Abstract
Background
Chronic rhinosinusitis (CRS) is a highly prevalent inflammatory condition, with significant effects on morbidity and quality of life. Given other chronic inflammatory conditions have been associated with increased mortality risk, we sought to evaluate the relationship between mortality and CRS including the influence of asthma. Our objective was to determine if CRS, with or without asthma, is associated with altered risk of mortality.
Methods
Using a statewide population database, we retrospectively identified 27,005 patients diagnosed with CRS between 1996 and 2012, and 134,440 unaffected controls matched 5:1 on birth year and sex. Risk of mortality was determined from Cox models and Kaplan-Meier curves were used to compare survival.
Results
A significant interaction between CRS and asthma status was observed in which CRS appeared to confer a protective effect in asthma patients. Asthma, when present, increased mortality in CRS-negative controls (P-interaction <0.0001). Independent of asthma status, CRS patients exhibited a decreased mortality risk (HR=0.80, 95%CI 0.74-0.85) compared to controls. However, in patients diagnosed at or before the median age of CRS onset (42y) independent of asthma status, survival was not improved (HR=0.98, 95%CI 0.81-1.18). Risk of mortality was greater in CRS with nasal polyps (n=1643) compared to 25,362 polyp-negative CRS patients (HR=1.38, 95%CI 1.09–1.77).
Conclusions
CRS was associated with lower risk of mortality compared to controls, and appeared to mitigate increased mortality from asthma. We posit that better survival conferred by CRS may be secondary to treatment. However, the etiology of this relationship and the effect of CRS treatment on mortality are unknown.
Keywords: Chronic rhinosinusitis, Asthma, Quality of Life
Introduction and Objectives
Chronic rhinosinusitis (CRS) is a chronic inflammatory condition of the nasal cavity and paranasal sinuses.1 This inflammation is associated with common rhinologic symptoms of congestion, nasal drainage, facial pressure/pain, and loss of smell which results in dramatic reductions in overall well-being and quality-of-life (QOL). Current treatments for CRS are aimed at reducing inflammation and its associated local effects with improvements in QOL. Recent investigations suggest that the health impacts of CRS extend beyond the sinuses and can impact basic functions including sleep,2,3 cognition,4 and mood.5 It has been hypothesized that these extra-rhinologic symptoms are due to a sophisticated and intertwined communication between the central nervous system and the immune system.6 While it is clear that sinonasal inflammation in CRS is associated with far-ranging effects, the underlying mechanisms of these effects remain unknown.
Chronic inflammation plays a significant role in CRS and many other chronic inflammatory diseases including asthma, inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). Long lasting uncontrolled inflammation in these conditions appears to have systemic consequences; patients with asthma, RA, and IBD have an increased risk of stroke, myocardial infarction (MI), and increased mortality.7-9 There is also an increased risk of cardiovascular death during periods of uncontrolled inflammation, suggesting a systemic effect is driving the increased mortality risk.10 It is unknown if patients with CRS have increased mortality, although, several studies have shown an increased risk of stroke and MI in patients with CRS.11,12
In as much as CRS is a chronic inflammatory state with symptoms and comorbidity that extend beyond the sinuses, we hypothesized that CRS would be associated with increased mortality. Moreover, we hypothesized that this increase in mortality would be independent of the increased mortality that would be expected from concomitant asthma. We also questioned whether the two most commonly recognized phenotypes in CRS, CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), had differing impacts on mortality.
Methods
The Utah Population Database (UPDB)
The UPDB at The University of Utah consists of over 27 million data records representing the life course of 8 million individuals in Utah. It is the only database of its kind in the United States (http://healthcare.utah.edu/huntsmancancerinstitute/research/updb/). The resource includes several decades of statewide vital records and medical diagnoses since the mid-1990's, linked to extensive genealogy data. Approvals to conduct this study were received from The University of Utah Institutional Review Board and the Resource for Genetic and Epidemiologic Research.
Study population
Using the UPDB, we identified a case-cohort of 33,023 CRS patients using International Classification of Diseases, ninth revision, (ICD-9) and Current Procedural Terminology (CPT) codes from all inpatient and ambulatory surgery facilities in Utah. Of these patients, 27,005 were age 18 and older and had adequate follow up over the study period. To form an unaffected cohort for comparison, 134,440 controls with no history of CRS (defined below) were randomly selected from the Utah population and matched to CRS cases at a target ratio of 5:1 on birth year, sex, and born in Utah or outside of the state. To appropriately match exposure periods, a control had to have follow-up (known to reside in Utah) at least as long as the diagnosis date of their respective case as previously described.
