Abstract
MAPK pathways play a critical role in the activation of monocytes and macrophages by pathogens, signaling molecules and environmental cues and in the regulation of macrophage function and plasticity. MAPK phosphatase 1 (MKP-1) has emerged as the main counter-regulator of MAPK signaling in monocytes and macrophages. Loss of MKP-1 in monocytes and macrophages in response to metabolic stress leads to dysregulation of monocyte adhesion and migration, and gives rise to dysfunctional, proatherogenic monocyte-derived macrophages. Here we review the properties of this redox-regulated dual-specificity MAPK phosphatase and the role of MKP-1 in monocyte and macrophage biology and cardiovascular diseases.
Keywords: monocyte, macrophage, redox signaling, MAPK, atherosclerosis
I. INTRODUCTION
The mitogen-activated protein kinase (MAPK) signaling pathways are evolutionally highly conserved [1] and involved in diverse cellular functions, including cell proliferation, differentiation and stress responses. A wide variety of extracellular stimuli induce phosphorylation and activation of MAPKs [2, 3]. For immune cells, these stimuli commonly include cytokines, chemoattractants, reactive oxygen species, antigen–antibody complexes, and pathogen-associated molecules that engage toll-like receptors. MAPKs are serine/threonine kinase activated via phosphorylation of both the threonine and tyrosine residues within the conserved TXY sequence. The three main arms of the MAPK pathway cascade are ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase) and p38. They mediate immune cell functional responses to a wide array of stimuli [4, 5]. Activated MAPKs are inactivated through dephosphorylation of threonine and/or tyrosine residues within the activation loop [6]. MAPK dephosphorylation can be mediated by serine/threonine phosphatases, tyrosine phosphatases, and/or dual-specificity phosphatases [7]. However, by far the largest group of protein phosphatases dedicated to the specific regulation of MAPK activity in mammalian cells and tissues are the dual-specificity MAPK phosphatases (MKPs). These phosphatases dephosphorylate both threonine and tyrosine residues within the substrates they target [8].
MAPK pathways play a critical role in the activation of monocytes and macrophages by pathogens, signaling molecules and environmental cues [9–11]. Human and murine monocytes and macrophages express six MKPs (MKP-1, MKP-2, MKP-3, MKP-5, MKP-6 and MKP-7), although MKP-6 has only been reported in murine macrophages [12–20]. However, our understanding of the specific roles of these MKPs in monocyte and macrophages is still very limited. Recent evidence from our group and others suggests that MKP-1 is a central regulator of monocyte and macrophage activation, function and phenotypic fate, and loss of MKP-1 activity in these cells may play an important role in dysregulated inflammatory responses and the onset and development of metabolic and chronic inflammatory diseases, including atherosclerosis [21–25]. The focus of this review will therefore be on MKP-1 and its role in monocyte and macrophage biology in the context of cardiovascular disease.
II. MKP-1 – STRUCTURE, FUNCTIONS AND REGULATION
A. Structure
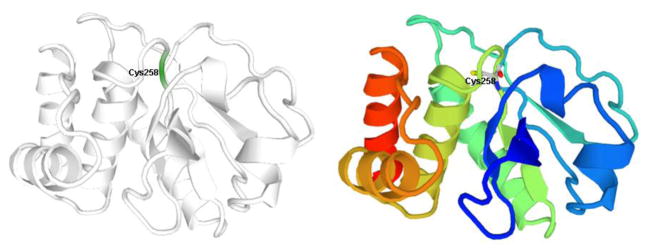
Although a three dimensional (3D) structure of MKP-1 has not been reported so far, its structure can be predicted with homology modeling using the X-ray crystal structure of MKP-2 (PDB code: 3EZZ) [26] as the template since the sequence identity between the two MKPs is 86% [27], and they have the same amino acid sequence (C–Q–A-G-I–S) in the PTP loop (residues 258–264 for MKP-1), the key component of the active site. The predicted 3D structure for MKP-1 (residues 172–314) based on calculations with ModPipe (https://salilab.org/modpipe/) is shown in figure 1.
Figure 1. Calculated 3D Structure of MKP-1 (residues 172–314).
The position of the catalytic residue Cys258 is indicated. This figure was adopted from ModBase. (https://modbase.compbio.ucsf.edu, Database ID: P28562).
B. Transcriptional Regulation
MKP-1 expression and activity can be regulated at several levels, including gene transcription, protein stability, and phosphatase activity. This multi-level regulation allows for tight control of MAPKs’ activities. MKP-1, the first MKP discovered, was identified as an immediate early gene that is induced rapidly after exposure to growth factors, heat shock and oxidative stress [28–30]. As MKP-1 functions to deactivate MAPKs, it was proposed that MAPKs may activate MKP-1 transcription, as part of a negative feedback mechanism [31, 32]. Indeed, in vascular smooth muscle cells, platelet-derived growth factor (PDGF), phorbol ester, and angiotensin II, which activate ERK, but not JNK or p38, and anisomycin, a potent stimulus for JNK and p38, all induced the transient expression of MKP-1 [33]. In C3H 10T1/2 murine fibroblasts, MKP-1 induction by heat shock and H2O2 is primarily dependent on ERK, whereas MKP-1 induction by arsenite and UVC is primarily mediated by p38 [34]. However, in NIH 3T3 fibroblasts, MKP-1 is highly induced by stress through a JNK-mediated process, while ERK has little effect on MKP-1 induction. MKP-1 induction in macrophages by LPS involves all three MAPK subfamilies [35–38]. In addition to the MAPK pathways, members of the protein kinase C (PKC) family have also been shown to regulate MKP-1 induction in a number of systems. In cardiomyocytes treated with angiotensin II, PKC inhibitors or intracellular calcium chelation decrease MKP-1 expression while calcium ionophores increase MKP-1 mRNA levels [39]. PKCε plays a critical role in MKP-1 induction in macrophages [40, 41]. While it is possible that the PKC pathways cross-talk with the MAPK cascades to regulate MKP-1 induction, MAPK-independent pathways may also be involved.
C. Epigenetic Regulation
Epigenetic mechanism has been also suggested to modulate MKP-1 expression. The mRNA expression levels of MKP-1 gene are down-regulated in both prostate cancer and breast cancer, and this downregulation appears to involve DNA methylation [42, 43]. In addition, phosphorylation and acetylation of histone H3 alters the chromatin at the MKP-1 gene locus, increasing the association of RNA polymerase II to the MKP-1 gene promoter and promoting MKP-1 transcription [34].
D. Post-Transcriptional Regulation
Because of the short half-life of MKP-1 mRNA (1 – 2 h) [44], it was generally assumed that MKP-1 expression is primarily transcriptionally regulated. This variation in half-life may stem from the different mechanisms that determine the amount of MKP-1 mRNA that accumulates. Tristetraprolin (TTP), a zinc-finger-containing AU-rich elements (ARE)-binding protein, binds to and destabilizes MKP-1 mRNA [45]. Recently, other post-transcriptional regulatory mechanisms have been shown to regulate MKP-1 levels. RNA-binding proteins HuR (also known as ELAV1) [45, 46], and NF90 [47] were found to associate with the MKP-1 3′ untranslated region. HuR both stabilizes the MKP-1 mRNA and promotes its translation [48, 49]. While NF90 also stabilizes the MKP-1 mRNA, binding of NF90 appears to suppress MKP-1 translation [48].
