Abstract
The clinical course of patients with recently diagnosed early stage chronic lymphocytic leukemia (CLL) is highly variable. We examined the relationship between CLL-cell birth rate and treatment-free survival (TFS) in 97 patients with recently diagnosed, Rai stage 0–II CLL in a blinded, prospective study, using in vivo 2H2O labeling. Birth rates ranged from 0.07–1.31% new cells per day. With median follow-up of 4.0 years, 33 subjects (34%) required treatment by NCI criteria. High birth rate was observed in 44% of subjects and was significantly associated with shorter TFS, unmutated IGHV status, and expression of ZAP70 and of CD38. In multivariable modeling considering age, gender, Rai stage, expression of ZAP70 or CD38, IGHV mutation status and FISH cytogenetics, only CLL-cell birth rate and IGHV mutation status met criteria for inclusion. Hazard ratios were 3.51 (p=0.002) for high birth rate and 4.93 (p<0.001) for unmutated IGHV. The association between elevated birth rate and shorter TFS was observed in subjects with either mutated or unmutated IGHVs, and the use of both markers was a better predictor of TFS than either parameter alone. Thus, an increased CLL birth rate in early stage disease is a strong predictor of disease progression and earlier treatment.
INTRODUCTION
Chronic lymphocytic leukemia (CLL), the most frequent adult leukemia in the Western world, is notable for its highly variable clinical course. Some patients may progress early, while others may have an indolent course and not require therapy for years, if at all.1, 2 Current guidelines do not recommend therapy for patients with recently diagnosed early stage CLL unless they demonstrate disease-related symptoms or complications or clear evidence of progressive disease. An ongoing clinical challenge is to identify patients who will develop aggressive disease and thus potentially might benefit from earlier treatment.
The staging systems of Rai3 and Binet4 remain the mainstays for classifying patient prognosis and survival. However, both systems have limited ability to predict early disease-progression.5 With an increasing array of treatment options for CLL6, 7 and with the majority of patients diagnosed with early stage disease, it is imperative to better understand the factors that put patients at highest risk for disease progression.
Prognostic markers based on CLL biology, including CLL-cell expression of ZAP708 CD389 and CD49d10, leukemia-cell chromosomal aberrations11, and IGHV mutational status9, 12 have shown promise in early stage CLL and have advanced our understanding of the disease process.
In CLL, as with any malignancy, disease progression represents an imbalance between cell birth and death, ultimately leading to an accumulation of leukemia cells. Measures of the rates of in vivo CLL cell birth (proliferation) and disappearance from blood (true cell death plus recirculation to solid tissues) have provided important insights into the pathophysiology of the disease.13–15 Although the classic view of CLL involved failed cell death in the setting of minimal cell proliferation,16 data from several small studies demonstrate that some patients with CLL can have birth rates greater than those seen for normal B-cells.13–15 Moreover, in a cross-sectional study that included patients with both early and late stage CLL, cell birth rates above 0.35% per day were associated with more aggressive disease, even in the setting of a low lymphocyte doubling time.13 An unanswered question that is addressed in this study is the extent to which an increased birth rate in early stage CLL associates with more rapid disease progression and shorter treatment free survival (TFS).
MATERIALS AND METHODS
The study protocol was approved by the institutional review boards of all the participating clinical sites as well as by Western IRB.
Patients
Between 2005 and 2009, 119 subjects with CLL provided written informed consent, were enrolled at CLL Research Consortium (CRC) institutions and had CLL cell kinetics measured using orally administered 2H2O. Eligibility criteria included age > 18 years, CLL diagnosis within 3 years of enrollment, and early stage disease (Rai stage 0, I, or II). Patients with current or prior CLL treatment or those anticipated to need treatment within 16 weeks after enrollment were excluded, as were patients with serious co-morbid medical conditions and pregnant women.
Treatment Free Survival (TFS)
Clinical follow up information available through November 2013 was used for analysis. TFS was defined as the time from enrollment and initiation of 2H2O (deuterated or “heavy water”) ingestion to the initiation of therapy according to the NCI criteria.17 Patients who were not treated were censored at the time they were last known to remain untreated. Patients who enrolled in intervention studies testing strategies to delay initial treatment were censored at the time of enrollment in the intervention study. Patients were to be followed for a minimum of 2 years from the end of2H2O ingestion. The study was blinded with cellular kinetics and clinical outcomes linked only after all data were finalized.
Analysis of Prognostic Markers
Analyses for leukemia cell expression of ZAP70 and of CD38 and IGHV mutation status were performed on peripheral blood leukemia-cell samples by the CRC Tissue Core as described.8, 18 ZAP70 and CD38 levels were considered positive when >20% or >30%, respectively, of the circulating CLL cells expressed the marker; IGHV mutation status was considered unmutated when B cells expressed an IGHV gene with ≥98% homology to the most similar germline gene. Fluorescence in situ hybridization (FISH) was performed at participating CRC institutional clinical laboratories and evaluated using the hierarchical Dohner classification11. If subjects had multiple time points for a marker, data obtained closest to the time of enrollment in the current study were used.
