Abstract
Antibodies against intact type II collagen (CII) are a feature of rheumatoid arthritis (RA) but have limited diagnostic value. Here we assess whether either of the two major cyanogen bromide fragments of CII, namely CB10 or CB11, are more sensitive substrates for the detection of antibodies in RA. Cleavage of bovine CII with cyanogen bromide yielded CB10 and CB11; these were purified by column chromatography for use in an enzyme-linked immunosorbent assay. Serum antibodies were measured in patients with RA, psoriatic arthritis (PsA), osteoarthritis (OA) and blood donors. Results were compared with those using intact CII. Antibodies against CB10 were found in as many as 88% of 96 patients with long-standing RA, but only 12% of 33 patients with PsA, 6% of 34 patients with OA and 3% of 93 control sera. Lower frequencies for these diseases were obtained on testing for antibodies against CB11: 50%, 6%, 21% and 2%, respectively. The sensitivity of detection in RA of antibodies against CB10 compared with antibodies against intact CII (88% versus 24%) was not at the expense of specificity, which remained high at 94%. The much higher frequency of antibodies against CB10 in RA than in other rheumatic diseases or control sera indicates that CB10 is clearly a more sensitive substrate than the intact collagen molecule and, combined with other assays (rheumatoid factor, anti-cyclic citrullinated peptide [anti-CCP]), might comprise a panel with a highly reliable predictive value. Moreover, our findings should encourage renewed interest in the role of collagen autoimmunity in the pathogenesis of RA.
Keywords: antibodies, cyanogen bromide peptides, rheumatoid arthritis, type II collagen
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic articular inflammation and progressive joint destruction. Autoimmunity to type II collagen (CII), an abundant molecule in articular cartilage, is implicated in pathogenesis because the immunization of susceptible strains of rats, mice and nonhuman primates using native CII in adjuvant results in collagen-induced arthritis (CIA), which has close immunologic and pathologic similarities to RA [1]. CIA is dependent on immune responses to CII by both T and B lymphocytes, with arthritis being initiated by complement-fixing autoantibodies that bind to CII in the cartilage matrix [2]. In humans, IgG-producing B cells specific for CII are present in rheumatoid synovium and synovial fluid [3,4], suggesting an intra-articular antigen-driven immune process. Serum autoantibodies against native and denatured type II collagen (anti-NCII and DCII) are detected in 24–30% of patients with RA, as shown by cross-sectional studies on patients with well-established disease [5-8]. However, this relatively low frequency in RA of anti-CII, together with seropositivity (albeit at even lower frequency) in other rheumatological and inflammatory diseases [7] including psoriatic arthritis (PsA) [7,9], osteoarthritis (OA) [7,10,11], juvenile arthritis [12], Paget's disease [10,11], and systemic lupus erythematosus [6,7,10,13], has negated the diagnostic utility and pathogenetic significance of anti-CII in RA. However, several studies have reported that the frequency of anti-NCII might be as high as 60–75% in patients with RA when tested very early in the disease [14-16], and thereafter frequencies tend to fall to those ascertained in the earlier cross-sectional studies [17], suggesting that their presence might provide a sensitive predictive marker for cases of early RA.
Our interest in the reactivity of RA sera with various cleavage products of CII led to the present study, in which we assess whether purified preparations of the two major cyanogen bromide (CNBr) fragments of CII, namely CB10 and CB11, would serve as superior substrates for the detection of antibodies in RA. It was found that the frequency of antibodies against CB10 in cases of well-established RA was markedly increased, from 24% to 88%, comparing the frequency using the intact CII molecule; this was not at the expense of the specificity, which remained at the high level of 94%.
