Summary
Objective
Neonates with hypoxic-ischemic encephalopathy (HIE) managed with therapeutic hypothermia (TH) often experience acute symptomatic seizures, prompting treatment with anti-seizure medications (ASM). Because the risk of seizure occurrence after hospital discharge is unknown, the optimal ASM treatment duration is unclear. We aimed to determine the risk of seizure occurrence after hospital discharge and the impact of ASM treatment duration on this outcome.
Methods
We performed a single-center, retrospective study of consecutive neonates with HIE managed with TH who received ASM for acute symptomatic seizures from June 2010 through December 2014. Neonates were monitored with continuous EEG during TH.
Results
Follow-up data were available for 59/72 (82%) neonates who survived to discharge, with a median follow-up period of 19 months (interquartile range 11, 25). Acute symptomatic seizures occurred in 35 (59%), including electrographic seizures in 21 (36%). ASM were continued upon discharge in 17 of 35 neonates (49%). Seizures occurred in follow-up in 4 neonates (11%). No patient for whom ASM were discontinued prior to discharge experienced seizures during the follow-up period.
Significance
Among neonates with HIE, seizures after hospital discharge were rare in those with acute symptomatic seizures and did not occur in neonates without acute symptomatic seizures. ASM discontinuation prior to discharge did not increase the risk of seizures during the follow-up period, suggesting that ASM may be discontinued in many neonates prior to discharge.
Keywords: hypoxic-ischemic encephalopathy, therapeutic hypothermia, neonatal seizures, anti-seizure medication, outcomes
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) occurs in 3–5 newborns per 1000 live births1. Historically, HIE resulted in significant morbidity and mortality2; however, in recent years, therapeutic hypothermia (TH) has emerged as the standard of care, leading to reductions in death or major neurodevelopmental disability3–9. In addition, TH may reduce seizures in neonates with moderate HIE10. Despite these improvements, the incidence of acute symptomatic seizures remains high, and many neonates experience a high seizure burden11,12. Neonates treated with TH for HIE most commonly experience seizures on the initial 1–2 days of TH and during rewarming on day 412,13.
Despite the high incidence of acute symptomatic seizures, the risk of future epilepsy in this population may be low5. In addition, there is growing concern about the sedating and potentially neurotoxic effects of anti-seizure medications (ASM), particularly phenobarbital14–17. For these reasons, at some institutions, care has evolved toward ASM discontinuation prior to hospital discharge18. However, the impact of this practice on future seizures is unknown. Among neonates treated with TH for HIE, we aimed to determine the risk of seizure occurrence after hospital discharge and whether shorter ASM treatment duration was associated with this outcome.
Patients and Methods
The Children’s Hospital of Philadelphia (CHOP) Institutional Review Board approved this study. We performed a single-center, retrospective study of consecutive neonates treated with TH for HIE from June 2010 through December 2014. All neonates treated with TH for HIE during this period were included. No neonates were excluded. Subjects were identified using a database maintained by the neonatal intensive care unit (NICU) that catalogues clinical data, including medications at discharge. Based on our institutional pathway, TH was initiated within 6 hours of birth. All neonates underwent continuous EEG monitoring (cEEG) during TH and through rewarming, or for 24–48 hours after the cessation of the last seizure. MRI scans were performed within 24–48 hours of rewarming in nearly all neonates, except when neonates were not medically stable for transport to MRI at that time. All neonates ultimately had MRI scans, except for those neonates who died prior to discharge. EEG monitoring was performed using Grass-Telefactor video EEG equipment and a standard neonatal 10–10 electrode montage. All EEG data were interpreted by trained pediatric electroencephalographers. Clinical management was performed by the neonatal and neonatal neurology consultation services. There was no institutional pathway determining whether neonates continue ASM after discharge; therefore, the decision to discontinue ASM was made by the clinical team on an individual basis using patient characteristics such as seizure burden, responsiveness of seizures to ASM treatment, and severity of injury on MRI. Data regarding cEEG and MRI findings were obtained by reviewing reports in the electronic medical record. The occurrence of clinical or electrographic seizures during follow-up was determined by reviewing clinical care notes and EEG reports from routine follow-up appointments with neurology and from structured follow-up appointments with the neonatal follow-up clinic at 3, 6, 12, 18, and 24 months, and additionally as needed.