2H2O Labeling Protocol
Measurement of CLL-cell kinetics was done at study entry. The protocol for oral administration of 2H2O and blood and saliva sampling was reported previously19. Briefly, subjects drank 2H2O (70% enriched) for 6 weeks: 50 ml thrice daily for a 5-day loading period followed by a 60 ml once daily maintenance phase for a total of six weeks. Target plateau body 2H2O enrichment was 1–1.5%. Saliva samples were obtained at weeks 2 and 4 to determine body water enrichment and ensure compliance with 2H2O intake. Six subjects provided weekly saliva samples (weeks 0–12) for more detailed water modeling. Blood samples were obtained at weeks 0, 3, and 6 (2H2O labeling phase) and at weeks 9, 12 and 16 (2H2O washout phase).
CLL Cell Isolation
CLL cells were isolated as described previously using the RosetteSep™ method.19 Cell purity was determined by flow cytometry by gating on CD19+CD5+ cells. For samples that did not achieve 95% purity, cells were fixed and isolated by FACS19 to achieve 95% purity for CD19+CD5+ cells.
Measurement and Calculation of Body 2H2O Enrichment and 2H-Incorporation into Purine dR of DNA
Body water 2H2O enrichment was determined from saliva and serum using cycloidal mass spectrometry as described.20, 19 2H enrichment (atom percent excess, APE) in purine dR from DNA used for the calculation of CLL-cell kinetics was determined by GC-P-IRMS as described.21, 19
Cell Birth and Disappearance Rate Calculations
The fraction of cells that divided during the labelling period (f) was calculated for each time point using the precursor-product ratio based on isotopic enrichment in cellular DNA (product) and the time-averaged enrichment of 2H2O in body water (precursor, p) (See Supplementary Appendix).21 Birth rate (f/day) was calculated by dividing f by the time interval up to each specific time point with a maximum value of 6 weeks corresponding to the duration of 2H2O ingestion. The highest birth rate of CLL cells observed across the time points for each individual was the birth rate used for subsequent analyses. Cell disappearance rate, representing the loss of labeled cells from the vascular compartment through cell death or recirculation to solid tissues, was calculated as described.22, 23 We also assessed for “lag” or delayed release of previously divided,2H-labelled cells into the sampled (vascular) compartment from a sequestered pool such as lymph nodes or spleen. This lag, previously observed by Messmer et al.13 and by Van Gent et al.,13, 15 becomes apparent when there is a delay in the initial rate of labeling and/or a continued increase in label enrichment (APE) in DNA from sampled CLL-cells long after subjects have ceased drinking 2H2O and 2H2O has washed out from body water pools (e.g. Supplemental Figure S3C). These labeled cells must have previously divided in a non-vascular compartment when 2H2O was present and later were released into the vascular compartment where they were sampled. Because of the potential biological significance of this cell trafficking, the presence or absence of a lag was assessed. Detailed descriptions of the calculation of 2H2O exposure, fractional DNA synthesis and birth and disappearance rates, as well as determination of lag and comparison with other methods for determining the birth rate can be found in the Supplementary Appendix.
Statistics
Associations between each candidate prognostic marker and outcome were evaluated using Fisher's exact test for categorical data and the Wilcoxon or Kruskal-Wallis rank-sum tests for continuous data comparing two or more groups.
Due to few events leading to sparse data on the primary endpoint, disease progression to treatment within two years of study entry, Firth’s penalized logistic regression was used to obtain robust model estimates and 95% confidence intervals. This analysis included only those patients who were followed for at least two years or experienced progression within two years. Step-wise variable selection of candidate prognostic markers was used to identify factors associated with disease course.
A pre-specified exploratory TFS endpoint was assessed using a Cox proportional hazards model. Using recursive partitioning and regression trees, an optimal cut-point for birth rate was obtained, which best distinguished those needing treatment from those remaining untreated for these data.
Analyses were performed using R version 3.2.0 with packages Hmisc, MASS, rpart, and survival for analyses and knitr for reproducibility. P-values were two-sided. Corrections for multiple testing were applied using the Holm method.
RESULTS
Patients
CLL patients (n=119) were enrolled from six CRC institutions. As per protocol eligibility criteria, we excluded patients who had received prior treatment (N=2) or who had a date of CLL diagnosis more than 3 years prior to enrollment (N=10). Six subjects did not complete the 2H2O labeling protocol (1 progressed, 3 entered early intervention trials, 1 cited travel commitments, and 1 for an unknown reason) requiring withdrawal from the study. We were unable to determine kinetic rates for 4 additional subjects for analytical reasons related to cell purity and subsequent isotopic analyses. Clinical and demographic characteristics for the remaining 97 subjects are presented in Table 1.
Table 1.
