Abstract
Background
While tacrolimus is the basis of most maintenance immunosuppression regimens for kidney transplantation, concerns about toxicity have made alternative agents, such as belatacept, attractive to clinicians. However, limited data exists to directly compare outcomes with belatacept-based regimens to tacrolimus.
Methods
We performed a propensity score matched cohort study of adult kidney transplant recipients transplanted between May 1, 2001 and December 31, 2015 using national transplant registry data to compare patient and allograft survival in patients discharged from their index hospitalization on belatacept versus tacrolimus-based regimens.
Results
In the primary analysis, we found that belatacept was not associated with a statistically significant difference in risk of patient death (HR 0.84, 95% CI 0.61-1.15, p=0.28) or allograft loss (HR 0.83, 95% CI 0.62-1.11, p=0.20) despite an increased risk of acute rejection in the first year posttransplant (OR 3.12, 95% CI 2.13-4.57, p<0.001). These findings were confirmed in additional sensitivity analyses that accounted for use of belatacept in combination with tacrolimus, transplant center effects, and differing approaches to matching.
Conclusions
Belatacept appears to have similar longitudinal risk of mortality and allograft failure compared to tacrolimus-based regimens. These data are encouraging but require confirmation in prospective randomized controlled trials.
Introduction
Calcineurin inhibitors (CNIs) are the backbone of contemporary immunosuppressive regimens for renal transplantation, however these medications have many undesirable secondary effects including hypertension (1), posttransplant diabetes (2), hyperlipidemia (3), neurotoxicity (4) and nephrotoxicity (4). Despite the improvements in acute rejection rates and short-term graft survival afforded by the use of CNIs, long term allograft survival has stagnated (5), and late graft losses are often attributed to recipient death from comorbid conditions with a functioning kidney (6). It has been posited that development of an alternative to maintenance CNI-based immunosuppression is the key to improving long term allograft survival.
Belatacept is a costimulation blocker, targeting the binding of CD80/86 with CD28 on T cells that is required for T cell activation. Belatacept was approved for use in renal transplant recipients in the United States in 2011 after 2 randomized, multicenter noninferiority trials compared belatacept-based regimens to cyclosporine with basiliximab induction and adjuvant mycophenolate mofetil and prednisone (7, 8). A recently published study (9) reported outcomes for 316 belatacept and 131 cyclosporine treated patients who achieved 84 months of follow up and demonstrated superior patient and allograft survival compared to cyclosporine (HR 0.57, p=0.02). Glomerular filtration rate was higher in the belatacept-treated patients, and rates of de novo donor specific antibody development were lower, despite an increase in acute rejection observed with belatacept use.
The main criticism of trials employing belatacept-based immunosuppression has been the comparison with cyclosporine. Tacrolimus, approved for use in renal transplant recipients since 1997, has overtaken cyclosporine as the standard for modern immunosuppressive regimens; more than 85% of renal transplant recipients are discharged from their index hospitalization on a tacrolimus based regimen (10). Only 2 small studies have directly compared maintenance immunosuppression with belatacept to tacrolimus, both of which were limited to 1 year of posttransplant follow up (11, 12). We undertook this study to compare the effectiveness of belatacept versus tacrolimus with regard to acute rejection, long-term allograft survival, and long-term patient survival, using a propensity score matched cohort.
Methods
Study Design
We performed a retrospective cohort analysis using national registry data collected by the United Network for Organ Sharing (UNOS); this study is based on Organ Procurement and Transplantation Network (OPTN) data as of March 4, 2016. The database includes information on all transplant recipients and donors in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The study met eligibility criteria for institutional review board exemption authorized by 45 CFR §46.101, category 4, as confirmed by the Institutional Review Board at the University of Pennsylvania (protocol # 824575).
Subjects
We included patients who were transplanted between May 1, 2001 (the first recorded use of belatacept in the UNOS dataset) and December 31, 2015. Patient follow up was through March 4, 2016. The cohort was restricted to adult recipients (≥ age 18 years), receiving their first kidney transplant; recipients of multi-organ transplants were excluded.
Exposures and Outcome Measures
The primary exposure was defined as treatment with “only belatacept” versus “only tacrolimus” in conjunction with an anti-metabolite and with/without steroids as the maintenance immunosuppression regimen recorded at discharge from the index hospitalization for renal transplantation. Secondary analyses were performed where patients discharged on both belatacept and tacrolimus at their index hospitalization were assigned to the belatacept exposure group (“all belatacept”). The primary outcome was all-cause mortality. The secondary outcomes examined were all-cause allograft failure and treated rejection at 1 year. These outcomes were determined based on mortality and allograft loss data provided in the UNOS dataset. The Social Security Master Death File (provided in dataset by UNOS) was used to corroborate the mortality data.
