Abstract
Background
Spontaneous intraventricular hemorrhage (IVH) is associated with high rates of morbidity and mortality despite critical care and other advances. An important step in clinical management is to confirm/rule out an underlying vascular lesion, which influences further treatment, potential for further bleeding and prognosis. Our aim is to compare demographic and clinical characteristics between IVH patients with and without an underlying vascular lesion, and among cohorts with different vascular lesions.
Methods
We analyzed prospectively collected data of IVH patients screened for eligibility as part of the Clot Lysis: Evaluation Accelerated Resolution of Intraventricular Hemorrhage-CLEAR Phase III clinical trial. The trial adopted a structured screening process to systematically exclude patients with an underlying vascular lesion as etiology of IVH. We collected age, sex, ethnicity and primary diagnosis on these cases and vascular lesions were categorized prospectively as aneurysm, vascular malformation (arteriovenous malformation, dural arteriovenous fistula, cavernoma), Moyamoya disease or other vascular lesion. We excluded cases < 18 or > 80 years of age. Baseline characteristics were compared between the CLEAR group (IVH screened without vascular lesion) and the group of IVH patients screened and excluded from CLEAR because of an identified vascular lesion. We further analyzed the differential demographic and clinical characteristics among subcohorts with different vascular lesions.
Results
10,538 consecutive IVH cases were prospectively screened for the trial between 2011 and 2015. 496 cases (4.7%) screened negative for underlying vascular lesion, met the inclusion criteria and were enrolled in the trial (no vascular etiology group), and 1,205 cases (11.4%) were concurrently screened and excluded from the trial because of a demonstrated underlying vascular lesion (vascular etiology group). Cases with vascular lesion were less likely to be older than 45 years of age (OR 0.28 CI 0.20–0.40), African-American (OR 0.23 CI 0.18–0.31) or male (OR 0.48 CI 0.38–0.60), and more likely to present with primary IVH (OR 1.85 CI 1.37–2.51) compared to those with no vascular etiology (p<0.001). Other demographic factors were associated with specific vascular lesion etiologies. A combination of demographic features increases the association with the absence of vascular lesion, but not with absolute reliability (OR 0.1, CI 0.06–0.17, p<0.001).
Conclusion
An underlying vascular lesion as etiology of intraventricular hemorrhage cannot be excluded solely by demographic parameters in any patient. Some form of vascular imaging is necessary in screening patients before contemplating interventions like intraventricular fibrinolysis, where safety may be impacted by the presence of vascular lesion.
Keywords: Intraventricular hemorrhage, Risk Factors, Etiology, Screening, Cerebral angiography
INTRODUCTION
Intraventricular hemorrhage (IVH) is a distinct subtype of intracerebral hemorrhage that results from blood gaining access to (or, less commonly, arising within) the brain ventricles [1]. The condition is invariably associated with high risk of mortality and poor functional outcome despite advances in clinical practice [2–8].
The presence of an underlying vascular lesion is important for prognosis, estimating risk of re-bleeding which consequently dictates further management in ICH/IVH such as the decision and timing for surgical and/or endovascular interventions. Other aspects of patient management are also influenced by the presence or absence of an unsecured vascular lesion, including blood pressure control, and prophylactic or therapeutic anticoagulation for venous or cardiogenic thromboembolism[9–14].
Guidelines for the selection of patients with ICH/IVH for vascular etiology screening are lacking. Recommendations by the American Heart Association and European Stroke Organization are based on low levels of evidence [15–17]. The decision about whether and how to deploy vascular imaging varies greatly in current practice [18–20]. The relative contribution of demographic factors to the likelihood of an underlying vascular lesion has not been carefully assessed. We hypothesize that patients with IVH secondary to a vascular lesion have distinctive demographic characteristics which differ according to the pathological nature of the underlying vascular etiology.
