Abstract
There are 5 Ubiquilin proteins (UBQLN1-4, UBQLN-L), which are evolutionarily conserved and structurally similar. UBQLN proteins have 3 functional domains: N-terminal ubiquitin-like domain (UBL), C-terminal ubiquitin-associated domain (UBA) and STI chaperone-like regions in the middle. Alterations in UBQLN1 gene have been detected in a variety of disorders ranging from Alzheimer’s disease to cancer. UBQLN1 has been largely studied in neurodegenerative disorders in the context of protein quality control. Several studies have hypothesized that the UBA domain of UBQLN1 binds to poly-ubiquitin chains of substrate and shuttles it to the proteasome via its UBL domain for degradation. UBQLN1 either facilitates degradation (Ataxin3, EPS15) or stabilizes (PSEN1/2, BCLb) substrates it binds to. The signal that determines this fate is unknown and there is conflicting data to support the existing working model of UBQLN1. Using BCLb as a model substrate, we characterized UBQLN1-substrate interaction. We identified the first two STI domains of UBQLN1 as critical for binding to BCLb. Interaction of UBQLN1 with BCLb is independent of ubiquitination of BCLb, but interaction with ubiquitin via UBA domain is required for its stabilization. Similarly, we showed that UBQLN1 interacts with IGF1R and ESYT2 through the STI domains and stabilizes these proteins through its UBA domain. Interactions that are not dependent on STI domains, for example UBL mediated interaction with PSMD4 and BAG6, do not appear to be stabilized by UBQLN1. We conclude that fate of substrates that UBQLN1 associates with, is interaction domain specific.
Keywords: UBQLN, UBL, STI, UBA, BCLb, IGF1R, ESYT2, PSMD4, BAG6, proteostasis
Introduction
The Ubiquilin (UBQLN) family of proteins (UBQLN1-4, UBQLN-L) is evolutionarily conserved and structurally similar. UBQLN1 is ~63 kDa protein and all its family members have 3 functional domains: ubiquitin-like domain (UBL) at the N-terminus, ubiquitin-associated domain (UBA) at the C-terminus and STI chaperone-like regions in the middle (Fig. 1 A). UBQLN1 is ubiquitously expressed in all tissues. Mutations and loss of the UBQLN1 gene have been detected in a variety of disorders ranging from neurodegenerative disorders like Alzheimer’s disease [Zheng et al., 2014] to cancers like non-small cell lung adenocarcinoma [Shah et al., 2015]. Initially, UBQLN1 was discovered as a protein that interacted with IAP (CD47) in ovarian cancer cells, and upon loss, increased cell spreading [Wu et al., 1999]. However, since then UBQLN1 has been largely studied in the context of neurodegenerative disorders and protein quality control. UBQLN1 assists in proteasomal degradation of ubiquitinated substrates and also participates in ERAD (Endoplasmic Reticulum Associated Degradation) [Shah et al., 2015], autophagy [Lee et al., 2013; N’Diaye et al., 2009], apoptosis [Lu et al., 2009] and receptor trafficking [Zhang et al., 2015]. As a UBL-UBA protein, UBQLN1 is hypothesized to bind to poly-ubiquitin chains of substrate through its UBA domain and shuttle it to the 19s proteasome via its UBL-S5a cap interaction. The central region of UBQLN1 consists of STI domains that are called so because of their similarity to STI-1 proteins (STress Inducible proteins or Hsp70-Hsp90 organizing protein). This region acts as a co-chaperone and mediates hydrophobic interactions with other proteins [Mueller et al., 2004; Zhao and Ackerman, 2006]. There is conflicting data to support the existing working model of UBQLN1. Several studies have reported that UBQLN1 facilitates proteolysis of substrates that it binds. Dsk2p, the yeast homolog of UBQLN1, forms a trimeric complex with Rad23 and Cdc48 and enables degradation of misfolded proteins via the proteasomal system [Medicherla et al., 2004]. In human cells, the UBL domain of UBQLN1 has been shown to regulate degradation of misfolded proteins like Ataxin3, a deubiquitinase enzyme, HSJ1a, a protein that stimulates activity of Hsp70, and EPS15, a protein important in endosomal trafficking [Heir et al., 2006]. UBQLN1 expression aids in poly-ubiquitination of viral polymerase NS5B, decreasing its half-life in HCV infected hepatocytes [Gao et al., 2003]. In contrast, UBQLN1 stabilizes proteins like Presenilin1/2 [Mah et al., 2000; Massey et al., 2004], IκBα [Feng et al., 2004], and BCLb [Beverly et al., 2012] when bound to them. These data indicate that UBQLN1 either stabilizes or facilitates degradation of substrates that it binds to. The signal that determines this fate is unknown.