Exposure status
We identified patients age 18 or older at index diagnosis of CRS as previously described.13 Briefly, CRS patients were identified based on ICD-9 code 473.x appearing in the medical record. Patients without a diagnosis of nasal polyps, defined as an absence of ICD-9 code 471.x, were considered CRSsNP and those with this code were considered CRSwNP. We incorporated CPT codes for diagnostic endoscopy (CPT codes 31231 and 31233) and sinus surgery CPT codes, 31237, 31254, 31255, 31256, 31276, and 31287 to increase the probability of accurately identifying CRS. In addition, CPT 31237 (nasal polypectomy) was used to distinguish CRS with nasal polyps (CRSwNP) from CRS without nasal polyps (CRSsNP). Following identification of these patients, a chart review of 79 patients seen in The University of Utah Health Care system was performed to compare diagnoses based on diagnostic and procedural codes from medical claims data to the ‘gold standard’ of clinical records. The authors achieved a positive predictive value (PPV) of 90% for those patients with CRS with or without nasal polyps. The accuracy using billing codes available in the UPDB to identify CRSsNP from CRSwNP was 71% based on chart review.
Patients with a CRS diagnosis were excluded from the analysis if a predisposing condition was present in the diagnostic record. These relatively rare conditions included cystic fibrosis, inverted papilloma, facial trauma, intracranial or orbital abscess, injury to the blood vessels of the head and neck, cerebrospinal fluid (CSF) leak, granulomatosis with polyangitis, sarcoidosis, Churg-Strauss syndrome, any HIV illness, deficiency of humoral or cell-mediated immunity, combined or unspecified immunity deficiency, as well as autoimmune disease not classified elsewhere; see Supplemental Table 1 for conditions and corresponding ICD-9 codes (Table S1). The same exclusion criteria were applied to the control cohort.
Covariates
In addition to CRS and asthma status (presence of ICD-9 diagnosis code 493.x),14 we obtained data for potential covariates including age at index diagnosis of CRS (controls were assigned the same ‘diagnosis age’ as their respective case), race/ethnicity, and education level as proxy for socioeconomic status (SES). As age in general is associated with mortality, we controlled for age by matching controls to cases on birth year. The matching variable of sex was likewise controlled for in our analyses. During model specification, self-reported race was initially considered as a potential covariate in our primarily non-Hispanic and white population, but demonstrated no difference in CRS-related mortality between white and non-white patients and was not included in the model. Likewise, the majority of the Utah population is well educated (high-school graduate or above), so education level did not substantively impact estimates and was not incorporated.
Outcomes
The primary outcome evaluated was all-cause mortality based on Utah death certificates and other sources of death data in the UPDB. If deceased prior to year-end 2014, survival time was measured as time from an index diagnosis of CRS on or after January 1, 1996 until date of death. Otherwise, survival time was measured from diagnosis to last follow-up date known to be alive or study-end (December 31, 2014), whichever occurred earlier and observations were right-censored as the outcome did not occur. Survival times in controls were measured from the CRS diagnosis date of their corresponding matched case.
Comparisons
Comparisons were made between all CRS patients and controls, and in the subset of CRS patients and controls that had no history of an asthma diagnosis. Patients stratified by CRSwNP and CRSsNP compared to their respective matched controls, and CRSwNP compared to CRSsNP in a ‘case-case’ analysis. Stratified comparisons of cases to controls within quantiles and median diagnosis-age groups were also conducted. Cox models were used to compare mortality differences between groups as described below.
Statistical Analysis
Cox proportional hazards models were used to examine the relationship between survival time and CRS status (affected ‘case’ patients or unaffected controls). We divided the data into quantiles (Q1:18-31 Q2:32-42 Q3:43-54 Q4:55-96) based on age of diagnosis. Each quantile was allowed to have a different baseline hazard function, while the coefficients of the remaining covariates were assumed to be constant across quantiles. Hazards ratios (HR) and 95% confidence intervals (95%CI) were estimated from: a main-effects model, including covariate adjustment for sex and asthma status (diagnosis present or absent) (Model I); and, a model including sex, asthma status, and a multiplicative interaction variable of CRS and asthma status (Model II). We tested the proportional hazards assumption for each term in the model by creating a generalized linear regression of the scaled Schoenfeld residuals as a function of time. We examined p-values of the Pearson product-moment correlation between the scaled residuals and time for each covariate, with a P >0.05 indicating non-violation of the proportionality assumption. We verified the proportionality assumption for each covariate by plotting the corresponding scaled Schoenfeld residuals against transformation of time by the Kaplan-Meier (K-M) estimator, and visually inspected a plot of the residuals for a zero or near-zero slope. The K-M method was used to create all the survival curves to examine the estimated distribution of survival times. All statistical analysis was performed using R version 3.2.2 for Windows.