Clinical variables were defined as follows. HIE severity was determined prior to TH initiation by the admitting neonatologist using a structured clinical examination to assess the degree of encephalopathy. Initial EEG background was classified as: (1) mildly abnormal if the record demonstrated mild attenuation or mild excessive discontinuity, (2) moderately abnormal if the record demonstrated both attenuation and discontinuity or if there was discontinuity alone with interburst intervals that lasted < 30 seconds, or (3) severely abnormal if the record was severely attenuated/featureless or if there was discontinuity with interburst intervals that lasted > 30 seconds or if burst-suppression was present. Acute symptomatic seizures were classified as present if there were clinical seizures leading to a neonatologist administering an ASM and/or if electrographic seizures occurred during cEEG. Electrographic seizures were defined according to neonatal EEG standardized terminology from the American Clinical Neurophysiology Society19. Electrographic seizure exposure during admission was classified as: (1) rare if there were 1–2 seizures, (2) occasional if there were 3–10 seizures, (3) frequent if there were > 10 seizures, or (4) status epilepticus if individual seizures lasted > 5 minutes or if 50% of any one hour epoch contained electrographic seizure. MRI reports were reviewed by one clinician (MPF) blinded to clinical and EEG data on two occasions spaced more than one month apart. MRI injury distribution was scored using published methods, modified to include DWI signal abnormality in addition to abnormalities on T1 and T2 weighted images20. MRI images were reviewed when the reports were not sufficiently clear for scoring. Time to ASM discontinuation was measured from the date of NICU discharge to the date of ASM discontinuation.
Data were summarized by standard descriptive statistics, including median/interquartile range (IQR) or mean/standard deviation (SD) for continuous variables, or as percentages for categorical variables. Comparisons between outcome groups were made using Fisher’s exact test for categorical exposure variables and Kruskall-Wallis test for continuous exposure variables with non-parametric distributions. A Kaplan-Meier survivor function curve was generated to illustrate time to seizure recurrence. Further analysis of the influence of exposure variables on survivor function was curtailed due to a small number of outcome events.
Results
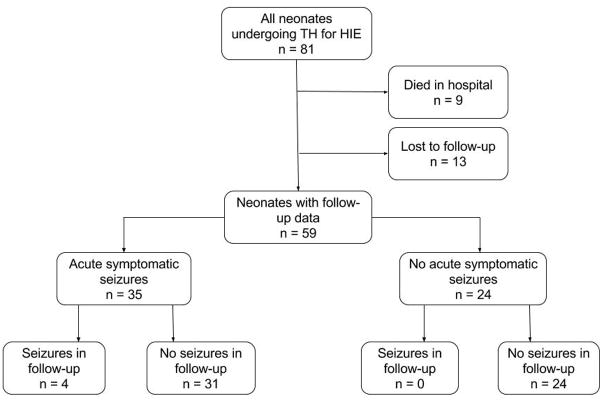
Eighty-one neonates underwent TH for HIE during the study period. Nine neonates (11%) died prior to discharge as a result of withdrawal of technological support, and 13 neonates (18%) were lost to follow-up, yielding 59 neonates (73%) in the remaining cohort (Figure 1). Table 1 summarizes the clinical characteristics of the neonates, and it demonstrates that the cohort with follow-up data is representative of the full cohort. Acute symptomatic seizures occurred in 35 of 59 neonates (59%). These included 21 neonates (36%) with electrographic seizures identified by cEEG during or after TH, and 14 neonates (24%) with clinical seizures that were treated without subsequent electrographic seizures on cEEG. ASM were continued on discharge for 17 of 35 neonates (49%) with acute symptomatic seizures, including 15 neonates with electrographic seizures identified by cEEG and 2 neonates with clinically apparent seizures prior to cEEG initiation. The majority of the seizures in the neonatal period were subclinical. Those with clinical signs were classified as focal with impaired awareness, often with lip smacking and eyelid fluttering, focal tonic with stiffening of extremities, or focal clonic.