E. Post-translational regulation
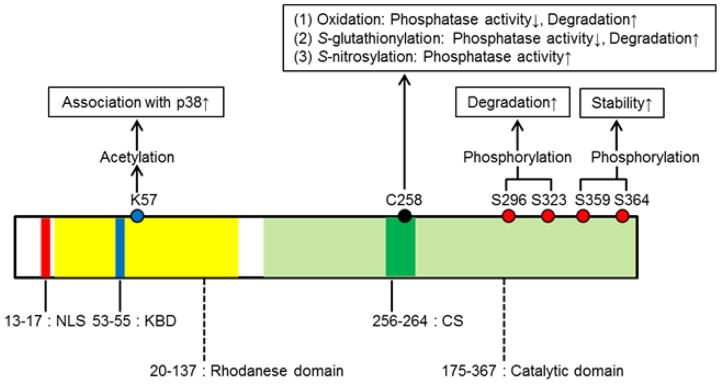
A number of post-translational modifications have been identified that modulate MKP-1 activity and stability. The binding of ERK, JNK, or p38 to recombinant MKP-1 increases the activity of the phosphatase [50, 51]. This increase in activity is induced by the interaction between domains in the amino terminus of the phosphatase and an acidic domain at the carboxyl terminus of the kinases. ERK-mediated phosphorylation of the C-terminal residues Ser359 and Ser364 in MKP-1 increases protein stability, thereby reinforcing phosphatase activity and establishing autoregulatory negative feedback control [52]. By contrast, ERK-mediated phosphorylation of the distinct residues Ser296 and Ser323 within MKP-1 results in the recruitment of the ubiquitin ligase SCFskp2, and increases the rate at which MKP-1 is degraded [53, 54]. In addition, MKP-1 is acetylated by p300 on lysine residue (Lys57) within its substrate-binding domain. Acetylation of MKP-1 enhances its interaction with p38, thereby increasing its phosphatase activity and interrupting MAPK signaling [55]. Intestinally, Lys57 is located in close proximity of MKP-1’s nuclear localization sequence (NLS: aa 53–55; Fig. 2), but whether acetylation of MKP-1 affects its cellular localization is not known. Little is known about the enzymes regulating MKP-1 deacetylation. However, a recent report suggests that in macrophages class I HDACs (HDAC1, 2, and 3) can deacetylate MKP-1, and that inhibition of HDAC1, -2, and -3 blocks LPS-induced expression of TNF-α, IL-1β, iNOS (NOS2), and nitrite synthesis [56]. Like protein tyrosine phosphatases (PTPs) [57, 58], MKP-1 contains an essential cysteine residue (Cys258) in the catalytic domain. This site is identical in both murine and human MKP-1. Reactive oxygen species (ROS) have been shown to oxidize the catalytic cysteine and inactivate the enzyme activities in MKP-1 [59, 60]. In addition, MKP-1, once oxidized, undergoes rapid degradation in the proteasome [60]. Moreover, we recently reported that S-glutathionylation of MKP-1 catalytic cysteine residue (Cys258), inhibits phosphatase activity, and targets the protein for proteasomal degradation [22]. Interestingly, Guan et al. reported that S-nitrosylation of MKP-1 on Cys258 enhances MKP-1 protein stability in cancer cells [61]. Therefore, MKP-1 activity and function appear to be regulated by redox-sensitive mechanisms. Together these data show that the different domains of MKP-1 and their post-translational modifications play distinct roles in substrate affinity, stability, and the catalytic activity (Fig. 2).
Figure 2. Structural Features and Sites of Post-Translational Regulation of the Human MKP-1 Protein.
The N-terminal domain of MKP-1 is responsible for both nuclear localization, driven by a leucine-rich nuclear localization sequence (NLS), and for binding of MAPK through its specific arginine-rich kinase binding domain (KBD). The highly-conserved C-terminal domain of MKP-1 contains the catalytic domain and its active site sequence (CS) that catalyzes the dephosphorylation of tyrosine/threonine residues of target substrates.
F. MKP-1 Substrates
MKPs are highly specific in their ability to recognize and bind to MAPK. However, recent evidence suggests that MKPs may also dephosphorylate non-MAP kinase proteins. For example, based on anti-sense mRNA knockdown experiments, it had been suggested that MKP-1 interacts with and dephosphorylate the signal transducers and activators of transcription (STAT) 1 protein, thus potentially directly regulating transcriptional responses to interferon γ [62], angiotensin II [63] and LPS [64]. However, subsequent in vitro assays demonstrated that MKP-1 fails to bind to recombinant STAT1, nor is STAT1 able to stimulate the catalytic activity of MKP-1 towards the chromogenic substrate para-nitrophenyl phosphate [51]. Together, these results strongly suggest that STAT1 is not a MKP-1 substrate and that this phosphatase plays no direct role in the regulation of STAT1-dependent transcriptional regulation in response to interferon γ. Instead, MKP-1 was found to inhibit miR155 expression thereby inducing SOCS-1, which in turn attenuates STAT1 activation [65]. How MKP-1 regulates miR-155 levels remains to be elucidated. More recently, it was reported that histone H3 also interacts with and is dephosphorylated by MKP-1 to mediate epigenetic regulation on vascular endothelial growth factor (VEGF)-induced gene transcription [66]. In agreement with this report, we found that histone H3 phosphorylation (Ser10) was increased in the MKP-1-deficient macrophage exposed to metabolic stress [21], making H3 the only known bona fide non-MAPK substrate candidate for MKP-1.
G. MKP-1 Inhibitors and Inducers
The search for MKP-1 selective inhibitors with cellular activity has been challenging because the X-ray structure of MKP-1 has not been solved, but a number of MKP-1 inhibitors have been identified. The benzo[c]phenanthridine alkaloid, Sanguinarine, was identified as a MKP-1 inhibitor from a cell-based high content screen of a collection of 720 pure natural products and their derivatives [67]. The benzofuran NU-126 was also identified as a MKP-1 inhibitor and showed some selectivity for MKP-1 over MKP-3. However, with an IC50 value for MKP-1 of almost 30 μM, NU-126 is not very potent [68]. Both PSI2106 and MDF2085 were identified from a pyrrole-2-carboxamide library, and have IC50 values for MKP-1 below 10 μM, showed greater than 5-fold selectivity for human MKP-1 over MKP-3, and a much greater preference for MKP-1 over Cdc25B, PTP1B, or VHR [69].
Increased MKP-1 expression has been reported in response to a variety of extracellular signals, including growth factors, cytokines, bacterial products such as LPS, as well as micronutrients and phytochemicals (Table 1). Note that in some instances the apparent induction of MKP-1 may be due to mRNA stabilization or reduced MKP-1 degradation [70, 71].
Table 1.
