Abstract
There is a growing appreciation that reductive stress represents a disturbance in the redox state that is harmful to biological systems. On a cellular level, the presence of increased reducing equivalents and the lack of beneficial fluxes of reactive oxygen species can prevent growth factor-mediated signalling, promote mitochondrial dysfunction, increase apoptosis, and decrease cell survival. In this review, we highlight the importance of redox balance in maintaining cardiovascular homeostasis and consider the tenuous balance between oxidative and reductive stress. We explain the role of reductive stress in models of protein aggregation-induced cardiomyopathies, such as those caused by mutations in αB-crystallin. In addition, we discuss the role of NADPH oxidases in models of heart failure and ischemia-reperfusion to illustrate how oxidants may mediate the adaptive responses to injury. NADPH oxidase 4, a hydrogen peroxide generator, also has a major role in promoting vascular homeostasis through its regulation of vascular tone, angiogenic responses, and effects on atherogenesis. In contrast, the lack of antioxidant enzymes that reduce hydrogen peroxide, such as glutathione peroxidase 1, promotes vascular remodeling and is deleterious to endothelial function. Thus, we consider the role of oxidants as necessary signals to promote adaptive responses, such as the activation of Nrf2 and eNOS, and the stabilization of Hif1. In addition, we discuss the adaptive metabolic reprogramming in hypoxia that lead to a reductive state, and the subsequent cellular redistribution of reducing equivalents from NADH to other metabolites. Finally, we discuss the paradoxical ability of excess reducing equivalents to stimulate oxidative stress and promote injury.
Keywords: redox, reductive stress, oxidative stress, Nrf2, αBgr-crystallin, Nox4, antioxidant, cardiovascular, hypoxia
The physiological flux of reactive oxygen species (ROS) regulates cellular processes essential for cell survival, differentiation, proliferation, and migration. In contrast, excess ROS is a hallmark of many pathological states. Nonetheless, antioxidant strategies and oxidant inhibitors have not always proved to be beneficial in treating diseases. Thus, there is a growing understanding that a lack of necessary ROS is detrimental to cells, and that redox imbalance characterized by an excess of oxidizing or reducing equivalents creates oxidative stress or reductive stress, respectively, states (redox stress states) that are harmful to biological systems.
The pyridine nucleotides, NADH and NADPH, play a major role in the generation of ROS and in their removal by antioxidant enzymes. Their oxidized forms, NAD+ and NADP+, accept electrons during substrate oxidation: NADH is formed during glycolysis by glyceraldehyde 3- phosphate and by enyzmes in the tricarboxylic acid (TCA) cycle, whereas NADPH is formed by glucose-6-phosphate dehydrogenase (G6PD) and other enzymes in the pentose phosphate pathway, as well as isocitrate dehydrogenase in mitochondria. NADH provides electrons to complex I of the mitochondrial electron transport chain, a major source of cellular superoxide. NADPH and NADH are sources of electrons in the NADPH oxidase (Nox) reactions that generate superoxide and/or hydrogen peroxide [1, 2]. Hydrogen peroxide reducing enzymes, such as catalase, glutathione peroxidases (GPx), and peroxiredoxins (Prx), are dependent on NADPH for their activities. NADPH maintains catalase in an active state [3], and NADPH is necessary to recylce the reducing equivalents used by GPx and Prx enzymes. The enzyme glutathione reductase (Gsr) is an NADPH-dependent enzyme that reduces oxidized glutathione (GSSG) to its reduced form (GSH) thereby providing canonical GPx enzymes with the GSH cofactor necessary to reduce hydrogen and lipid peroxides [4, 5]. Similarly, the redox-active thioredoxin (Trx) proteins are electron donors for some of the Prx enzymes [6], and oxidized Trxs are reduced by NADPH-dependent thioredoxin reductases (Txnrd). Additionally, the redox-active factors, GSH and thioredoxin, can modulate the redox state of cysteine residues in proteins. Notably, some glutathione-S-transferases can facilitate protein S-glutathionylation, a process that may also be promoted by the GSH-dependent glutaredoxin (Grx) in certain circumstances [7]. Grx, as well as the Trx/Txnrd system, can reduce protein disulfide bonds, including mixed disulfides between protein thiols and GSH [8].
The redox environment of the cell can be monitored in several ways. NADPH, NADH, GSH, and their respective oxidized forms represent three major redox couples in cells that are used to define the redox environment [9]. Fluxes in their oxidized and reduced states affect metabolism, ROS production and removal, enzyme activities, and other protein functions [7, 10]. ROS, especially hydrogen peroxide, play an important role in the formation of necessary structural disulfides in proteins and in the redox state of functional thiols, the oxidation and reduction of which regulate protein activity [11]. Thus, oxidation of redox-active cysteines in Trx, Prx, and other proteins can modulate anti-oxidant functions, protein-protein interactions, cell signaling, and other protein functions. In addition, changes in redox status can activate or inactivate nuclear factors, such as the transcription factors Ap-1 and Nrf2 [12]. Once activated, Nrf2 can promote a reductive environment by upregulating the expression of several antioxidant enzymes, including enzymes involved in GSH metabolism [13]. The expression and activity of enzymes that regulate NADH and NADPH formation and intracellular shuttling can also affect the intracellular redox environment.
Interestingly, the redox state of various intracellular compartments can be substantially different. This separation is maintained via multiple mechanisms, including specific transporters and metabolic shuttles that facilitate the exchange of biomolecules between intracellular compartments, and the cellular distribution and activities of ROS generating and catabolizing enzymes. The end result of these systems is distinct redox environments that may be differentially regulated. One example of this segregation is the relative oxidative environment of the endoplasmic reticulum compared to other subcellular compartments [14]. Thus, in the cytosol, GSH is in a highly reduced state with a ratio of GSH: GSSG of approximately 100:1, whereas in the oxidative environment of the endoplasmic reticulum (ER), the GSH:GSSG ratio is in the range of 1:1 −3:1. In addition, mitochondria typically have more NADH than the cytosol. The malate-aspartate shuttle promotes the mitochondrial transfer of NADH from the cytosol to mitochondria to decrease cytoplasmic NADH and regenerate the NAD+ needed for glycolysis [15]. Other shuttles, such as the pyruvate-malate shuttle, use the reducing equivalents of mitochondrial NADH to produce cytosolic NADPH.
In this review, we focus on how changes in the redox potential caused by reductive stress may contribute to cardiovascular dysfunction. We review the literature on protein aggregation cardiomyopathies and the redox mechanisms by which they mediate cardiac dysfunction. We also discuss the role of Nox enzymes in modulating oxidants in cells, and the paradoxical effects of their suppression and overexpression on cardiac and vascular function. Similarly, we discuss the role of antioxidant enzymes in reductive stress and how loss of necessary ROS affects cellular function, growth, and proliferation. We also address new findings regarding adaptive responses to the reductive environment in hypoxia, discuss the basis for ‘pseudo’-hypoxic phenotypes in diabetes mellitus, and address how excess NADH may affect NAD+-dependent actions. Finally, as ROS play an essential role in normal cellular functions and in the adaptive responses to cellular stress, we consider how prolonged reductive stress may cause cellular dysfunction, perhaps by causing increases in harmful ROS.
Protein aggregation-induced cardiomyopathies and reductive stress
The small heat shock protein, αB-crystallin (CryAB), is a molecular chaperone of desmin and actin, and it maintains cytoskeletal integrity in cardiac and skeletal muscle. Mutations in desmin, an intermediate myofibrillar protein that is essential for the structure and contractile function of myofibrils, were shown to lead to a skeletal myopathy and cardiomyopathy characterized by protein misfolding and accumulation of cytoplasmic aggregates [16]. Interestingly, mutations in the chaperone CryAB were found to cause a similar desmin-related myopathy with protein misfolding [17]. The pathogenesis of this cardiomyopathy was studied in mice, where expression of CryAB with the R120G mutation caused protein aggregation and cardiac dysfunction [18, 19]. Concurrent with this phenotype, there was an upregulation in the expression and activity of antioxidant enzymes, such as catalase and GPx-1. In addition, the NADPH-dependent enzyme Gsr, which recycles oxidized GSSG, and glucose 6-phosphate dehydrogenase (G6PD), which is the rate-limiting enzyme of the pentose phosphate pathway and an important cytosolic source for NADPH, were both upregulated in transgenic R120GCryAB hearts. Taken together, these findings suggested a redox imbalance in these hearts, favoring increased reducing potential. To show that the reductive state contributed to pathogenesis in this model, transgenic R120GCryAB mice were crossed to male hemizygous G6pd deficient mice that have a mutation limiting G6PD activity to approximately 20% that of normal mice. (As G6pd is on the X-chromosome, male mice have only a single copy of this gene; therefore, they are hemizygous for either wildtype or mutant G6pd.) In the context of the R120GCryAB mutation, deficiency of G6PD lessened cardiac hypertrophy, confirming a role for excess reducing equivalents in the pathobiology of aggregation-induced cardiomyopathies.
The R120GCryAB transgenic mouse also had an upregulation of heat shock proteins (Hsp), including Hsp25, Hsp90, and Hsp70 [18]. Heat shock proteins have been shown to mitigate myocyte dysfunction by preserving cytoskeletal structure and preventing apoptotic signaling [20]; however, additional studies have linked overexpression of some heat shock proteins with reductive stress. Thus, in cell culture systems, overexpression of Hsp25 or Hsp27 increased GSH levels and reduced cellular ROS to provide increased protection against subsequent oxidative stress [21, 22]. In mice, high levels of overexpression of Hsp27 were detrimental, causing cardiac hypertrophy and reduced contractile function. The hypertrophy was characterized by a reductive stress phenotype with increased GSH/GSSG ratio, increased GPx1 expression and activity, and decreased ROS [23]. In this model, treatment with mercaptosuccinate, an inhibitor of GPx1, attenuated cardiac hypertrophy and improved contractile function, suggesting that the lack of ROS and increased GPx1 activity were detrimental to the heart.