Covariates
Covariates used for matching were selected a priori that were known to be risk factors for mortality or allograft loss based on clinical judgment and previously published literature. As recipients of both living and deceased donor kidneys were included in the analyses, all components of the kidney donor profile index (KDPI) (13) were incorporated into the models as individual covariates, with the exception of donor cause of death, rather than using KDPI directly. Donor-associated covariates included donor type (living or deceased), age, race height, weight, diabetes mellitus status, hypertension, hepatitis C virus status, and cold ischemia time (13). Recipient-associated covariates included age, ethnicity, gender, dialysis vintage time, diabetes mellitus status, and percent panel reactive antibody (14-16). Transplant-related covariates included induction immunosuppression (characterized as lymphodepleting, nonlymphodepleting [basiliximab and daclizumab], steroids, or none), and maintenance immunosuppression (mycophenolate mofetil versus azathioprine and steroids versus “steroid free”). Sensitivity analyses were performed in which patients were matched on transplant center. We also performed sensitivity analyses restricting the cohort to only include patients transplanted at centers that took part in the BENEFIT trials (“Belatacept study centers only” in Tables 3 and 4) (7-9). All covariates included in the final models had < 5% missingness.
Table 3.
Cox regression models for patient death.
| Matching* | Number treated | Centers | Cohort | HR | P value | 95% CI |
|---|---|---|---|---|---|---|
| 1:5 | 657 | All | Tacrolimus only/belatacept only | 0.84 | 0.28 | 0.61-1.15 |
| 1:5 | 1,192 | All | Tacrolimus only/all belatacept | 0.90 | 0.45 | 0.70-1.17 |
| 1:5 | 214 | Belatacept study centers only | Tacrolimus only/belatacept only | 0.97 | 0.91 | 0.52-1.79 |
| 1:5 | 703 | Belatacept study centers only | Tacrolimus only/all belatacept | 0.94 | 0.72 | 0.65-1.35 |
| 1:5 | 648 | Matched on center | Tacrolimus only/belatacept only | 0.85 | 0.33 | 0.61-1.18 |
| 1:5 | 1,182 | Matched on center | Tacrolimus only/all belatacept | 0.88 | 0.35 | 0.68-1.14 |
Matched on recipient age, recipient ethnicity, male sex, dialysis vintage, recipient diabetes, donor age, donor height, donor weight, donor race, donor hypertension, donor diabetes, donor HCV, deceased donor, and induction.
Table 4.
Cox regression models for allograft loss and logistic regression models for acute rejection.
| Matching* | Outcome | Number treated | Centers | Cohort | HR | OR | P value | 95% CI |
|---|---|---|---|---|---|---|---|---|
| 1:5 | GS | 602 | All | Tacrolimus only/belatacept only | 0.83 | - | 0.20 | 0.62-1.11 |
| 1:5 | GS | 1,128 | All | Tacrolimus only/all belatacept | 0.87 | - | 0.26 | 0.69-1.10 |
| 1:5 | AR | 602 | All | Tacrolimus only/belatacept only | - | 3.12 | <0.001 | 2.13-4.57 |
| 1:5 | AR | 1,128 | All | Tacrolimus only/all belatacept | - | 2.56 | <0.001 | 1.93-3.41 |
| 1:5 | GS | 197 | Belatacept study centers only | Tacrolimus only/belatacept only | 0.77 | - | 0.31 | 0.46-1.28 |
| 1:5 | GS | 683 | Belatacept study centers only | Tacrolimus only/all belatacept | 0.85 | - | 0.32 | 0.61-1.17 |
| 1:5 | AR | 197 | Belatacept study centers only | Tacrolimus only/belatacept only | - | 2.74 | 0.001 | 1.49-5.04 |
| 1:5 | AR | 683 | Belatacept study centers only | Tacrolimus only/all belatacept | - | 2.09 | <0.001 | 1.46-2.98 |
| 1:5 | GS | 597 | Matched on center | Tacrolimus only/belatacept only | 0.94 | - | 0.66 | 0.69-1.26 |
| 1:5 | GS | 1,124 | Matched on center | Tacrolimus only/all belatacept | 0.98 | - | 0.88 | 0.78-1.24 |
| 1:5 | AR | 597 | Matched on center | Tacrolimus only/belatacept only | - | 2.68 | <0.001 | 1.87-3.85 |
| 1:5 | AR | 1,124 | Matched on center | Tacrolimus only/all belatacept | - | 2.21 | <0.001 | 1.69-2.89 |
Matched on recipient age, recipient ethnicity, male sex, dialysis vintage, recipient diabetes, donor age, donor height, donor weight, donor race, donor hypertension, donor diabetes, donor HCV, deceased donor, induction, cold ischemia time and PRA.
GS: graft survival; AR: acute rejection
Statistical Analysis
Statistical analyses were performed using STATA version 14.0 (Statacorp LP, College Station, TX) with 2-sided hypothesis testing and p-value of < 0.05 as the criteria for statistical significance. Descriptive statistics were used to describe baseline donor and recipient clinical and demographic characteristics comparing patients exposed to belatacept versus tacrolimus. Continuous variables were compared using Student's t-test, or ranksum test for nonnormally distributed variables. Categorical and binary variables were compared using chi-square test.