Inducers of MKP-1 Expression
| Inducer | Cell Type/Tissue | mRNA/Protein | Effect/Function | References |
|---|---|---|---|---|
| Glucocorticoid | Endothelial cell, macrophages | mRNA | Inflammation ↓ | [72–74] |
| Vitamin D | Monocytes, macrophages | mRNA | Inflammation ↓ | [75, 76] |
| Curcumin | Hippocampal cells | Protein | Ethanol-induced toxicity ↓ | [71] |
| Ursolic acid | Monocytes | Protein | Metabolic stress-induced priming ↓ | [70] |
| Nitroalkenes | Macrophages | mRNA | Inflammation ↓ | [64] |
| VEGF | Endothelial cell | mRNA | Migration ↓ | [77] |
| Thrombin | Endothelial cell | mRNA | Activation ↓ | [78] |
| Atrial natriuretic peptide (ANP) | Endothelial cell | mRNA | Activation ↓ | [78, 79] |
| Retinoids | Mesangial cells | mRNA | Apoptosis ↓ | [80] |
| Trichostatin A, HDAC inhibitor | Macrophages | Protein | Osteoclastogenesis ↓ | [81] |
| Rapamycin, mTOR inhibitor | Macrophages | mRNA | Inflammation ↓ | [82] |
| Clozapine | Frontal cortex (rat) | mRNA | Psychotic action ↓ | [83] |
| High molecular weight hyaluronic acid (HA) | Chondrocytes | Protein | MMP production ↓ | [84] |
| ETYA, peroxisome proliferator-activated receptor (PPAR)-α activator | Astrocytes | mRNA | Inflammation ↓ | [85] |
| Rolipram (PDE4 inhibitor) | Macrophages | mRNA | Inflammation ↓ | [86] |
A better understanding of the physiological roles of the MKP-1, particularly in monocytes and macrophages, will pave the way for developing new immunomodulatory therapies for cancer and (chronic) inflammatory diseases. In cancer, MKP-1 inhibitors may prove beneficial, as MKP-1 is overexpressed in tumor cells and is considered responsible for the resistance of JNK-driven apoptotic pathways to activation by chemotherapeutics. Conversely, in inflammatory diseases such as asthma and arthritis, MKP-1 counter-regulates pro-inflammatory MAPK-mediated signaling. Developing novel ligands to upregulate MKP-1 levels and activity would make for a therapeutically attractive anti-inflammatory strategy.
III. MKP-1 – ROLE IN MONOCYTES AND MACROPHAGES
Monocytes and macrophages are essential for tissue and metabolic homeostasis, but in the context of metabolic disorders become dysfunctional and promote chronic inflammatory diseases, including vascular inflammation and atherosclerosis [87–89]. However, the underlying mechanisms are not well-understood. These cells belong to the mononuclear phagocyte system and function as part of the innate immunity to detect and clear invading pathogens as well as dying cells and tissue debris [90]. Macrophages are extremely diverse and adaptive to their microenvironment. They are potent secretory cells of various cytokines in response to “activation” by infectious agents, danger signals and other environmental cues, and these cytokines have paracrine and autocrine effects within the tissue [91]. Macrophages originate from at least two different sources and the majority of tissue-resident macrophage populations, including splenic red pulp and alveolar macrophages, microglia, Kupffer cells, osteoclasts, Langerhans cells and large peritoneal macrophages, originate from embryonic yolk-sac-derived progenitor cells [92–96]. However, in response to tissue injury there is a vast infiltration of monocyte-derived macrophages that originate from bone marrow hematopoietic stem cells [97–99]. This recruitment of blood monocytes to discrete anatomical locations followed by their differentiation into mature macrophages is a rate-limiting step in multiple physiological processes, including wound repair, pathogen clearance, and replacement of tissue-resident macrophages [100, 101], as well as pathophysiological processes such as atherosclerosis [102].
We and others showed that maintaining protein thiol redox homeostasis is critical for the accurate programming and proper functioning of monocytes and macrophages [99, 103, 104]. Our data demonstrate that chronic exposure of monocytes to metabolic stress disrupts thiol redox homeostasis, resulting in monocyte dysfunction and the conversion of blood monocytes into a pro-inflammatory, pro-atherogenic phenotype, hyper-sensitive to chemoattractant [105, 106]. MKP-1 appears to play a critical role in preventing monocyte and macrophage dysfunction.
A. Monocyte Adhesion and Migration
Recruitment of monocyte-derived macrophages to sites of tissue injury is a hallmark of acute inflammation [107]. The extent of monocyte recruitment and macrophage accumulation is generally believed to be controlled by local inflammatory processes within that tissue [108, 109]. Monocyte recruitment, adhesion and chemotaxis, is regulated by MAPK pathways [110–113]. We previously reported that MKP-1 deficiency induced by metabolic stress results in the hyperactivation of ERK and p38 MAPK and increased monocyte adhesion and migration in response to monocyte chemoattractant protein-1 (MCP-1) and other chemokines, a process we coined “metabolic priming” [22]. With our discovery that chronic metabolic stress primes monocytes for dramatically enhanced responsiveness to chemoattractants and increased monocyte recruitment [22, 105, 106], we identified a completely novel mechanism by which metabolic disorders promote atherosclerosis, and possibly other chronic inflammatory diseases associated with metabolic disorders such as obesity, liver steatosis, kidney diseases and possibly cancer. A report by Grimshaw et al. suggests that early induction (< 90 min) of MKP-1 during monocyte migration by hypoxia or TNF-α treatment, decreases MAPK activation and inhibits monocyte chemotaxis [114]. This mechanism may result in the trapping of newly recruited monocyte-derived macrophages at sites of inflammation or hypoxia. Together, these data suggest that MKP-1 plays a crucial role in monocyte transmigration and the recruitment monocyte-derived macrophages to sites of tissue injury and inflammation.
B. Macrophage Activation and Inflammatory Responses
Macrophage activation and polarization plays a critical role in both inflammation and subsequent inflammation resolution. Dysregulation of macrophage activation and plasticity has been proposed to play a major role in impaired inflammation resolution and the conversion of local acute inflammation into a chronic process [115]. The classification of macrophage activation states into classical (M1) polarization and alternative (M2) polarization is to a large extent based on the ex vivo response of macrophages to Th1 (e.g., IFN-γ and TNF-α) and Th2 (e.g., IL-4, IL-10, and IL-13) cytokines and inadequate to capture the complexity and diversity of macrophage activation states [116]. However, it continues to be broadly utilized to characterize in vivo macrophage phenotypes [117]. We reported that MKP-1 deficient macrophages exhibit severally skewed activation profiles, demonstrating an exaggerated pro-inflammatory “M1-like” phenotype in response to INFγ+TNFα and a severely suppressed “M2-like” inflammation resolving phenotype after IL-4 stimulation [21].
MKP-1 was originally described as a stress and growth factor-inducible non-receptor phosphatase able to dephosphorylate all three MAPKs ERK, p38 and JNK [29, 31, 32, 118]. Subsequent studies established that MKP-1 preferentially dephosphorylates p38 and JNK [119, 120]. Consistent with these findings, studies using macrophages from MKP-1 knockout mice and MKP-1 silencing by siRNA in cell lines have shown that impaired MKP-1 leads to increased and prolonged p38 and JNK phosphorylation [24, 121–123]. Despite MKP-1’s substrate preference for p38 and JNK, in macrophages activation of ERK is also regulated by MKP-1. For example, in mouse macrophages, inhibition of MKP-1 expression using siRNA prolongs ERK phosphorylation and blocks of M-CSF-dependent proliferation [124]. And as mentioned above, MKP-1 deficiency results in the hyperactivation ERK and increased adhesion in in MCP-1-stimulated monocytes [22].