In other model systems, such as doxorubicin-mediated cardiotoxicity in the cardiomyocyte line H9c2, excess antioxidants in the form of N-acetylcysteine (NAC) increased GSH and the GSH/GSSG ratio, and reduced ROS but failed to protect against cell death [24]. In contrast in vivo, cardiac specific GPx1 overexpression is protective against doxorubicin-induced cardiac injury in a manner that preserves mitochondrial complex I activity [25]. Most likely, increased GPx1 expression effectively removed hydrogen and lipid peroxides produced by doxorubicin exposure without changing GSH/GSSG redox potential, avoiding the reductive stress found in the cell culture model. Additional studies have found that NAC treatment paradoxically promotes an oxidative shift in cellular redox state. In H9c2 cells, NAC exposure increased the oxidation of mitochondrial redox biosensors, including the endogenous mitochondrial protein Trx2. Targeted overexpression of gamma-glutamylcysteine ligase enzymes, Gclm or Gclc, also increased cellular GSH and GSSG to cause Trx2 oxidation and a decrease in the GSH/GSSG ratio. [26]. Gclm and Gclc are the modulatory and catalytic subunits, respectively, of the first and rate-limiting enzyme in GSH biosynthesis. Similarly, in myoblasts, 24 h NAC treatment also caused reductive stress by decreasing the NAD+/NADH ratio. These changes in redox state correlated with impaired mitochondrial function and increased mitochondrial free radical leak [27]. In the R120GCryAB model, reductive stress appears to be the result of the compensatory efforts of the cell to remove an (initial) oxidant stress. Ultimately, reductive stress augments protein aggregation [28], likely by altering cysteinyl side chain redox potential and limiting disulfide bond formation.
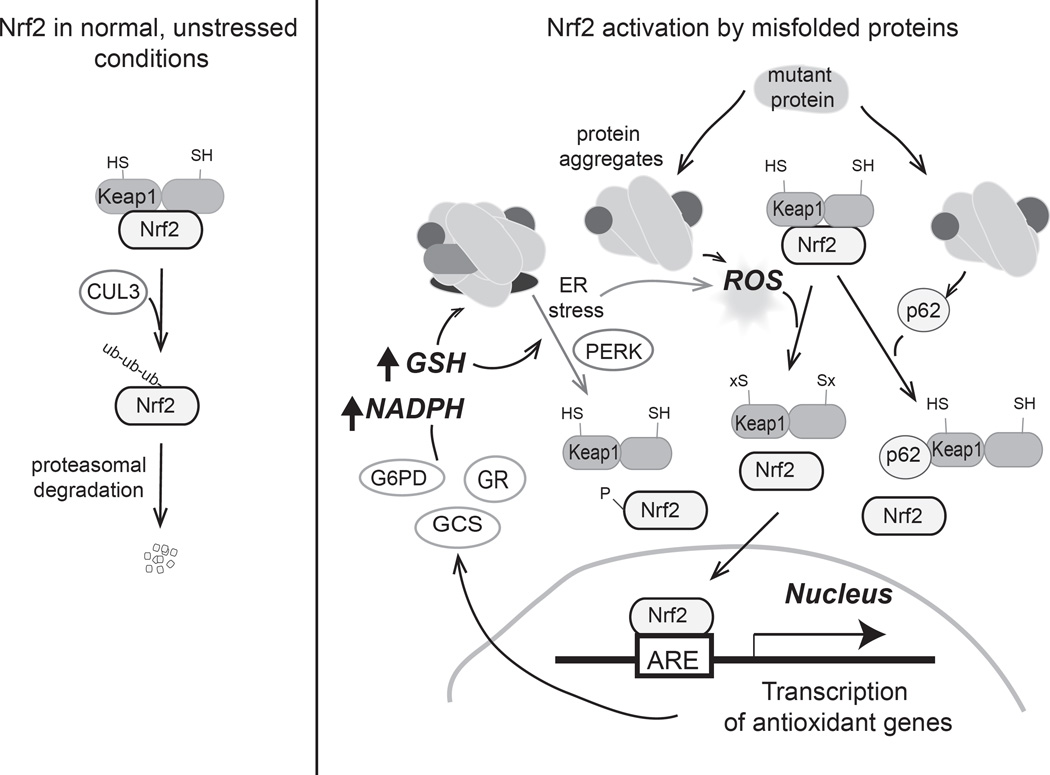
In the R120GCryAB hearts, reductive stress stemmed from an activation of the redox-sensitive nuclear erythroid 2-related factor 2 (Nrf2) transcription factor [29]. In the basal state, Nrf2 is kept in the cytoplasm by its interactions with Keap1 (kelch-like ECH-associated protein1), which targets Nrf2 for ubiquitination by the CUL3 E3 ligase leading to its subsequent degradation in the proteasome [13, 30]. Modification of Keap1 at redox-sensitive cysteinyl residues leads to a release of Nrf2, allowing this transcription factor to shuttle to the nucleus to initiate an antioxidant program by binding to genes with antioxidant/electrophile response elements (Fig.1). In the transgenic R120GCryAB hearts, nuclear Nrf2 was upregulated, a sign of its activation by redox-dependent mechanisms. Consistent with sustained activation of Nrf2, several Nrf2 target genes were also upregulated, including the antioxidant genes G6pd and catalase, and genes involved in GSH biosynthesis, Gclm and Gclc. In support of a role for Nrf2 in mediating the pathological increase in reductive stress, Nrf2 deficiency attenuated cardiac hypertrophy in transgenic R120GCryAB mice, decreasing GSH and GSH/GSSG ratios, lessening cardiac hypertrophy, and improving survival of the mice [28]. In addition, the protein levels of antioxidants such as Gsr, G6PD, and catalase were normalized and protein aggregate formation was partially decreased by Nrf2 deficiency.
Figure 1.
Nrf2 activation by misfolded proteins. The left panel illustrates the pathway by which Nrf2 is ubiquitinated and degraded under normal, unstressed conditions. Redox-sensitive Keap1 promotes the association with E3-ubiquitin ligase proteins that modify Nrf2 leading to its subsequent degradation. Under stressed conditions, such as in the cardiac and skeletal myopathies that are associated with protein aggregation phenotypes, Nrf2 is activated by a variety of means to promote reductive stress. Protein aggregation complexes include the mutant protein, heat shock proteins, and other cellular proteins. In the case of the mutant αΒ-crystallin, excess oxidants were proposed to activate Nrf2 by an increase in reactive oxygen species (ROS) that cause oxidative modifications (−Sx) to the regulatory cysteine residues on Keap1, promoting Nrf2 release. In the case of the mutant lamin proteins, activation of Nrf2 was proposed to involve the association of p62 with Keap1 to release Nrf2. Reductive stress is characterized by the increased expression of antioxidant genes involved in glutathione (GSH) biosynthesis and reduction, as well as an upregulation of enzymes such as G6PD, an enzyme involved in NADPH production, leading to increased GSH and NADPH. An environment with increased reducing equivalents may further increase protein aggregation by interfering with normal protein folding. These conditions can further promote ER stress, a condition that will activate Nrf2 by PERK-mediated phosphorylation of Nrf2. Although PERK activation of Nrf2 has not been examined in the protein aggregation disorders discussed above, ER stress proteins were elevated in these models. Notably, reductive stress or even ER stress can augment ROS production to perpetuate Nrf2 signaling.
A similar upregulation of Nrf2 caused reductive stress in a related protein aggregation disorder involving mutations in lamin [31]. Although the primary effect of mutations in human lamin A (LMNA) is a muscular dystrophy, there is often associated cardiac dysfunction in these patients. In the Drosophila model for this disease, mutant lamins were shown to aggregate in the cytoplasm of the Drosophila larval body-wall muscle, and their expression resulted in reductive stress, characterized by an upregulation of GSH and NADPH. In this model, the presence of the mutant lamin did not alter G6PD activity in muscle; rather, there was a significant increase in the activity of isocitrate dehydrogenase, another NADPH-producing enzyme [31]. The role of Nrf2 in disease pathology was supported by the detection of active, nuclear located Nrf2 in human muscle biopsies from patients with various dystrophic lamin mutations. Similarly, Nrf2 was also found in nuclei of mutant Drosophila larvae muscle. Interestingly, at least in the Drosophila model system, reductive stress occurred within 24–48 h of expression of the mutant lamins. Although this study did not examine ROS as a possible activator of Nrf2, it found evidence for an alternative mechanism that activates Nrf2 involving upregulation of the autophagy adaptor protein p62 (Fig 1). Previous studies have shown that accumulation of p62 competes for the Nrf2 binding sites on Keap1, allowing Nrf2 to translocate to the nucleus [32]. High levels of p62 have been found under circumstances where autophagy is inhibited. There may, however, be a complex relationship between autophagy and Nrf2, as overexpression of Nrf2 was found to stimulate autophagy, contributing to its protection in models of cardiac pressure overload induced by trans-aortic constriction (TAC) [33].
Endoplasmic reticulum (ER) stress may also activate Nrf2. Reductive stress can lead to endoplasmic reticulum (ER) stress, for example, by disrupting the normal oxidative environment of the ER. In the ER, an oxidative redox environment is necessary for proper protein folding and the formation of native protein disulfides. Reductive changes to the environment can lead to an accumulation of unfolded proteins in the ER, activating the unfolded protein response (UPR) [34]. UPR pathways can also be activated by other mechanisms, including oxidative stress [35]. The UPR is an adaptive mechanism devised to restore ER homeostasis; however, chronic activation can lead to apoptosis. Three ER-mediated signaling cascades participate in the UPR that are initiated by the activation of IRE1 (inositol-requiring kinase 1), PERK (double-stranded RNA-activated protein kinase-like ER kinase), and the transcription factor ATF6 (activating transcription factor 6). PERK, in particular, can activate Nrf2, as phosphorylation of Nrf2 by PERK promotes the dissociation of the Nrf2-Keap1 complex [36] (Fig.1). Although to date the activation of PERK has not been shown in the R120CryAB cardiomyopathy, other ER stress markers, such as the chaperones, Grp94, Bip, and XBP1 [28, 37], are increased in R120GCryAB mice, providing evidence for ER stress in these hearts.