Materials and methods
Patients
Sera were studied from 96 of 114 consecutively selected patients with the diagnosis of RA, according to the 1987 criteria of the American College of Rheumatology [18], who were attending the rheumatology clinic at the Royal Melbourne Hospital, Melbourne; clinical and immunogenetic data have been reported on these patients [8,19,20]. Of the 96 patients, 70% were female, 87% had been seropositive for rheumatoid factor at some stage, 86% had the RA HVR3 susceptibility motif, and 86% had erosive disease. At entry to the study, 78% had active disease, and the median Ritchie Articular Index was 5 (range 0–25). The median age at onset was 45 years (range 15–77), and the median duration of disease was 13 years (range 3–51). Patients were receiving various drugs including corticosteroids, non-steroidal anti-inflammatory drugs, or disease-modifying anti-rheumatic drugs, including methotrexate.
Sera were also obtained from 33 patients with PsA and from 34 patients with OA attending rheumatology clinics at the Royal Melbourne Hospital and Monash Medical Centre, Melbourne. Sera from 93 blood donors attending the Red Cross Blood Bank in Melbourne were used as normal controls. The use of these sera for the present study was approved by the Standing Committee on Ethics in Research involving Humans of Monash University.
Preparation of type II collagen
CII was prepared from human rib cartilage and bovine nasal septum cartilage by digestion with pepsin and by differential salt precipitation [12], and the preparations appeared pure according to an overloaded 5% SDS-PAGE gel stained with 0.2% (w/v) Coomassie blue (Bio-Rad, Hercules, CA, USA); no contamination with other collagen types or with proteoglycans was evident (data not shown).
CNBr digestion of bovine CII and purification of CB10 and CB11 peptides
CNBr digestion of bovine CII was performed with 3 mg of purified bovine CII. This was dissolved in 1 ml of 70% (v/v) formic acid, containing 50 mg of CNBr (Sigma, St Louis, MO, USA) and cleavage was achieved by the method of Scott and Veis [21].
CB10 and CB11 were then purified as described by Miller and Lunde [22], with slight modifications. The CB peptides were initially resolved into two major fractions by chromatography on a Bio-Gel P-2 column (100–200 mesh, exclusion limit 2600 Da, globular proteins; Bio-Rad), equilibrated with 0.1 M acetic acid. The larger peptides that were eluted in the excluded volume were pooled, freeze-dried and re-chromatographed on a cation-exchange column of carboxymethylcellulose (Whatman CM-52), equilibrated with 0.02 M sodium citrate, pH 3.6, containing 0.01 M NaCl (start buffer). Chromatography was performed at 42°C for 10 hours with a linear salt gradient obtained by the addition of 1% 0.16 M NaCl in 0.02 M sodium citrate, pH 3.6, to the start buffer every 6 min, with a flow rate of 1.75 ml/min, using the Bio-Rad Econo system (Bio-Rad). Fractions were collected at 10 min intervals, run on 15% Tricine gels and stained with 0.2% (w/v) Coomassie blue. Fractions containing CB10 or CB11 were pooled separately, desalted on the Bio-Gel P-2 column and freeze-dried. To purify CB10 from CB8 and CB9,7, and CB11 from CB12, each fraction was applied to a P-30 column (100–200 mesh, exclusion limit 40 kDa, globular proteins; Bio-Rad), equilibrated in 0.1 M acetic acid. To determine the purity of the peptides after each column run, fractions were run on 15% Tricine gels and stained with 0.2% (w/v) Coomassie blue. Purified CB10 and CB11 were renatured by stepwise cooling, as described by Terato and colleagues [23], from 20 to 0.2°C.
Circular dichroism
We confirmed that renaturation of the CB peptides had occurred by performing circular dichroism spectroscopy on a Jasco J-810 spectropolarimeter equipped with a PFD 423S/L Peltier-type temperature controller, with constant flushing with N2. CII and CB peptides were dissolved at 1 mg/ml in 0.1 M acetic acid. Renaturation was performed as described previously [23] and the protein concentration was adjusted to 70–80 μg/ml. When required, denaturation was performed immediately before analysis by heating samples to 45°C for 5 min before placement on ice. Circular dichroism spectra were recorded from 190 to 250 nm at 5°C in a cuvette with a 1 mm light path. Each spectrum represents an average of three scans performed at 50 nm/min with a bandwidth of 1 nm. Thermal melting profiles were followed at 222 nm as the temperature increased from 5 to 40°C at a heating rate of 1°C/min.