Given the crucial role of p38 and JNK in the regulation of cytokine biosynthesis and the substrate preference of MKP-1 for these two MAPKs, it was long suspected that MKP-1 may play an important role in the control of cytokine biosynthesis [36]. Compared with wild-type mice, MKP-1–deficient mice produced substantially greater amounts of TNF, IL-1β, CC-chemokine ligand 2 (CCL2; also known as MCP1), granulocyte/macrophage colony-stimulating factor (GM-CSF), IL-6, IL-10 and IL-12p70 and showed a considerably higher incidence of multi-organ failure and mortality after LPS challenge in vivo compared with wild-type mice [25, 121, 122]. These findings are consistent with our report mentioned above that MKP-1 deficiency induced by metabolic stress predisposing macrophages to a hyper-inflammatory M1-like polarization state [21].
Overall, these studies support the concept that MKP-1 is a critical negative regulator of the innate immune response. By modulating the activities of both p38 and JNK as well as ERK, MKP-1 limits both strength and duration of signals triggering the production of inflammatory cytokines. Interestingly, the half-live of the mRNA of several cytokines, including IL-6, IL-10, and TNF, also appears to be controlled by MKP-1 by promoting the translocation of RNA binding proteins from the nucleus to the cytosol [125]. More recent data shows that MKP-1 also modulates the activity of the cytokine mRNA by destabilizing the phosphorylation status of tristetraprolin (TTP), an RNA-destabilizing protein [126].
In addition to regulating macrophage cytokine, chemokine and growth factor synthesis and release, accumulating evidence suggests that MAPKs also regulate autophagy and apoptosis in macrophages [127–129]. We recently reported that both processes are regulated by MKP-1 and that both genetic and metabolic stress-induced MKP-1 deficiency impairs macrophage autophagy and sensitizes macrophages to oxysterol-induced caspase 3/7 activation and programmed cell death [21]. Moreover, it is known that cigarette smoke decreases MKP-1 activity in the lung [130]. Correspondingly, alveolar macrophages of smokers accumulate both autophagosomes and p62, a marker of autophagic flux acute cigarette smoke exposure was shown to induce apoptosis of alveolar macrophages [131, 132]. Interestingly, MKP-1 induction with Malvidin, a major red wine polyphenol, attenuates PARP activation by LPS in RAW 264.7 macrophages further supporting the notion that MKP-1 plays a critical role in regulating macrophage autophagy and cell death.
IV. MKP-1 – ROLE IN CARDIOVASCULAR DISEASES
A. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease characterized by the infiltration and accumulation of monocyte-derived macrophages and lipid-laden foam cells in the vessel wall [108, 133, 134]. The dysregulation of macrophage activation and functions appears to be a major contributor to the conversion of acute inflammation into a chronic process and to the development and progression of atherosclerotic lesions [87]. Macrophage numbers and functionalities within atherosclerotic lesions and their removal from plaques are controlled by four key processes: recruitment, autophagy, apoptosis, and macrophage polarization. As we have reviewed above, macrophage chemotaxis [110, 111], autophagy [129], apoptosis [127, 128] and activation [135–137] are mediated by mitogen-activated protein kinase (MAPK) pathways, which in turn are counter-regulated by MKP-1 [21, 22]. Two earlier reports had suggested that complete MKP-1 deficiency in apolipoprotein E-null (apoE−/−) mice is atheroprotective [138, 139]. While it is possible that these partial atheroprotective properties of complete MKP-1 deficiency reported in apoE−/− mice are related specifically to apoE deficiency, a more likely explanation is that MKP-1 plays different roles in different cell types. While MKP-1 deficiency in vascular cells may protect against the formation of atherosclerotic lesions, loss of MKP-1 activity in monocytes and macrophages clearly promotes inflammatory responses, macrophage dysfunction and accelerates atherogenesis.
B. Heart failure
Monocytes and macrophages are known to be the major drivers of inflammatory and fibrotic processes in heart failure [140, 141]. However, to date no study has addressed the role of macrophage MKP-1 in heart failure. However, cardiomyocyte apoptosis triggered by RAFTK/pyk2 via p38 was shown to be blocked by MKP-1 overexpression [142]. Heart failure induced by alpha(1B)-adrenergic receptor (alpha(1B)-AR) overexpression was accompanied by reduced MKP-1 expression [143]. Human cardiac tissues from patients with heart failure secondary to rheumatic heart disease show a marked increase p38 activity, and MKP-1 levels are reduced [144]. In contrast, increased MKP-1 levels and reduced JNK and p38 activities were reported in heart tissue from end-stage heart failure patients with idiopathic dilated cardiomyopathy [145]. The reason for these diverging observations is not yet clear.
C. Myocardial infarction
Macrophages are also critical players in the pathophysiological processes induced by myocardial infarction (MI). Shortly after initial MI, a large number of macrophages exhibiting a pro-inflammatory M1-like profile are rapidly recruited to the cardiac tissue, where they contribute to cardiac remodeling [146]. After this initial period, inflammation resolution is initiated in the wound, with the infiltrated macrophages display a predominant anti-inflammatory/pro-resolution M2-like activation profile, promoting cardiac repair by mediating pro-fibrotic responses [141, 147]. Since macrophages in myocardial infarction play a similar role as they do in wound healing, we can speculate the role of macrophage MKP-1 will be similar too. By restricting p38 MAPK activation, in the early phase of tissue repair MKP-1 facilitates the transition of macrophages from a pro- to an inflammation resolving state, and during later stages the progression of macrophages into an “exhaustion-like state” characterized by cytokine silencing, thereby permitting full resolution of inflammation and tissue recovery [148]. In the absence of MKP-1, p38-induced AKT activity suppresses the acquisition of an anti-inflammatory gene program and final cytokine silencing in macrophages, resulting in impaired tissue healing [148].
Figure 3. Role of MKP-1 in Monocyte Adhesions, Chemotaxis and The Molecular Mechanisms of Monocyte Priming by Metabolic Stress.