Study Participant Demographics and Treatment Outcome
| Treated CLL | |||||
|---|---|---|---|---|---|
| Total n = 97 |
No n = 64 |
Yes n = 33 |
|||
| Sex – no.(%) | |||||
| Female | 39 (40%) | 26 (41%) | 13 (39%) | ||
| Male | 58 (60%) | 38 (59%) | 20 (61%) | ||
| Age –yr | |||||
| Median (range) | 57 (40 – 85) | 58 (40 – 85) | 56 (41 –78) | ||
| Race – no. (%) | |||||
| White | 92 (95%) | 60 (94%) | 32 (97%) | ||
| Asian | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Multi-Racial | 1 (1%) | 1 (2%) | 0 (0%) | ||
| Unknown | 3 (3%) | 2 (3%) | 1 (3%) | ||
| Rai stage at enrollment – no. (%) | |||||
| 0: Lymphocytosis only | 36 (37%) | 32 (50%) | 4 (12%) | ||
| I: Lymphocytosis with lymphadenopathy | 44 (45%) | 24 (38%) | 20 (61%) | ||
| II: Lymphocytosis with hepatomegaly or splenomegaly |
17 (18%) | 8 (12%) | 9 (27%) | ||
| Enrollment site – no. (%) | |||||
| Dana-Farber Cancer Institute | 5 (5%) | 3 (5%) | 2 (6%) | ||
| North Shore - Long Island Jewish Health System |
19 (20%) | 11 (17%) | 8 (24%) | ||
| Mayo Clinic | 18 (19%) | 12 (19%) | 6 (18%) | ||
| MD Anderson Cancer Center | 14 (14%) | 9 (14%) | 5 (15%) | ||
| Ohio State University | 10 (10%) | 9 (14%) | 1 (3%) | ||
| University of California San Diego | 31 (32%) | 20 (31%) | 11 (33%) | ||
| CLL diagnosis to study entry -months | |||||
| Median (range) | 12 (1 – 36) | 11 (1 – 36) | 12 (1 – 36) | ||
Birth Rate and Cellular Kinetics
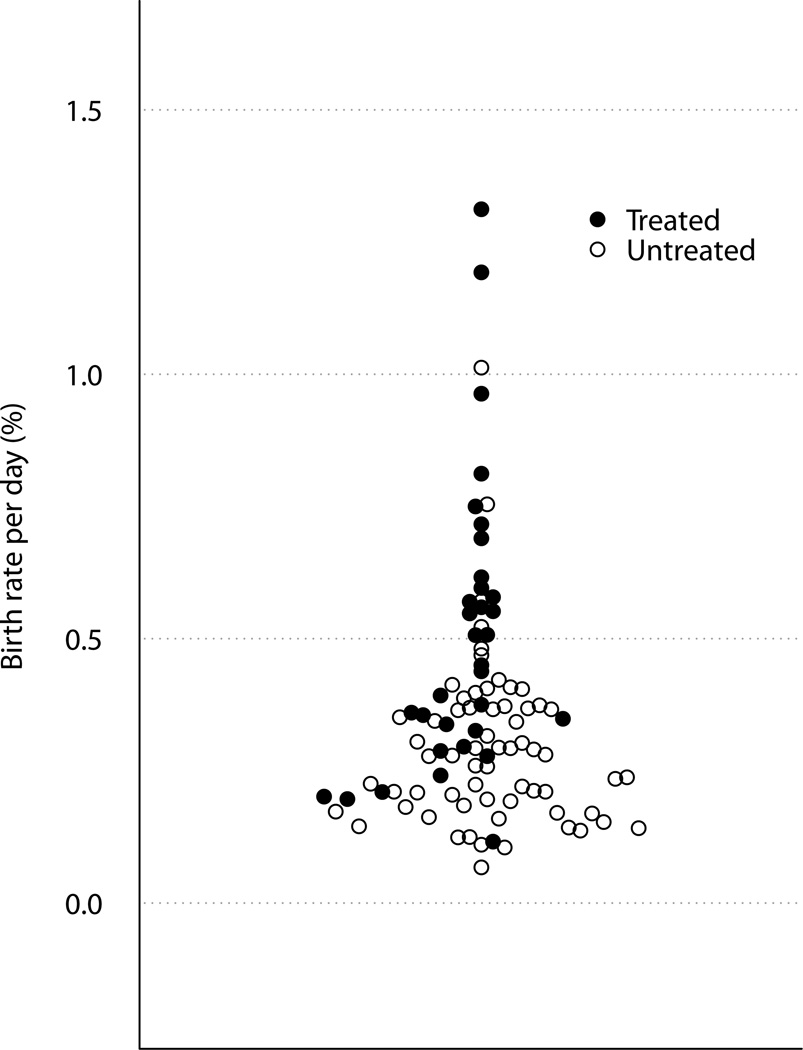
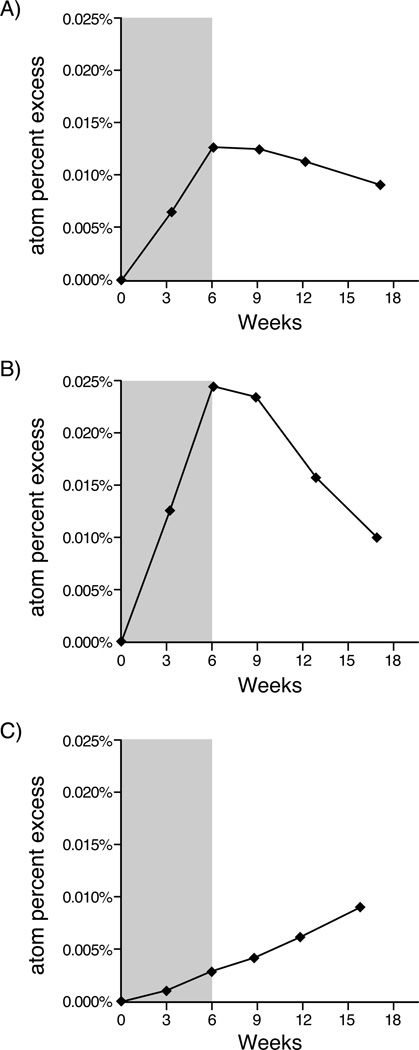
The median birth rate was 0.32% per day (range 0.07–1.31% per day, Figure 1). Median disappearance rate was 0.55% per day (range 0.00–3.60% per day). The kinetic profiles generally fell into one of three patterns: (1) equivalent birth and disappearance rates (Figure 2a), (2) more rapid disappearance than birth rates suggesting that the death/recirculation rate exceeded the birth rate (Figure 2b), and (3) continued increase in 2H enrichment in cellular DNA after the 6-week labeling period indicative of a delayed release of previously divided cells from tissue compartments into the blood, “lag” (Figure 2c).