METHODS
CLEAR III Trial
The “Clot Lysis: Evaluation of Accelerated Resolution of Intraventricular Hemorrhage Phase III” (CLEAR III) clinical trial is an international multicenter double-blinded phase III clinical trial investigating the effect of an intraventricular recombinant tissue plasminogen activator (alteplase) on outcomes in patients with IVH. The methodology and results of the trial have been published [21,22]. Approximately 75 study centers throughout the U.S., Canada and Europe participated in enrolling 500 subjects for the trial between April 2009 and January 2015. Patients presenting with supratentorial IVH who met the inclusion criteria were recruited through sites’ emergency departments, neurological ICUs and clinical stroke services and underwent a detailed screening process to confirm the absence of other exclusion criteria, including a structural vascular lesion as etiology of hemorrhage. This utilized computed tomographic angiography (CTA) as the default modality, or magnetic resonance angiography (MRA)/magnetic resonance imaging (MRI) in cases unable to receive iodinated contrast agent, as per CLEAR III protocol. A catheter cerebral angiogram was used in young patients, atypical bleeds, or with suspect findings on CTA, MRA or MRI with two step decision making by the site and then under advisement from the CLEAR III Surgical Center. Each participating site recorded inclusion/exclusion information about all screened patients into the VISION web-based electronic case report form (eCRF) system, which was then confirmed centrally by the trial’s Surgical and Reading centers [21].
Patients and Methods
This is a retrospective case-controlled analysis of prospectively collected data of IVH patients screened for eligibility as part of the CLEAR III clinical trial. The study was approved by the institutional review board of respective participating sites and conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA). We considered all patients who tested negative on vascular imaging and subsequently enrolled in the trial as the group with no vascular etiology. We identified cases where reason(s) of exclusion was documented as “Aneurysm, mycotic aneurysm, Moyamoya, vascular malformation, etc.” or “Other” (Reason for exclusion is recorded as “Other” when a patient failed to satisfy one or more inclusion criteria and/or had one or more exclusion criteria). Other than harboring a structural vascular lesion, exclusionary criteria from CLEAR included infra-tentorial hemorrhage, systemic coagulopathy, ongoing internal bleeding, pregnancy, participation in another medical investigation or clinical trial and lack of subject or legal representative to give written informed consent. All cases were then manually screened to exclude patients with no documentation of an identified vascular lesion confirmed on further imaging studies through the screening process (Figure 1). The group with vascular etiology was ultimately defined as IVH patients who were excluded from the trial after confirmation of a distinct structural etiology on vascular imaging studies. We included one case enrolled into the trial and later found on subsequent imaging to harbor Moyamoya disease. In both groups, subjects outside the age range 18–80 years (n=24) were excluded for consistency, resulting in 496 cases in the group with no vascular etiology group and 1,205 cases in the group with vascular etiology (4.7% and 11.4% of 10, 538 patients screened for the trial, respectively).
Figure 1.
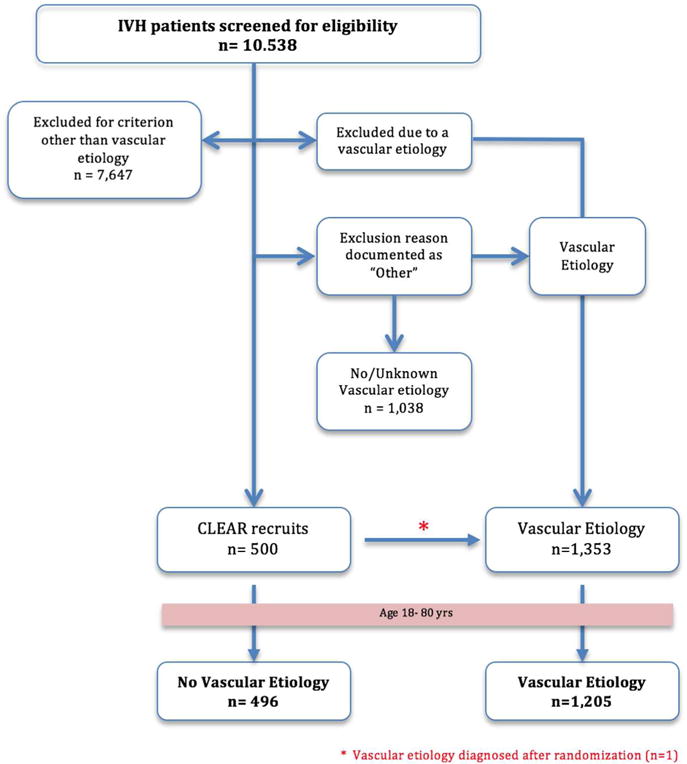
Breakdown of groups involved in the analysis
For all patients in our analysis we collected age, sex, ethnicity and primary diagnosis (i.e. primary IVH or IVH with ICH). Age was used as a continuous variable in comparing baseline demographic characteristics and as a binary variable for logistic regression with a cut-off age of less than or equal to 45 years in accordance with relevant literature [19]. Vascular lesions were categorized as aneurysm, vascular malformations (arteriovenous malformation, dural arteriovenous fistula, cavernoma or non-specified vascular malformation), or Moyamoya disease. Baseline characteristics of the two groups were directly compared to identify any associated demographic patterns. In the vascular etiology group, we further analyzed the baseline characteristics between subgroups with different vascular lesions.