The protective effect of (some) oxidants in the heart
NADPH oxidases (Nox) are a major source of cellular ROS. To date, seven NADPH oxidases have been described that consist of a NOX catalytic subunit (Nox1–5, Duox-1 and Duox-2) and various regulatory molecules, such as p22phox, and p47phox [2]. Nox4 is unique in that it only requires p22phox for its activity, and is considered to be constitutively active. Recent findings, however, suggest that Nox4 activity may be negatively regulated post-translationally via phosphorylation by a Src family tyrosine kinase, FYN [38]. Other Nox, such as Nox1 and Nox2, are regulated by various post-transcriptional mechanisms, including phosphorylation of regulatory subunits. Nonetheless, Nox4 expression can be upregulated at the transcriptional level in response to various stimuli, such as pressure overload in the heart, myocardial infarction, or hypoxia [39], and its expression has been shown to change in development [39]. Catalytically, Nox4 is also unique because it can utilize both NADPH and NADH as substrates in its reduction of oxygen [1]. In addition, whereas other Nox enzymes produce superoxide, Nox4 conducts a 2-electron reduction of oxygen to release hydrogen peroxide. Compared with superoxide, hydrogen peroxide is less reactive, has a greater ability to transverse membranes, and has a longer half-life.
In the heart, Nox2 and Nox4 are the major Nox enzymes. Nox2 is a plasma-membrane protein, whereas Nox4 has been found in perinuclear sites, the ER, and mitochondria, as well as the plasma membrane. Whereas Nox2 has been characterized as producing harmful ROS in the context of many models of cardiac dysfunction, including pressure overload, angiotensin II-dependent hypertrophy, and myocardial infarction [40–42], the effects of Nox4 on cardiac function are more complicated with evidence to suggest that its loss as well as its overexpression may be detrimental to cardiac function, depending on the pathological stress.
In unstressed hearts, cardiac-specific overexpression of Nox4 activated Nrf2 to upregulate the expression of antioxidant genes (including Gst, Txnrd1, Gclc, Gsr, the NADH quinone reductase or Nqo1, and the inducible heme oxygenase-1 or Hmox1), resulting in significant increases in the levels of cardiac GSH and the GSH/GSSG ratio [43]. Crosses with the Nrf2 knockout mice attenuated the upregulation of this ‘reductive’ program. In contrast, Nox2 transgenic mice had neither the Nrf2-mediated oxidant gene expression nor an elevation of cardiac GSH. It is unclear, however, whether overexpression of Nox2, an NADPH oxidase that is not constitutively active, would increase ROS production in these mice. In this study, ROS levels were not measured in either of these transgenic lines. Previous findings, however, indicate that cardiac-specific overexpression of Nox4 increases hydrogen peroxide generation in the myocardium [39], suggesting that excess ROS in Nox4 transgenic hearts may account for the increase in basal Nrf2. The role of Nox4 in cardiac function has been assessed in several models of cardiac disease, including the chronic-load stress model of heart failure and ischemia-reperfusion models of myocardial infarction; however, the activation of Nrf2 has not been assessed in the context of these models. The mechanisms by which Nox4-mediated hydrogen peroxide may affect cardiovascular function are summarized in Figure 2 and discussed further below.
Figure 2.
Effects of Nox4 in cardiovascular systems. Hydrogen peroxide generated by Nox4 appears to have many beneficial effects: it modulates basal expression of Nrf2, and promotes vascular tone by increasing membrane hyperpolarization and activation of eNOS. The activation of eNOS has other beneficial effects on vascular homeostasis that are anti-atherogenic and pro-angiogenic. Nox4-dependent hydrogen peroxide is also thought to preserve endothelial and cardiac function by promoting Hif1α stabilization to initiate pro-angiogenic and metabolic reprogramming. Furthermore, oxidants produced by Nox4 contribute to cell signaling, and differentiation. It is not entirely clear why oxidants generated by Nox4 are protective rather than harmful; however, its excess expression has been associated with dysfunction under some circumstances.
In vivo, in a model of heart failure induced by suprarenal banding of the aorta, Nox4 overexpression protected mouse hearts against chronic-load stress, with improved systolic and diastolic function and reduced hypertrophy and fibrosis compared to hearts from control banded mice [39]. Consistent with the protective role of Nox4 in pressure-overload stress, Nox4 deficiency enhanced cardiac dysfunction decreasing the ejection fraction and augmenting cardiomyocyte hypertrophy and fibrosis compared to hearts from control-banded mice. The preservation of cardiac function by Nox4 overexpression in cardiomyocytes appears to be due, in part, to the enhancement of angiogenesis in the heart by mechanisms involving augmented hypoxia inducible factor-1 (Hif1α) protein in Nox4 transgenic hearts leading to upregulation of VEGF-A levels. This finding is consistent with the preservation of myocardial capillary density in the Nox4 transgenic hearts. It was hypothesized that the stabilization of the Hif1α in these hearts was due to inhibition of prolyl hydroxylases by ROS [39]. Hif1α (and Hif2α) is (are) oxygen sensitive proteins that each can form active heterodimeric transcription factors by binding to the oxygen stable Hif1β [44, 45]. Under oxygen-replete conditions, Hifα-factors are hydroxylated at conserved proline residues by oxygen-, 2-oxoglutarate-, iron- and ascorbate-dependent dioxygenases. Hydroxylated Hifα proteins are subsequently targeted for ubiquitination and proteasomal degradation. Under decreased oxygen tension, the prolyl hydroxylases (PHD) proteins are inactive, thereby leading to stabilization of the Hifα factors. Other mediators, such as ROS and iron chelators, can inactivate the PHD enzymes, ultimately augmenting Hif1α-mediated gene transcription.
The role of Nox4 in cardio-protection remains unclear: in another heart failure model, the results suggest that knockdown of Nox4 is protective, whereas its overexpression is detrimental [46]. In this study, transverse aortic constriction was used to promote pressure overload and the knockout and transgenic models were both cardiac-specific. In the suprarenal banding model discussed above, the Nox4 knockdown was global, suggesting that loss of Nox4 in other cell types could contribute to the detrimental effects of pressure overload in this model [39]. (As discussed in the next section, endothelial expression of Nox4 appears to be protective.) Regardless of the knockout results, a similar MHC-driven cardiac-specific promoter was used to overexpress Nox4 in in the study reporting a protective mechanism as well as the study reporting that Nox4 augments cardiac dysfunction. One possible explanation is that the time-course for cardiac dysfunction may differ in these models [38], allowing for compensatory, protective actions that are Nox4-dependent in the suprarenal banding model, or there may be other mechanistic differences between these pressure-overload models that account for the distinct outcomes. Alternatively, the level of Nox4 overexpression may differ in the context of these transgenic lines established by different investigators.
The mechanism by which Nox4 can alter cardiomyocyte function warrants further exploration. Nox4 modulation by FYN, a Src family tyrosine kinase, suggests the existence of additional ROS-mediated feedback mechanisms that may play a role in cardiac stress responses [38]. FYN was shown specifically to mitigate Nox4-mediated ROS production by phosphorylating tyrosine 566 on Nox4. Furthermore, FYN is activated by ROS and was found to be upregulated in the early phases of transverse aortic constriction (TAC), although in the later stages of TAC (4–6 weeks) its expression was diminished. In addition, in chronic heart failure caused by dilated cardiomyopathy in patients, FYN expression was also decreased. FYN was shown to be important in modulating cardiac damage in heart failure as knockdown of FYN augmented cardiac dysfunction, ROS production, and cardiomyocyte apoptosis in TAC, whereas overexpression of FYN mitigated the effects of TAC. FYN overexpression was also able to attenuate the enhanced cardiac dysfunction caused by Nox4 overexpression in this pressure overload model.
In other models of cardiac injury, cardiac specific elimination of Nox4 was also found to be protective. Thus, in ischemia-reperfusion models (I/R) of injury in mice, cardiac-specific knockout (KO) of Nox4 or global knockout of Nox2 reduced injury and decreased myocardial apoptosis. In contrast, the simultaneous deficiency of both Nox4 and Nox2 was found to increase I/R injury, suggesting that although some decrease in ROS may be beneficial in I/R injury, eliminating too much ROS impedes recovery [47]. Interestingly, the protective effects in I/R were also proposed to involve ROS-mediated stabilization of Hif1α, a response that was lacking in mice with deficiency in both Nox4 and Nox2. Hearts lacking both Nox2 and Nox4 had reduced NADP+/NADPH ratios, suggesting an altered redox state. After I/R, hearts from mice lacking both Nox2 and Nox4 produced less superoxide than those from wildtype, cardiac specific Nox4 KO, or Nox2 KO. The double-deficiency of Nox proteins was also associated with decreased hydrogen peroxide production and decreased lipid peroxidation after the 20 m ischemia as well as after 24 h reperfusion. The effects of combined Nox2 and Nox4 deficiency were assessed in double knockout mice as well as in mice with a cardiac specific Nox4 dominant negative (DN) construct: both models showed similar phenotypes. The DN construct appears to sequester p22phox, and to inactivate both Nox4 and Nox2. The DN mice were used for the mechanistic analysis in this study. Reduced levels of Hif1α protein in the DN Nox2/4-deficient hearts following I/R attenuated the adaptive responses to I/R, resulting in diminished upregulation of genes involved in glycolysis, and augmenting the expression of genes involved in fatty acid metabolism, such as PPARα. These changes were reversed in the DN hearts by introducing a deficiency of PHD2. Thus, deficiency of PHD2 augmented Hif1α stabilization, increased the expression glycolytic genes, suppressed PPARα expression, and reduced infarct size. Strikingly, the metabolic changes caused by upregulation of PPARα in the DN after I/R increased triglyceride deposition in the heart. Downregulation of PPARα in the context of Nox2/4 deficiency also decreased infarct size, reduced triglyceride deposition, and suppressed apoptotic mechanisms, suggesting that excess triglycerides may contribute to cardiac lipotoxicity. Interestingly, studies in non-cardiac cells have linked reductive stress caused by decreased NAD+/NADH ratios with enhanced triglyceride deposition, as well [48]. Cardiac-specific targeted overexpression of PPARα recapitulates these changes in triglyceride deposition, with increased fatty acid oxidation and decreased glucose utilization [49]. These studies highlight how cellular redox state can regulate metabolic adaptations to influence cardiac function and apoptosis.