Figure 1. CLL Cell Birth Rate.
Birth rate (% new cells per day) in treated (closed circles) and untreated (open circles) for early stage CLL patients (n=97).
Figure 2. CLL Cell Kinetics.
Atom percent excess representing 2H enrichment in DNA of CLL cells over time in three individual patients; six week period of 2H2O labeling is indicated by the shaded area. A) pattern consistent with similar birth and disappearance rates compatible with a simple single pool system B) rapid birth rate and more rapid disappearance rate C) delayed release (lag) of previously labeled cells into the vascular compartment many weeks after discontinuation of 2H2O.
Eighteen subjects (19%) were determined to have a lag. Eleven subjects (11%) had a substantially greater disappearance rate than birth rate, and the remainder of subjects (70%) had roughly equivalent birth and disappearance rates or an indeterminate pattern.
Association of Birth Rate with Other Prognostic Measures
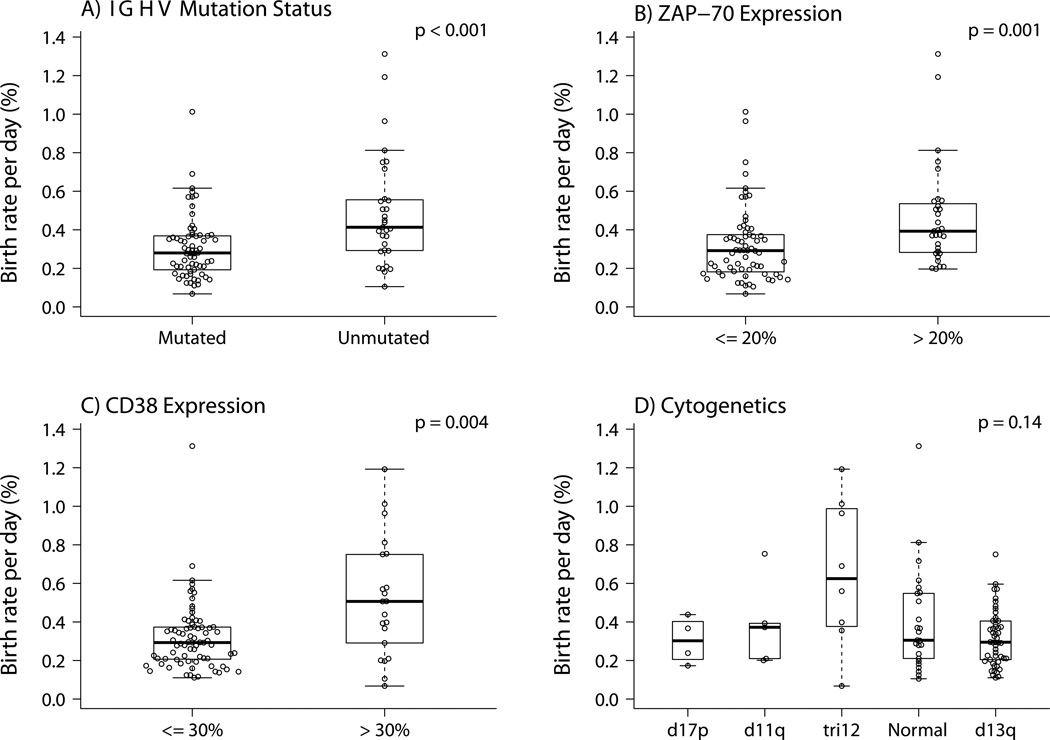
We compared birth rate with published markers of earlier treatment (ZAP70+, CD38+, and unmutated IGHV). Each was significantly associated with higher birth rates (Figure 3a–c). There was no significant association observed between birth rate and the few deleterious chromosome abnormalities (e.g. del(17p13.1) and del(11q23.3)) seen in this early stage cohort (Figure 3d; Table 2).
Figure 3. Birth Rate in Relation to Other Prognostic Factors.
A) Shows birth rate is significantly higher for patients with unmutated versus mutated IGHVs (n=97). B) Shows birth rate is significantly higher for patients with high levels of ZAP70 expression (n=97). C) Shows birth rate is significantly higher for patients with high levels of CD38 expression (n=96). D) Shows the relationship between birth rate and cytogenetic findings by FISH (n=94). With relatively small numbers for the most deleterious mutations (e.g. 17p and 11q deletions), there was no significant association with birth rate.
Table 2.