Collagen antibody detection by enzyme-linked immunosorbent assay (ELISA)
Antibodies against native human and native bovine CII were measured by ELISA as described previously [7,8]. Microtitre plates were coated overnight with 10 μg/ml human or bovine CII, at 4°C. Sera, diluted 1:50, were tested in duplicate in the presence or absence of antigen. Antibodies were detected with horseradish peroxidase-conjugated anti-human IgG (Chemicon International, Temecula, CA, USA), with 0.5 mg/ml 2,2,-azino-di-[3-ethyl-benzthiazoline sulfonate (6)] (Boehringer, Mannheim, Germany) as substrate in 0.03 M citric acid, 0.04 M Na2HPO4, 0.003% H2O2, pH 4. To compensate for non-specific binding of immunoglobulins to the plates, the optical density obtained for wells lacking collagen was subtracted from that obtained for wells coated with collagen. Results were expressed as levels of reactivity based on the number of standard deviations (SD) above the mean for 10 normal blood donor controls tested in the same assay; the level was considered to be raised when greater than 3 SD above the mean; that is, an antibody level of 3 or more.
CB10 and CB11 antibody detection by ELISA
Antibodies against the purified CB peptides of bovine type II collagen, namely CB10 and CB11, were measured by ELISA as described above, with the following modifications. Plates were coated with 10 μg/ml purified peptide and sera were diluted 1:200. All incubations were performed at 4°C and washes were done with cold Tris-buffered saline containing 0.05% Tween 20. Before addition of the substrate, plates were left at 22°C for 10 min. Results are expressed as SD above the mean for 10 controls, as described above.
The monoclonal antibody (mAb) M2139 recognizing native CB10, and the mAb CII-CI recognizing native CB11 [24], donated by Professor R Holmdahl (Lund University, Lund, Sweden), were used to establish that the CB peptides of CII had renatured sufficiently to display a conformational epitope.
Statistics
The frequency of antibodies between individual groups was analysed with the χ2 or Fisher exact probability test, and levels of antibodies were compared with the Mann–Whitney U-test. Antibody levels to human NCII and bovine NCII from the same sera were compared using the Pearson product moment correlation coefficient. P ≤ 0.05 was taken as significant.
Results
Comparison of antibodies against human CII and bovine CII by ELISA
We have previously reported that IgG antibodies against NCII are detectable in 24% of patients with RA, 12% of those with OA and 13% of those with other chronic inflammatory diseases [7]. As the CB peptides were purified from bovine CII, because of the amount of collagen required for purification, we initially selected random sera from patients with RA, PsA, OA, and normal controls, to establish that levels of antibodies against intact bovine CII were equivalent to those obtained with human CII.
In the present study, IgG antibodies against native bovine CII were present in 23 (24%) of 94 patients with RA, 9 (27%) of 33 with patients with PsA, 3 (10%) of 31 patients with OA, and 1 (1%) of 93 control sera. The levels of reactivity to bovine CII compared with levels with human CII tended to be higher for patients with PsA, but not for patients with RA (data not shown). However, the frequency of positivity of antibodies against bovine or human CII in the different diseases was not significantly different and was equivalent to frequencies we have reported previously [7,8]. Overall, for the 251 sera that were compared, there was a highly significant correlation (R = 0.777, P < 0.001) between levels of antibodies against human CII and antibodies against bovine CII (Fig. 1). The correlation was higher for patients with RA (R = 0.875) than for patients with PsA or OA (R = 0.556).
Figure 1.