Results
After applying our inclusion and exclusion criteria, we identified a total of 27,005 patients with CRS, as well as 134,440 unaffected controls matched 5:1 on birth year and sex. Of those identified to have CRS, 1,643 had diagnosed polyps (CRSwNP) and 25,362 had no diagnosis of polyps (CRSsNP). The median age at diagnosis of CRS was 42 years. A slightly higher proportion of patients with CRS were high school graduates or above (77.0%) compared to controls (67.2%), for those in which data were available. Characteristics of CRS patients and controls are shown in Table 1.
Table 1. Characteristics of CRS patients and 5:1 controls.
| Characteristic | CRS cases | Controls |
|---|---|---|
| Total, N. | 27,005 | 134,440 |
| Men, N (%) | 13,039 (48.3) | 64,885 (48.3) |
| Women, N (%) | 13,966 (51.7) | 69,555 (51.7) |
| Race, Na | 26,032 | 92,489 |
| White, N (%) | 25,447 (97.8) | 89,666 (96.9) |
| Nonwhite, N (%) | 585 (2.3) | 2823 (3.1) |
| Education Levelb | ||
| High School and above | 20,782 (99.5) | 90,344 (98.5) |
| Less than High School | 112 (0.5) | 1392 (1.5) |
| Asthma, N (%) | 7,047 (26.1) | 10,067 (7.5) |
| CRSsNP, N (%) | 25,362 (93.9) | |
| CRSwNP, N (%) | 1,643 (6.1) | |
| Age at diagnosis/selection, mean (SD), y | 42.91 (15.0) | 42.92 (15.0) |
| Follow-up time, mean (SD), y | 8.58 (4.4) | 8.53(4.4) |
| Dead on 12/31/2014, N (%) | 884 (3.3) | 5,679 (4.2) |
Abbreviations: CRS, chronic rhinosinusitis; y, years; SD, standard deviation.
Number is less than total due to missing race/ethnicity data for some patients.
Based on highest level attained, available for 20,894 cases and 91,736 controls.
In patients with CRS compared to population controls, we observed a reduction in all-cause mortality in both a main effects (HR = 0.70, 95%CI 0.66-0.74) and interaction model (HR = 0.80, 95%CI 0.74-0.85); see Figure 1. As a multiplicative interaction term was significant (P<0.0001), we will focus on the results of Model II in Figure 1 as follows. CRS patients diagnosed up to the median age of 42 compared to age-matched controls (see Table 2 for characteristics of study subjects stratified by diagnosis age) did not exhibit a reduced mortality (Figure 1, Model II.a.1: HR 0.98, 95%CI 0.81 – 1.18). After accounting for an association of diagnosis age to mortality in our model, asthma was independently associated with increased risk of mortality (HR = 1.75, 95% CI 1.65 – 1.87) as expected, as was male gender (HR = 1.39, 95%CI 1.34-1.45). This relationship was further explored by removing subjects with a history of asthma from both CRS cases and controls, and in asthma-negative patients a similar reduction in mortality was observed (Model I.a: HR = 0.76, 95%CI 0.71-0.81. In a ‘case-only’ analysis, there was a significant difference in mortality risk between CRSwNP and CRSsNP (Model II.b: HR = 1.39, 95%CI 1.09 – 1.77).
Figure 1.
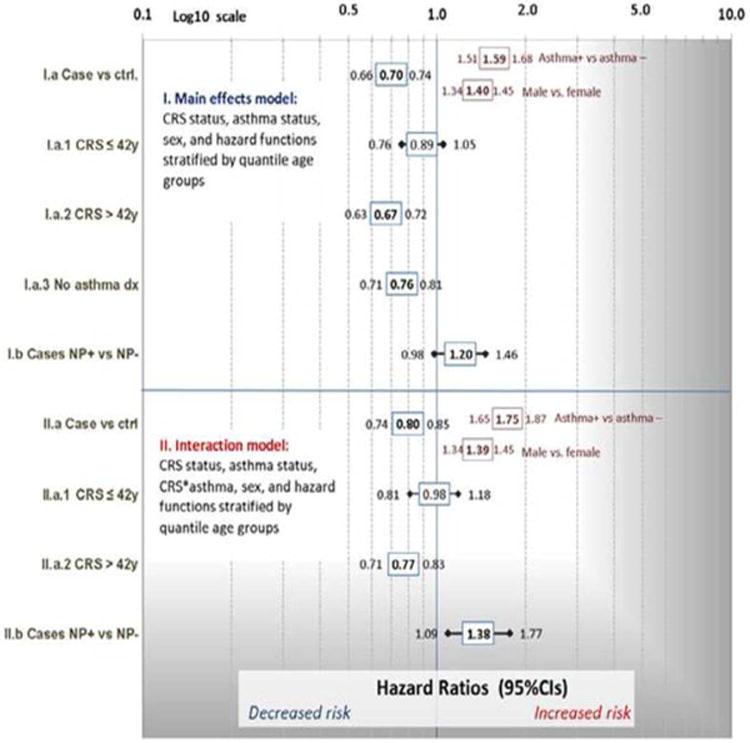
Estimated risk of mortality in chronic rhinosinusitis (CRS) cases compared to controls, from Cox models, hazard ratios for: (I) main effects only, and (II) model including multiplicative interaction of CRS and asthma status. Case-case comparison (CRS with and without polyps) is also shown. All bolded values were significant at P<0.0001. Independent risk factors of asthma and sex covariates are shown for models I.a and II.a. Red highlight indicates model variables that were significant independent of CRS status (or in the interaction model, independent of CRS status AND interaction of CRS and asthma).