Figure 1.
Study Design. Eighty-one neonates were eligible and 59 had follow-up data available. Thirty-five neonates experienced acute symptomatic seizures, while 24 did not. Of those with acute symptomatic seizures, 4 had seizures in follow-up. Of those without acute seizures, none had seizures in follow-up.
Table 1.
Clinical characteristics of the study cohort, stratified based on (1) presence or absence of seizures during the follow-up period, and (2) anti-seizure medication (ASM) on discharge. Continuous variables were analyzed with the Kruskall-Wallis test, while categorical variables were analyzed with Fisher’s exact test.
| Variable | Full cohort (n=81) | Follow-up cohort (n=59) | Neonates with acute symptomatic seizures (n=35) | Neonates with acute symptomatic seizures (n=35) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Seizures in follow-up (n=4) | No seizures in follow-up (n=31) | p-value | ASM on discharge (n=17) | No ASM on discharge (n=18) | p-value | |||
| Sex, n (%) | ||||||||
| Male | 44 (54%) | 33 (56%) | 4 (100%) | 20 (65%) | 0.15 | 11 (65%) | 13 (72%) | 0.63 |
| Female | 37 (46%) | 26 (44%) | 0 (0%) | 11 (35%) | 6 (35%) | 5 (28%) | ||
| Gestational age (weeks), mean (SD) | 38.8 (1.7) | 38.9 (1.7) | 38.3 (0.9) | 38.6 (1.7) | 0.51 | 38.8 (1.3) | 38.4 (1.9) | 0.6 |
| Birth weight (grams), mean (SD) | 3367 (694) | 3389 (671) | 3046 (758) | 3314 (580) | 0.36 | 3351 (552) | 3220 (644) | 0.62 |
| pH, cord blood, mean (SD) | 6.93 (0.20) | 6.96 (0.18) | 6.69 (0.11) | 7.00 (0.2) | 0.02 | 6.89 (0.21) | 7.00 (0.23) | 0.4 |
| pH, 1hr gas, mean (SD) | 6.97 (0.14) | 6.93 (0.15) | 6.88 (0.14) | 7.02 (0.14) | 0.18 | 6.95 (0.13) | 7.02 (0.16) | 0.27 |
| APGAR 1min, median (IQR) | 1 (1,2) | 1 (1,2) | 1 (0,2) | 1 (0,2) | 0.49 | 1 (0,2) | 1 (0,2) | 0.51 |
| APGAR 5min, median (IQR) | 3 (2,5) | 3 (2,5) | 2 (1,3) | 2 (2,5) | 0.24 | 3 (1,7) | 2 (2,3) | 0.21 |
| APGAR 10min, median (IQR) | 4 (3,6) | 4 (3,6) | 4 (3,6) | 4 (3,6) | 0.93 | 4 (3,6) | 4 (3,5) | 0.68 |
| Admission length (days), median (IQR) | 18 (11, 27) | 19 (12,27) | 34 (27,50) | 18 (13,26) | 0.02 | 27 (16,32) | 18 (11,23) | 0.02 |
| HIE Severity, n (%) | ||||||||
| Mild | 15 (19%) | 13 (22%) | 0 (0%) | 3 (10%) | 0.71 | 1 (6%) | 2 (11%) | 0.68 |
| Moderate | 45 (56%) | 36 (61%) | 2 (50%) | 20 (65%) | 10 (59%) | 12 (67%) | ||
| Severe | 21 (26%) | 10 (17%) | 2 (50%) | 8 (26%) | 6 (35%) | 4 (22%) | ||
| EEG seizures, n (%) | ||||||||
| Yes | 26 (32%) | 21 (36%) | 3 (75%) | 18 (58%) | 0.52 | 15 (88%) | 6 (33%) | <0.01 |
| No | 55 (68%) | 38 (64%) | 1 (24%) | 13 (42%) | 2 (12%) | 12 (67%) | ||
| Seizure exposure, n (%) | ||||||||
| 1–2 Seizures (rare) | 8 (10%) | 7 (12%) | 1 (33%) | 6 (33%) | 0.62 | 5 (33%) | 2 (33%) | 0.91 |