The consequences of the reductive environment fostered by Nox2/4 deficiency was studied further in isolated perfused hearts subject to low-flow ischemia for 25 m followed by 1 h of reperfusion (I/R) [50]. At baseline, the DN heart was found to have significantly higher cardiac GSH/GSSG ratio and lower NAD+/NADH and NADP+/NADPH ratios compared to hearts from wildtype mice or those from mice overexpressing wild type Nox4 in cardiomyocytes (TG), consistent with reductive stress in the DN heart. However, both the overexpression of Nox4 and the deficiency of Nox impaired functional recovery to I/R in the isolated perfused heart studies. DN hearts showed the most severe impairment of cardiac function and energetics, with no recovery of heart beat after ischemia that corresponded with the attenuated recovery of ATP, phosphocreatine, and intracellular pH in these hearts. Paradoxically, mitochondrial ROS was upregulated during I/R in the DN hearts compared to TG and wildtype hearts, although both TG and DN produced higher levels of cytoplasmic ROS. The increased dysfunction in the perfused DN heart is consistent with data from the in vivo I/R experiments, as simultaneous deficiency of Nox2 and Nox4 augmented cardiac dysfunction in vivo. In the in vivo I/R procedures, however, ROS was lower in the DN whereas in the perfused model, ROS was paradoxically higher at the end of perfusion. Obviously, the in vivo and isolated heart studies were performed over a different time frame and with different environments. However, in the perfused heart, the controlled environment allowed for manipulations that metabolically decrease reductive stress. Thus, limiting substrate availability during the I/R procedure to glucose and pyruvate (i.e., eliminating fatty acids, lactate, ketone and insulin from the perfusate) was sufficient to raise the NAD+/NADH ratio in DN hearts at baseline and during ischemia. These changes also decreased ROS, improved cardiac function, and normalized cardiac bioenergetics. Taken together, these data suggest that increased reductive stress promotes mitochondrial dysfunction in the stressed heart to increase mitochondrial dysfunction and enhance maladaptive responses.
Oxidant generators and antioxidants in vascular dysfunction
Vascular Nox4 expression is altered in a number of cardiovascular diseases, including atherosclerosis, diabetes mellitus, restenosis, and hypertension, suggesting an important role of Nox4 in the response to injury and stress [51]. Endothelium-specific overexpression of Nox4 in mice leads to lower blood pressure, despite the excess generation of hydrogen peroxide in endothelial cells [52]. In fact, in this model, enhanced endothelium-dependent vasodilation was shown to depend on production of hydrogen peroxide by Nox4. ROS is generally considered to inhibit vasodilatory responses by reducing bioavailable NO. Antioxidant enzymes, such as GPx-1, have been shown to mitigate this response [53]; however, some vasoactive substances, such as arachidonic acid, have been shown to promote hydrogen peroxide-dependent vasodilation [54, 55] that is inhibited by overexpression of GPx-1 [56]. In the case of endothelial Nox4 overexpression, excess hydrogen peroxide was suggested to alter vascular tone by regulating membrane hyperpolarization. In fact, plasma nitrite/nitrate concentrations, aortic cGMP, and phosphorylated vasodilator-stimulated phosphoprotein, markers of NO bioavailability, and protein kinase G activity were not different between Nox4 overexpressing and control mice [52]. Other evidence implicates Nox4-mediated hydrogen peroxide in mediating vascular tone at least in the context of low-density lipoprotein receptor (Ldlr) deficiency [57]. Although the effect of Nox4 deficiency on blood pressure was not measured in these mice, Ldlr knockout mice had normal endothelium-dependent vasodilation whereas Ldlr/Nox4 double knockout mice had endothelial dysfunction, suggesting an important role of Nox4 on vascular function in these mice.
In contrast to the studies suggesting that the vasoprotective effects of Nox4 are not dependent on eNOS [52, 57], several other studies suggest that Nox4 mediates its effects on the vasculature, in part, by regulating eNOS expression and nitric oxide production [58–60]. Thus, Nox4 knockout mice were found to have decreased levels of eNOS, decreased NO production, and decreased neoangiogenesis in a hind limb ischemia model [58]. Nox4 knockout also decreased the upregulation of Hmox1, a gene that is known to be upregulated by Nrf2. Experiments in cultured endothelial cells showed a role for Nox4 in basal Nrf2 activation and Hmox1 expression. Although Hmox1 may prevent apoptosis in some settings [58], it is unclear whether its expression or Nrf2 upregulation contributes to neoangiogenesis in the hind limb ischemia model as Nrf2 deficiency was shown to promote recovery from hind limb ischemia, presumably due the enhancement of ROS production in these mice [61]. Consistent with the concept that excess Nox4 may be protective, endothelial Nox4 overexpression improved recovery in hind limb ischemia with an upregulation of eNOS at baseline and in response to hind limb ischemia [59]. Similarly, endothelial targeted overexpression of Nox4 enhances, whereas overexpression of its dominant negative form in endothelium inhibits recovery in a hind limb ischemia model, in part, due to mechanisms regulating eNOS expression [60].
Although upregulation of endogenous Nox4 expression by stimuli such as TNF-α and angiotensin II has been associated with oxidative stress, apoptosis, and fibrosis [62, 63], many studies report that upregulation of endogenous Nox4 improves cell survival and increases angiogenic responses. Thus, the upregulation of Nox4 was found to be essential in the angiogenic responses to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [64]. In vivo, TRAIL deficiency decreased neovascularization in a hind limb ischemia model concurrent with a reduction in Nox4 expression. In vitro, TRAIL-induced proliferation, migration, and tubule formation was attenuated by knockdown of Nox4, the removal of hydrogen peroxide from the system, or inhibition of eNOS, suggesting that the TRAIL-mediated pro-angiogenic responses require Nox4-mediated hydrogen peroxide production and eNOS activation. Nox4 expression was also upregulated by hypoxia/reoxygenation in isolated cardiac microvascular endothelial cells [65]. In these cells, increased Nox4 expression was suggested to play a role in promoting the survival and angiogenic responses by promoting Hif1α/VEGF signaling, similar to the pathways described in cardiomyocytes [39]. Thus, in endothelial cells, knockdown of Nox4 blocked the normal adaptive responses to hypoxia/reoxygenation stress by attenuating the stabilization of Hif1α and the subsequent VEGFA upregulation, increasing apoptosis, decreasing cell survival, and reducing angiogenic responses [65]. These findings and those with transgenic and knockout mice suggest that hydrogen peroxide generated by Nox4 preserve vascular tone and promote pro-angiogenic responses that are beneficial in the setting of ischemia and that a loss of protective oxidants are harmful.
In some conditions, however, upregulation of Nox4 may contribute to pathological proliferation of vascular cells [66–68]. Thus, in oxygen-induced retinopathy, upregulation of Nox4 stimulates VEGF-mediated neovascularization that can be attenuated by scavenging excess hydrogen peroxide or by knockdown of Nox4 [66]. In hypoxia-induced pulmonary hypertension, vascular remodeling contributes to right ventricular hypertrophy and the elevation of right ventricular pressure. In both hypoxia-induced pulmonary hypertension, as well as in the model of persistent pulmonary hypertension of the newborn (PPHN), Nox4 upregulation was associated with increased hydrogen peroxide, proliferation of vascular cells, and the vascular remodeling associated with disease [67, 68]. Taken together, these findings suggest that excess Nox4 may promote disease pathology in some biological systems, indicating that its inhibition or antioxidants therapies may be beneficial in these states.
Nox4 may also have an anti-atherogenic effect. In control C57Bl6 mice, a Western diet increased the expression of Nox4, whereas a Western diet reduced the expression of Nox4 in atherosclerosis-susceptible, ApoE knockout, mice [69]. Interestingly, plaques from patients with cardiovascular events or diabetes had decreased levels of Nox4, a condition that was replicated in streptozotocin-treated ApoE knockout mice 20 weeks after developing diabetes [70]. In the context of ApoE deficiency, endothelial specific overexpression of Nox4 reduced late stage (24 wk) aortic lesion area in response to a Western diet, with the greatest effect on lesions in the abdominal aorta [69]. In addition, Nox4 overexpression in the ApoE model specifically affected the expression of T cell markers within the plaque, suggesting decreased presence of inflammatory T effector cells and an increased presence of T regulatory cells that may suppress inflammation. Another approach that examined the loss of Nox4 by using an inducible Nox4 to create Nox4/ApoE double knock mice reported that after 9 months the double knockout mice on a normal chow diet developed an approximately two-fold increase in plaque burden, with an increase in fibrosis and inflammatory infiltration of macrophages [71]. The Nox4/ApoE double knockout mice produced significantly less hydrogen peroxide in both the thoracic aorta and abdominal aorta compared to the ApoE knockout mice, although isolated aortic rings from the double knockout mice produced significantly more superoxide [71]. In addition, following 20 weeks on a high fat diet, the Nox4/Ldlr double knock out mice developed a greater plaque burden and more fibrosis in the aortic arch compared with Ldlr-deficient mice [57]. Similarly, in the context of the diabetic ApoE deficient model, absence of Nox4 enhanced plaque development, with increased inflammation, as characterized by augmented MCP-1 release and increased macrophage infiltration [70]. Interestingly, the Nox4/ApoE diabetic double knockout also had decreased levels of aortic hydrogen peroxide generation, but the levels of superoxide production were similar to those found in the diabetic ApoE knockout mice. Nox4 deficiency also augmented fibrosis caused by the diabetic phenotype and caused an upregulation of TGFβ, a known pro-fibrotic activator. In contrast, Nox1 deficient ApoE knockout diabetic mice developed less atherosclerosis, produced less superoxide production, and had less inflammation than ApoE deficient diabetic mice, suggesting that the source and type of ROS generated will influence disease progression.
