeuchi O, Akira S, Simon MM, Muhlradt PF. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol. 2002;32:3337–3347. doi: 10.1002/1521-4141(2002012)32:12<3337::AID-IMMU3337>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14:1065–1074. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- Imler JL, Hoffmann JA. Toll signaling: the TIReless quest for specificity. Nat Immunol. 2003;4:105–106. doi: 10.1038/ni0203-105. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Wanted: a molecular basis for specificity in toll-like receptor signal transduction. Mol Cell. 2002;10:969–971. doi: 10.1016/S1097-2765(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Kane LP, Lin J, Weiss A. It's all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 2002;23:413–420. doi: 10.1016/S1471-4906(02)02264-0. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Mackey MF, Barth RJ, Jr, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J Exp Med. 1997;186:757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel T, Breloer M, Kohler H, von Bonin A, Weltzien HU. Partial agonism and independent modulation of T cell receptor and CD8 in hapten-specific cytotoxic T cells. Eur J Immunol. 1998;28:3706–3718. doi: 10.1002/(SICI)1521-4141(199811)28:11<3706::AID-IMMU3706>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Steinberg AD. Retroviruses and autoimmunity. J Autoimmun. 1990;3:137–166. doi: 10.1016/0896-8411(90)90137-H. [DOI] [PubMed] [Google Scholar]
- Rook GA, Stanford JL. Slow bacterial infections or autoimmunity? Immunol Today. 1992;13:160–164. doi: 10.1016/0167-5699(92)90119-R. [DOI] [PubMed] [Google Scholar]
- Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. doi: 10.1034/j.1600-065X.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Valdez JC, Chandrasekaran R, Eibel H, Mikecz K, Glant TT, Finnegan A. Th1 and Th2 cytokines regulate proteoglycan-specific autoantibody isotypes and arthritis. Arthritis Res. 2002;4:54–58. doi: 10.1186/ar383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Siegert S, Neure L, Grolms M, Liu L, Eggens U, Radbruch A, Braun J, Sieper J. The elevated ratio of interferon gamma-/interleukin-4-positive T cells found in synovial fluid and synovial membrane of rheumatoid arthritis patients can be changed by interleukin-4 but not by interleukin-10 or transforming growth factor beta. Rheumatology (Oxford) 1999;38:1058–1067. doi: 10.1093/rheumatology/38.11.1058. [DOI] [PubMed] [Google Scholar]
- Johnston YE, Duray PH, Steere AC, Kashgarian M, Buza J, Malawista SE, Askenase PW. Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am J Pathol. 1985;118:26–34. [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Gay S, Museteanu C, Kramer MD, Zimmer G, Eichmann K, Museteanu U, Simon MM. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kramer MD, Wallich R, Tran T, Simon MM. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- Whitmire WM, Garon CF. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect Immun. 1993;61:1460–1467. doi: 10.1128/iai.61.4.1460-1467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- Steere AC. Clinical definitions and differential diagnosis of Lyme arthritis. Scand J Infect Dis Suppl. 1991;77:51–54. [PubMed] [Google Scholar]
- Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, Huber BT. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- Trollmo C, Meyer AL, Steere AC, Hafler DA, Huber BT. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1 alpha L is a partial agonist for outer surface protein A-reactive T cells. J Immunol. 2001;166:5286–5291. doi: 10.4049/jimmunol.166.8.5286. [DOI] [PubMed] [Google Scholar]
- Steere AC, Falk B, Drouin EE, Baxter-Lowe LA, Hammer J, Nepom GT. Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 2003;48:534–540. doi: 10.1002/art.10772. [DOI] [PubMed] [Google Scholar]
- Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- Kalish RS, Wood JA, Golde W, Bernard R, Davis LE, Grimson RC, Coyle PK, Luft BJ. Human T lymphocyte response to Borrelia burgdorferi infection: no correlation between human leukocyte function antigen type 1 peptide response and clinical status. J Infect Dis. 2003;187:102–108. doi: 10.1086/346059. [DOI] [PubMed] [Google Scholar]
- Mackay CR, Marston W, Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992;22:2205–2210. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Migration pathways and immunologic memory among T lymphocytes. Semin Immunol. 1992;4:51–58. [PubMed] [Google Scholar]