We used propensity score matching to balance important baseline characteristics between the treatment groups. We generated the propensity scores using logistic regression with key covariates that were determined a priori (see Covariates, described above, and Tables 2, 3 and Supplemental Tables 2, 3 for the list of covariates for each model) (17). We applied a nearest neighbor matching algorithm using a caliper of 0.01 with common support and replacement to create 1:5 matches between the belatacept and tacrolimus exposure groups (18). Sensitivity analyses were performed employing 1:1 matching, 1:10 matching, and unmatched models of the overall cohort adjusted by propensity score weight to assess for differences in bias, variance, and the outcome measures (see Supplemental Tables 4). We assessed for balance between the matched cohorts using t-testing for equality of the means in the 2 groups, standardized percentage bias between the 2 groups, the variance ratio between the 2 groups (for continuous covariates) (19), and visual examination of histograms of propensity scores between the 2 exposure groups (Supplemental Figure 1). After performing the propensity score matching, Cox proportional hazards regression was used to estimate HRs and 95% CIs for mortality and all-cause allograft failure. Robust sandwich estimation of the variance of the regression coefficient was used to account for clustering within the matched groups (20, 21). The proportional hazards assumption was assessed via weighted versions of Kaplan-Meier curves using log-log plots as well as statistical testing and graphical displays based on the Schoenfeld and scaled Schoenfeld residuals (22). Logistic regression was used to estimate the odds of treated rejection at 1 year based on treatment.
Results
Cohort assembly
After applying the exclusion criteria, we identified 160,398 adult, first kidney only transplant recipients discharged on either belatacept or tacrolimus-based immunosuppression from their index hospitalization, Figure 1. Of these, 158,864 were discharged on tacrolimus, 901 were discharged on belatacept-based immunosuppression, and 633 were discharged on both tacrolimus and belatacept. We identified 88 distinct transplant centers who reported at least 1 patient discharged on a belatacept maintenance regimen. Among the 1534 patients who ever received belatacept, 700 (46%) of these patients were transplanted at 1 center, with the next most frequently prescribing transplant center reporting data on 112 patients.
Figure 1.
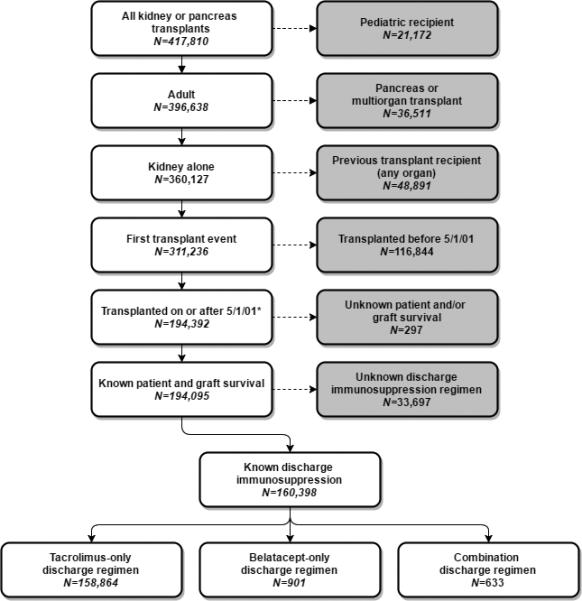
Creation of the patient cohort. We identified 158,864 patients in the tacrolimus cohort, 901 patients in the “belatacept only” cohort and 633 patients discharged on both belatacept and tacrolimus.
When we compared the clinical and demographic characteristics of the “belatacept only” patients (n=901) with the tacrolimus patients (n=158,864), we observed significant differences (Table 1). Patients discharged on belatacept were older (p<0.001), more often male (p=0.04) and were less sensitized (median PRA 0%, IQR 0-2 v. 0%, IQR 0-10 p<0.001; PRA>30% 9% versus 15%, p<0.001). Belatacept patients were less likely to have received a transplant from a deceased donor (55% v 62%, p<0.001) and were less likely to have received a lymphodepleting induction agent than patients on tacrolimus (55% v. 59%, p < 0.001). Clinical and demographic characteristics of all patients discharged on belatacept (n=1534) compared to all patients discharged on tacrolimus were similar and are presented in Supplemental Table 1.
Table 1.