Statistical Analyses
All statistical analyses were performed using STATA12 (StataCorp LP, College Station, Texas). To compare baseline demographic characteristics, Student’s t test was used for continuous variables and Chi square test was used for categorical variables. Logistic regression was used to investigate the association between vascular etiology and variables of interest. Multinomial logistic regression was applied to test the association between vascular lesions as etiology and variables of interest. Forward stepwise method was performed to assess combined effects with a significance level of 0.05 for both variable entry and retention.
RESULTS
Study and Patient Characteristics
Our final analysis was therefore based on 1,701 IVH patients comprising a group with no structural vascular etiology (No Vascular Etiology, n=496) and those harboring a culprit vascular lesion (Vascular etiology, n=1,205). Demographic characteristics of the two groups are summarized in Table 1. The most common lesions diagnosed throughout the screening process were cerebral aneurysms (n=835) followed by vascular malformations (n=320) and Moyamoya disease (n=35). In three cases the vascular etiology could not be classified further. Imaging modalities used for vascular etiology screening of enrolled subjects comprised CTA (n=404, 81%), MRI (n=45, 9%), MRA/V (n=37, 7%) and formal catheter angiography (n=65, 13%).
Table 1.
Demographic features of cases with and without vascular lesion as etiology.
| No Vascular Lesion n=496 |
Vascular Lesion n=1,205 |
P | |
|---|---|---|---|
| Age (mean) | 58.4 | 53.9 | 0.001 |
| Male (n, %) | 275 (55.4) | 483 (40.0) | <0.001 |
| Ethnicity (n, %) | |||
| • AA | 168 (33.6) | 145 (13.1) | <0.001 |
| • Asian | 14 (2.8) | 44 (4.0) | 0.38 |
| • Hispanic | 60 (12.0) | 141 (12.8) | 0.69 |
| • White | 252 (50.4) | 765 (69.40) | <0.001 |
| • Other | 6 (1.2) | 8 (0.7) | 0.34 |
| Primary IVH (n, %) | 72 (14.4) | 260 (23.7) | <0.001 |
| Etiology screening: | |||
| Imaging technique (n, %) | |||
| • CTA | 404 (81) | ||
| • MRI | 45 (9) | ||
| • MRA | 37 (7) | ||
| • Catheter Angiography | 65 (13) | ||
| Etiology (n, %) | |||
| • Aneurysm | 835 (68.0) | ||
| • Vascular Malformation | |||
| ○ AVM | 320 (26.1) | ||
| ○ dAVF | 13 (1.0) | ||
| ○ CCM | 11 (0.9) | ||
| ○ VM/Galen | 3 (0.2) | ||
| ○ VM:NOS | 3 (0.2) | ||
| • Moyamoya | 35 (2.9) | ||
| • Other | |||
| ○ Vasculitis | 1 (0.08) | ||
| ○ Mycotic Aneurysm | 4 (0.3) |
AA: African-American, Other: American Indian/Alaskan Native, Native Hawaiian/other Pacific Islander, mixed or unknown ethnicity, AVM = arteriovenous malformation, dAVF= dural arteriovenous fistula, CCM = cerebral cavernous malformation, VM = venous malformation, Galen = vein of Galen malformation, VM: NOS = unspecified vascular malformations.