| 3–10 Seizures (occasional) | 6 (7%) | 6 (10%) | 0 (0%) | 6 (33%) | 5 (33%) | 1 (17%) | ||
| > 10 Seizures (frequent) | 8 (10%) | 6 (10%) | 2 (67%) | 4 (23%) | 4 (27%) | 2 (33%) | ||
| Status Epilepticus | 4 (5%) | 2 (3%) | 0 (0%) | 2 (11%) | 1 (7%) | 1 (17%) | ||
| Initial EEG background, n (%) | ||||||||
| Normal | 14 (17%) | 8 (14%) | 0 (0%) | 3 (10%) | 0.31 | 1 (6%) | 2 (11%) | 0.32 |
| Mildly abnormal | 28 (35%) | 23 (39%) | 0 (0%) | 11 (35%) | 3 (18%) | 8 (44%) | ||
| Moderately abnormal | 15 (19%) | 14 (24%) | 1 (25%) | 9 (29%) | 6 (35%) | 4 (22%) | ||
| Severely abnormal | 24 (30%) | 14 (24%) | 3 (75%) | 8 (26%) | 7 (41%) | 4 (22%) | ||
| MRI injury distribution, n (%) | ||||||||
| Normal | 35/72 (49%) | 28/57 (49%) | 1 (25%) | 13 (43%) | 0.08 | 3 (19%) | 11 (61%) | 0.03 |
| Deep Grey Only | 7/72 (10%) | 5/57 (9%) | 2 (50%) | 2 (7%) | 2 (13%) | 2 (11%) | ||
| Cortical Only | 20/72 (28%) | 19/57 (33%) | 0 (0%) | 11 (37%) | 6 (38%) | 5 (45%) | ||
| Cortical + Deep Grey | 7/72 (10%) | 4/57 (7%) | 1 (25%) | 3 (10%) | 4 (25%) | 0 (0%) | ||
| Extensive Injury | 3/72 (4%) | 1/57 (2%) | 0 (0%) | 1 (3%) | 1 (6%) | 0 (0%) | ||
| Discharged on ASM, n (%) | ||||||||
| Yes | 18/72 (22%) | 17 (29%) | 4 (100%) | 13 (42%) | 0.05 | NA | NA | NA |
| No | 63/72 (78%) | 42 (71%) | 0 (0%) | 18 (58%) | ||||
| Length of ASM therapy after discharge (days), mean (SD) | 144 (95) | 144 (95) | 121 (221) | 120 (75) | 0.08 | NA | 144 (95) | NA |
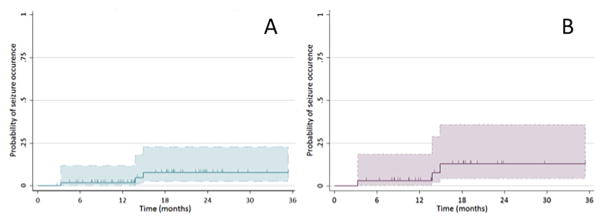
The median follow-up time was 19 months (IQR 11,25). Four neonates experienced seizures during the follow-up period, all of whom had acute symptomatic seizures. Four neonates represent 7% of the 59 neonates with available follow-up data and 11% of the 35 neonates with acute symptomatic seizures. All four neonates were discharged from the hospital on an ASM. None of these neonates had ongoing seizures at the time of discharge. The first unprovoked seizure in these patients occurred at ages 4 months, 15 months, 16 months, and 48 months (Figure 2). The infants with seizure occurrence at 4 months, 15 months, and 16 months were on standard ASM doses at the time, whereas the child with seizures at 48 months had discontinued ASM several years prior. These four patients were diagnosed with epilepsy because they had two or more unprovoked seizures in follow-up. Their epilepsy was classified as focal with impaired awareness, with hemiclonic or dyscognitive seizures. One patient developed infantile spasms. None of the neonates with acute symptomatic seizures who discontinued ASM prior to discharge experienced seizures during the follow-up period. Additionally, all neonates who were seizure free during the acute period remained seizure free during the follow-up period.