Reductive stress was also found to contribute to neointimal cell proliferation following angioplasty and stenting in a manner dependent on deficiency of the antioxidant enzyme, GPx1. Interestingly, GPx1 expression was decreased in human coronary atherectomy samples from patients with in-stent stenosis, a condition that may involve reductive stress. In GPx1/ApoE double knockout mice, aortic levels of GSH were elevated and, following balloon angioplasty, there was a further time-dependent increase in GSH and the GSH/GSSG ratio [53]. Interestingly, in the double knockout mice there was a significant decrease in endothelial regeneration, suggesting that loss of GPx1 is detrimental for endothelial cell growth. This finding is consistent with previous work showing that GPx1 deficiency promotes endothelial progenitor-like cell dysfunction and decreases neoangiogenesis [72]. In addition, GPx1/ApoE double knockout mice had increased expression of the Gcs genes that regulate GSH biosynthesis, as well as the GSH synthetase and Grx1 genes. The genes encoding these GSH metabolizing enzymes were upregulated in human neointima from in-stent stenosis samples, as well. Although the GPx1/ApoE double knockout mice have evidence for vascular reductive stress, superoxide generation was elevated in their vessels, primarily in the medial layer. Furthermore, reductive stress in this model was shown to promote vascular smooth muscle proliferation specifically via activation of Ros1, a membrane bound, orphan receptor tyrosine protein kinase, and this activation may be dependent, in part, on the S-glutathionylation of SHP-2 phosphatases. Thus, it is possible that in the context of in-stent restonosis, GPx1 deficiency increases oxidative stress to activate pathways leading to excess GSH production. There findings are consistent with oxidant-mediated upregulation of Nrf2; however, the activation of Nrf2 was not analyzed in this study.
Hypoxia-induced reductive stress
The adaptive responses to hypoxia are found in all metazoan species. The activity of the heterodimeric nuclear factors, Hif1 and Hif2, formed following hypoxia-mediated stabilization of the Hif1α and Hif2α allow for their association with Hif1β to form these transcriptionally active nuclear factors to coordinate the cellular response to low oxygen tension [45]. Hif1α is ubiquitously expressed and, in general, is stabilized in a cell within hours of exposure to hypoxia. In contrast, Hif2α, which is also known as the endothelial PAS domain protein 1 (EPAS1), has a limited cell distribution, although it can be found in cardiomyocytes, vascular smooth muscle cells, endothelial cells, and fibroblasts. Its temporal stabilization lags behind that of Hif1α, although it has been shown to be upregulated in chronic hypoxia. Hif1 and Hif2 nuclear factors bind to the same hypoxia response element sequence; however, for reasons that are not entirely clear, the sets of genes regulated by Hif1 and Hif2 overlap, but are not identical. Hif1α, in particular, regulates the metabolic energy shift from oxidative phosphorylation to anerobic glycolysis, whereas Hif2α may play a role in other adaptive mechanisms.
The PHD enzymes are the oxygen sensors that are inactivated under reduced oxygen tension [44]. Interestingly, PHD enzymes also rely on 2-oxoglutarate, a TCA cycle intermediary, and they can be inhibited by the accumulation of other TCA metabolites, such as succinate and fumarate, as well as the glycolytic metabolites, pyruvate and lactate. Thus, the function of PHD is linked to normal energy metabolism. D(R)-2-hydroxyglutarate, an oncometabolite that is produced by mutant isocitrate dehydrogenases (IDH1 and IDH2), can also inhibit PHD enzymes [73]. PHD enzymes can be inhibited by ROS, as well as by hydrogen sulfide. Inflammatory mediators, such as TNF-α and lipopolysaccharide, have been shown to activate Hif-mediated pathways, most likely, via their stimulation of cellular ROS production to inhibit PHD enzymes. Alternatively, cytokines and LPS may augment normoxic Hif1-signaling by other means [74]. In the context of the cardiac models discussed above, ROS were proposed to play a role in the stabilization of Hif1α. It is unclear whether Hif2α was also upregulated in these studies, as only Hif1α was analyzed, although other studies indicate that Hif2α is detectable in the ischemic myocardium, as well [75].
In the context of decreased oxygen tension, the mitochondrial transport chain becomes less efficient, resulting in increased leak of electrons to molecular oxygen to increase superoxide production. The production of ROS, early in hypoxia, appears to be essential to initiate the hypoxic reprograming of the cells [76]. Stabilization of Hif1α mediates a metabolic shift to increase anaerobic glycolysis, thereby decreasing the TCA cycle. In addition, other adaptive mechanisms suppress mitochondrial function. In particular, the hypoxamir miR-210 concurrently suppresses the expression of the iron sulfur-cluster scaffold proteins ISCU1/2 to decrease the activity of iron sulfur cluster-containing enzymes, such as complex I of the mitochondrial electron transport chain and aconitase of the TCA cycle [77]. The mitochondrial complexes II and III also contain iron sulfur-complex subunits that are essential for electron transport, and miR-210 targets other genes, such as NDUFA4, a complex I component, SDHD, a complex II subunit, and COX10, a complex IV component, to suppress mitochondrial electron flow. Nonetheless, loss of oxidative phosphorylation in hypoxia leads to an increase in NADH, and a reduction in the NAD+/NADH ratio in mitochondria and in the cytosol.
Recent work by us and others suggests additional adaptive mechanisms that may attenuate NADH accumulation during hypoxia [78, 79]. We found that hypoxic cells reduce 2-oxoglutarate to form the L(S)-enantimer of 2-hydroxyglutarate (L2HG) using NADH as the source of reducing equivalents. Malate dehydrogenases or lactate dehydrogenase may mediate this reaction, depending on the cell type. Additionally, accumulation of L2HG redirects cellular metabolism by inhibiting glycolysis and increasing the pentose phosphate pathway [79, 80]. The switch to the pentose phosphate pathway (PPP) would lower the rate of NADH production via glycolysis, and enhance the production of NADPH. Other studies in rat heart suggest that increased activity of an aspartate-pyruvate shuttle under hypoxia contributes to the increase in cytosolic NADPH from mitochondrial NADH [81]. In addition, mitochondria transfer reducing equivalents from NADH to NADPH via the nicotinamide nucleotide transhydrogenase (NNT), which is driven by the mitochondrial proton gradient towards reduction of NADP+ [82], although it is unclear whether the expression and activity of NNT are affected by hypoxia. The accumulation of reducing equivalents as NADPH can maintain GSH and Trx pools in their reduced state in order to preserve antioxidant enzyme function. Reoxygenation (or reperfusion of ischemic tissues) has been shown to lead to an increase in the production of superoxide and other ROS species, due, in part, to the activity of NADPH oxidases, xanthine oxidases, as well as the electron transport chain. Thus, the preservation of multiple reductant pools (GSH, Trx, NADPH) may lessen injury caused by increased oxygen production. In support of this concept, deficiency of G6PD, the first and rate limiting enzyme of the PPP, increases cardiac dysfunction following ischemia-reperfusion, and these hearts show decreased regeneration of GSH with increased levels of GSSG and decreased GSH/GSSG ratios compared to control hearts [83]. Furthermore, chronic models of heart failure and myocardial infarction in the G6PD deficient mouse also augmented redox stress and cardiac dysfunction [84].
In diabetes, the excess abundance of substrates in the heart leads to an accumulation of NADH and a decrease in the NAD+/NADH ratio, similar to changes found under hypoxic conditions [85]. This reductive state has been referred to as pseudo-hypoxia. In diabetes, the excess NADH has been associated with oxidative stress, due, in part, to increased mitochondrial dysfunction and the excess production of ROS [86]. Similar pseudo-hypoxic changes that involve reductive stress and deficiencies in mitochondrial homeostasis were described in aging, as well [87]. These studies point to alterations in mitochondrial dynamics and turn over that lead to increased ROS production.
Studies in diabetes and aging implicate decreased function of NAD+- dependent enzymes, such as the sirtuin lysine-deacetylases that regulate metabolic responses, in the pathogenic responses to lower NAD+ levels [85, 87]. Sirt1, in particular, is upregulated with calorie restriction, and mediates mitochondrial biogenesis by PGC1α-independent means. These functions are suppressed with decreased NAD+. In contrast, increased NADH augments the repressor function of carboxy-terminal binding protein (CtBP), a redox sensitive transcriptional corepressor [88]. Importantly, CtBP can antagonize Sirt1 activity by repressing its transcription [85], indicating that both a decrease in NAD+ and and an increase in NADH can potentially suppress Sirt1. Furthermore, CtBP can be activated under hypoxia, and was shown to suppress the expression of bone morphogenetic protein target genes [89]. Although only a handful of CtBP repression targets have been studied, these findings highlight the role of both NAD+ and NADH in regulating metabolism and cell signaling by the effects of these dinucleotides on transcriptional pathways.
Redox changes: the importance of the target
Many questions remain unanswered about the harmful and protective effects of ROS on cell function and the mechanisms by which a lack of oxidants or excess of reductants may mediate dysfunction. Clearly, not all oxidant generation is equal. Thus, although excess ROS generated by Nox4 appears to be protective against atherosclerosis, a deficiency in antioxidant enzymes, such as GPx1, Prx1, or Prx2, that target hydrogen and lipid peroxides augment atherosclerotic disease progression in the context of ApoE deficiency [90–93]. Furthermore, overexpression of antioxidants enzymes or use of ebselen, a selenium-containing antioxidant drug that mimics the ability of GPx1 to reduce lipid and hydrogen peroxides, decreases atherosclerosis in the context of the ApoE model of disease [92, 94–96]. Although a variety of Nox4 knockout models were used, the Nox4 overexpression model that showed atherosclerotic protection used a targeted endothelial-specific expression system. In contrast, all of the antioxidant enzyme knockout models cited are global knockouts, and some of their disease-causing effects may be due to enhanced activation of leukocytes as well as dysfunction of endothelial cells brought about by deficiency of an antioxidant enzyme [90, 97]. Nonetheless, deficiency of GPx1 has been shown to cause excess production of ROS and a pro-inflammatory phenotype in endothelial cells grown in culture [98, 99]. Thus, the question remains as to why oxidants generated by Nox4 overexpression do not cause the same detrimental effects on the endothelium as excess oxidants that are produced as a result of antioxidant enzyme deficiencies. Perhaps it is due, in part, to the differences in their subcellular locations, and, therefore, differences in localized compartmental effects of hydrogen peroxide. Furthermore, although Nox4 is considered to be constitutively active, it has been suggested that this enzyme may differentially accumulate in certain cellular compartments under various stress conditions providing targeted increases in ROS in specific subcellular spaces [100]. Thus, after glucose deprivation, Nox4 protein levels were specifically increased in the ER, but not in other compartments of cardiomyocytes [100]. Local increases in ROS in the ER may explain the activation of the ER stress-related PERK/eIF-2α/ATF-4 signaling pathway in glucose deficiency. In this context, upregulation of ATF-4 allowed for adaptation to decreased substrate availability by upregulating autophagy.