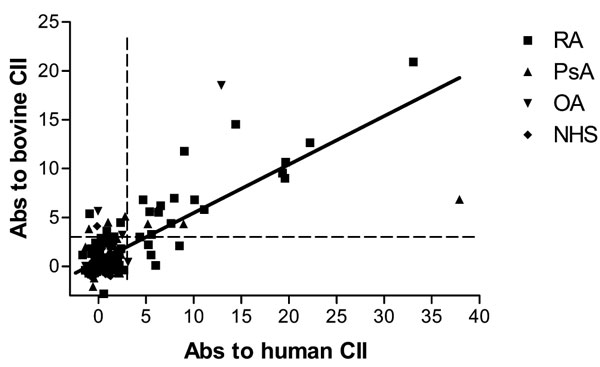
Correlation of antibodies against bovine type II collagen (CII) and human CII. Scatter plot of the levels of IgG antibodies against human CII and bovine CII in sera of patients with rheumatoid arthritis (RA; n = 94), psoriatic arthritis (PsA; n = 33), osteoarthritis (OA; n = 31), and normal human serum (NHS; n = 93). There is a highly significant correlation between the levels of antibodies against human CII and bovine CII (R = 0.777, P < 0.001). The points represent for individual sera the number of standard deviations above the mean of normal sera for which the mean is 0. A serum is regarded as positive if it is more than three standard deviations above the mean (dashed lines).
Purified CB peptides of bovine CII
After digestion of bovine CII with CNBr, the CB peptides were separated on a carboxymethylcellulose column, using an increasing salt gradient (Fig. 2a). The purity of the peptides after chromatography was then checked by SDS-PAGE (Fig. 2b). After purification and renaturation of the CB peptides by stepwise cooling, circular dichroism was used to confirm that, upon renaturation, the CB peptides had adopted a correctly folded triple-helical conformation. Circular dichroism analysis of CB10 showed a large positive ellipticity peak at 220–225 nm, as reported for the native CII triple helix, that was lost on denaturation by heat (Fig. 3a) [25]. Unfolding of CB10 started at about 20°C, as shown by a decrease in the positive peak at 222 nm with increasing temperature (Fig. 3b). These data confirm that, after renaturation, CB10 assumes a triple-helical, collagen-like conformation. Similar results were obtained for CB11 (data not shown). By ELISA, mAb M2139, which recognizes native CB10 of CII, reacted strongly with the renatured CB10 but not renatured CB11 (Fig. 4a), whereas mAb CII-C1, which recognizes native CB11 of CII, reacted strongly with CB11 but not CB10 (Fig. 4b). On denaturation of CB10 and CB11, there was a loss of reactivity of mAbs M2139 and CII-CI (Fig. 4).
Figure 2.

Purification of CB peptides of type II collagen (CII). (a) Carboxymethylcellulose chromatogram and corresponding Coomassie blue protein stain illustrating the separation of the CB peptides of bovine CII with a linear salt gradient (dotted line). (b) Coomassie blue staining of purified CB peptides of bovine CII separated on a 15% Tricine gel.
Figure 3.

Circular dichroism (CD) spectroscopy of CB10. (a) CD analysis of CB10 (bold) showing positive ellipticity peak at 220–225 nm that was lost after heat denaturation (non-bold). (b) Thermal melting profile of CB10 followed at 222 nm. At about 20°C, unfolding of CB10 started.
Figure 4.

Reactivity of monoclonal antibodies (mAbs) to CB10 and CB11. (a) mAb M2139, reactive with native CB10; (b) mAb CII-C1, reactive with native CB11. mAbs were tested by enzyme-linked immunosorbent assay for reactivity on purified renatured CB10 (CB10), purified renatured CB11 (CB11), denatured CB10 (dCB10), and denatured CB11 (dCB11).
Antibodies against the purified CB10 and CB11 of CII as measured by ELISA
IgG antibodies against bovine CB10 were present in 84 (88%) of 96 patients with RA, 4 (12%) of 33 patients with PsA, 2 (6%) of 34 patients with OA, and 3 (3%) of 93 control sera (Fig. 5a). Moreover, levels of antibodies against CB10 were significantly higher in RA sera than in PsA, OA, and control sera (P < 0.0001). In fact, the four anti-CB10-positive PsA patients had levels between 3 and 4 SD above the mean. The frequency of IgG antibodies against bovine CB11 was lower, but still substantial, at 48 (50%) of 96 patients with RA (Fig. 5b), but only 2 (6%) of 33 patients with PsA, 7 (21%) of 34 patients with OA, and 2 (2%) of 93 control sera (Fig. 5b). The concentrations of antibodies against CB11 were significantly higher in RA than in other diseases (P < 0.0001).