Figure 2.
Kaplan-Meier curve illustrating the probability of seizure occurrence after discharge for (A) all 59 neonates with follow-up data for review, and (B) the 35 neonates who experienced acute symptomatic seizures. Hashmarks indicated individuals censored. The 95% confidence interval is shown in color.
The characteristics that distinguished neonates who experienced seizures during the follow-up period from those who did not included lower cord gas pH (6.69 +/− 0.11 versus 7.00 +/−0.20, p=0.02), longer median admission length (34 days [IQR 27,50] versus 18 days [IQR 13,26], p=0.02), and higher likelihood of discharge from the hospital on an ASM (100% versus 42%, p=0.05, see Table 1). The presence of MRI injury was associated with a higher risk of seizures during the follow-up period, but the difference was not statistically significant (75% versus 57%; p=0.08). Table 2 summarizes the clinical characteristics of neonates who experienced seizures during the follow-up period. Because of the low prevalence of seizure occurrence in follow-up, a multivariate predictive model for seizure occurrence could not be constructed.
Table 2.
Clinical characteristics for neonates with seizures during follow-up. Patient 3 had early clinical seizures and was started on phenobarbital prior to placement on cEEG. No electrographic seizures were captured; however, cEEG was epileptiform, as was a repeat EEG prior to discharge, thus phenobarbital was continued.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Sex | Male | Male | Male | Male |
| Gestational Age (weeks) | 37 | 38 | 39 | 39 |
| Race | Caucasian | Caucasian | Caucasian | Other |
| Apgar 1 min | 0 | 3 | 0 | 1 |
| Apgar 5 min | 1 | 4 | 0 | 2 |
| Apgar 10 min | 3 | 4 | 3 | 7 |
| NICU length of stay (days) | 63 | 21 | 32 | 36 |
| Hospital length of stay (days) | 63 | 21 | 49 | 38 |
| pH, cord blood | 6.67 | 6.59 | 6.8 | NA |
| pH, 1hr blood gas | 6.8 | 7.04 | 6.8 | NA |
| Acute symptomatic seizures | Yes (EEG) | Yes (EEG) | Yes (clinical) | Yes (EEG) |
| EEG background abnormality | Severe | Severe | Moderate | Severe |
| Number of seizures on cEEG | 15 | 2 | 0 | 104 |
| MRI injury distribution | Deep gray | Deep gray | Normal | Cortical + deep gray |
| HIE severity | Moderate | Severe | Moderate | Severe |
| ASM on discharge | Phenobarbital | Phenobarbital | Phenobarbital | Levetiracetam |
| Age of seizure occurrence in follow-up (months) | 48 | 4 | 16 | 15 |
| Age at last follow-up (months) | 54 | 46 | 48 | 21 |
| Developmental delay at last follow-up | Global | Global | Gross motor | Global |
Characteristics which distinguished neonates who continued ASM after discharge from those who did not included longer median admission length (27 days [16,32] versus 18 days [11,23], p=0.02), higher likelihood of having electrographic seizures (88% versus 33%, p<0.01), and lower likelihood of having a normal brain MRI (19% versus 61%, p=0.03, see Table 1).