Chromosomal aberration by FISH for 97 eligible patients.
| Abnormality | |||||||
|---|---|---|---|---|---|---|---|
|
Treatment for CLL |
d17p | d11q | tri12 | Normal | d13q | ND† | Total |
| No | 3 | 2 | 3 | 16 | 37 | 3 | 64 |
| Yes | 1 | 3 | 5 | 10 | 14 | 0 | 33 |
| Total | 4 | 5 | 8 | 26 | 51 | 3 | 97 |
Three patients who did not have FISH data are noted as ND (not determined)
Progression
During a median follow-up of 4.0 (0.0–6.9) years, 33 patients (34%) required treatment (Table 1) with treatment occurring within 2 years for 14 of the 33 patients. Six patients were followed for less than two years (one died of pneumonia at day 148 and 5 were lost to follow-up before two years) and were excluded from the logistic regression but were retained for the analyses of TFS.
Association of Individual Prognostic Markers with Disease Progression
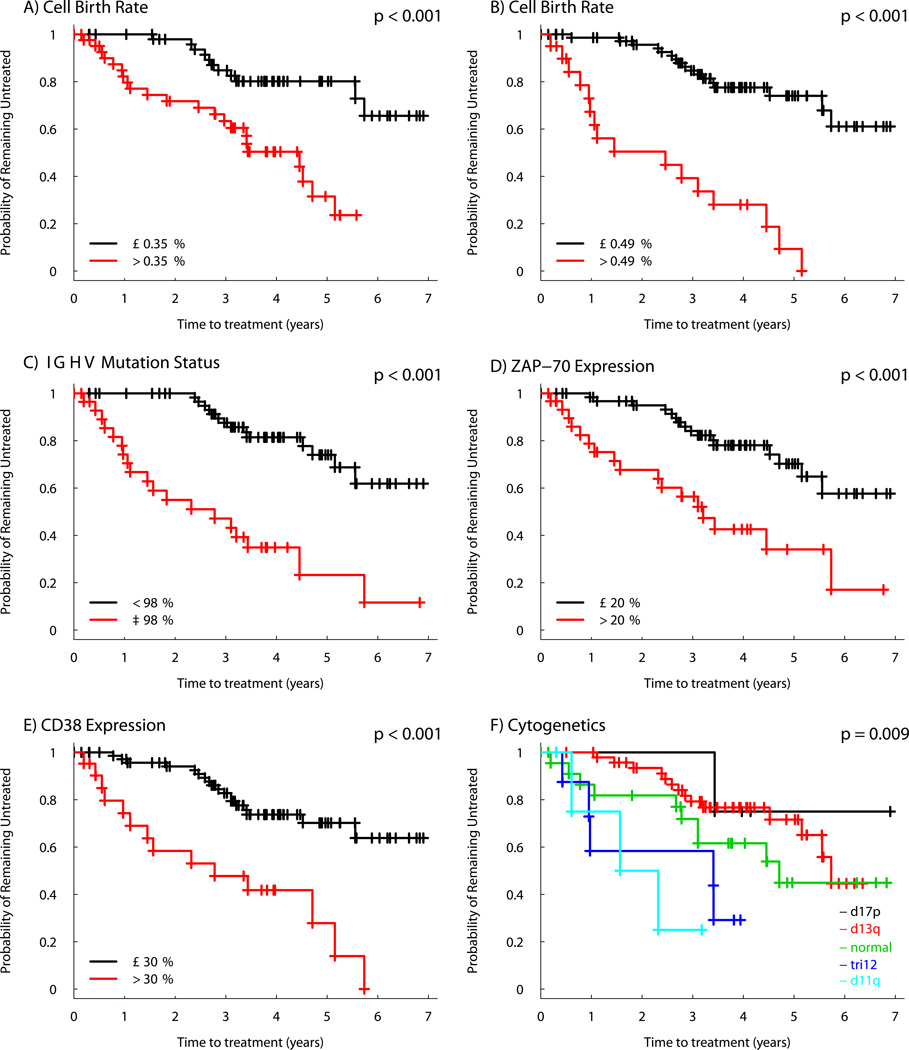
Univariable TFS Kaplan-Meier curves illustrate a significant association with TFS for all prognostic markers studied (Figure 4). A higher birth rate was a significant predictor of TFS using both the previously established cut point of 0.35% cell/d13 (Figure 4a) and the optimal predictive cut-point for birth rate determined in the current study (0.49% cells/d, Figure 4b). Forty-three subjects (44%) had birth rates above the 0.35% cells per day threshold. Distribution of the prognostic markers and birth rates among patients who did and did not progress to treatment are shown in Supplemental Table 1. Cellular disappearance/death rate did not associate with progression and thus was omitted from further modeling.
Figure 4. Relationship between Prognostic Markers and Time from Measurement to Initial Therapy.
Kaplan-Meier curves depict the proportion of untreated patients with CLL according to the time from measurement of the prognostic marker for A) birth rate > 0.35% per day, B) birth rate > 0.49% per day, C) IGHV ≥ 98% homology, D) ZAP70 expression > 20%, E) CD38 expression > 30%, F) Dohner hierarchical categorization of FISH cytogenetics.