Figure 5.

Levels of antibodies against CB10 (a) and CB11 (b) of bovine type II collagen in patients with rheumatoid arthritis (RA; n = 96), psoriatic arthritis (PsA; n = 33), osteoarthritis (OA; n = 34) and in normal human serum (NHS; n = 93). The points represent, for individual sera, the number of standard deviations (SD) above the mean of normal sera, for which the mean is 0. A serum is regarded as positive if it is 3SD or more above the mean (dashed line).
The sensitivity of the test for antibodies in RA was increased from 24% to 88% when the intact CII molecule was replaced by CB10 and, notably, this was not at the expense of specificity, which was 94% when CB10 was used.
Discussion
The most significant finding from our study was that in RA the use in an ELISA of fragments of the intact CII molecule, particularly CB10, as antigen markedly increased the sensitivity of detection of antibodies in serum against NCII from 24% to 88%. Moreover, this increase in sensitivity was not at the expense of the specificity, which remained high at 94%. The current study used CB peptides purified from bovine CII. Although obtaining sufficient human CII precluded studies on the relative efficacy of human versus bovine CB10, our data on the use of intact CII suggest that human CB10 might be an even more precise reactant. There have been several studies reporting that the frequency of anti-NCII might be as high as 60–75% in patients with RA when tested very early in the disease [14-16], and thereafter frequencies tend to fall to those ascertained in the earlier cross-sectional studies [17]. If the same applies to anti-CB10, it is likely that collagen–related autoimmunity occurs during early initiating events in the development of RA, rather than later as a result of new epitopes being exposed as a result of articular damage. Our observations should therefore be applicable to routine diagnostic testing for anti-CII in articular diseases and, in particular, to those cases of early undifferentiated arthritis that do not fulfil criteria for RA but might later evolve to typical disease. Moreover, tests for anti-CB10 in conjunction with other assays including rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) could add further to diagnostic precision, although this remains to be tested.
During analysis, epitopes of CII are detected usually by Western blotting with CNBr digests of collagen. In RA, studies using CB peptides of CII showed reactivity to multiple components [6,16,26,27], and there was no apparent difference in the patterns of recognition of different CB peptides with sera from, for example, patients with RA or systemic lupus erythematosus [27], although anti-CII sera from patients with relapsing polychondritis react uniquely with CB9,7 [6]. Analytical methods alternative to immunoblotting on CB peptides have included epitope scanning of CB11 by using overlapping sequential synthetic peptides. This revealed as many as 21 different antibody-binding sites on CB11 with the use of individual sera from rats immunized with CII [28]. More recently, the use of recombinant chimeric preparations of CII has allowed the detection of conformation-dependent epitopes; it is of interest that antibodies against intact CII from patients with RA recognized several epitopes known to be arthritogenic for murine CIA, whereas anti-CII from patients with diverse diseases, OA, systemic lupus erythematosus, and relapsing polychondritis, reacted with other sites that lacked arthritogenic epitopes [29].