The vascular etiology group was significantly younger and more likely to be female. While an ICH was very common in both groups, IVH secondary to vascular etiology was less likely to be associated with intracerebral hemorrhage (primary IVH was more prevalent in cases with vascular etiology). Racial distribution revealed a marked prevalence of African-American patients in the IVH group lacking a structural etiology (Table 1).
Differential demographic characteristics were compared within the vascular etiology group among different vascular lesions (Table 2). Patients with cerebral aneurysm were older compared to vascular malformation and Moyamoya disease (mean age 57.9 vs 46.9 vs 44.2, p<0.001); they also exhibited the strongest female predominance (66.2% vs 45.4% vs 60.0%, p<0.001). Racial distribution among patients with cerebral aneurysm and vascular malformation were very similar. In both groups there was a predominance of Whites, constituting a little over two thirds of cases, followed by a very close prevalence of patients of African-American and Hispanic ethnicity. Excluding other minor ethnicities, Asian patients were the least prevalent, comprising roughly one-thirtieth of patients. Moyamoya patients in contrast had a distinctively different racial distribution. Compared to other vascular etiologies, Moyamoya had a significantly higher prevalence of Asians (OR 8.4 p<0.0001) and, to a lesser extent, African-Americans (OR 2.3 p=0.03). Conversely the Moyamoya showed the least prevalence of Hispanic (OR 0.4 p=0.22) and White patients (OR 0.4, p=0.003). Finally, patients with Moyamoya disease were significantly more likely to present with primary IVH compared to other vascular lesions (45.5% vs 21.0% and 23.4%, p=0.01). (Table 2)
Table 2.
Demographic features in cases with different vascular lesions as etiology of IVH
| Cerebral Aneurysm n=835 |
Vascular Malformation n=350 |
Moyamoya disease n=35 |
p | |
|---|---|---|---|---|
| Age (mean) | 57.96 | 46.85 | 44.14 | <0.001 |
| Male (n, %) | 282 (33.8) | 191 (54.6) | 14 (40.0) | <0.001 |
| Ethnicity (n, %) | ||||
| • AA | 90 (11.9) | 46 (15.2) | 9 (25.7) | 0.06 |
| • Asian | 27 (3.6) | 9 (3.0) | 8 (22.8) | <0.001 |
| • Hispanic | 91 (12.0) | 45 (14.9) | 2 (5.7) | 0.01 |
| • White | 542 (71.6) | 204 (67.6) | 16 (45.7) | 0.002 |
| • Other | 7 (0.9) | 1 (0.3) | 0 | 0.23 |
AA: African-American, Other: American Indian/Alaskan Native, Native Hawaiian/other Pacific Islander, mixed or unknown ethnicity
Logistic regression analysis for the presence of structural vascular etiology revealed a strong association with some of the demographic characteristics, p=0.001 (Table 3). Such association was strongest with age < 45, female gender, and non-AA ethnicities. Whites, on the other hand, exhibited a subtle predilection to harboring a vascular lesion. Likewise, subjects presenting with primary IVH were more likely to have an underlying lesion compared to those with associated overt ICH. These variables retained their significance after multivariate modeling. Baseline characteristics having the weakest correlation with vascular etiology were combined to identify a cohort of IVH patients with the least likelihood of harboring an underlying structural etiology. The combined cohort consisted of African-American, male patients, aged > 45 years and presenting with overt ICH associated IVH. This group was found to have the least likelihood of harboring a structural vascular etiology. Nevertheless, even within this group of combined demographics, there were still non-negligible odds of harboring a vascular etiology (Figure 2).
Table 3.