Logistic Model
For statistical modeling we used a cut point of >0.35% cells/d, which was previously associated with more aggressive disease13. Also, while not yet validated, we modeled using the optimal predictive cut point determined in this study, which was 0.49% cells/d. Firth’s penalized likelihood was used for the logistic regression. Predictor variables at hand for the model included sex, age at study entry, Rai stage, birth rate, presence of a delayed appearance of labeled cells in blood (lag), CD38 or ZAP70 expression, IGHV mutation status and chromosomal abnormalities by FISH. In the final model, only CLL birth rate and IGHV mutation status contributed significantly. Odds ratios were 7.59 (95% CI: 1.24–31.15) for birth rate >0.35% cells/d and 67.07 (95% CI: 7.98–9100) for unmutated IGHV. Using the optimal cut point of birth rate defined in this study (>0.49% cells per day) the odds ratios were 15.53 (95% CI: 2.92–117.15) for birth rate and 82.12 (95% CI: 8.62–1100) for umuntated IGHV (Supplemental Table 2). The area under the receiver operation characteristic curve of the model showed an extremely good prediction accuracy of 0.94 with the 0.35% per day cut point and of 0.96 with the 0.49% per day cut point.
Time to Event Model
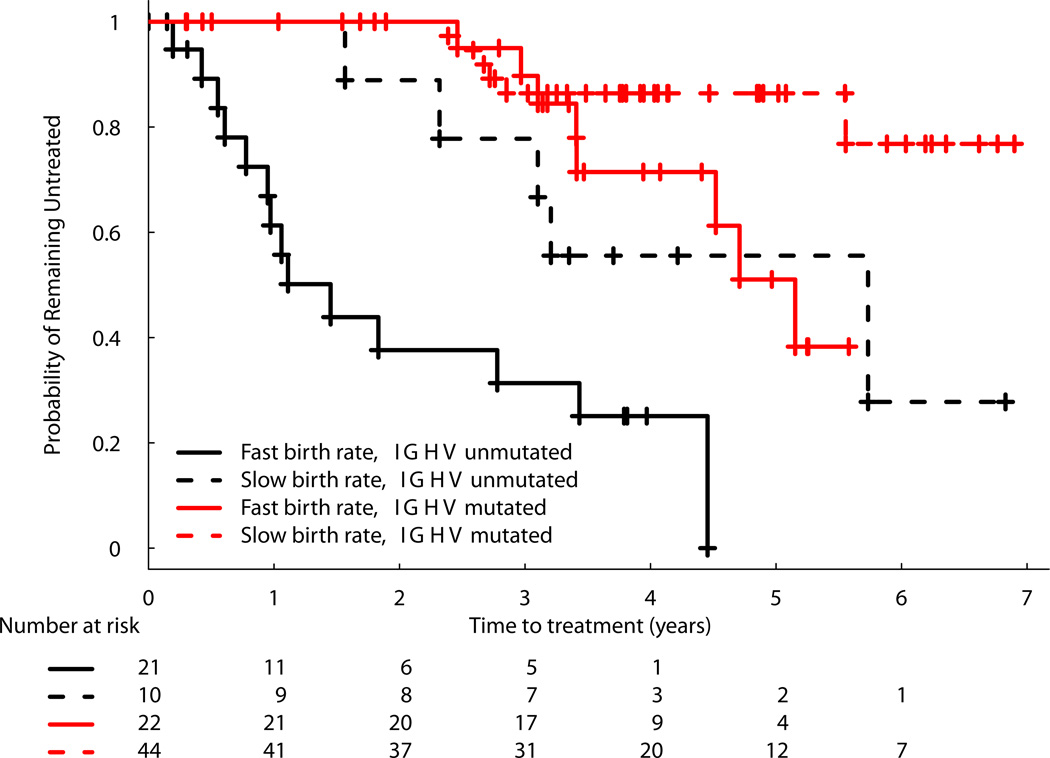
A stepwise Cox proportional hazards model using all available data was used to investigate the relationships with TFS. Again, only cell birth rate and IGHV mutation status contributed significantly. Hazard ratios were 3.51 (95% CI: 1.61–7.67; p=0.002) for CLL birth rate >0.35% cells/d and 4.93 (95% CI: 2.41–10.12; p <0.001) for unmutated IGHV. Using the optimal cut point of birth rate of >0.49% cells per day, the hazard ratios were 6.43 (95% CI: 3.10–13.34) for fast birth rate and 4.87 (95% CI: 2.37–10.00) for unmuated IGHV (Supplemental Table 3). Kaplan-Meier analyses using 0.35% per day depict four separable curves and show that higher birth rate is associated with shorter TFS in both the unmutated and the mutated IGHV groups (Figure 5).
Figure 5. Kaplan-Meier Curve of TFS Model Stratified by IGHV Mutation Status and CLL-Cell Birth Rate.
The curves shown represent the proportion of untreated patients with CLL since time of entry into the study. Patients are stratified into 4 categories: fast birth rate (>0.35% per day), unmutated IGHV (≥ 98% homology); slow birth rate, unmutated IGHV; fast birth rate, mutated IGHV; and slow birth rate unmuated IGHV.
DISCUSSION
In this blinded, prospective study of recently diagnosed patients with early stage CLL, we found that a higher CLL-cell birth rate significantly associated with a shorter TFS, thereby providing a new prognostic marker in early stage patients that is linked directly to the biology of the disease. Though the cut-point of 0.35% was from a cross-sectional study with a heterogeneous patient population13, it discriminated well between shorter and longer TFS. The optimal predictive cut-point of 0.49% cells/d determined in the present data set may be a better discriminator in patients with early stage disease, although this will need to be validated in future studies.