Table 2. Comparison of characteristics between CRS and control patients diagnosed at age ≤42 years (median), and those diagnosed at age >42 years.
| Characteristic | Diagnosis Age: ≤ or > median (42 yo) | |||
|---|---|---|---|---|
| ≤42 yo | >42 yo | |||
| CRS | Control | CRS | Control | |
| Total, N | 13,579 | 67,670 | 13,426 | 66,770 |
| Men, N (%) | 6360 (46.8) | 31,685(46.8) | 6679 (49.7) | 33,200 (49.7) |
| Women, N (%) | 7219 (53.2) | 35,985(53.2) | 6747 (50.3) | 33,570 (50.3) |
| Race, Na | 13,117 | 46,774 | 13,426 | 66,770 |
| White, N (%) | 12,769(97.3) | 45,129(96.5) | 12,678(98.2) | 44,537(97.4) |
| Nonwhite, N (%) | 348 (2.7) | 1645 (3.5) | 237 (1.8) | 1178 (2.6) |
| Age at diagnosis/selection, mean (SD), y | 31.0 (6.80) | 31.1 (6.76) | 56.1 (9.71) | 56.1 (9.67) |
| Cohort time, mean (SD), y | 9.03 (4.43) | 9.05 (4.43) | 8.49 (4.37) | 8.37 (4.41) |
| Dead on 12/31/2014, N (%) | 202 (1.5) | 911 (1.3) | 1107 (8.2) | 6744 (10.1) |
Abbreviations: CRS, chronic rhinosinusitis; y, years; yo, years old;
Number is less than total due to missing race/ethnicity data for some patients.
Kaplan-Meier curves comparing survival between CRS cases and unaffected controls over a nearly 20-year period are shown in Figures 2 and 3. In Figure 2, subjects without a history of asthma (affected CRS cases and CRS-negative controls) stratified by median diagnosis age are shown. Including all subjects with a diagnosis of asthma, the same comparison stratified by age at diagnosis is shown in Figure 3.
Figure 2. Kaplan-Meier survival plot by diagnosis age above or below the median age without asthma.
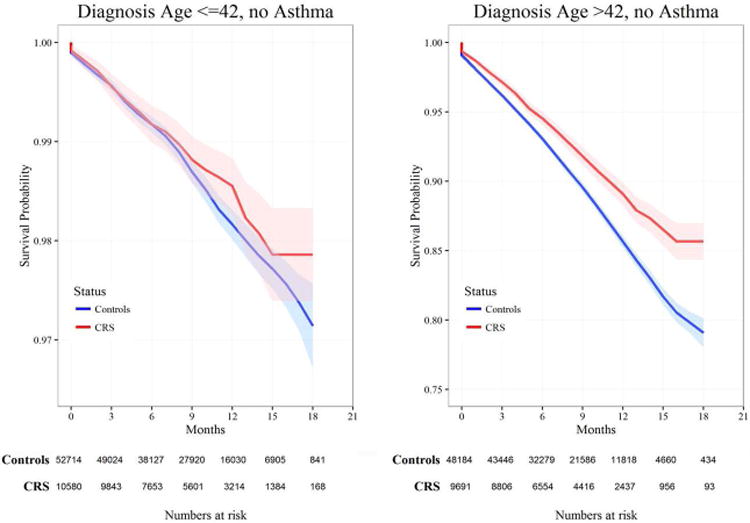
The percent survival of patients is indicated by the y-axis and years of time followed is indicated on the x-axis. Left panel demonstrates diagnosis at age ≤42 years, versus right panel diagnosis at age >42 years. Red line indicates the CRS cases and the blue line indicates the unaffected controls. Light colored shading area around the lines indicates the range of the 95%CI for the respective group.