Recent studies highlight how rapidly stress can alter the redox state of cardiomyocytes and provide additional proof that redox changes may be localized to specific cellular compartments [101, 102]. In these studies, a biosensor consisting of a redox-sensitive green fluorescent protein 2 coupled to glutaredoxin (Grx1-roGFP2) was targeted to either mitochondrial or cytosolic compartments in a cardiac-specific manner in transgenic mice to allow for measurement of the glutathione redox potential. In isolated cardiomyocytes, known ROS-generators, isoprenaline and angiotensin II, caused a rapid (within minutes) increase in glutathione oxidation in the cytoplasm but caused a small reduction in the mitochondrial redox potential. Interestingly, exposure of cultured cells to hypoxia (1% or 0.1% oxygen) for 15 minutes caused a decrease in glutathione redox potential with a restoration to normal upon reoxygenation in both cytosolic and mitochondrial compartments; however, the changes in redox state were more rapid in the cytosolic compartment. In vivo 3, 7 and 14 days after myocardial infarction, the cytosolic glutathione redox state was not altered, whereas at 7 and 14 days, the mitochondrial glutathione redox state was more oxidized, suggesting that mitochondrial dysfunction (and ROS production) is contributing to the pathogenic remodeling following ischemic injury. Additional studies are necessary to determine the role that shifts in the redox state may play in disease pathogenesis and to clarify whether reductive stress leads to oxidative stress [102].
We have previously discussed the upregulation of Nrf2, which is promoted, in part, by redox-dependent modification of Keap1. This pathway responds to oxidants and then alters cellular redox state by activating an antioxidant response. Although over-oxidation of protein targets is detrimental, reductive stress also adversely affects many cellular responses by preventing requisite cysteine oxidation. Necessary structural disulfides are formed in the ER during protein synthesis. There also is a subset of free cysteine residues in proteins that have functional, redox-active thiols with low pKa, that exist as thiolates, and that are readily oxidized to sulfenic acid or disulfides in response to hydrogen peroxide [11]. Over oxidation of these sites to sulfinic or sulfonic acid may result in a loss of their redox activity and alter their cellular function. It is well recognized that ROS are necessary to promote growth factor mediated-signaling. Production of hydrogen peroxide following growth factor interaction with its cognate receptor reversibly oxidizes protein phosphatases at these redox-sensitive thiols in order to allow for the unopposed action of protein tyrosine kinases.
Mitochondrial production of ROS has been shown to be important to promote receptor-mediated cell signaling [103, 104], as has Nox4 as a source of ROS [105, 106]. Overexpression of antioxidant enzymes, such as catalase, Prx, or GPx1, eliminates necessary hydrogen peroxide to attenuate growth factor receptor-mediated signaling and cellular proliferation [103, 104]. Thus, lack of ROS can preserve the reduced state of redox-sensitive phosphatases and decrease protein kinase activation to attenuate growth factor receptor-mediated signaling. Experiments illustrating the role of Nox4-mediated hydrogen peroxide on insulin receptor signaling suggest that there may be thresholds for a necessary oxidant level to promote signaling. Thus, overexpression of a wildtype Nox4 did not augment insulin signaling; however, excess Nox4 could overcome the attenuated signaling responses of insulin that were caused by overexpression of a PTP1B phosphatase. In addition, overexpression of an inactive dominant negative form of Nox4 or its knockdown attenuated insulin-mediated signal transduction [105, 106], similar to the suppressive effects caused by overexpression of antioxidant enzymes. Consistent with cell culture experiments, overexpression of GPx1 in mice decreased the insulin-mediated activation of the insulin receptor and Akt, suggesting that a loss of intracellular ROS preserves phosphatase activity [107]. Furthermore, these attenuated signaling responses to insulin contributed to insulin resistance and obesity, producing a type 2 diabetes-like phenotype in mice overexpressing antioxidant enzymes [107, 108].
Many other protein redox targets have been described in other reviews [109, 110]; however, we will mention a few redox targets here because they affect vascular activity. Oxidant-induced formation of disulfide bonds between subunits of the protein kinase G can stimulate activity independent of cGMP [111]. In smooth muscle cells, protein kinase G mediates vasorelaxation in response to nitric-oxide mediated production of cGMP by the soluble guanlyl cyclease (sGC). Interestingly, ROS-mediated oxidation of aredox-active cysteinyl thiol residues in sGC, the enzyme that synthesizes cGMP, decreases sGC activity to disrupt smooth muscle cells responses to nitric oxide [112]. It is unclear, though, whether these opposing responses would occur under the same conditions, such that direct activation of protein kinase G can compensate for the oxidative inactivation of sGC; however, the ability to form disulfide bonds in protein kinase G was shown to be important in mediating endothelium-dependent vasodilation and blood pressure regulation in vivo [111]. In addition, the presence of disulfides at redox-sensitive sites in SERCA are important for vascular homeostasis. In particular, oxidant-mediated glutathionylation of SERCA at Cys-674 has been shown to increase its activity, and promote angiogenesis [113]. Taken together, these findings illustrate how some oxidative modifications to proteins can be beneficial while others can be harmful.
Our previous work highlighted the role of mitochondrial-produced ROS for the global formation of disulfide bonds in proteins [11]. Overexpression of antioxidant enzymes such as GPx1 and catalase suppress global disulfide bond formation; in the case of GPx1 overexpression, this effect was linked to reductions in mitochondrial activity with a suppression of cellular ATP production and mild uncoupling, which may contribute to the decreased cellular proliferation and survival in these cells [103]. Taken together, these findings illustrate how the loss of necessary oxidants can be detrimental to cells. Additional studies, however, detailing specific molecular targets that are affected by the loss of necessary oxidants are needed to clarify redox-sensitive targets that define reductive stress.
Two recent editorials consider whether the underlying effects of reductive stress occur via oxidative stress, stressing the importance of both in cardiac dysfunction, and highlighting the need for new approaches to understand better the role of redox homeostasis in disease pathogenesis [102, 114]. One of the most difficult questions to address is how an excess of reducing equivalents can lead to oxidative stress. Perhaps new approaches, including the use of biosensors, will help to resolve this conundrum. In the mitochondria, theoretically, more NADH can lead to more superoxide production via an increase in electron flow and, therefore, an increase in electron leak. This mechanism, however, does not explain how excess GSH and increased GSH/GSSG ratio can lead to excess ROS or the increased oxidation of redox-sensitive protein targets. It has been suggested that GSH may increase mitochondrial membrane potential by increasing redox recycling of ubiquinone or that it may increase reverse electron flow from succinate to NAD+ to augment mitochondrial ROS production [115]. Increased GSH/GSSG ratio may also increase NADH and NADPH to drive the electron transport chain or to increase ROS production by Nox enzymes. In fact, in the case of G6PD deficiency, a decrease in cellular NADPH was suggested to be protective under certain conditions due to decreased superoxide oxide generation [116]. Another unexpected source of ROS may come from the antioxidant enzymes, Gsr or Txnrd. It has been proposed that under reductive stress, NADPH could supply electrons to the FAD unit of Gsr or Txnrd and thence to oxygen to produce superoxide [117]. This mechanism of converting the antioxidant enzymes to oxidant generators would only take place when the natural electron acceptors, GSSG or oxidized Trx, are in limited supply; however, these examples emphasize the fundamental chemical fact that all redox reactions result in oxidation and reduction, depending on the specific redox couples involved and their relative redox potentials [114]. The effect on the cell depends on the final byproducts produced.
It is apparent that alterations to the cellular redox state is harmful. Although the antioxidant program regulated by Nrf2 may be protective against oxidative stress, pathogenic disorders that promote continual stimulation of the Nrf2 response cause reductive stress that contributes to disease progression. Furthermore, reductive stress can promote metabolic reprogramming in a multitude of ways by regulating nuclear factors such as the NAD+-dependent Sirt1 or the NADH-dependent CtBP to alter metabolic adaptations. Excess reducing equivalents can lead to a lack of necessary oxidants, ultimately suppressing responses that involve protein-thiol modification. In addition, lack of oxidants under certain stress conditions may also eliminate necessary adaptive responses, such as the stabilization of Hif1α to promote angiogenic and metabolic reprogramming. Reductive stress can lead to ROS generation, by driving mitochondria or other oxidant generators to utilize the abundance of reducing equivalents or by affecting protein folding and ER function, processes that also produce ROS. The challenge is to develop a better understanding of these processes in order to restore redox balance.
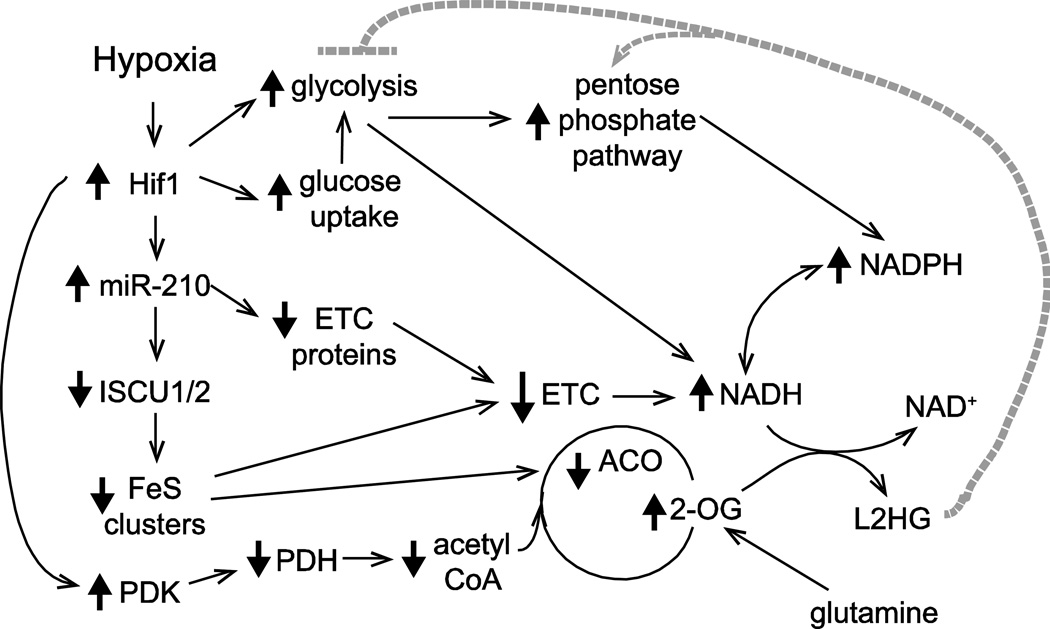
Figure 3.