Clinical and demographic characteristics of the “belatacept only” and “tacrolimus-only cohorts”.
| Belatacept only N=901 | Tacrolimus only N=158,864 | P value | |
|---|---|---|---|
| Median Age at Transplant (IQR) | 55 (44-64) | 53 (42-62) | <0.001 |
| Male | 605 (67%) | 96,134 (61%) | <0.001 |
| Race | <0.001 | ||
| Caucasian | 535 (59%) | 81,052 (51%) | |
| African American | 213 (24%) | 41,819 (26%) | |
| Latino | 82 (9%) | 23,997 (15%) | |
| Asian | 61 (7%) | 8.944 (6%) | |
| Other | 10 (1%) | 3,052 (2%) | |
| Cause of ESRD | 0.001 | ||
| Diabetes | 279 (31%) | 42,962 (27%) | |
| Hypertension | 200 (22%) | 38,641 (24%) | |
| Glomerular disease | 164 (18%) | 29,521 (19%) | |
| Cystic disease | 105 (12%) | 16,532 (10%) | |
| Other | 123 (14%) | 21,170 (13%) | |
| Missing | 30 (3%) | 10,038 (6%) | |
| Median years on dialysis (IQR) | 2.9 (1.4-5.0) | 2.9 (1.3-5.2) | 0.92 |
| Pretransplant diabetes | 323 (36%) | 52,813 (33%) | 0.09 |
| HCV+ | 9 (1%) | 5,386 (3%) | <0.001 |
| HIV+ | 1 (<1%) | 778 (<1%) | 0.10 |
| Median PRA (IQR) | 0 (0-2) | 0 (0-10) | <0.001 |
| PRA > 30% | 78 (9%) | 24,188 (15%) | <0.001 |
| Deceased donor | 498 (55%) | 98,305 (62%) | <0.001 |
| Expanded criteria donor | 128 (14%) | 17,222 (11%) | 0.001 |
| Median donor age | 45 (32-54) | 41 (28-51) | <0.001 |
| Median donor weight, kg (IQR) | 78 (67-91) | 77 (66-90) | 0.27 |
| Median donor height, cm (IQR) | 170 (163-178) | 170 (163-178) | 0.92 |
| African American donor | 106 (12%) | 20,973 (13%) | 0.20 |
| Donor hypertension | 182 (20%) | 28,551 (18%) | 0.08 |
| Donor diabetes | 36 (4%) | 6,999 (4%) | 0.55 |
| Donor HCV+ | 2 (<1%) | 2,574 (2%) | 0.001 |
| Median cold ischemia time, hours (IQR) | 11 (1-19) | 12 (2-20) | <0.001 |
| Induction | <0.001 | ||
| Lymphodepleting | 498 (55%) | 94,345 (59%) | |
| Non-lymphodepleting | 338 (38%) | 35,802 (23%) | |
| Steroids only | 23 (2%) | 10,310 (6%) | |
| None | 42 (5%) | 18,407 (12%) | |
Propensity score matching
We then assembled a propensity score-matched cohort in order to compare the 158,864 tacrolimus patients to the 901 belatacept patients, using 5:1 matching. There were no statistically significant differences noted between the 2 cohorts in any of the matching variables (Tables 2a and 2b).
Table 2a.
Balance table after propensity score matching (1:5) for patient death.
| Belatacept only N=657 | Tacrolimus only N=3,210 | % Bias | T-test P value | |
|---|---|---|---|---|
| Mean Age at Transplant | 54 | 54 | −0.7 | 0.89 |
| Male | 70% | 71% | −0.8 | 0.88 |
| Race | ||||
| Caucasian | 53% | 54% | NR | NR |
| African American | 28% | 28% | −0.7 | 0.90 |
| Latino | 10% | 9% | 2.1 | 0.68 |
| Asian | 8% | 7% | 1.1 | 0.85 |
| Other | 1% | 2% | −2.7 | 0.62 |
| Mean years on dialysis | 3.7 | 3.7 | 1.3 | 0.82 |
| Pretransplant diabetes | 39% | 39% | 0.5 | 0.93 |
| Deceased donor | 65% | 64% | 1.4 | 0.79 |
| Mean donor age | 43 | 43 | −1.4 | 0.80 |
| Mean donor weight, kg | 80 | 80 | −0.7 | 0.91 |
| Mean donor height, cm | 169 | 169 | −1.0 | 0.85 |
| African American Donor | 13% | 13% | −1.3 | 0.82 |
| Donor hypertension | 25% | 25% | −0.8 | 0.89 |
| Donor diabetes | 5% | 6% | −2.8 | 0.65 |
| Donor HCV+ | 0.3% | 0.5% | −2.5 | 0.50 |
| Induction | ||||
| Lymphodepleting | 60% | 62% | NR | NR |
| Non-lym phodepleting | 37% | 36% | 1.7 | 0.77 |
| Steroids only | 3% | 2% | 0.4 | 0.92 |
NR= Not reported
Table 2b.