Figure 3. Kaplan-Meier survival plot by diagnosis age above or below the median age with asthma.
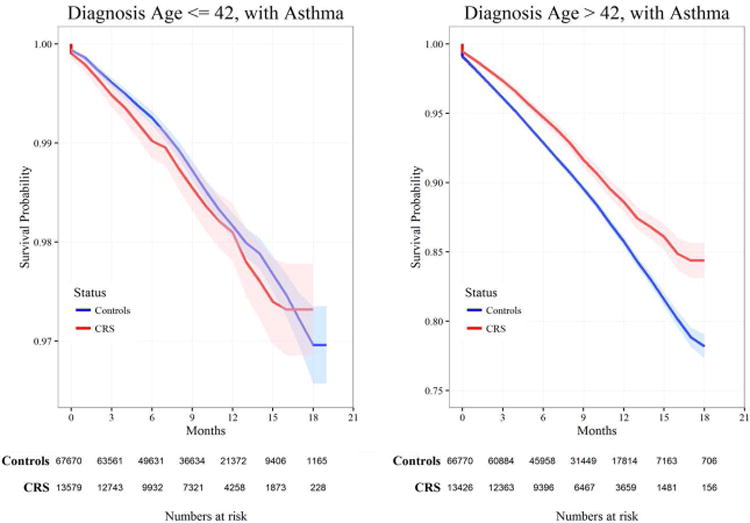
The percent survival of patients is indicated by the y-axis and years of time followed is indicated on the x-axis. Left panel demonstrates diagnosis at age ≤42 years, versus right panel diagnosis at age >42 years. Red line indicates the CRS cases and the blue line indicates the unaffected controls. Light colored shading area around the lines indicates the range of the 95%CI for the respective group.
To demonstrate the characteristics of the interaction between CRS status and asthma status, the Cox model coefficients corresponding to the HR for the main effects of CRS or asthma and their interaction compared to the reference group (CRS-negative controls who were asthma-negative) are shown in Figure 4. In unaffected controls, plotting the line between point-coefficients for asthma-negative and asthma-positive status shows a sharply increased slope (greater mortality risk) whereas in CRS cases, the line between coefficients for asthma-negative and asthma-positive exhibits a much less pronounced decrease in slope, indicating slightly lower mortality risk. A test of the difference in the slopes between CRS cases and controls was significant (P<0.05).
Figure 4.
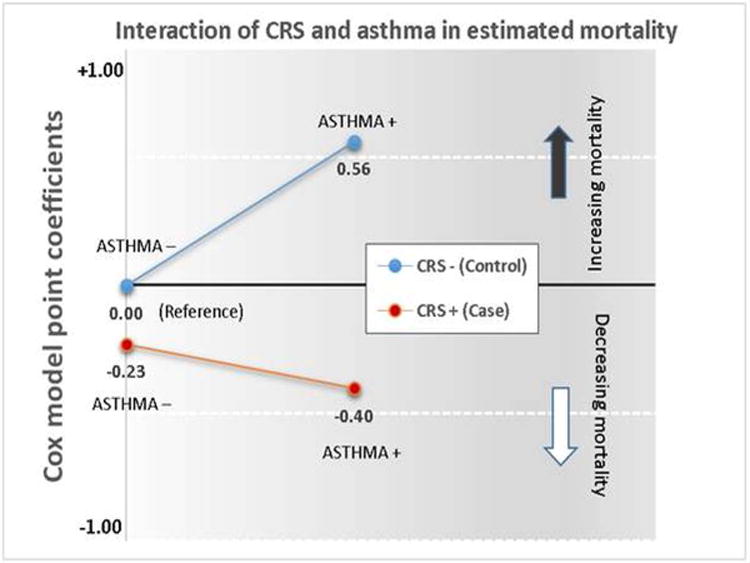
Point coefficients corresponding to an interaction of CRS and asthma status (negative or positive). Reference point is (0.00), CRS-negative (controls) and asthma-negative. A comparison between cases and controls is shown of a line representing the slope from asthma-negative to asthma-positive status.
Diagnosis age was also further stratified into quantiles to further refine the effect of diagnosis age on mortality. The two quantiles below the median both showed no change in mortality of CRS patients compared to controls while both quantiles above the median demonstrated a decrease in mortality for CRS patients compared to controls. Diagnosis age quantiles: 1) 18-31 years old, HR = 0.98 (95% CI 0.70 – 1.36); 2) ages 32-42 years, HR = 0.98 (95% CI 0.78 – 1.23); 3) ages 43-54 years, HR = 0.80 (95% CI 0.68 – 0.94); 4) and ages 55 years or older, HR = 0.76 (95% CI 0.70 – 0.83). Again, these results held true even when CRS patients and controls with asthma were removed from the analysis.