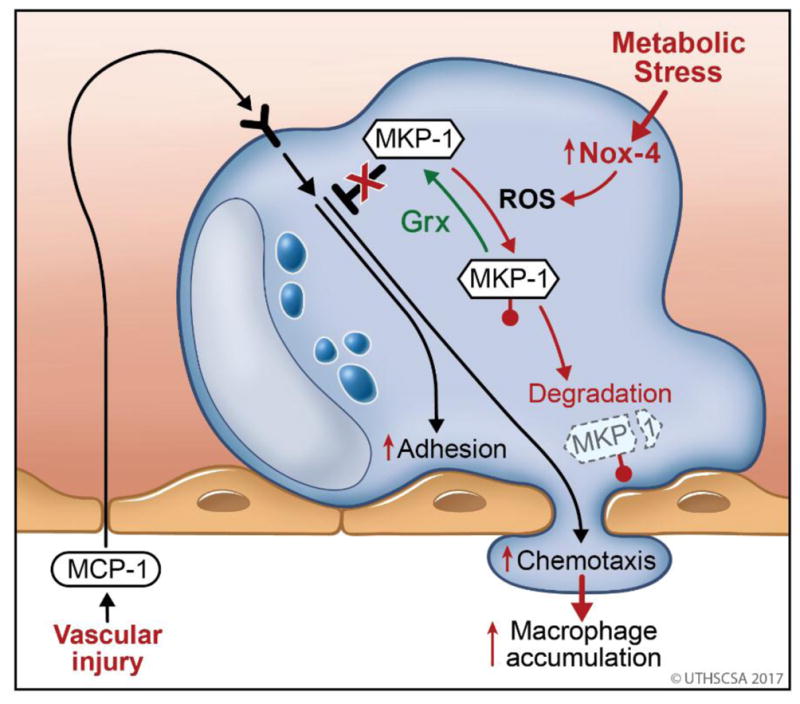
Chemoattractants, such as MCP-1 released by the vessel wall activate of monocytes (blue) in the blood stream and initiate monocyte recruitment in response to vascular injury. Monocyte transmigration and involves adhesion to and chemotaxis through the endothelial layer (pink). Both processes are initiated by protein kinase-dependent signaling pathways and counter-regulated by protein phosphatases (MKP-1). MKP-1 is sensitive to S-glutathionylation (
 ) of the catalytic cysteine residue which is reversed by the thiol transferase glutaredoxin (Grx). Metabolic disorders induce the expression of Nox4 in monocytes, an enzyme that generates reactive oxygen species (ROS) capable of catalyzing the S-glutathionylation of MKP-1. In the absence of adequate antioxidant protection, S-glutathionylated MKP-1 is rapidly degraded, promoting in MKP-1 deficiency, hyper-activation of monocyte adhesion and migration and subsequently the increased accumulation of monocyte-derived macrophages in the injured vessel wall.
) of the catalytic cysteine residue which is reversed by the thiol transferase glutaredoxin (Grx). Metabolic disorders induce the expression of Nox4 in monocytes, an enzyme that generates reactive oxygen species (ROS) capable of catalyzing the S-glutathionylation of MKP-1. In the absence of adequate antioxidant protection, S-glutathionylated MKP-1 is rapidly degraded, promoting in MKP-1 deficiency, hyper-activation of monocyte adhesion and migration and subsequently the increased accumulation of monocyte-derived macrophages in the injured vessel wall.
Figure 4. Role of MKP-1 in Macrophage Dysfunction induced by Metabolic Stress.
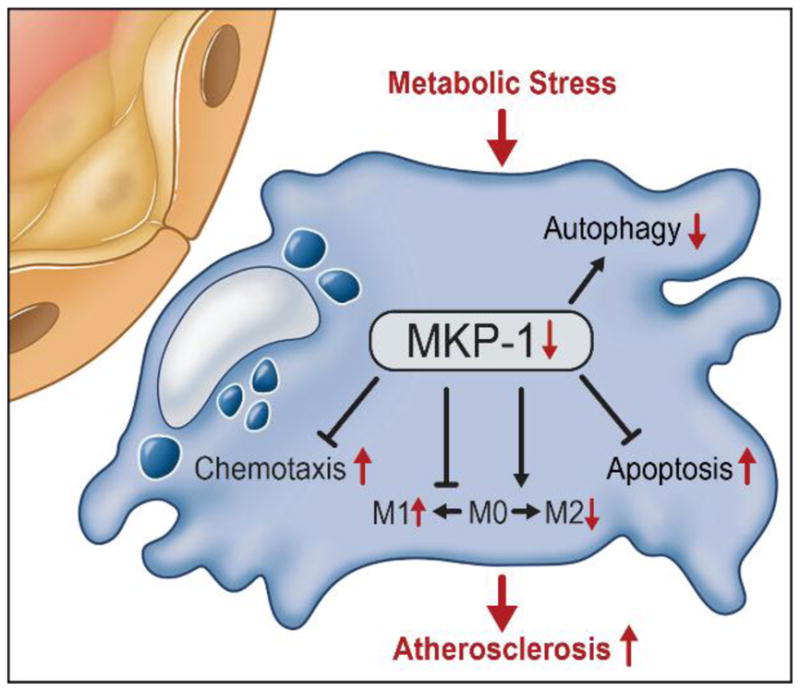
Metabolic stress induces MKP-1 S-glutathionylation and the subsequent degradation of the phosphatase, and the overall loss of MKP-1 activity. Loss of MKP-1 activity leads to the hyper-responsiveness to chemokines, accelerated chemotaxis and increase recruitment and accumulation of dysfunction monocyte-derived macrophages, characterized by dysregulated activation profiles (M1 versus M2), impaired autophagy and increased sensitivity to oxysterol induced programmed cell death.
HIGHLIGHTS.
MKP-1 is a critical counter-regulator of MAPK signaling in monocytes and macrophages
MKP-1 regulates monocyte adhesion and migration
MKP-1 regulates macrophage activation, inflammatory responses and survival.
MKP-1 activity is regulated at the level of transcription, translation and by posttranslational modifications
Acknowledgments
FUNDING
Reto Asmis is supported by grants form the National Institutes of Health (RO1 AT006885 and RO1 HL115858). Hong Seok Kim is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2014R1A5A2009392 and NRF-2016K2A9A1A01947271).
ABBREVIATIONS
- ANP
Atrial natriuretic peptide
- ARE
AU-rich elements
- CS
catalytic sequence
- DUSP
dual-specificity phosphatases
- ERK
extracellular signal-regulated kinase
- Grx
glutaredoxin
- HA
hyaluronic acid
- IFN
Interferon
- IL
Interleukin
- JNK
c-Jun N-terminal kinase
- KBD
kinase binding domain
- LPS
Lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MI
myocardial infarction
- MKP-1
MAPK phosphatase 1
- M-CSF
macrophage-colony stimulating factor
- NLS
nuclear targeting sequence
- PDGF
platelet-derived growth factor
- PPAR
peroxisome proliferator-activated receptor
- PTP
protein tyrosine phosphatase
- ROS
Reactive oxygen species
- SOCS
Suppressor of cytokine signaling
- STAT
signal transducers and activators of transcription
- TNF
Tumor necrosis factor
- TTP
Tristetraprolin
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 2.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 5.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 7.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 8.Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 9.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Jaworowski A, Wilson NJ, Christy E, Byrne R, Hamilton JA. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J Biol Chem. 1999;274:15127–15133. doi: 10.1074/jbc.274.21.15127. [DOI] [PubMed] [Google Scholar]
- 11.Valledor AF, Sanchez-Tillo E, Arpa L, Park JM, Caelles C, Lloberas J, Celada A. Selective roles of MAPKs during the macrophage response to IFN-gamma. J Immunol. 2008;180:4523–4529. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- 12.Krautwald S, Buscher D, Dent P, Ruthenberg K, Baccarini M. Suppression of growth factor-mediated MAP kinase activation by v-raf in macrophages: a putative role for the MKP-1 phosphatase. Oncogene. 1995;10:1187–1192. [PubMed] [Google Scholar]
- 13.Valledor AF, Xaus J, Marques L, Celada A. Macrophage colony-stimulating factor induces the expression of mitogen-activated protein kinase phosphatase-1 through a protein kinase C-dependent pathway. J Immunol. 1999;163:2452–2462. [PubMed] [Google Scholar]
- 14.Al-Mutairi MS, Cadalbert LC, McGachy HA, Shweash M, Schroeder J, Kurnik M, Sloss CM, Bryant CE, Alexander J, Plevin R. MAP kinase phosphatase-2 plays a critical role in response to infection by Leishmania mexicana. PLoS Pathog. 2010;6:e1001192. doi: 10.1371/journal.ppat.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods S, Schroeder J, McGachy HA, Plevin R, Roberts CW, Alexander J. MAP kinase phosphatase-2 plays a key role in the control of infection with Toxoplasma gondii by modulating iNOS and arginase-1 activities in mice. PLoS Pathog. 2013;9:e1003535. doi: 10.1371/journal.ppat.1003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tephly LA, Carter AB. Asbestos-induced MKP-3 expression augments TNF-alpha gene expression in human monocytes. Am J Respir Cell Mol Biol. 2008;39:113–123. doi: 10.1165/rcmb.2007-0356OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–797. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]
- 18.Qian F, Deng J, Gantner BN, Flavell RA, Dong C, Christman JW, Ye RD. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L866–874. doi: 10.1152/ajplung.00277.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano Y. Novel function of DUSP14/MKP6 (dual specific phosphatase 14) as a nonspecific regulatory molecule for delayed-type hypersensitivity. Br J Dermatol. 2007;156:848–860. doi: 10.1111/j.1365-2133.2006.07708.x. [DOI] [PubMed] [Google Scholar]