Balance table after propensity score matching (1:5) for graft loss.
| Belatacept only N=602 | Tacrolimus only N=2,930 | % Bias | T-test P value | |
|---|---|---|---|---|
| Mean Age at Transplant | 53 | 53 | −0.5 | 0.93 |
| Male | 70% | 69% | 1.7 | 0.76 |
| Race | ||||
| Caucasian | 51% | 51% | NR | NR |
| African American | 30% | 30% | −0.5 | 0.94 |
| Latino | 10% | 10% | −0.5 | 0.92 |
| Asian | 8% | 8% | 0.8 | 0.90 |
| Other | 1% | 1% | 0.8 | 0.86 |
| Mean years on dialysis | 3.9 | 3.9 | 0.4 | 0.94 |
| Pretransplant diabetes | 39% | 38% | 1.6 | 0.79 |
| Mean PRA | 7 | 7 | 2.6 | 0.56 |
| Deceased donor | 71% | 70% | 1.1 | 0.84 |
| Mean donor age | 44 | 43 | 1.1 | 0.86 |
| Mean donor weight, kg | 80 | 80 | 0.8 | 0.89 |
| Median donor height, cm | 169 | 169 | 2.0 | 0.73 |
| African American donor | 13% | 12% | 4.5 | 0.44 |
| Donor hypertension | 27% | 26% | 1.6 | 0.80 |
| Donor diabetes | 6% | 6% | −1.6 | 0.81 |
| Donor HCV+ | <1% | <1% | 0 | 1.00 |
| Mean cold ischemia time, hours | 13 | 13 | 1.7 | 0.76 |
| Induction | ||||
| Lymphodepleting | 59% | 61% | NR | NR |
| Non-lym phodepleting | 38% | 37% | 1.6 | 0.79 |
| Steroids only | 3% | 2% | 0.8 | 0.86 |
NR = Not reported
Patient and allograft survival
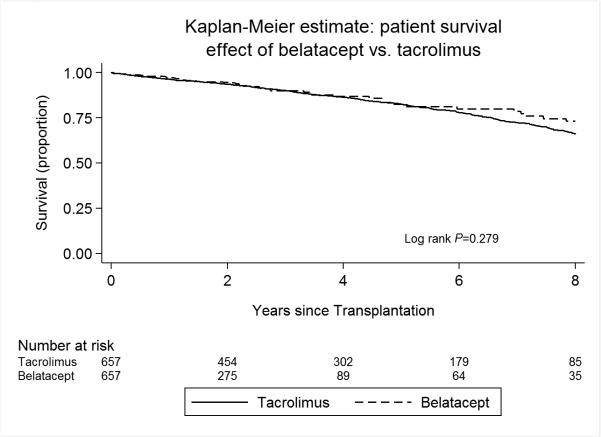
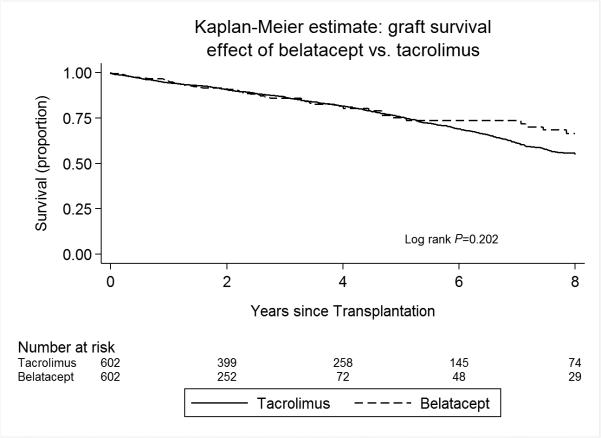
In our primary propensity score matched cohort (657 “belatacept only” versus 3210 tacrolimus in a 1:5 match), the use of a belatacept-based immunosuppressive regimen was associated with no statistically different risk of mortality compared to patients discharged on a tacrolimus-based regimen (HR 0.84, 95% CI 0.61-1.15, p=0.28, Table 3 and Figure 2). The risk of allograft loss was also not statistically different in patients discharged on a belatacept-based regimen (HR 0.83, 95% CI 0.62-1.11, p=0.20) compared to their propensity score-matched tacrolimus counterparts (Table 4 and Figure 3). This effect on patient and allograft survival was despite an increased risk of acute rejection in the first year after transplant observed in the belatacept cohort compared to patients treated with tacrolimus (OR 3.12, 95% CI 2.13-4.57, p<0.001).
Figure 2.
Kaplan Meier estimates of patient survival, stratified by discharge immunosuppression with “belatacept only” or tacrolimus.
Figure 3.
Kaplan Meier estimates of allograft survival, stratified by discharge immunosuppression with “belatacept only” or tacrolimus.