The above observations prompt inferences on the role of antibodies against CII in the pathogenesis of RA. Considering the CIA model, anti-CII are directly pathogenic, well exemplified by transfer experiments in mice [30-32]. In addition, CII-specific B cells in the diseased synovium, and in synovial fluids of patients with RA, produce IgG autoantibodies that react with native CII, suggesting an intra-articular antigen-driven immune process [3,4,33]. Presumably IgG antibodies form immune complexes with CII in the cartilage matrix and initiate the complement cascade to provoke an inflammatory reaction within the joint that causes degradation of the cartilage matrix, noting that purified anti-CII from RA serum activates complement C5 to C5a when bound to human cartilage in vitro [2]. Anti-CII in RA are predominantly of the complement-fixing IgG subclasses IgG1 and IgG3 [7,34], as found in CIA-susceptible mouse strains [35], whereas in the other rheumatic diseases, in which anti-NCII are present, the non-complement fixing subclasses IgG2 and IgG4 are predominant. Furthermore, anti-CII, when bound to cartilage in vitro, induces degradation and phagocytosis by leukocytes of the collagen/proteoglycan matrix [36]. These observations, including the C5 activation analyses, provide persuasive evidence that cartilage-bound anti-CII can participate in the initiation or perpetuation of RA. In view of the particular attention given to the role of T lymphocytes among effector mechanisms in RA, these observations on antibody-dependent damage are germane; further, they are not limited to anti-CII, because antibody transfer with sera induces a profound inflammatory arthritis in naive recipients in the murine K/BxN model of RA in which an entirely different autoantigenic molecule is implicated [37].
Conclusion
Current immunoassays to detect antibodies against intact CII have insufficient specificity and/or sensitivity to be useful for the diagnosis of RA. However, by using as substrate the specific cleavage product CB10 of the CII molecule in an ELISA, antibodies were detectable in as many as 88% of patients with RA, without any loss of specificity. CB10 is therefore a more sensitive substrate than the intact collagen molecule and, combined with other assays (rheumatoid factor, anti-cyclic citrullinated peptide) could well comprise a panel with a highly reliable predictive value. Moreover, it might also aid our understanding of the role of collagen autoimmunity in the pathogenesis of RA.
Competing interests
MR has been paid by ThermElectron Corporation to perform further work directed to kit development over the past 2 years. Results reported in this manuscript are the subject of Australian Patent No. 762471 Montech Medical Developments Pty Ltd and Rondole Pty Ltd: 'Method for the diagnosis of rheumatoid arthritis'; inventors: AC, MR and IM.
Abbreviations
CIA = collagen-induced arthritis; CII = type II collagen; CNBr = cyanogen bromide; ELISA = enzyme-linked immunosorbent assay; mAb = monoclonal antibody; NCII = native type II collagen; NHS = normal human serum; OA = osteoarthritis; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SD = standard deviation.
Acknowledgments
Acknowledgements
We thank Ms Veronica Glattauer (CSIRO) for her assistance in the purification of the collagen fragments, and the rheumatologists at the Royal Melbourne Hospital and Monash Medical Centre for providing the sera used in this study. Financial support was from Rondole Pty Ltd, under the Australian Federal Government Syndicated Research and Development Scheme.
Contributor Information
Andrew D Cook, Email: adcook@unimelb.edu.au.
Robyn Gray, Email: Robyn.Gray@med.monash.edu.au.
John Ramshaw, Email: John.Ramshaw@csiro.au.
Ian R Mackay, Email: Ian.Mackay@med.monash.edu.au.
Merrill J Rowley, Email: Merrill.Rowley@med.monash.edu.au.