Treatment effect was examined to further define the effect surgery (CPT codes 31254, 31255, 31256, 31276, and 31287) had on mortality compared to controls. Patients with CRS who had surgery had significant improved mortality compared to controls HR = 0.78 (95% CI 0.73 – 0.82). For those patients who elected medical management (31231 and 31233) there was no significant improvement in mortality compared to controls HR = 1.11 (95% CI 0.84 – 1.46).
Discussion
Chronic inflammatory states have been associated with increased mortality. We hypothesized that CRS would be similarly associated with increased mortality. Contrary to our hypothesis, patients with CRS who were treated by an otolaryngologist (i.e. received nasal endoscopy and sinus surgery) had a reduced risk of mortality. Furthermore, this effect was more pronounced at later age of diagnosis and even appeared to counteract the increased mortality of asthma.
A reduction in mortality was seen for patients diagnosed with CRS at older age. There is no biologically plausible explanation for a protective effect of CRS at an older age, though there are multiple possible explanations for this relationship based on correlations with SES, healthcare access and utilization. Patients diagnosed with CRS in our study required both an ICD-9-CM diagnosis code of CRS as well as a CRS-related procedure. This selects for patients who have access to, and utilize care services, in order to have nasal endoscopy or other CRS-related procedures performed. Educational level is an established proxy measure of SES and a higher education level attained may be the best predictor of good health.15 Controls in our cohort were found to be less likely to graduate from high school, and therefore may have worse overall health than cases. However, the Utah population is highly educated, and of those whom education data was available, over 99% of cases and over 98% of controls had at least a high-school education. Although beyond the scope of this investigation, future studies to comprehensively assess SES and adequately control for differences between comparison groups may attenuate the mortality differences we observed between CRS and unaffected subjects. Primary care attributes of comprehensiveness, patient-centeredness, and enhanced access have been associated with decreased mortality.16 Longer wait times for healthcare have also been associated with increased mortality.17 Diagnosis later in life may be due to late onset of the disease, or mild disease, which was not severe enough to be diagnosed or prompt attention from an otolaryngologist earlier. Diagnosis later in life may also be associated with diagnosis and treatment for other medical conditions associated with an increased mortality risk, and therefore CRS diagnosis later in life may simply imply increased healthcare utilization.
Epidemiological and clinical studies have shown that CRS and asthma frequently coexist with a reported prevalence up to 38%.18,19 We found a prevalence of asthma in patients with CRS to be 26% compared to controls at 7.5%. Asthma is commonly associated with CRS and often symptom severity of the upper airways will parallel that of the lower airways. It is believed the etiology and pathogenic mechanisms underlying the development and progression of these 2 conditions largely overlap. Asthma is independently associated with increased risk of mortality due to inflammatory attacks, sustained long-term effects that result in chronic obstructive pulmonary disease, and cardiovascular disease.20 As expected, our data corroborate that asthma significantly increases mortality risk as seen both in the main effects and interaction models (p<0.0001). Surprisingly, however, we found that patients with both CRS and asthma had lower mortality than controls with asthma. Even more unexpectedly, we found a significant decrease in mortality for patients with CRS and asthma compared to those with CRS alone. It appears from these data that the presence of CRS exerted a “protective” effect, blunting and even reversing the increase in mortality attributable to asthma.
Because of the strict inclusion criteria, many of the CRS patients had sinus surgery defined by CPT codes (98% CRSsNP and 100% CRSwNP). With regard to the possible mechanisms whereby CRS appears to have overcome the mortality risk of asthma in controls, we cannot exclude the possibility of treatment effect. Effect of treatment, either surgical or medical, may be the most plausible explanation for our findings. Although preliminary, our data suggest that those patients whom elect surgical management have a significant improvement in mortality compared to controls. This protective effect was not seen in those who were grouped as medical management. The fact that patients underwent sinus surgery demonstrates access to medical care and attention to their upper (and most likely lower) airways. Inasmuch as the current study involves a database of medical records with diagnoses codes for asthma, all of these patients would have had some asthma treatment. Unfortunately, this population-level database investigation cannot differentiate among the intensity of asthma treatments among patients. It is possible that the asthmatics with CRS had more intensive management of their asthma than non-CRS asthmatics. CRS treatment may have had a sufficiently salutatory effect on the asthma that the increased risk associated with asthma was reversed. It has been demonstrated that sinus care improves asthma status in unified airway patients.21 This explanation seems more plausible than an unknown systemic effect of CRS that enhances longevity. Further work is needed to better understand these interactions.