We observed similar results in our sensitivity analysis that included patients discharged on both tacrolimus and belatacept in the belatacept group (“all belatacept” in Tables 3 and 4 as well as balance tables in Supplemental Tables 2 and 3), as well as sensitivity analyses in which patients were propensity score matched by transplant center (“Matched on center” in Tables 3 and 4). The risk of patient death was not significantly different in the “all belatacept” cohort (HR 0.90, 95% CI 0.70-1.17, p=0.45; Supplemental Figure 2), nor was the risk of allograft loss (HR 0.87, 95% CI 0.69-1.10, p=0.26; Supplemental Figure 3). We again noted an increased risk in acute rejection in the “all belatacept” group (OR 2.56, 95% CI 1.93-3.41, p<0.001). After restricting the cohort to only include patients who were transplanted at belatacept study centers, the results were similar (belatacept patient survival HR 0.97, 95% CI 0.52-1.79 p=0.91; belatacept allograft survival HR 0.77, 95% CI 0.46-1.28, p=0.31).
Additional sensitivity analyses were performed to assess the effects of matching, propensity score weights, and inclusion of other discharge maintenance immunosuppressive agents on calculated hazard ratios (Supplemental Table 4). Matching in ratios of 1:1 and 1:10 did not have a notable impact on the outcomes of death and graft loss as previously observed. In unmatched analyses in which we adjusted for propensity score weight in the model, there was no appreciable change in hazard ratios obtained for death or graft loss. 1:10 matching and propensity score weighting did not have a notable impact on the 95% CIs, supporting that sample size was not a major factor in the statistically insignificant difference between the exposure groups. We tested models that included additional elements of maintenance immunosuppression at discharge (use of mycophenolate mofetil versus azathioprine and use of steroids versus “steroid free”); likewise the addition of these variables to our analysis did not impact the results. Our models consistently demonstrated that belacept was associated with a statistically similar hazard of both death and allograft loss when compared to tacrolimus. Further sensitivity analyses demonstrated similar outcomes among patients discharged on belatacept only and patients discharged on both belatacept and tacrolimus compared to patients discharged on tacrolimus only (using multivariable models without propensity scores, due to the comparison of 3 exposure groups; see Supplemental Table 5).
We assessed how many patients started on belatacept or tacrolimus during their index hospitalization were continued on the same medication during their posttransplant follow-up. Despite the fact that UNOS collects data regarding immunosuppression regimen at posttransplant follow up visits, these data were frequently missing; only 60% of the belatacept patients and 87% of tacrolimus patients had this field completed in the follow-up file. Among the limited group of patients with follow up data available, 79% of patients on belatacept only at discharge were continued on belatacept at follow up; 97% of patients on tacrolimus only at discharge were continued on tacrolimus. Likewise we explored the serum creatinine data reported in the follow-up file; 74% of tacrolimus patients and 71% of belatacept patients had serum creatinine reported at 1 year posttransplant. 1 year posttransplant estimated glomerular filtration rate (eGFR; calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (23)) was slightly lower among tacrolimus patients compared to belatacept patients (tacrolimus median eGFR 58.5 mL/min/1.73m2, IQR 45.9-72.9 versus belatacept median eGFR 62.3 mL/min/1.73m2, IQR 49.1-76.2, ranksum p-value <0.001).
Additionally, we explored differences in causes of death and allograft loss for patients with these data reported in the UNOS dataset. The most common causes of death in both groups were cardiovascular disease, (18% in the tacrolimus group and 15% in the belatacept group) and infection (13% in the tacrolimus group and 15% in the belatacept group). We also assessed incidence of posttransplant lymphoproliferative disease (PTLD). PTLD status was missing in 92% of tacrolimus patients and 95% of belatacept patients. Among those in whom PTLD data were reported, there was no significant difference in incident PTLD between tacrolimus and belatacept patients (8% versus 13%, respectively, among 15,965 patients with reported PTLD data, chi-square p-value 0.237; 0.15% versus 0.20%, respectively, assuming all patients with missing data were negative for PTLD, chi-square p-value 0.457). Allograft loss was most often reported to be due to rejection in both cohorts, with 29% of graft losses in the belatacept group and 33% of graft losses in the tacrolimus group attributed to rejection.
Discussion
In this study, we present the results of our retrospective, propensity score matched cohort analysis comparing outcomes for patients discharged from their index transplant hospitalization on maintenance belatacept versus tacrolimus. Belatacept was associated with an equivalent risk of patient death and allograft loss, despite an increased risk of acute rejection in the first year after renal transplant. These findings were consistent regardless of whether the cohort was restricted to patients discharged on belatacept alone or included patients discharged on both belatacept and tacrolimus. This equivalent risk for death and allograft loss was maintained even when we restricted our cohort to centers that used belatacept, varied the number of tacrolimus match patients, accounted for other maintenance immunosuppression or included propensity score weights in the adjusted models.
The published data comparing belatacept to tacrolimus is extremely limited. There is only 1 published prospective study which directly compares belatacept to tacrolimus (11); in this study 33 patients were randomized to belatacept plus mycophenolate and 30 to tacrolimus with mycophenolate after rabbit anti-thymocyte globulin induction. Patients were followed for 1 year, at which time 91% of the belatacept/mycophenolate group and 100% of the tacrolimus/mycophenolate group were alive with functioning kidneys, which was not significantly different; acute rejection rates were notably higher in the belatacept/mycophenolate group (12% versus 3%). However, with the small number of patients and short period of follow-up the authors were not powered to detect a difference in patient or allograft survival.