Discussion
This study evaluated the risk of seizure occurrence after hospital discharge in neonates with HIE treated with TH, with attention to whether ASM discontinuation prior to discharge impacted the risk of seizures during the follow-up period. There are clinically meaningful findings. First, the risk of seizure occurrence after discharge in this population is low. Seizures occurred in follow-up in only 7% of neonates with available follow-up data (including neonates who did and did not experience acute symptomatic seizures) and 11% of neonates with acute symptomatic seizures. This is consistent with the TOBY trial for TH, in which 10% of subjects required ASM for treatment of epilepsy at 18 months of age5. Second, infants for whom ASM were discontinued prior to hospital discharge did not have an increased risk of seizure occurrence during the follow-up period.
Determining how long to treat a neonate after resolution of acute symptomatic seizures with an ASM is an important clinical question. Phenobarbital, the most frequently administered ASM, may negatively impact brain development, and it and can induce neuronal apoptosis in animal models even after a single dose16,17,21. Studies of human children exposed in utero to ASM have also demonstrated an increased risk of cognitive impairments in later childhood22–25. Additionally, phenobarbital has been associated with lower intelligence quotient and lasting cognitive impairments in children who were treated for prevention of febrile seizures14,26. Given these concerns, ASM should be used judiciously in neonates already at risk for neurodevelopmental impairments due to HIE. Thus, if effective control of acute symptomatic seizures can be obtained with limited ASM exposure and without an increased risk of future seizures, then it is important to precisely define the optimal duration of ASM treatment. This study represents an initial step toward this goal, and it provides evidence that ASM can be discontinued for many neonates prior to hospital discharge.
This study has several strengths. First, this cohort is one of the largest outside of the pivotal clinical trials of TH for HIE. Second, these data are generalizable to other neonatal referral centers since they are derived from consecutively treated neonates. Further, key data are consistent with prior reports related to acute symptomatic seizures, including a 36% incidence of electrographic seizures10–13,27–29. Notably, the cohort contains neonates who experienced clinical seizures but who did not have electrographic seizures on cEEG. Despite the fact that neonatal seizures are prone to misidentification30, we included these neonates because clinical seizures are an indication for initiating therapeutic hypothermia, and a cohort including them reflects the patient population typically encountered by practicing neonatologists and pediatric neurologists. Third, relatively few patients were lost to follow-up, which improves our confidence in the conclusion that the risk of seizure occurrence in the initial two years after hospital discharge is indeed low.
The study has several limitations. First, because of its retrospective design, some of the clinical variables cannot be defined optimally. For example, seizure exposure measurement is restricted to the number of seizures present on cEEG, which is not the most accurate measure of seizure exposure. Other studies have quantified seizure exposure in terms of the time spent experiencing electrographic seizures10,13,27,28,31 or have calculated a seizure score that encompasses multiple parameters11. The most precise manner of quantifying seizure exposure is not clear; however, the optimal measure would likely need to account for both time spent seizing and the area of cortex involved. These considerations will be important in the design of future prospective studies. Second, despite our large cohort, the limited number of neonates experiencing future seizures prohibits multivariate analysis to identify risk factors for seizure occurrence. Our study did identify some potentially important commonalities among those patients who experienced seizures after discharge, including low cord gas pH, prolonged hospitalization, and the presence of injury on MRI scan. Additionally, neonates discharged on an ASM were more likely to have a prolonged hospital admission, electrographic seizures on cEEG, and an abnormal brain MRI. These findings likely reflect an increased clinical concern about the risk of future seizures in these neonates; however, the extent these parameters predict seizure recurrence remains unclear. Third, while our median follow-up interval of 19 months may not be long enough to capture the entire period of risk for developing epilepsy, no study has reported on long-term risk for seizure occurrence after TH for HIE. A larger, multi-center, prospective study is needed to better evaluate risk factors for later epilepsy over the longer term, with the aim of providing guidance to clinicians deciding whether to prescribe an ASM upon discharge. However, if a lengthy period exists in which ASM are not needed, avoiding unnecessary ASM exposure could still have neurodevelopmental benefits even if seizures recur later.