More than 90% of the CRS patients in this study were CRSsNP. In the current investigation, CRSwNP patients were found to have an increase in mortality risk relative to that seen in CRSsNP patients. There is biologic plausibility for a relationship between the chronic inflammatory state of CRS and increased mortality risk seen in patients with CRSwNP. It is believed prolonged exposure to increased levels of pro-inflammatory cytokines results in systemic inflammation, and the increased mortality we observed. This is not a new concept as it is accepted that chronic inflammation in IBD, RA, and asthma is associated with increased cardiovascular risk, stroke, and death.7-9 A key player in inflammation seen in other inflammatory conditions is the cytokine interleukin-6 (IL-6).22,23 While IL-6 plays a protective role in the acute phase response, prolonged exposure and elevated levels tips the balance and causes a pro-inflammatory environment, which can cause apoptosis and cell death, with an increased risk of mortaity.24,25 Differential levels of IL-6 have been observed between CRSwNP, CRSsNP, and controls with a significant increase in IL-6 in those patients with CRSwNP.26,27 Nonetheless, it should be noted that others have shown lower levels of IL-6 protein in CRSwNP compared to CRSsNP.28 It is still unknown how the underlying pathophysiology of CRS including pro-inflammatory cytokines affects either morbidity and/or mortality.
As in any observational study, there is a potential for selection bias. We attempted to minimize any bias by matching controls to cases on sex and birth year, and by applying identical exclusion criteria to both groups. Although stringent criteria were used to define CRS cases in order to maximize accuracy, we do acknowledge that this method is associated with potential misclassification bias. Because CRS cases were required to have undergone a nasal endoscopy or a sinus surgery, the patients included in our study may have a more severe CRS phenotype and/or better access to surgical and/or specialty care. However, prior investigations have suggested that there are no differences in demographic characteristics (age, gender, or race/ethnicity), economic cofactors (income and medical payment coverage), clinical cofactors (smoking, depression, prior sinus surgery, allergic rhinitis, aspirin sensitivity, nasal polyps), measure of social support, personality, or the physician–patient relationship between those patients who elect surgical management versus those who elect medical management.29
It should be noted that the diagnoses of CRS using the methodology from Oakley et al., was determined using a subset (<1%) of the study population (25,838) and may not reflect the true accuracy of diagnosis in the entire patient cohort. Furthermore, the accuracy ((true positive + true negative) / (# positive + # negative)) of using billing codes available in the UPDB to define CRSwNP from CRSsNP was found to be 71% ((48+8) / (62+79))13 and should be considered when interpreting these results. We acknowledge that the true concordance between the CPT/ICD-9 code-based diagnosis and CRS as defined by clinical practice guidelines in our cohort is inherently limited by this methodology.
Conclusions
In patients with CRS defined by commonly used Otolaryngology CPT/ICD billing codes (i.e. nasal endoscopy and sinus surgery), CRS is associated with lower risk of mortality compared to controls, with a greater effect seen at later age of diagnosis. The lower risk of mortality seen with CRS appeared to mitigate and even overcome the increased mortality from asthma, a common CRS comorbidity. Surgical treatment was associated with a significant improvement in mortality compared to controls. We hypothesize that better survival conferred by CRS may be secondary to treatment. Further study is needed to elicit underlying clinical, socioeconomic, and/or biological mechanisms underlying this association between CRS and reduced mortality.
Supplementary Material
Acknowledgments
This study was supported with funding from The University of Utah School of Medicine, Department of Surgery, Division of Otolaryngology. The Pedigree and Population Resource funded by the Huntsman Cancer Foundation, supported use of the Utah Population Database (UPDB). The UPDB has also received support from the National Cancer Institute (NCI), but the content of this study is the responsibility of the authors and does not necessarily represent the views of the NCI or the National Institutes of Health. No additional outside funding or support was used.
Jeremiah A. Alt, is supported by grants from the University of Utah Program in Personalized Health and the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number KL2TR001065 and from the National Institute of Allergy and Infectious Diseases under Award Number 1R43AI126987, and a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R01 DC005805; PI/PD: TL Smith). Richard R. Orlandi is a consultant for IntersectENT, Inc. Jeremiah A. Alt and Richard R. Orlandi are consultants for Medtronic, Inc. Jeremiah A. Alt is a consultant for GlycoMira Therapeutics Inc. and Spirox. Luke Rudmik, Scientific advisory board for BioInspire Inc. and 480 Biomedical Inc.