- 20.Niedzielska M, Bodendorfer B, Munch S, Eichner A, Derigs M, da Costa O, Schweizer A, Neff F, Nitschke L, Sparwasser T, Keyse SM, Lang R. Gene trap mice reveal an essential function of dual specificity phosphatase Dusp16/MKP-7 in perinatal survival and regulation of Toll-like receptor (TLR)-induced cytokine production. J Biol Chem. 2014;289:2112–2126. doi: 10.1074/jbc.M113.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Tavakoli S, Piefer LA, Nguyen HN, Asmis R. Monocytic MKP-1 is a Sensor of the Metabolic Environment and Regulates Function and Phenotypic Fate of Monocyte-Derived Macrophages in Atherosclerosis. Scientific Reports. 2016;6:34223. doi: 10.1038/srep34223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc Natl Acad Sci U S A. 2012;109:E2803–E2812. doi: 10.1073/pnas.1212596109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imasato A, Desbois-Mouthon C, Han J, Kai H, Cato AC, Akira S, Li JD. Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of toll-like receptor 2. J Biol Chem. 2002;277:47444–47450. doi: 10.1074/jbc.M208140200. [DOI] [PubMed] [Google Scholar]
- 24.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 26.Jeong DG, Jung SK, Yoon TS, Woo EJ, Kim JH, Park BC, Ryu SE, Kim SJ. Crystal structure of the catalytic domain of human MKP-2 reveals a 24-mer assembly. Proteins. 2009;76:763–767. doi: 10.1002/prot.22423. [DOI] [PubMed] [Google Scholar]
- 27.Baker D, Sali A. Protein structure prediction and structural genomics. Science. 2001;294:93–96. doi: 10.1126/science.1065659. [DOI] [PubMed] [Google Scholar]
- 28.Charles CH, Abler AS, Lau LF. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–190. [PubMed] [Google Scholar]
- 29.Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359:644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi T, Metz R, Chen L, Mattei MG, Carrasco D, Bravo R. Structure, mapping, and expression of erp, a growth factor-inducible gene encoding a nontransmembrane protein tyrosine phosphatase, and effect of ERP on cell growth. Mol Cell Biol. 1993;13:5195–5205. doi: 10.1128/mcb.13.9.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 33.Bokemeyer D, Lindemann M, Kramer HJ. Regulation of mitogen-activated protein kinase phosphatase-1 in vascular smooth muscle cells. Hypertension. 1998;32:661–667. doi: 10.1161/01.hyp.32.4.661. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol. 2001;21:8213–8224. doi: 10.1128/MCB.21.23.8213-8224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG, Iversen L, Arthur JS. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–1036. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 36.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 37.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Tillo E, Comalada M, Xaus J, Farrera C, Valledor AF, Caelles C, Lloberas J, Celada A. JNK1 Is required for the induction of Mkp1 expression in macrophages during proliferation and lipopolysaccharide-dependent activation. J Biol Chem. 2007;282:12566–12573. doi: 10.1074/jbc.M609662200. [DOI] [PubMed] [Google Scholar]
- 39.Hiroi Y, Hiroi J, Kudoh S, Yazaki Y, Nagai R, Komuro I. Two distinct mechanisms of angiotensin II-induced negative regulation of the mitogen-activated protein kinases in cultured cardiac myocytes. Hypertens Res. 2001;24:385–394. doi: 10.1291/hypres.24.385. [DOI] [PubMed] [Google Scholar]
- 40.Kar S, Ukil A, Sharma G, Das PK. MAPK-directed phosphatases preferentially regulate pro- and anti-inflammatory cytokines in experimental visceral leishmaniasis: involvement of distinct protein kinase C isoforms. J Leukoc Biol. 2010;88:9–20. doi: 10.1189/jlb.0909644. [DOI] [PubMed] [Google Scholar]
- 41.Valledor AF, Xaus J, Comalada M, Soler C, Celada A. Protein kinase C epsilon is required for the induction of mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2000;164:29–37. doi: 10.4049/jimmunol.164.1.29. [DOI] [PubMed] [Google Scholar]
- 42.Rauhala HE, Porkka KP, Tolonen TT, Martikainen PM, Tammela TL, Visakorpi T. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int J Cancer. 2005;117:738–745. doi: 10.1002/ijc.21270. [DOI] [PubMed] [Google Scholar]
- 43.Guenard F, Bouchard L, Tchernof A, Deshaies Y, Hould FS, Lebel S, Marceau P, Perusse L, Vohl MC. DUSP1 Gene Polymorphisms Are Associated with Obesity-Related Metabolic Complications among Severely Obese Patients and Impact on Gene Methylation and Expression. Int J Genomics. 2013;2013:609748. doi: 10.1155/2013/609748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin NY, Lin CT, Chang CJ. Modulation of immediate early gene expression by tristetraprolin in the differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2008;365:69–74. doi: 10.1016/j.bbrc.2007.10.119. [DOI] [PubMed] [Google Scholar]
- 46.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6:1288–1292. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- 47.Shim J, Lim H, JRY, Karin M. Nuclear export of NF90 is required for interleukin-2 mRNA stabilization. Mol Cell. 2002;10:1331–1344. doi: 10.1016/s1097-2765(02)00730-x. [DOI] [PubMed] [Google Scholar]
- 48.Kuwano Y, Kim HH, Abdelmohsen K, Pullmann R, Jr, Martindale JL, Yang X, Gorospe M. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD. Genetic background determines the extent of atherosclerosis in ApoE- deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1960–1968. doi: 10.1161/01.atv.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 50.Hutter D, Chen P, Barnes J, Liu Y. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. Biochem J. 2000;352(Pt 1):155–163. [PMC free article] [PubMed] [Google Scholar]
- 51.Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- 52.Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 53.Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–21541. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- 54.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 55.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeong Y, Du R, Zhu X, Yin S, Wang J, Cui H, Cao W, Lowenstein CJ. Histone deacetylase isoforms regulate innate immune responses by deacetylating mitogen-activated protein kinase phosphatase-1. J Leukoc Biol. 2014;95:651–659. doi: 10.1189/jlb.1013565. [DOI] [PubMed] [Google Scholar]
- 57.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 58.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 59.Liu RM, Choi J, Wu JH, Gaston Pravia KA, Lewis KM, Brand JD, Mochel NS, Krzywanski DM, Lambeth JD, Hagood JS, Forman HJ, Thannickal VJ, Postlethwait EM. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem. 2010;285:16239–16247. doi: 10.1074/jbc.M110.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 61.Guan W, Sha J, Chen X, Xing Y, Yan J, Wang Z. S-Nitrosylation of mitogen activated protein kinase phosphatase-1 suppresses radiation-induced apoptosis. Cancer Lett. 2012;314:137–146. doi: 10.1016/j.canlet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Scafidi J, Prada AE, Zahedi K, Davis AE., III Nuclear phosphatases and the proteasome in suppression of STAT1 activity in hepatocytes. Biochem Biophys Res Commun. 2002;299:574–580. doi: 10.1016/s0006-291x(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 63.Venema RC, Venema VJ, Eaton DC, Marrero MB. Angiotensin II-induced tyrosine phosphorylation of signal transducers and activators of transcription 1 is regulated by Janus-activated kinase 2 and Fyn kinases and mitogen-activated protein kinase phosphatase 1. J Biol Chem. 1998;273:30795–30800. doi: 10.1074/jbc.273.46.30795. [DOI] [PubMed] [Google Scholar]