Logistic regression analysis of demographic features with likelihood of vascular lesion as etiology of IVH.
| Univariate logistic regression | Multivariable logistic regression | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (>45) | 0.37 (0.27, 0.50) | 0.001 | 0.28 (0.20, 0.40) | <0.001 |
| Gender (Male) | 0.54 (0.44, 0.67) | <0.001 | 0.48 (0.38, 0.60) | <0.001 |
| Primary IVH | 1.82 (1.37, 2.42) | <0.001 | 1.85 (1.37, 2.51) | <0.001 |
| AA | 0.27 (0.21, 0.34) | <0.001 | 0.23 (0.18, 0.31) | <0.001 |
| White | 1.65 (1.34, 2.04) | <0.001 | ||
| Asian | 1.32 (0.71, 2.41) | 0.38 | ||
| Hispanic | 0.94 (0.68, 1.29) | 0.69 | ||
| Combination | 0.10 (0.06, 0.17) | <0.001 | ||
AA: African-American, Combination: Represents the Group with least likelihood (Age >45, Male, African American, IVH associated with ICH),
Figure 2.
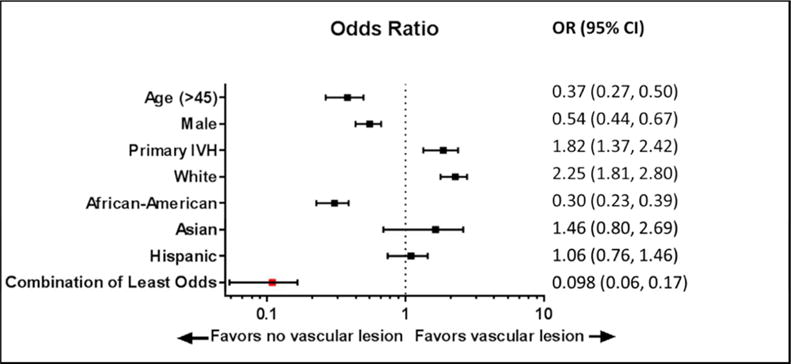
Forest plot illustration of the impact of different demographic features among cohorts with and without underlying vascular lesion as etiology of IVH
DISCUSSION
A systematic review of published literature recently compiled by Cordonnier, et al. in 2010 included 20 studies (n=1,933) and addressed the utilization of vascular imaging in the investigation of ICH [23]. The review highlighted the paucity of data, preponderance of selection bias, and small sample sizes, limiting the generalizability of most studies. Yet roughly one third of cases in that review had an AVM or aneurysm. Decisions about whether and how to deploy vascular imaging after diagnostic plain CT to determine ICH cause were generally based on patient’s age, ICH location and pre-stroke hypertension, hence introducing significant biases regarding true rates of vascular etiologies and their demographic associations. Other studies have advocated that harboring a vascular etiology is exceptionally rare in the elderly, especially in the context of a deep ICH and history of hypertension [24,25]. Our study aimed primarily at addressing the relative prevalence of demographic features (age, sex and ethnicity) in cases with and without vascular etiology in association with IVH, subjected to systematic screening for vascular etiology (Figure 3).
Figure 3. Arteriovenous malformation diagnosed on catheter angiogram during screening in a 78 year old female patient.
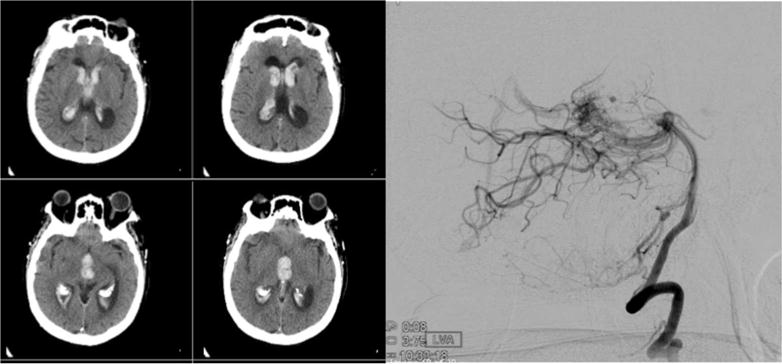
Angiography was performed in view of suspicious finding on less invasive computed tomographic angiogram (CTA). The appearance of IVH on diagnostic CT scan alone would not have raised suspicion of underlying vascular etiology
Pattern of bleeding on initial non-contract CT scan is pivotal in triaging patients for angiography but remains far from being conclusive. In a study by Delgado Almandoz et al, 179 Plain CT scans were categorized as “low-probability” for vascular etiology by 2 blinded neuroradiologists, 4 of which (2.2%) were later found to harbor a lesion. More than two thirds of plain CT scans in the study were deemed indeterminate (n=421), 17.1% of these cases were later found to have an underlying vascular etiology [19]. In our study, diagnostic and stability CT images of all cases enrolled in the CLEAR III trial were reviewed through the trial Surgical Center led by the senior author (IAA) to recommend cases for more rigorous vascular etiology screening if there was a suspicious pattern of bleeding. But we could not assess the relative roles of individual screening modalities (CTA versus MRA, nor the role of MRI and catheter DSA) as a range of options were allowed in the study protocol, deployed per clinician judgment, with guidance by the trial’s Surgical Center, and we did not have access to all imaging features of cases excluded from the trial.