Footnotes
Presented at the annual American Rhinologic Society meeting during the 119th annual Combined Otolaryngologic Spring Meeting (COSM) in Chicago, IL, May 18-22nd, 2016.
Conflict of Interest: None of which were affiliated with this investigation
References
- 1.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 2.Alt JA, Smith TL, Schlosser RJ, et al. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123:2364–2370. doi: 10.1002/lary.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarasidis GS, DeConde AS, Mace JC, et al. Cognitive dysfunction associated with pain and quality of life in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015 doi: 10.1002/alr.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox DR, Ashby S, DeConde AS, et al. Dyad of pain and depression in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:308–314. doi: 10.1002/alr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alt JA, Smith TL. Chronic rhinosinusitis and sleep: a contemporary review. Int Forum Allergy Rhinol. 2013;3:941–949. doi: 10.1002/alr.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. doi: 10.1371/journal.pone.0056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogdie A, Haynes K, Troxel AB, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Annals of the rheumatic diseases. 2014;73:149–153. doi: 10.1136/annrheumdis-2012-202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iribarren C, Tolstykh IV, Miller MK, et al. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. American journal of epidemiology. 2012;176:1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen SL, Lindhardsen J, Ahlehoff O, et al. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014;16:477–484. doi: 10.1093/europace/eut312. [DOI] [PubMed] [Google Scholar]
- 11.Kang JH, Wu CS, Keller JJ, Lin HC. Chronic rhinosinusitis increased the risk of stroke: a 5-year follow-up study. Laryngoscope. 2013;123:835–840. doi: 10.1002/lary.23829. [DOI] [PubMed] [Google Scholar]
- 12.Wang PC, Lin HC, Kang JH. Chronic rhinosinusitis confers an increased risk of acute myocardial infarction. Am J Rhinol Allergy. 2013;27:e178–182. doi: 10.2500/ajra.2013.27.3952. [DOI] [PubMed] [Google Scholar]
- 13.Oakley GM, Curtin K, Orb Q, et al. Familial risk of chronic rhinosinusitis with and without nasal polyposis: genetics or environment. Int Forum Allergy Rhinol. 2015;5:276–282. doi: 10.1002/alr.21469. [DOI] [PubMed] [Google Scholar]
- 14.Teerlink CC, Hegewald MJ, Cannon-Albright LA. A genealogical assessment of heritable predisposition to asthma mortality. Am J Respir Crit Care Med. 2007;176:865–870. doi: 10.1164/rccm.200703-448OC. [DOI] [PubMed] [Google Scholar]
- 15.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. American journal of public health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jerant A, Fenton JJ, Franks P. Primary care attributes and mortality: a national person-level study. Ann Fam Med. 2012;10:34–41. doi: 10.1370/afm.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice JC, Pizer SD. Delayed access to health care and mortality. Health Serv Res. 2007;42:644–662. doi: 10.1111/j.1475-6773.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(7):S1–11. doi: 10.1002/lary.24418. [DOI] [PubMed] [Google Scholar]
- 19.Benninger MS, Holy CE. The impact of endoscopic sinus surgery on health care use in patients with respiratory comorbidities. Otolaryngol Head Neck Surg. 2014;151:508–515. doi: 10.1177/0194599814536369. [DOI] [PubMed] [Google Scholar]
- 20.Appleton SL, Ruffin RE, Wilson DH, et al. North West Adelaide Cohort Health Study T. Cardiovascular disease risk associated with asthma and respiratory morbidity might be mediated by short-acting beta2-agonists. J Allergy Clin Immunol. 2009;123:124–130. e121. doi: 10.1016/j.jaci.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy. 2016;30:44–47. doi: 10.2500/ajra.2016.30.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houssiau FA, Devogelaer JP, Van Damme J, et al. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 23.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Bettencourt R, Brenner D, et al. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7:e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:165–173. doi: 10.1093/gerona/glt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaffar O, Lavigne F, Kamil A, et al. Interleukin-6 expression in chronic sinusitis: colocalization of gene transcripts to eosinophils, macrophages, T lymphocytes, and mast cells. Otolaryngol Head Neck Surg. 1998;118:504–511. doi: 10.1016/s0194-5998(98)70209-8. [DOI] [PubMed] [Google Scholar]
- 27.Peters AT, Kato A, Zhang N, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. e310. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sejima T, Holtappels G, Kikuchi H, et al. Cytokine profiles in Japanese patients with chronic rhinosinusitis. Allergol Int. 2012;61:115–122. doi: 10.2332/allergolint.10-OA-0290. [DOI] [PubMed] [Google Scholar]
- 29.Soler ZM, Rudmik L, Hwang PH, et al. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123:2341–2346. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