- 64.Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, Freeman BA, Chen YE, Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–4094. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Zhao Q, Matta R, Meng X, Liu X, Liu CG, Nelin LD, Liu Y. Inducible nitric-oxide synthase expression is regulated by mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2009;284:27123–27134. doi: 10.1074/jbc.M109.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinney CM, Chandrasekharan UM, Yang L, Shen J, Kinter M, McDermott MS, DiCorleto PE. Histone H3 as a novel substrate for MAP kinase phosphatase-1. Am J Physiol Cell Physiol. 2009;296:C242–249. doi: 10.1152/ajpcell.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
- 68.Lazo JS, Nunes R, Skoko JJ, Queiroz de Oliveira PE, Vogt A, Wipf P. Novel benzofuran inhibitors of human mitogen-activated protein kinase phosphatase-1. Bioorg Med Chem. 2006;14:5643–5650. doi: 10.1016/j.bmc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 69.Lazo JS, Skoko JJ, Werner S, Mitasev B, Bakan A, Koizumi F, Yellow-Duke A, Bahar I, Brummond KM. Structurally unique inhibitors of human mitogen-activated protein kinase phosphatase-1 identified in a pyrrole carboxamide library. J Pharmacol Exp Ther. 2007;322:940–947. doi: 10.1124/jpet.107.122242. [DOI] [PubMed] [Google Scholar]
- 70.Ullevig SL, Kim HS, Nguyen HN, Hambright WS, Robles AJ, Tavakoli S, Asmis R. Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of Nox4. Redox Biol. 2014;2:259–266. doi: 10.1016/j.redox.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pae HO, Jeong SO, Zheng M, Ha HY, Lee KM, Kim EC, Kim DH, Hwang SY, Chung HT. Curcumin attenuates ethanol-induced toxicity in HT22 hippocampal cells by activating mitogen-activated protein kinase phosphatase-1. Neurosci Lett. 2009;453:186–189. doi: 10.1016/j.neulet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Furst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J. 2007;21:74–80. doi: 10.1096/fj.06-6752com. [DOI] [PubMed] [Google Scholar]
- 73.Furst R, Zahler S, Vollmar AM. Dexamethasone-induced expression of endothelial mitogen-activated protein kinase phosphatase-1 involves activation of the transcription factors activator protein-1 and 3′,5′-cyclic adenosine 5′-monophosphate response element-binding protein and the generation of reactive oxygen species. Endocrinology. 2008;149:3635–3642. doi: 10.1210/en.2007-1524. [DOI] [PubMed] [Google Scholar]
- 74.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Leung DY, Goleva E. Anti-inflammatory and corticosteroid-enhancing actions of vitamin D in monocytes of patients with steroid-resistant and those with steroid-sensitive asthma. J Allergy Clin Immunol. 2014;133:1744–1752 e1741. doi: 10.1016/j.jaci.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. Am J Physiol Cell Physiol. 2008;294:C241–250. doi: 10.1152/ajpcell.00187.2007. [DOI] [PubMed] [Google Scholar]
- 78.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. J Biol Chem. 2004;279:46678–46685. doi: 10.1074/jbc.M406441200. [DOI] [PubMed] [Google Scholar]
- 79.Kiemer AK, Weber NC, Furst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 2002;90:874–881. doi: 10.1161/01.res.0000017068.58856.f3. [DOI] [PubMed] [Google Scholar]
- 80.Xu Q, Konta T, Furusu A, Nakayama K, Lucio-Cazana J, Fine LG, Kitamura M. Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids. Selective roles of nuclear receptors and contribution to the antiapoptotic effect. J Biol Chem. 2002;277:41693–41700. doi: 10.1074/jbc.M207095200. [DOI] [PubMed] [Google Scholar]
- 81.Williams PJ, Nishu K, Rahman MM. HDAC inhibitor trichostatin A suppresses osteoclastogenesis by upregulating the expression of C/EBP-beta and MKP-1. Ann N Y Acad Sci. 2011;1240:18–25. doi: 10.1111/j.1749-6632.2011.06286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rastogi R, Jiang Z, Ahmad N, Rosati R, Liu Y, Beuret L, Monks R, Charron J, Birnbaum MJ, Samavati L. Rapamycin induces mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) expression through activation of protein kinase B and mitogen-activated protein kinase kinase pathways. J Biol Chem. 2013;288:33966–33977. doi: 10.1074/jbc.M113.492702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim SH, Yu HS, Park HG, Park S, Seo MS, Jeon WJ, Ahn YM, Ha K, Shin SY, Kim YS. Role of MKP-1 (DUSP1) in clozapine-induced effects on the ERK1/2 signaling pathway in the rat frontal cortex. Psychopharmacology (Berl) 2013;230:425–437. doi: 10.1007/s00213-013-3165-y. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403:184–189. doi: 10.1016/j.bbrc.2010.10.135. [DOI] [PubMed] [Google Scholar]
- 85.Lee JH, Kim H, Woo JH, Joe EH, Jou I. 5, 8, 11, 14-eicosatetraynoic acid suppresses CCL2/MCP-1 expression in IFN-gamma-stimulated astrocytes by increasing MAPK phosphatase-1 mRNA stability. J Neuroinflammation. 2012;9:34. doi: 10.1186/1742-2094-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korhonen R, Hommo T, Keranen T, Laavola M, Hamalainen M, Vuolteenaho K, Lehtimaki L, Kankaanranta H, Moilanen E. Attenuation of TNF production and experimentally induced inflammation by PDE4 inhibitor rolipram is mediated by MAPK phosphatase-1. Br J Pharmacol. 2013;169:1525–1536. doi: 10.1111/bph.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 90.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 93.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators of inflammation. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, Varol C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 99.Tavakoli S, Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal. 2012;17:1785–1795. doi: 10.1089/ars.2012.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 102.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ullevig SL, Kim HS, Short JD, Tavakoli S, Weintraub ST, Downs K, Asmis R. Protein S-Glutathionylation Mediates Macrophage Responses to Metabolic Cues from the Extracellular Environment. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2015.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Short JD, Downs K, Tavakoli S, Asmis R. Protein Thiol Redox Signaling in Monocytes and Macrophages. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiao M, Zhao Q, Lee CF, Tannock L, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, Asmis R. Thiol Oxidative Stress Induced by Metabolic Disorders Amplifies Macrophage Chemotactic Responses and Accelerates Atherogenesis and Kidney Injury in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–1786. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ullevig S, Zhao Q, Lee CF, Kim HS, Zamora D, Asmis R. NADPH Oxidase 4 Mediates Monocyte Priming and Accelerated Chemotaxis Induced by Metabolic Stress. Arterioscler Thromb Vasc Biol. 2012;32:415–426. doi: 10.1161/ATVBAHA.111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ryan GB, Majno G. Acute inflammation. A review. Am J Pathol. 1977;86:183–276. [PMC free article] [PubMed] [Google Scholar]