Metabolic shifts in hypoxia: the role of Hif1 and miR-210 in reductive stress. The Hif1 nuclear factor mediates the metabolic response to hypoxia. Hif1 upregulates genes involved in glucose uptake and glycolysis and decreases the entry of pyruvate into the citric acid cycle by the upregulation of pyruvate dehydrogenase kinase, a suppressor of pyruvate dehydrogenase. The Hif-mediated upregulation of miR-210 reinforces the decrease carbon flux through the TCA cycle and the reduction of oxidative phosphorylation under limited oxygen. miR-210 decreases the expression of the iron sulfur-cluster assembly enzyme (ISCU1/2) to suppress the synthesis of iron-sulfur clusters (FeS), decreasing the activity of enzymes such as aconitase, a TCA cycle enzyme. FeS clusters are also important for electron transfer in subunits of complex I, complex II, and complex III. Furthermore, miR-210 directly targets genes involved in the electron transport chain (ETC) complexes, such as NDUFA4, a complex I component; SDHD, a complex II subunit; and COX10, a complex IV component. Decreased complex I activity leads to accumulation of NADH. In addition, multiple pathways augment the electron flux from NADH to NADPH. Under hypoxia, compensatory pathways (such as glutamine utilization) lead to an increase in 2-oxoglutarate and its subsequent reduction to L(S)-2-hydroxyglutarate (L2HG) by malate dehydrogenase and other enzymes. Experimental evidence suggests that L2HG inhibits glycolysis and that the pentose phosphate pathway (PPP) is upregulated in hypoxia.
Highlights.
Reductive stress and oxidative stress are harmful to biological systems.
Reductive stress plays a role in protein aggregation-induced cardiomyopathies.
Oxidant generators, such as Nox4, are essential for cardiovascular homeostasis.
Oxidants mediate adaptive responses, including Nrf2, eNOS and Hif1α activation.
Excess reducing equivalents may paradoxically cause oxidative stress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation research. 2010;106(7):1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(4):653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. The Journal of biological chemistry. 1999;274(20):13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 4.Flohe L, Loschen G, Gunzler WA. E. Eichele. Glutathione peroxidase, V. The kinetic mechanism, Hoppe-Seyler’s Zeitschrift fur physiologische Chemie. 1972;353(6):987–999. doi: 10.1515/bchm2.1972.353.1.987. [DOI] [PubMed] [Google Scholar]
- 5.Pannala VR, Bazil JN, Camara AK, Dash RK. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free radical biology & medicine. 2013;65:1385–1397. doi: 10.1016/j.freeradbiomed.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free radical biology & medicine. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Kalinina EV, Chernov NN, Novichkova MD. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry. Biokhimiia. 2014;79(13):1562–1583. doi: 10.1134/S0006297914130082. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Kim SM, Lee RT. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxidants & redox signaling. 2013;18(10):1165–1207. doi: 10.1089/ars.2011.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free radical biology & medicine. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 10.Oka S, Hsu CP, Sadoshima J. Regulation of cell survival and death by pyridine nucleotides. Circulation research. 2012;111(5):611–627. doi: 10.1161/CIRCRESAHA.111.247932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Song Y, Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. The Journal of biological chemistry. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. The Journal of biological chemistry. 2003;278(24):21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 14.Jessop CE, Bulleid NJ. Glutathione directly reduces an oxidoreductase in the endoplasmic reticulum of mammalian cells. The Journal of biological chemistry. 2004;279(53):55341–55347. doi: 10.1074/jbc.M411409200. [DOI] [PubMed] [Google Scholar]
- 15.Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends in endocrinology and metabolism: TEM. 2012;23(9):420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalakas MC, Park KY, Semino-Mora C, Lee HS, Sivakumar K, Goldfarb LG. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. The New England journal of medicine. 2000;342(11):770–780. doi: 10.1056/NEJM200003163421104. [DOI] [PubMed] [Google Scholar]
- 17.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nature genetics. 1998;20(1):92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 18.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, Zhang XQ, Stevenson TJ, Peshock RM, Leopold JA, Barry WH, Loscalzo J, Odelberg SJ, Benjamin IJ. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130(3):427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G–alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circulation research. 2001;89(1):84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. American journal of physiology. Heart and circulatory physiology. 2006;291(2):H638–H647. doi: 10.1152/ajpheart.00144.2006. [DOI] [PubMed] [Google Scholar]
- 21.Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. Journal of cellular physiology. 2000;183(1):100–107. doi: 10.1002/(SICI)1097-4652(200004)183:1<100::AID-JCP12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxidants & redox signaling. 2005;7(3–4):414–422. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Min X, Li C, Benjamin IJ, Qian B, Zhang X, Ding Z, Gao X, Yao Y, Ma Y, Cheng Y, Liu L. Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension. 2010;55(6):1412–1417. doi: 10.1161/HYPERTENSIONAHA.109.147066. [DOI] [PubMed] [Google Scholar]
- 24.Shi R, Huang CC, Aronstam RS, Ercal N, Martin A, Huang YW. N-acetylcysteine amide decreases oxidative stress but not cell death induced by doxorubicin in H9c2 cardiomyocytes. BMC pharmacology. 2009;9:7. doi: 10.1186/1471-2210-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Y, Liu X, Lee CP, Chua BH, Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free radical biology & medicine. 2006;41(1):46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Limphong P, Pieper J, Liu Q, Rodesch CK, Christians E, Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(4):1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh F, Charles AL, Schlagowski AI, Bouitbir J, Bonifacio A, Piquard F, Krahenbuhl S, Geny B, Zoll J. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochimica et biophysica acta. 2015;1853(7):1574–1585. doi: 10.1016/j.bbamcr.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Kannan S, Muthusamy VR, Whitehead KJ, Wang L, Gomes AV, Litwin SE, Kensler TW, Abel ED, Hoidal JR, Rajasekaran NS. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovascular research. 2013;100(1):63–73. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, Zweier JL, Benjamin IJ. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxidants & redox signaling. 2011;14(6):957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dialynas G, Shrestha OK, Ponce JM, Zwerger M, Thiemann DA, Young GH, Moore SA, Yu L, Lammerding J, Wallrath LL. Myopathic lamin mutations cause reductive stress and activate the nrf2/keap-1 pathway. PLoS genetics. 2015;11(5):e1005231. doi: 10.1371/journal.pgen.1005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Li S, Wang H, Li B, Shao L, Lai Y, Horvath G, Wang Q, Yamamoto M, Janicki JS, Wang XL, Tang D, Cui T. Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins. Journal of molecular and cellular cardiology. 2014;72:305–315. doi: 10.1016/j.yjmcc.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. The Journal of cell biology. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eletto D, Chevet E, Argon YC. Appenzeller-Herzog, Redox controls UPR to control redox. Journal of cell science. 2014;(Pt 17):3649–3658. doi: 10.1242/jcs.153643. [DOI] [PubMed] [Google Scholar]
- 36.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and cellular biology. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao Q, Sanbe A, Zhang X, Liu JP, Minamisawa S. alphaB-Crystallin R120G variant causes cardiac arrhythmias and alterations in the expression of Ca(2+) -handling proteins and endoplasmic reticulum stress in mice. Clinical and experimental pharmacology & physiology. 2014;41(8):589–599. doi: 10.1111/1440-1681.12253. [DOI] [PubMed] [Google Scholar]
- 38.Matsushima S, Kuroda J, Zhai P, Liu T, Ikeda S, Nagarajan N, Oka S, Yokota T, Kinugawa S, Hsu CP, Li H, Tsutsui H, Sadoshima J. Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. The Journal of clinical investigation. 2016;126(9):3403–3416. doi: 10.1172/JCI85624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18121–1816. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circulation research. 2003;93(9):802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 41.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51(2):319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 42.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circulation research. 2007;100(6):894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 43.Brewer AC, Murray TV, Arno M, Zhang M, Anilkumar NP, Mann GE, Shah AM. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free radical biology & medicine. 2011;51(1):205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Science’s STKE : signal transduction knowledge environment. 2007;2007(407):cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(35):15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circulation research. 2013;112(8):1135–1149. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Q, Wang M, Petucci C, Gardell SJ, Han X. Rotenone induces reductive stress and triacylglycerol deposition in C2C12 cells. The international journal of biochemistry & cell biology. 2013;45(12):2749–2755. doi: 10.1016/j.biocel.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 2010;121(3):426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. Journal of the American Heart Association. 2014;3(1):e000555. doi: 10.1161/JAHA.113.000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Touyz RM, Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circulation research. 2012;110(9):1159–1161. doi: 10.1161/CIRCRESAHA.112.269068. [DOI] [PubMed] [Google Scholar]
- 52.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(6):1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 53.Ali ZA, de Jesus Perez V, Yuan K, Orcholski M, Pan S, Qi W, Chopra G, Adams C, Kojima Y, Leeper NJ, Qu X, Zaleta-Rivera K, Kato K, Yamada Y, Oguri M, Kuchinsky A, Hazen SL, Jukema JW, Ganesh SK, Nabel EG, Channon K, Leon MB, Charest A, Quertermous T, Ashley EA. Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. The Journal of clinical investigation. 2014;124(12):5159–5174. doi: 10.1172/JCI77484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovascular research. 2007;73(1):73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Oltman CL, Kane NL, Miller FJ, Jr, Spector AA, Weintraub NL, Dellsperger KC. Reactive oxygen species mediate arachidonic acid-induced dilation in porcine coronary microvessels. American journal of physiology. Heart and circulatory physiology. 2003;285(6):H2309–H2315. doi: 10.1152/ajpheart.00456.2003. [DOI] [PubMed] [Google Scholar]