Two systematic reviews have attempted to compare belatacept and tacrolimus. The meta-analysis by Talawila and Pengel (24) used data from 5 trials that compared belatacept to cyclosporine in addition to the Ferguson study (11); they found that 12 month patient and allograft survival was not significantly different for the belatacept versus CNI groups, but almost all of the studies used in their analysis compared belatacept to cyclosporine, not tacrolimus. Muduma and colleagues (25) performed an “indirect treatment comparison” of belatacept to tacrolimus; they first performed 2 meta-analyses comparing tacrolimus to cyclosporine and cyclosporine to belatacept and then compared the results of these 2 analyses to each other in order to generate a comparison of tacrolimus to belatacept. Their study failed to demonstrate a significant difference between belatacept and tacrolimus for the outcomes for death and allograft loss, but did detect a decreased risk for acute rejection with tacrolimus. However, their approach does not permit a true direct comparison of the 2 immunosuppressive agents, which our study offers.
Our study has several strengths. It is 1 of the few studies to directly compare intermediate term outcomes for belatacept to tacrolimus based maintenance immunosuppression for renal transplant recipients. As our study was performed using national registry data, we were able to assess the national experience using belatacept and the effects in the “real world” setting beyond the BENEFIT trials (7-9). The use of propensity score matching facilitated assessment of comparative effectiveness between the 2 medications with regard to posttransplant outcomes. Our propensity score matching was robust, with well-balanced groups that did not differ significantly from each other. We performed multiple sensitivity analyses, including propensity score weighting and various matching ratios that confirmed our original results. Our findings are supported by a very recent smaller study (12) that used registry data to assess outcomes for patients on tacrolimus versus belatacept. In contrast to our study, this analysis examined only 1 year outcomes using a composite endpoint of death, allograft loss and rejection, did not incorporate any matching techniques, and thus did not attempt to account for selection bias or confounding by indication.
There are several limitations to our study. The belatacept group was small compared to the number of contemporary patients discharged on tacrolimus. We only analyzed patients on the basis of discharge maintenance immunosuppression regimen from their initial transplant admission; the quality of follow up immunosuppression data in the UNOS registry is poor and the data very incomplete. Therefore, we cannot comment accurately upon the number of patients who may have switched from belatacept or tacrolimus to another regimen during their follow up period nor the reasons for doing so. Furthermore, based on limitations within the existing data, we cannot address issues related to medication dosing nor serum concentrations of tacrolimus. Additionally, many patients who were on belatacept may have been enrolled in trials, representing a group of patients who are more carefully selected and closely observed than the general population of transplant patients, and potentially introducing bias. While propensity score matching and sensitivity analyses restricting the cohort to centers enrolled in belatacept trials were used to attempt to address issues related to selection bias and unmeasured confounding, these methods result in restriction of the study to a select group of patients; matching potentially reduces the generalizability of the results to the broad population of transplant patients. Accordingly, we performed additional sensitivity analyses in which we adjusted by propensity score in the overall cohort, rather than restricting the cohort to patients who met criteria for matching. These sensitivity analyses consistently demonstrated no notable differences in the results, which also corroborated our sample size calculations, and supported that sample size was not a major contributing factor to the statistically insignificant difference between the treatment groups. We intended to perform analyses divided by propensity score strata, as well, however we were underpowered to do so. Another limitation of using registry data is we cannot assess precise differences in long-term renal function on the basis of regimen used (belatacept versus tacrolimus), nor can we elucidate if rejection episodes were cellular, antibody-mediated or both. Likewise, there is no data on de novo donor specific antibodies, which are important risk factors for antibody mediated rejection and allograft loss. While our results are encouraging, they still needs to be confirmed by prospective trials of belatacept versus tacrolimus which can provide the necessary granularity to fully evaluate the risks and benefits of either maintenance regimen.
In our retrospective propensity score matched cohort study, belatacept-based maintenance immunosuppression regimens were associated with an equivalent hazard of death and allograft loss for renal transplant recipients when compared to standard of care tacrolimus-based immunosuppression. Randomized prospective trials are required to confirm these results and to clarify the mechanism by which belatacept, while associated with an increased risk of acute rejection, leads to similar patient and allograft outcomes.