Previous reports on the prevalence of vascular malformation in ICH range from 5% to as high as 23% depending on the study design and target population [26,27]. In our study almost 11% of screened patient were excluded from CLEAR III for harboring a vascular malformation (7.9% Aneurysms, 3% AVM and 0.3% Moyamoya disease). Though in keeping with previous studies, these figures should be interpreted with caution since vascular imaging was not systemically applied to all screened cases, and some cases were excluded for other reasons (e.g. death, poor functional scores, withdrawal of consent, etc.) before undergoing etiology screening. Our study recruited subjects from multiple centers around the world, hence the threshold of vascular screening and the choice of modalities were undoubtedly influenced by different local practices.
We also did not have access to systematic past medical histories of excluded cases so we could not address other potential factors such as history of hypertension (and its treatment), smoking, and drug or anticoagulant use, in the excluded cohort. Other cases excluded during the screening process, for causes other than vascular etiology, were not included in our analysis, hence our demographic associations only apply to cases who met all criteria for CLEAR III (with definite exclusion of vascular etiology) versus those excluded for definite vascular etiology. And as with all such studies, screening may not have identified angiographically occult vascular etiologies such as cavernous malformations.
Despite these limitations, the strength of this study lies primarily in the number of cases of IVH with and without etiology, identified concurrently at 73 sites worldwide, based on a common strategy of screening all potential trial cases for vascular etiology. The differences in demographic features between groups with and without vascular etiology, and among cohorts with vascular etiology, could not otherwise be gleaned in smaller or single site series.
We demonstrated that being younger, female sex, white race and primary IVH are significantly more prevalent among patients with vascular etiology than without. Our findings also suggest that, in IVH with a structural vascular etiology, patient demographics can help predict the pathological nature of the underlying lesion, particularly Asian ethnicity in association with Moyamoya disease. In this large multinational cohort of IVH patients, there was no combination of demographic features which would predict the presence or absence of vascular etiology with better than 90% reliability. Structural vascular lesions were still encountered in hypertensive elderly patients presenting with deep ICH. An underlying vascular etiology for IVH therefore, cannot be ruled out solely on demographic features in any patient.
The relatively rare primary IVH (without an overt ICH on CT scan), believed to represent 3% of ICH cases [28,29], presents an additional challenge due to a rather similar CT picture regardless of bleeding etiology. Hence clinical suspicion of vascular etiology in primary IVH is more dependent on baseline demographic features than the distribution of blood. Our findings suggest that primary IVH cases have higher odds of harboring an underlying structural etiology compared to IVH with an intraparenchymal ICH component. Results are in keeping with previous series in literature reporting higher etiologic diagnostic yield in primary IVH ranging between 29–65% [24,30–32].
We have also illustrated that when vascular etiology screening is judiciously utilized, structural vascular lesions can be found in groups of patients formerly believed to be at low-risk of harboring a vascular etiology. No demographic risk factor(s) can be deemed fully exclusionary for vascular etiology. Our findings advocate that alongside sound clinical judgment, some form of routine vascular imaging is likely needed when screening IVH patients before contemplating interventions like intraventricular fibrinolysis.