While failed cell death no doubt remains an important contributor to CLL,16 our data confirm findings from smaller studies13–15 demonstrating that a proliferative compartment exists in CLL clones and that the cellular birth rates within this compartment differ among subjects (~20-fold here). Birth rates for the early stage, untreated patients studied here ranged from 0.07% to 1.31% per day, with the latter birth rates being greater than those observed for normal B cells14, 15.
We calculated birth rate using a simplified clinical protocol and assumed a single homogeneous pool of cells with a steady-state pool size. This is an oversimplification as cellular kinetics for both normal polyclonal B lymphocytes and for CLL cells have multiple potential and established biologic complexities.22 As noted in 1968 by Zimmer et al24, and confirmed more recently25, CLL cell populations are heterogeneous with distinct immunologic and anatomic pools having different kinetic rates. Although we sampled cells from the vascular compartment, lymph nodes, spleen and bone marrow contain a large number of cells that can traffic into and out of the vascular compartment. Indeed, it has been suggested that these anatomic sites vary in the numbers of proliferating cells within them26. Moreover, the potential delay in entry of newly proliferated cells into the vascular compartment and the transit time or lag from birth to mobilization from tissue (Figure 2c) introduces another variable, which was observed in almost 20% of the subjects in this study. Despite these biological complexities, the relatively simple method presented here for calculation of birth rate provided results that aligned closely with more complex mathematical modeling and has the advantage of more accurately calculating birth rates in patients with a lag. Most importantly, the use of a previously established method for the calculation of birth rate allowed us to use the previously published cut point for birth rate of 0.35% cells per day,13 for prospective binary classification of subjects as slow or fast proliferators in the current clinical outcomes trial.
The strong association of short TFS with elevated proliferation rate of the total pool of CLL cells and the lack of association with lag or disappearance rate suggest that total cell proliferation per se is a significant factor in CLL disease progression. A relatively high rate of DNA replication may enable more rapid clonal evolution, allowing for leukemia-cell acquisition of new genetic aberrations conducive to more aggressive disease27. Future studies designed to explore the complexities of CLL kinetics and associated new genomic abnormalities may provide a more detailed understanding of the biology of CLL.
Each of the prognostic markers studied here was a predictor of TFS in univariable modeling and, with the exception of FISH, was significantly associated with leukemia cell birth rate. The strong association between higher birth rate and unmutated IGHV gene status in subjects with newly diagnosed early-stage disease is consistent with results from a cross-sectional study of 9 subjects with Rai stage 0–IV disease where an inverse correlation was observed between birth rate and IGHV mutation levels15.
In multivariable modeling, however, we found that only birth rate >0.35% cells/d and unmutated IGVH mutation status were significant contributors to disease progression with hazard ratios for shorter TFS of 3.5 and 4.9, respectively. The lack of other prognostic markers as independent contributors to the model is consistent with the hypothesis that prognostic markers based on the immunobiology of CLL cells (intracellular ZAP70 and surface membrane CD38 expression) are real time indicators of clonal dynamics and disease aggressiveness28. These markers therefore may overlap, at least partially, with the leukemia cell proliferation rates measured in this study. IGHV mutation status, on the other hand, is a static marker at the clonal level that represents the maturation pathway the cell travelled in its transition to leukemia.29 Recent studies showing greater long-term disease free survival with treatment (fludarabine/cyclophosphamide/rituximab) in IGHV-mutated as opposed to unmutated disease30–32 suggest that these early disease markers also may have implications for treatment response.
Strengths of this study include its prospective and blinded nature that focused on patients within only a few years of diagnosis that as yet did not have clinical findings suggestive of disease progression. It is this clinical group for whom determination of prognosis is most relevant. In addition, TFS was defined from the time of the measurement of the prognostic markers, including birth rate, rather than from time of diagnosis of the disease. A potential limitation is that the patients in this study were enrolled at major referral centers and had a median age at diagnosis of 57 years, which is substantially younger than that of patients evaluated in the community setting (~70 years). Nonetheless, the decision for early intervention can be a more pressing concern for younger patients, who might benefit most from the knowledge that they are at risk for a more aggressive disease course.
The protocol for 2H2O labeling and sample collection used here represents a considerable improvement in terms of clinical feasibility compared with more complex previous approaches.13, 19 Strategies such as the use of stable isotope labeled glucose may further reduce labeling time and blood sampling, thereby improving patient compliance and resulting in a more clinically usable assay.33
In conclusion, increased birth rate of CLL cells early in the disease course is a strong predictor of the need for earlier initial treatment reinforcing the concept that enhanced cell proliferation is an important driver in the biology of disease progression. Future studies monitoring proliferation rates over time, their response to treatment, and relation to survival will further expand our understanding of the role of CLL-cell kinetics in this disease.
Supplementary Material
Acknowledgments
Marc Hellerstein is on the Board of Directors of KineMed Inc.
Sources of Support: This work was supported in whole by federal funding from the National Cancer Institute, NIH, PO1 CA081534 for the CLL Research Consortium (T Kipps, PI) and R44 CA100506 (E Murphy and G Hayes, PI).
The authors thank the study participants, referring physicians and study coordinators at all six clinical sites where patients were recruited. We also acknowledge Robert Bush for important guidance in the development of protocols for CLL cell isolation necessary for this work, Dan Holquist for cell processing, and Mohammed Awada and Tim Riff for IRMS analyses.