References
- Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Watson WC, Brown PS, Pitcock JA, Townes AS. Passive transfer studies with type II collagen antibody in B10.D2/old and new line and C57Bl/6 normal and beige (Chediak–Higashi) strains: evidence of important roles for C5 and multiple inflammatory cell types in the development of erosive arthritis. Arthritis Rheum. 1987;30:460–465. doi: 10.1002/art.1780300418. [DOI] [PubMed] [Google Scholar]
- Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989;32:1087–1092. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- Rudolphi U, Rzepka R, Batsford S, Kaufmann SH, von der Mark K, Peter HH, Melchers I. The B cell repertoire of patients with rheumatoid arthritis. II. Increased frequencies of IgG+ and IgA+ B cells specific for mycobacterial heat-shock protein 60 or human type II collagen in synovial fluid and tissue. Arthritis Rheum. 1997;40:1409–1419. doi: 10.1002/art.1780400808. [DOI] [PubMed] [Google Scholar]
- Morgan K, Clague RB, Collins I, Ayad S, Phinn SD, Holt PJ. Incidence of antibodies to native and denatured cartilage collagens (types II, IX, and XI) and to type I collagen in rheumatoid arthritis. Ann Rheum Dis. 1987;46:902–907. doi: 10.1136/ard.46.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terato K, Shimozuru Y, Katayama K, Takemitsu Y, Yamashita I, Miyatsu M, Fujii K, Sagara M, Kobayashi S, Goto M, et al. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990;33:1493–1500. doi: 10.1002/art.1780331006. [DOI] [PubMed] [Google Scholar]
- Cook AD, Mackay IR, Cicuttini FM, Rowley MJ. IgG subclasses of antibodies to type II collagen in rheumatoid arthritis differ from those in systemic lupus erythematosus and other connective tissue diseases. J Rheumatol. 1997;24:2090–2096. [PubMed] [Google Scholar]
- Cook AD, Stockman A, Brand CA, Tait BD, Mackay IR, Muirden KD, Bernard CC, Rowley MJ. Antibodies to type II collagen and HLA disease susceptibility markers in rheumatoid arthritis. Arthritis Rheum. 1999;42:2569–2576. doi: 10.1002/1529-0131(199912)42:12<2569::AID-ANR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Kammer GM, McCune WJ, David JR. Autoimmunity to collagen: a shared feature of psoriatic and rheumatoid arthritis. Arthritis Rheum. 1981;24:1363–1369. doi: 10.1002/art.1780241105. [DOI] [PubMed] [Google Scholar]
- Choi EK, Gatenby PA, McGill NW, Bateman JF, Cole WG, York JR. Autoantibodies to type II collagen: occurrence in rheumatoid arthritis, other arthritides, autoimmune connective tissue diseases, and chronic inflammatory syndromes. Ann Rheum Dis. 1988;47:313–322. doi: 10.1136/ard.47.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charriere G, Hartmann DJ, Vignon E, Ronziere MC, Herbage D, Ville G. Antibodies to types I, II, IX, and XI collagen in the serum of patients with rheumatic diseases. Arthritis Rheum. 1988;31:325–332. doi: 10.1002/art.1780310303. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Gershwin ME, Mackay IR. Collagen antibodies in juvenile arthritis and adult rheumatoid arthritis: differences in levels and type-specificity. J Rheumatol. 1988;15:289–294. [PubMed] [Google Scholar]
- Choi EK, Gatenby PA, Bateman JF, Cole WG. Antibodies to type II collagen in SLE: a role in the pathogenesis of deforming arthritis? Immunol Cell Biol. 1990;68:27–31. doi: 10.1038/icb.1990.4. [DOI] [PubMed] [Google Scholar]
- Pereira RS, Black CM, Duance VC, Jones VE, Jacoby RK, Welsh KI. Disappearing collagen antibodies in rheumatoid arthritis. Lancet. 1985;2:501–502. doi: 10.1016/S0140-6736(85)90436-2. [DOI] [PubMed] [Google Scholar]
- Fujii K, Tsuji M, Kitamura A, Murota K. The diagnostic significance of anti-type II collagen antibody assay in rheumatoid arthritis. Int Orthop. 1992;16:272–276. doi: 10.1007/BF00182710. [DOI] [PubMed] [Google Scholar]
- Cook AD, Rowley MJ, Stockman A, Muirden KD, Mackay IR. Specificity of antibodies to type II collagen in early rheumatoid arthritis. J Rheumatol. 1994;21:1186–1191. [PubMed] [Google Scholar]