Footnotes
Conflict of interests: MF: None, AP: None, HAZ: none, SM: None, AS: None, MJ: None, LZ: None, RD: None, WZ: none, IAA and DH have testified in legal proceedings. DH and Johns Hopkins University hold a patent for intracranial use of Alteplase. CLEAR Phase III trial was funded by Grant 5U01NS062851 from the National Institutes of Health. Alteplase was donated by Genentech, Inc
References
- 1.Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. 2009;40:1533–1538. doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruscalleda J, Peiro A. Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology. 1986;28:34–37. doi: 10.1007/BF00341763. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Diringer MN, Schneider D, Mayer SA, Begtrup K, Broderick J, Skolnick BE, Davis SM. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 4.Mayfrank L, Hutter BO, Kohorst Y, Kreitschmann-Andermahr I, Rohde V, Thron A, Gilsbach JM. Influence of intraventricular hemorrhage on outcome after rupture of intracranial aneurysm. Neurosurgical review. 2001;24:185–191. doi: 10.1007/s101430100160. [DOI] [PubMed] [Google Scholar]
- 5.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD, STICH Investigators Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 6.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical care medicine. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 7.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. 1990;40:616–619. doi: 10.1212/wnl.40.4.616. [DOI] [PubMed] [Google Scholar]
- 8.Chan E, Anderson CS, Wang X, Arima H, Saxena A, Moullaali TJ, Heeley E, Delcourt C, Wu G, Wang J, Chen G, Lavados PM, Stapf C, Robinson T, Chalmers J, Huang Y, INTERACT2 Investigators Significance of intraventricular hemorrhage in acute intracerebral hemorrhage: intensive blood pressure reduction in acute cerebral hemorrhage trial results. Stroke. 2015;46:653–658. doi: 10.1161/STROKEAHA.114.008470. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G, Special Writing Group of the Stroke Council ASA AHA Scientific Statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458–1471. doi: 10.1161/01.str.32.6.1458. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med. 2010;77:791–799. doi: 10.3949/ccjm.77a.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarlov N, Norbash AM, Nguyen TN. The safety of anticoagulation in patients with intracranial aneurysms. J Neurointerv Surg. 2013;5:405–409. doi: 10.1136/neurintsurg-2012-010359. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Lukovits T, Friedman JA. “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage. Neurocritical care. 2006;4:68–76. doi: 10.1385/NCC:4:1:068. [DOI] [PubMed] [Google Scholar]
- 14.Meretoja A, Strbian D, Putaala J, Curtze S, Haapaniemi E, Mustanoja S, Sairanen T, Satopaa J, Silvennoinen H, Niemela M, Kaste M, Tatlisumak T. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–2597. doi: 10.1161/STROKEAHA.112.661603. [DOI] [PubMed] [Google Scholar]
- 15.European Stroke Initiative Writing C, Writing Committee for the EEC. Steiner T, Kaste M, Forsting M, Mendelow D, Kwiecinski H, Szikora I, Juvela S, Marchel A, Chapot R, Cognard C, Unterberg A, Hacke W. Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 16.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D, American Heart Association Stroke C, Council on C. Stroke N, Council on Clinical C Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 17.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M, European Stroke O European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 18.Dey M, Stadnik A, Awad IA. Spontaneous intracerebral and intraventricular hemorrhage: advances in minimally invasive surgery and thrombolytic evacuation, and lessons learned in recent trials. Neurosurgery. 2014;74(Suppl 1):S142–150. doi: 10.1227/NEU.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado Almandoz JE, Schaefer PW, Forero NP, Falla JR, Gonzalez RG, Romero JM. Diagnostic accuracy and yield of multidetector CT angiography in the evaluation of spontaneous intraparenchymal cerebral hemorrhage. AJNR Am J Neuroradiol. 2009;30:1213–1221. doi: 10.3174/ajnr.A1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hino A, Fujimoto M, Yamaki T, Iwamoto Y, Katsumori T. Value of repeat angiography in patients with spontaneous subcortical hemorrhage. Stroke. 1998;29:2517–2521. doi: 10.1161/01.str.29.12.2517. [DOI] [PubMed] [Google Scholar]