Conclusion
Most neonates managed with TH for HIE who experience acute symptomatic seizures do not have additional seizures in the 1.5 years after hospital discharge. Moreover, discontinuation of ASM prior to discharge is not associated with an increased risk of seizure occurrence later. Thus, many neonates treated with ASM for acute symptomatic seizures can have the ASM discontinued prior to discharge. This may be particularly applicable for neonates with few seizures that are easily controlled with ASM, if the EEG appears only mildly abnormal, or if the brain MRI does not show severe injury. Priorities for further research include elucidating which neonates with HIE are at the highest risk of seizures later in life, and whether more prolonged ASM administration after the period of acute illness may be indicated in a specific cohort.
Key Points.
Acute symptomatic seizures are common in neonates undergoing therapeutic hypothermia for hypoxic-ischemic encephalopathy.
Seizure occurrence after discharge for neonates who have acute symptomatic seizures is rare.
Discontinuing anti-seizure medications prior to discharge is not associated with an increased risk of future seizures.
Additional studies are needed to identify clinical factors that predict later seizures.
Acknowledgments
This research was supported by NINDS K23NS076550.
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose.
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Levene MI, Sands C, Grindulis H, et al. Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet (London, England) 1986;1:67–9. doi: 10.1016/s0140-6736(86)90718-x. [DOI] [PubMed] [Google Scholar]
- 2.Vannucci RC. Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1990;85:961–8. [PubMed] [Google Scholar]
- 3.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371:140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi DV, Strohm B, Edwards aD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 7.Simbruner G, Mittal Ra, Rohlmann F, et al. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S, Pappas A, McDonald SA, et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F267–72. doi: 10.1136/archdischild-2011-300716. [DOI] [PubMed] [Google Scholar]
- 11.Glass HC, Glidden D, Jeremy RJ, et al. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–23. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic Seizures During Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Child Neurol. 2011;26:724–8. doi: 10.1177/0883073810390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F219–24. doi: 10.1136/archdischild-2013-305206. [DOI] [PubMed] [Google Scholar]
- 14.Sulzbacher S, Farwell JR, Temkin N, et al. Late cognitive effects of early treatment with phenobarbital. Clin Pediatr (Phila) 1999;38:387–94. doi: 10.1177/000992289903800702. [DOI] [PubMed] [Google Scholar]
- 15.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103-114-124. doi: 10.1111/j.1749-6632.2003.tb07517.x. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res. 2010;88:11–22. doi: 10.1016/j.eplepsyres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Kaindl AM, Asimiadou S, Manthey D, et al. Antiepileptic drugs and the developing brain. Cell Mol Life Sci. 2006;63:399–413. doi: 10.1007/s00018-005-5348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massaro AN, Murthy K, Zaniletti I, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s Hospitals Neonatal Consortium HIE focus group. J Perinatol. 2014:1–7. doi: 10.1038/jp.2014.190. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- 20.Barkovich aJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103-14-4. doi: 10.1111/j.1749-6632.2003.tb07517.x. [DOI] [PubMed] [Google Scholar]
- 22.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–52. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings C, Stewart M, Stevenson M, et al. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child. 2011;96:643–7. doi: 10.1136/adc.2009.176990. [DOI] [PubMed] [Google Scholar]
- 25.Bromley RL, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia. 2010;51:2058–65. doi: 10.1111/j.1528-1167.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- 26.Farwell JR, Lee YJ, Hirtz DG, et al. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364–9. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG Seizures in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. Pediatrics. 2015;136:e1302–9. doi: 10.1542/peds.2014-3777. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasakumar P, Zempel J, Wallendorf M, et al. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163:465–70. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–5. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malone A, Anthony Ryan C, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101. doi: 10.1111/j.1528-1167.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 31.Lynch NE, Stevenson NJ, Livingstone V, et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53:549–57. doi: 10.1111/j.1528-1167.2011.03401.x. [DOI] [PubMed] [Google Scholar]