- Cook AD, Rowley MJ, Mackay IR, Gough A, Emery P. Antibodies to type II collagen in early rheumatoid arthritis. Correlation with disease progression. Arthritis Rheum. 1996;39:1720–1727. doi: 10.1002/art.1780391015. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Sherritt MA, Tait B, Varney M, Kanaan C, Stockman A, Mackay IR, Muirden K, Bernard CC, Rowley MJ. Immunosusceptibility genes in rheumatoid arthritis. Hum Immunol. 1996;51:32–40. doi: 10.1016/S0198-8859(96)00204-2. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Stockman A, Brand CA, Tait BD, Rowley GL, Sherritt MA, Mackay IR, Muirden KD, Bernard CC. The effect of HLA-DRB1 disease susceptibility markers on the expression of RA. Scand J Rheumatol. 1997;26:448–455. doi: 10.3109/03009749709065718. [DOI] [PubMed] [Google Scholar]
- Scott PG, Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4:107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Lunde LG. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973;12:3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Terato K, Cremer MA, Hasty KA, Kang AH, Hasty DL, Townes AS. Physicochemical and immunological studies of the renatured alpha 1(II) chains and isolated cyanogen bromide peptides of type II collagen. Collagen Relat Res. 1985;5:469–480. doi: 10.1016/s0174-173x(85)80001-7. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Goodman M, Bhumralkar , Jefferson EA, Kwak J, Locardi E. Collagen mimetics. Biopolymers. 1998;47:127–142. doi: 10.1002/(SICI)1097-0282(1998)47:2<127::AID-BIP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Buckee C, Morgan K, Ayad S, Collins I, Clague RB, Holt PJ. Diversity of antibodies to type II collagen in patients with rheumatoid arthritis: detection by binding to alpha-chains and to cyanogen bromide peptides. Br J Rheumatol. 1990;29:254–258. doi: 10.1093/rheumatology/29.4.254. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Mackay IR, Brand CA, Bateman JF, Chan D. Epitope specificity of antibodies to type II collagen in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int. 1992;12:65–69. doi: 10.1007/BF00300979. [DOI] [PubMed] [Google Scholar]
- Worthington J, Brass A, Morgan K. Identification of antibody epitopes within the CB-11 peptide of type II collagen. I. Detection of antibody binding sites by epitope scanning. Autoimmunity. 1991;10:201–207. doi: 10.3109/08916939109001890. [DOI] [PubMed] [Google Scholar]
- Burkhardt H, Koller T, Engstrom A, Nandakumar KS, Turnay J, Kraetsch HG, Kalden JR, Holmdahl R. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 2002;46:2339–2348. doi: 10.1002/art.10472. [DOI] [PubMed] [Google Scholar]
- Kerwar SS, Englert ME, McReynolds RA, Landes MJ, Lloyd JM, Oronsky AL, Wilson FJ. Type II collagen-induced arthritis. Studies with purified anticollagen immunoglobulin. Arthritis Rheum. 1983;26:1120–1131. doi: 10.1002/art.1780260910. [DOI] [PubMed] [Google Scholar]
- Loutis N, Bruckner P, Pataki A. Induction of erosive arthritis in mice after passive transfer of anti-type II collagen antibodies. Agents Actions. 1988;25:352–359. doi: 10.1007/BF01965042. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Larsson A, Jonsson R. Arthritis in DBA/1 mice induced with passively transferred type II collagen immune serum. Immunohistopathology and serum levels of anti-type II collagen auto-antibodies. Scand J Immunol. 1990;31:147–157. doi: 10.1111/j.1365-3083.1990.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Ronnelid J, Lysholm J, Engstrom-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–1029. doi: 10.1002/art.1780370707. [DOI] [PubMed] [Google Scholar]
- Collins I, Morgan K, Clague RB, Brenchley PE, Holt PJ. IgG subclass distribution of antinative type II collagen and antidenatured type II collagen antibodies in patients with rheumatoid arthritis. J Rheumatol. 1988;15:770–774. [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Jasin HE, Taurog JD. Mechanisms of disruption of the articular cartilage surface in inflammation. Neutrophil elastase increases availability of collagen type II epitopes for binding with antibody on the surface of articular cartilage. J Clin Invest. 1991;87:1531–1536. doi: 10.1172/JCI115164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]