Supplementary Material
Acknowledgments
Funding/Support
JC is supported by the NIH/NHLBI K23HL133843
KF is supported by the NIH/NIDDK K23DK090209
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute (JC: K23HL133843) and the National Institute of Diabetes and Digestive and Kidney Diseases (KF: K23DK090209) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported in part by Heath Resources and Service Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the view or policies of the Department of Health and Human Services nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Abbreviations
- CNI
calcineurin inhibitor
- eGFR
estimated glomerular filtration rate
- KDPI
Kidney Donor Profile Index
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Author Contributions
Jordana B. Cohen MD, MSCE: Conception or design of the work, analysis and interpretation of data, drafting and revision of the manuscript
Kevin C. Eddinger MD: Conception or design of the work, analysis and interpretation of data, drafting and revision of the manuscript
Kimberly A. Forde MD, MHS: Conception or design of the work, interpretation of data, drafting and revision of the manuscript
Peter L. Abt MD: Conception or design of the work, drafting and revision of the manuscript
Deirdre Sawinski MD: Conception or design of the work, interpretation of data, drafting and revision of the manuscript
Disclosures
The authors declare no conflicts of interest.
References
- 1.Ducloux D, Motte G, Kribs M, Abdelfatah AB, Bresson-Vautrin C, Rebibou JM, Chalopin JM. Hypertension in renal transplantation: donor and recipient risk factors. Clin Nephrol. 2002;57:409–413. doi: 10.5414/cnp57409. [DOI] [PubMed] [Google Scholar]
- 2.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N, Investigators D Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 3.Mathis AS, Dave N, Knipp GT, Friedman GS. Drug-related dyslipidemia after renal transplantation. Am J Health Syst Pharm. 2004;61:565–585. quiz 586-567. [PubMed] [Google Scholar]
- 4.Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database of Systematic Reviews. 2005 doi: 10.1002/14651858.CD003961.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 6.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2012 Annual Data Report. Department of Health and Human Services, Health Resources and Service Administration; Rockville, MD: 2014. [April 12, 2016]. [Google Scholar]
- 7.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 8.Pestana JOM, Grinyo JM, Vanrenterghem Y, Becker T, Campistol JM, Florman S, Garcia VD, Kamar N, Lang P, Manfro RC, Massari P, Rial MDC, Schnitzler MA, Vitko S, Duan T, Block A, Harler MB, Durrbach A. Three-Year Outcomes From BENEFIT-EXT: A Phase III Study of Belatacept Versus Cyclosporine in Recipients of Extended Criteria Donor Kidneys. American Journal of Transplantation. 2012;12:630–639. doi: 10.1111/j.1600-6143.2011.03914.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 10.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, Marks WH, Agarwal M, Wu D, Dong Y, Garg P. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11:66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Casey MJ, Santos AH, Hartzema A, Womer KL. Comparison of Utilization and Clinical Outcomes for Belatacept- and Tacrolimus-Based Immunosuppression in Renal Transplant Recipients. Am J Transplant. 2016 doi: 10.1111/ajt.13853. [DOI] [PubMed] [Google Scholar]
- 13.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JB, Bloom RD, Reese PP, Porrett PM, Forde KA, Sawinski DL. National outcomes of kidney transplantation from deceased diabetic donors. Kidney Int. 2015 doi: 10.1038/ki.2015.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faravardeh A, Eickhoff M, Jackson S, Spong R, Kukla A, Issa N, Matas AJ, Ibrahim HN. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation. 2013;96:1089–1096. doi: 10.1097/TP.0b013e3182a688e5. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan M, Pankewycz O, Shihab F, Wiland A, McCague K, Chan L. Long-term outcomes in African American kidney transplant recipients under contemporary immunosuppression: a four-yr analysis of the Mycophenolic acid Observational REnal transplant (MORE) study. Clin Transplant. 2014;28:184–191. doi: 10.1111/ctr.12294. [DOI] [PubMed] [Google Scholar]
- 17.Abadie A, Drukker D, Herr JL, Imbens GW. Implementing matching estimators for average treatment effects in Stata. Stata Journal. 2004;4:290–311. [Google Scholar]
- 18.Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Statist Assoc. 1989;84:1074–1078. [Google Scholar]
- 22.Therneau TM, Grambsch PM. Statistics for Biology and Health. Springer; New York, NY: 2001. Modeling Survival Data: Extending the Cox Model. [Google Scholar]
- 23.Keddis MT, Amer H, Voskoboev N, Kremers WK, Rule AD, Lieske JC. Creatinine-Based and Cystatin C-Based GFR Estimating Equations and Their Non-GFR Determinants in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2016;11:1640–1649. doi: 10.2215/CJN.11741115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talawila N, Pengel LHM. Does belatacept improve outcomes for kidney transplant recipients? A systematic review. Transplant International. 2015;28:1251–1264. doi: 10.1111/tri.12605. [DOI] [PubMed] [Google Scholar]
- 25.Muduma G, Hart WM, Patel S, Odeyemi AO. Indirect treatment comparison of belatacept versus tacrolimus from a systematic review of immunosuppressive therapies for kidney transplant patients. Curr Med Res Opin. 2016;32:1065–1072. doi: 10.1185/03007995.2016.1157463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.