- 21.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, Butcher K, Vespa P, Wright DW, Keyl PM, Mendelow AD, Kase C, Wijman C, Lapointe M, John S, Thompson R, Thompson C, Mayo S, Reilly P, Janis S, Awad I, Hanley DF. A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III) Int J Stroke. 2014;9:536–542. doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, Dawson J, Gandhi D, Ullman N, Mould WA, Mayo SW, Mendelow AD, Gregson B, Butcher K, Vespa P, Wright DW, Kase CS, Carhuapoma JR, Keyl PM, Diener-West M, Muschelli J, Betz F, Thompson CB, Sugar EA, Yenokyan G, Janis S, John S, Harnof S, Lopez G, Aldrich EF, Harrigan MR, Ansari S, Jallo J, Caron J, LeDoux D, Adeoye O, Zuccarello M, Adams HP, Rosenblum M, Thompson RE, Awad IA, CLEARIII Investigators Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. :2016. doi: 10.1016/S0140-6736(16)32410-2. DOI: http://dx.doi.org/10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed]
- 23.Cordonnier C, Klijn CJ, van Beijnum J, Al-Shahi Salman R. Radiological investigation of spontaneous intracerebral hemorrhage: systematic review and trinational survey. Stroke. 2010;41:685–690. doi: 10.1161/STROKEAHA.109.572495. [DOI] [PubMed] [Google Scholar]
- 24.Zhu XL, Chan MS, Poon WS. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature Stroke. 1997;28:1406–1409. doi: 10.1161/01.str.28.7.1406. [DOI] [PubMed] [Google Scholar]
- 25.Toffol GJ, Biller J, Adams HP, Jr, Smoker WR. The predicted value of arteriography in nontraumatic intracerebral hemorrhage. Stroke. 1986;17:881–883. doi: 10.1161/01.str.17.5.881. [DOI] [PubMed] [Google Scholar]
- 26.van Asch CJ, Velthuis BK, Rinkel GJ, Algra A, de Kort GA, Witkamp TD, de Ridder JC, van Nieuwenhuizen KM, de Leeuw FE, Schonewille WJ, de Kort PL, Dippel DW, Raaymakers TW, Hofmeijer J, Wermer MJ, Kerkhoff H, Jellema K, Bronner IM, Remmers MJ, Bienfait HP, Witjes RJ, Greving JP, Klijn CJ, Investigators D Diagnostic yield and accuracy of CT angiography, MR angiography, and digital subtraction angiography for detection of macrovascular causes of intracerebral haemorrhage: prospective, multicentre cohort study. BMJ. 2015;351:h5762. doi: 10.1136/bmj.h5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung J, Cord BJ, O’Rourke TK, Maina RM, Sommaruga S, Matouk CC. Macrovascular Lesions Underlying Spontaneous Intracerebral Hemorrhage. Semin Neurol. 2016;36:244–253. doi: 10.1055/s-0036-1581994. [DOI] [PubMed] [Google Scholar]
- 28.Guo R, Ma L, Shrestha BK, Yu Z, Li H, You C. A retrospective clinical study of 98 adult idiopathic primary intraventricular hemorrhage cases. Medicine (Baltimore) 2016;95:e5089. doi: 10.1097/MD.0000000000005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Jia B, Li H, You C, Hanley DF, Jiang Y. Primary intraventricular hemorrhage in adults: etiological causes and prognostic factors in Chinese population. J Neurol. 2016 doi: 10.1007/s00415-016-8367-x. [DOI] [PubMed] [Google Scholar]
- 30.Passero S, Ulivelli M, Reale F. Primary intraventricular haemorrhage in adults. Acta neurologica Scandinavica. 2002;105:115–119. doi: 10.1034/j.1600-0404.2002.1o118.x. [DOI] [PubMed] [Google Scholar]
- 31.Giray S, Sen O, Sarica FB, Tufan K, Karatas M, Goksel BK, Yerdelen D, Cekinmez M, Can U. Spontaneous primary intraventricular hemorrhage in adults: clinical data, etiology and outcome. Turk Neurosurg. 2009;19:338–344. [PubMed] [Google Scholar]
- 32.Weisberg LA, Elliott D, Shamsnia M. Intraventricular hemorrhage in adults: clinical-computed tomographic correlations. Computerized medical imaging and graphics. the official journal of the Computerized Medical Imaging Society. 1991;15:43–51. doi: 10.1016/0895-6111(91)90108-8. [DOI] [PubMed] [Google Scholar]