This work was a collaboration between KineMed Inc. and the CRC and was funded entirely by NIH grants to both institutions. MH is on the board of directors at KineMed, Inc.
Footnotes
Conflict of Interest: No other authors have a current financial conflict of interest.
Supplementary information is available at Leukemia’s website.
REFERENCES
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Kipps TJ. The pathogenesis of chronic lymphocytic leukemia. Annu Rev Pathol. 2014;9:103–118. doi: 10.1146/annurev-pathol-020712-163955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 4.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Binet JL, Caligaris-Cappio F, Catovsky D, Cheson B, Davis T, Dighiero G, et al. Perspectives on the use of new diagnostic tools in the treatment of chronic lymphocytic leukemia. Blood. 2006;107:859–861. doi: 10.1182/blood-2005-04-1677. [DOI] [PubMed] [Google Scholar]
- 6.Delgado J, Baumann T, Santacruz R, Montserrat E. New treatment options for chronic lymphocytic leukemia. Expert Opin Pharmacother. 2014;15:823–832. doi: 10.1517/14656566.2014.891017. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M. Chronic lymphocytic leukemia: 2015 Update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 8.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 9.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 10.Bulian P, Shanafelt TD, Fegan C, Zucchetto A, Cro L, Nuckel H, et al. CD49d is the strongest flow cytometry-based predictor of overall survival in chronic lymphocytic leukemia. J Clin Oncol. 2014;32:897–904. doi: 10.1200/JCO.2013.50.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 12.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 13.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defoiche J, Debacq C, Asquith B, Zhang Y, Burny A, Bron D, et al. Reduction of B cell turnover in chronic lymphocytic leukaemia. Br J Haematol. 2008;143:240–247. doi: 10.1111/j.1365-2141.2008.07348.x. [DOI] [PubMed] [Google Scholar]
- 15.van Gent R, Kater AP, Otto SA, Jaspers A, Borghans JA, Vrisekoop N, et al. In vivo dynamics of stable chronic lymphocytic leukemia inversely correlate with somatic hypermutation levels and suggest no major leukemic turnover in bone marrow. Cancer Res. 2008;68:10137–10144. doi: 10.1158/0008-5472.CAN-08-2325. [DOI] [PubMed] [Google Scholar]
- 16.Dameshek W. Chronic lymphocytic leukemia--an accumulative disease of immunolgically incompetent lymphocytes. Blood. 1967;29(Suppl):566–584. [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes GM, Busch R, Voogt J, Siah IM, Gee TA, Hellerstein MK, et al. Isolation of malignant B cells from patients with chronic lymphocytic leukemia (CLL) for analysis of cell proliferation: validation of a simplified method suitable for multi-center clinical studies. Leuk Res. 2010;34:809–815. doi: 10.1016/j.leukres.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JL, Peacock E, Samady W, Turner SM, Neese RA, Hellerstein MK, et al. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triacylglyceride glycerol measured by mass isotopomer distribution analysis. J Biol Chem. 2005;280:25396–25402. doi: 10.1074/jbc.M413948200. [DOI] [PubMed] [Google Scholar]
- 21.Voogt JN, Awada M, Murphy EJ, Hayes GM, Busch R, Hellerstein MK. Measurement of very low rates of cell proliferation by heavy water labeling of DNA and gas chromatography/pyrolysis/isotope ratio-mass spectrometric analysis. Nat Protoc. 2007;2:3058–3062. doi: 10.1038/nprot.2007.421. [DOI] [PubMed] [Google Scholar]
- 22.Asquith B, Debacq C, Macallan DC, Willems L, Bangham CR. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 2002;23:596–601. doi: 10.1016/s1471-4906(02)02337-2. [DOI] [PubMed] [Google Scholar]
- 23.Ganusov VV, Borghans JA, De Boer RJ. Explicit kinetic heterogeneity: mathematical models for interpretation of deuterium labeling of heterogeneous cell populations. PLoS Comput Biol. 2010;6:e1000666. doi: 10.1371/journal.pcbi.1000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman TS, Godwin HA, Perry S. Studies of leukocyte kinetics in chronic lymphocytic leukemia. Blood. 1968;31:277–291. [PubMed] [Google Scholar]
- 25.Calissano C, Damle RN, Hayes G, Murphy EJ, Hellerstein MK, Moreno C, et al. In vivo intraclonal and interclonal kinetic heterogeneity in B-cell chronic lymphocytic leukemia. Blood. 2009;114:4832–4842. doi: 10.1182/blood-2009-05-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau DA, Carter SL, Getz G, Wu CJ. Clonal evolution in hematological malignancies and therapeutic implications. Leukemia. 2014;28:34–43. doi: 10.1038/leu.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiorazzi N. Implications of new prognostic markers in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:76–87. doi: 10.1182/asheducation-2012.1.76. [DOI] [PubMed] [Google Scholar]
- 29.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126:1921–1924. doi: 10.1182/blood-2015-05-647925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PA, Tam CS, O'Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127:303–309. doi: 10.1182/blood-2015-09-667675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defoiche J, Zhang Y, Lagneaux L, Pettengell R, Hegedus A, Willems L, et al. Measurement of ribosomal RNA turnover in vivo by use of deuterium-labeled glucose. Clin Chem. 2009;55:1824–1833. doi: 10.1373/clinchem.2008.119446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.