- 56.Modrick ML, Didion SP, Lynch CM, Dayal S, Lentz SR, Faraci FM. Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(6):1130–1137. doi: 10.1038/jcbfm.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langbein H, Brunssen C, Hofmann A, Cimalla P, Brux M, Bornstein SR, Deussen A, Koch E, Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. European heart journal. 2016;37(22):1753–1761. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circulation research. 2012;110(9):1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 59.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF., Jr NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124(6):731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L, Xiao J, Kuroda J, Ago T, Sadoshima J, Cohen RA, Tong X. Both hydrogen peroxide and transforming growth factor beta 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines. Biochimica et biophysica acta. 2014;1842(12 Pt A):2489–2499. doi: 10.1016/j.bbadis.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Ichihara S, Yamada Y, Liu F, Murohara T, Itoh K, Yamamoto M, Ichihara G. Ablation of the transcription factor Nrf2 promotes ischemia-induced neovascularization by enhancing the inflammatory response. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(8):1553–1561. doi: 10.1161/ATVBAHA.110.204123. [DOI] [PubMed] [Google Scholar]
- 62.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. American journal of physiology. Cell physiology. 2009;296(3):C422–C432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee DY, Wauquier F, Eid AA, Roman LJ, Ghosh-Choudhury G, Khazim K, Block K, Gorin Y. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. The Journal of biological chemistry. 2013;288(40):28668–28686. doi: 10.1074/jbc.M113.470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Bartolo BA, Cartland SP, Prado-Lourenco L, Griffith TS, Gentile C, Ravindran J, Azahri NS, Thai T, Yeung AW, Thomas SR, Kavurma MM. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Promotes Angiogenesis and Ischemia-Induced Neovascularization Via NADPH Oxidase 4 (NOX4) and Nitric Oxide-Dependent Mechanisms. Journal of the American Heart Association. 2015;4(11) doi: 10.1161/JAHA.115.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Hong Z, Zeng C, Yu Q, Wang H. NADPH oxidase 4 promotes cardiac microvascular angiogenesis after hypoxia/reoxygenation in vitro. Free radical biology & medicine. 2014;69:278–288. doi: 10.1016/j.freeradbiomed.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Wang JJ, Zhang SX. NADPH oxidase 4-derived H2O2 promotes aberrant retinal neovascularization via activation of VEGF receptor 2 pathway in oxygen-induced retinopathy. Journal of diabetes research. 2015;2015:963289. doi: 10.1155/2015/963289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. American journal of respiratory cell and molecular biology. 2012;47(5):718–726. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxidants & redox signaling. 2013;18(14):1765–1776. doi: 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, Sugamura K, Fitzgibbons T, Keaney JF., Jr Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free radical biology & medicine. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, Calkin AC, Biessen EA, Touyz RM, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Reactive Oxygen Species Can Provide Atheroprotection via NOX4-Dependent Inhibition of Inflammation and Vascular Remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2016;36(2):295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 71.Schurmann C, Rezende F, Kruse C, Yasar Y, Lowe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, Schroder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. European heart journal. 2015;36(48):3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circulation research. 2006;98(2):254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell death and differentiation. 2008;15(4):635–641. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- 74.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M, Philipp S, Griethe W, Maxwell PH, Frei U, Bachmann S, Willenbrock R, Eckardt KU. Persistent induction of HIF-1alpha and −2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(12):1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 76.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell metabolism. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell metabolism. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, Thompson CB. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell metabolism. 2015;22(2):304–311. doi: 10.1016/j.cmet.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell metabolism. 2015;22(2):291–303. doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu L, Shah S, Fan J, Park JO, Wellen KE, Rabinowitz JD. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nature chemical biology. 2016;12(5):345–352. doi: 10.1038/nchembio.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peuhkurinen KJ, Takala TE, Nuutinen EM, Hassinen IE. Tricarboxylic acid cycle metabolites during ischemia in isolated perfused rat heart. The American journal of physiology. 1983;244(2):H281–H288. doi: 10.1152/ajpheart.1983.244.2.H281. [DOI] [PubMed] [Google Scholar]
- 82.Ronchi JA, Francisco A, Passos LA, Figueira TR, Castilho RF. The Contribution of Nicotinamide Nucleotide Transhydrogenase to Peroxide Detoxification Is Dependent on the Respiratory State and Counterbalanced by Other Sources of NADPH in Liver Mitochondria. The Journal of biological chemistry. 2016;291(38):20173–20187. doi: 10.1074/jbc.M116.730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109(7):898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 84.Hecker PA, Lionetti V, Ribeiro RF, Jr, Rastogi S, Brown BH, O’Connell KA, Cox JW, Shekar KC, Gamble DM, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circulation. Heart failure. 2013;6(1):118–126. doi: 10.1161/CIRCHEARTFAILURE.112.969576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxidants & redox signaling. 2007;9(7):931–942. doi: 10.1089/ars.2007.1630. [DOI] [PubMed] [Google Scholar]
- 86.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicology and applied pharmacology. 2007;225(2):214–220. doi: 10.1016/j.taap.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jack BH, Pearson RC, Crossley M. C-terminal binding protein: A metabolic sensor implicated in regulating adipogenesis. The international journal of biochemistry & cell biology. 2011;43(5):693–696. doi: 10.1016/j.biocel.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 89.Wu X, Chang MS, Mitsialis SA, Kourembanas S. Hypoxia regulates bone morphogenetic protein signaling through C-terminal-binding protein 1. Circulation research. 2006;99(3):240–247. doi: 10.1161/01.RES.0000237021.65103.24. [DOI] [PubMed] [Google Scholar]
- 90.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(4):850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 91.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115(16):2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 92.Kisucka J, Chauhan AK, Patten IS, Yesilaltay A, Neumann C, Van Etten RA, Krieger M, Wagner DD. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circulation research. 2008;103(6):598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park JG, Yoo JY, Jeong SJ, Choi JH, Lee MR, Lee MN, Hwa Lee J, Kim HC, Jo H, Yu DY, Kang SW, Rhee SG, Lee MH, Oh GT. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circulation research. 2011;109(7):739–749. doi: 10.1161/CIRCRESAHA.111.245530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chew P, Yuen DY, Stefanovic N, Pete J, Coughlan MT, Jandeleit-Dahm KA, Thomas MC, Rosenfeldt F, Cooper ME, de Haan JB. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes. 2010;59(12):3198–3207. doi: 10.2337/db10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Z, Ran Q, Roberts LJ, 2nd, Zhou L, Richardson A, Sharan C, Wu D, Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free radical biology & medicine. 2008;44(3):343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo X, Yamada S, Tanimoto A, Ding Y, Wang KY, Shimajiri S, Murata Y, Kimura S, Tasaki T, Nabeshima A, Watanabe T, Kohno K, Sasaguri Y. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxidants & redox signaling. 2012;17(10):1362–1375. doi: 10.1089/ars.2012.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng F, Torzewski M, Degreif A, Rossmann H, Canisius A, Lackner KJ. Impact of glutathione peroxidase-1 deficiency on macrophage foam cell formation and proliferation: implications for atherogenesis. PloS one. 2013;8(8):e72063. doi: 10.1371/journal.pone.0072063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lubos E, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, Handy DE. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. The Journal of biological chemistry. 2011;286(41):35407–35417. doi: 10.1074/jbc.M110.205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner AH, Kautz O, Fricke K, Zerr-Fouineau M, Demicheva E, Guldenzoph B, Bermejo JL, Korff T, Hecker M. Upregulation of glutathione peroxidase offsets stretch-induced proatherogenic gene expression in human endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(11):1894–1901. doi: 10.1161/ATVBAHA.109.194738. [DOI] [PubMed] [Google Scholar]
- 100.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circulation research. 2013;113(11):1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swain L, Kesemeyer A, Meyer-Roxlau S, Vettel C, Zieseniss A, Guntsch A, Jatho A, Becker A, Nanadikar MS, Morgan B, Dennerlein S, Shah AM, El-Armouche A, Nikolaev VO, Katschinski DM. Redox Imaging Using Cardiac Myocyte-Specific Transgenic Biosensor Mice. Circulation research. 2016;119(9):1004–1016. doi: 10.1161/CIRCRESAHA.116.309551. [DOI] [PubMed] [Google Scholar]
- 102.Touyz RM, Anagnostopoulou A, De Lucca Camargo L, Montezano AC. Novel Biosensors Reveal a Shift in the Redox Paradigm From Oxidative to Reductive Stress in Heart Disease. Circulation research. 2016;119(9):969–971. doi: 10.1161/CIRCRESAHA.116.309854. [DOI] [PubMed] [Google Scholar]
- 103.Handy DE, Lubos E, Yang Y, Galbraith JD, Kelly N, Zhang YY, Leopold JA, Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. The Journal of biological chemistry. 2009;284(18):11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling. 2011;15(7):1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxidants & redox signaling. 2005;7(7–8):1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Molecular and cellular biology. 2004;24(5):1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(24):8852–8827. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxidants & redox signaling. 2011;14(12):2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circulation research. 2012;111(8):1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 110.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nature medicine. 2012;18(2):286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. The Journal of biological chemistry. 2009;284(12):7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson MD, Mei Y, Weisbrod RM, Silver M, Shukla PC, Bolotina VM, Cohen RA, Tong X. Glutathione adducts on sarcoplasmic/endoplasmic reticulum Ca2+ ATPase Cys-674 regulate endothelial cell calcium stores and angiogenic function as well as promote ischemic blood flow recovery. The Journal of biological chemistry. 2014;289(29):19907–19916. doi: 10.1074/jbc.M114.554451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loscalzo J. Adaptions to Hypoxia and Redox Stress: Essential Concepts Confounded by Misleading Terminology. Circulation research. 2016;119(4):511–513. doi: 10.1161/CIRCRESAHA.116.309394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen D, Dalton TP, Nebert DW, Shertzer HG. Glutathione redox state regulates mitochondrial reactive oxygen production. The Journal of biological chemistry. 2005;280(27):25305–25312. doi: 10.1074/jbc.M500095200. [DOI] [PubMed] [Google Scholar]
- 116.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. Journal of molecular and cellular cardiology. 2006;41(2):340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 117.Korge P, Calmettes G, Weiss JN. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochimica et biophysica acta. 2015;1847(6–7):514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]