- 108.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 109.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 110.Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- 111.Arefieva TI, Kukhtina NB, Antonova OA, Krasnikova TL. MCP-1-stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–446. doi: 10.1016/j.cyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 112.Chuang SY, Yang SH, Pang JH. Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression. Biochem Biophys Res Commun. 2011;411:402–408. doi: 10.1016/j.bbrc.2011.06.163. [DOI] [PubMed] [Google Scholar]
- 113.Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR, Schratzberger P, Djanani AM, Mahata SK, Kirchmair R. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–111. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 114.Grimshaw MJ, Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation--a potential mechanism. Eur J Immunol. 2001;31:480–489. doi: 10.1002/1521-4141(200102)31:2<480::aid-immu480>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 115.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H. Macrophages in inflammation and its resolution. Front Immunol. 2012;3:324. doi: 10.3389/fimmu.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tavakoli S, Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal. 2012;17:1785–1795. doi: 10.1089/ars.2012.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 119.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 120.Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Korhonen R, Turpeinen T, Taimi V, Nieminen R, Goulas A, Moilanen E. Attenuation of the acute inflammatory response by dual specificity phosphatase 1 by inhibition of p38 MAP kinase. Mol Immunol. 2011;48:2059–2068. doi: 10.1016/j.molimm.2011.06.439. [DOI] [PubMed] [Google Scholar]
- 124.Valledor AF, Arpa L, Sanchez-Tillo E, Comalada M, Casals C, Xaus J, Caelles C, Lloberas J, Celada A. IFN-{gamma}-mediated inhibition of MAPK phosphatase expression results in prolonged MAPK activity in response to M-CSF and inhibition of proliferation. Blood. 2008;112:3274–3282. doi: 10.1182/blood-2007-11-123604. [DOI] [PubMed] [Google Scholar]
- 125.Yu H, Sun Y, Haycraft C, Palanisamy V, Kirkwood KL. MKP-1 regulates cytokine mRNA stability through selectively modulation subcellular translocation of AUF1. Cytokine. 2011;56:245–255. doi: 10.1016/j.cyto.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smallie T, Ross EA, Ammit AJ, Cunliffe HE, Tang T, Rosner DR, Ridley ML, Buckley CD, Saklatvala J, Dean JL, Clark AR. Dual-Specificity Phosphatase 1 and Tristetraprolin Cooperate To Regulate Macrophage Responses to Lipopolysaccharide. J Immunol. 2015;195:277–288. doi: 10.4049/jimmunol.1402830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gumireddy K, Reddy CD, Swamy N. Mitogen-activated protein kinase pathway mediates DBP-maf-induced apoptosis in RAW 264.7 macrophages. J Cell Biochem. 2003;90:87–96. doi: 10.1002/jcb.10615. [DOI] [PubMed] [Google Scholar]
- 128.Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- 129.Mei S, Gu H, Ward A, Yang X, Guo H, He K, Liu Z, Cao W. p38 mitogen-activated protein kinase (MAPK) promotes cholesterol ester accumulation in macrophages through inhibition of macroautophagy. J Biol Chem. 2012;287:11761–11768. doi: 10.1074/jbc.M111.333575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu C, Russell RM, Wang XD. Low dose beta-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38 MAPK, and p53 proteins. J Nutr. 2004;134:2705–2710. doi: 10.1093/jn/134.10.2705. [DOI] [PubMed] [Google Scholar]
- 131.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1392–1401. doi: 10.1152/ajplung.2001.281.6.L1392. [DOI] [PubMed] [Google Scholar]
- 133.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 134.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nature Medicine. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 135.Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J Immunol. 2011;187:1764–1777. doi: 10.4049/jimmunol.1002315. [DOI] [PubMed] [Google Scholar]
- 136.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 137.Sung NY, Yang MS, Song DS, Byun EB, Kim JK, Park JH, Song BS, Lee JW, Park SH, Park HJ, Byun MW, Byun EH, Kim JH. The procyanidin trimer C1 induces macrophage activation via NF-kappaB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur J Pharmacol. 2013;714:218–228. doi: 10.1016/j.ejphar.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 138.Shen J, Chandrasekharan UM, Ashraf MZ, Long E, Morton RE, Liu Y, Smith JD, DiCorleto PE. Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis. Circ Res. 2010;106:902–910. doi: 10.1161/CIRCRESAHA.109.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Imaizumi S, Grijalva V, Priceman S, Wu L, Su F, Farias-Eisner R, Hama S, Navab M, Fogelman AM, Reddy ST. Mitogen-activated protein kinase phosphatase-1 deficiency decreases atherosclerosis in apolipoprotein E null mice by reducing monocyte chemoattractant protein-1 levels. Mol Genet Metab. 2010;101:66–75. doi: 10.1016/j.ymgme.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res. 2010;85:649–660. doi: 10.1093/cvr/cvp327. [DOI] [PubMed] [Google Scholar]
- 141.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Melendez J, Turner C, Avraham H, Steinberg SF, Schaefer E, Sussman MA. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J Biol Chem. 2004;279:53516–53523. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- 143.Benoit MJ, Rindt H, Allen BG. Cardiac-specific transgenic overexpression of alpha1B-adrenergic receptors induce chronic activation of ERK MAPK signalling. Biochem Cell Biol. 2004;82:719–727. doi: 10.1139/o04-123. [DOI] [PubMed] [Google Scholar]
- 144.Su Q, Chen J, Fang Q. Modulation of the p38MAPK pathway in failing human myocardium secondary to rheumatic heart disease. J Heart Valve Dis. 2007;16:683–689. [PubMed] [Google Scholar]
- 145.Communal C, Colucci WS, Remondino A, Sawyer DB, Port JD, Wichman SE, Bristow MR, Singh K. Reciprocal modulation of mitogen-activated protein kinases and mitogen-activated protein kinase phosphatase 1 and 2 in failing human myocardium. J Card Fail. 2002;8:86–92. doi: 10.1054/jcaf.2002.32755. [DOI] [PubMed] [Google Scholar]
- 146.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 147.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 148.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardi M, Caelles C, Serrano AL, Munoz-Canoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]