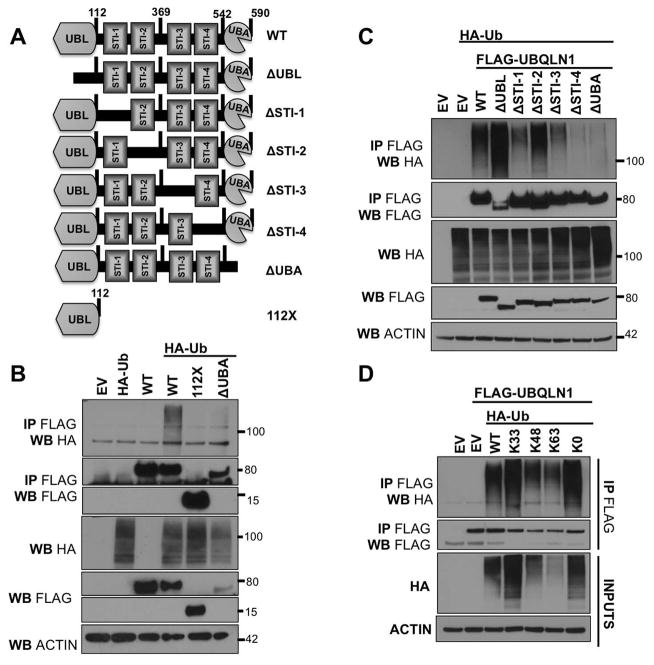
Figure 1. UBQLN1 interacts non-preferentially with diverse ubiquitin linkages through its UBA domain.
(A) Schematic of UBQLN1WT and engineered domain deletion constructs missing individual domains. (B,C), 293T cells were transfected with empty vector (EV), FLAG-UBQLN1WT, domain deletion constructs of UBQLN1 and HA-UbiquitinWT. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed. UBQLN1WT interacts with ubiquitin but UBQLN1112X and UBQLN1ΔUBA (both missing UBA domain) lose this interaction. Therefore, UBQLN1 interacts with ubiquitin through the UBA domain. (D) 293T cells were transfected with FLAG-UBQLN1WT and constructs of HA-UbiquitinWT that have all Lysines (K) mutated to Arginine (R) except one for example, HA-UbiquitinK33 has all K’s are mutated to R except K33 and therefore overexpressing and promoting formation of K33 ubiquitin chains on substrates. K0 indicates all K’s have been mutated to R and this ubiquitin molecule is can only conjugate to the substrate (mono-ubiquitination) and is incapable for forming ubiquitin linkages. UBQLN1 interacts with WT, K33-, K48-, K63- poly-ubiquitin chains and K0 mono-ubiquitinated substrates non-selectively.
In this study, we aimed to understand characteristics of substrates that bind to UBQLN1 and whether the nature of UBQLN1-substrate interaction decides the fate of substrates – stabilization or degradation. We show that Ubiquilin interacts with multiple substrates through its STI or UBL domains. We also show that UBQLN1 is capable of forming dimers and continues to interact with proteins in its dimerized form. We have previously published that UBQLN1 interacts with the anti-apoptotic BCL2 protein BCLb and stabilizes it in the cytoplasm. Here, we have used BCLb as a model to characterize the nature of its interaction with UBQLN1.
Materials and Methods
Tissue Culture and Protein Analysis
293T cells were cultured in DMEM supplemented with 10% FBS. DNA transfections were done using PEI (2.5:1). All cell extracts were prepared following scrape harvesting of 293T cells using CHAPS lysis buffer (1% CHAPS detergent, 150 mM NaCl, 50 mM Tris pH 7, 5 mM EDTA). Subcellular fractionation experiments were performed using the ProteoExtract Subcellular Proteome Extraction kit (Calbiochem/Merck), as per the manufacturer’s protocol. For immunoprecipitations, 400 ug of protein was incubated in 400 uL of total CHAPS buffer and incubated with indicated affinity matrix for 1 h at 4 °C. Following incubation, the matrix was washed three times in CHAPS buffer and then SDS loading buffer was added directly to washed matrix, boiled, and loaded directly into the wells of a PAGE gel. Drug treatments were performed as described in the text using 20 uM Cycloheximide, 25 uM proteasome inhibitor MG132, 360nM Epoximide, 1uM Bortezomib.
Mass Spectrometric Analysis of Immunoprecipitated FLAG-UBQLN1
FLAG-UBQLN1 was transfected into 293T cells and 48 hours later lysates were prepared. Immunoprecipiatations and LC-MS/MS was performed exactly as previously described in Beverly et al 2012.
Plasmids
All experiments using expression of BCLb proteins were performed with vectors engineered for this study. The following accession numbers were used to design oligos for PCR based cloning from cDNA libraries generated from a “universal human RNA” library (Stratagene/Agilent Technologies): BCLb #NM_020396. FLAG-tagged BCL2 constructs were generated by performing PCR of the MIG-BCL constructs using an oligo that fused the FLAG coding sequences to the amino terminus of each BCL2-protein. This product was then subcloned back into the MIGRX vector. The following plasmids were obtained from Addgene; FLAG-Ubqln (Addgene plasmid 8663, deposited by Peter Howley), FLAG-Ubqln-112X (Addgene plasmid 8664, deposited by Peter Howley), pRK5-HA-Ubiquitin-WT (Addgene plasmid 17608, deposited by Ted Dawson), pRK5-HA-Ubiquitin-K0 (Addgene plasmid 17603, deposited by Ted Dawson), pRK5-HA-Ubiquitin-K48 (Addgene plasmid 17605, deposited by Ted Dawson). Lysine-less BCLb construct was engineered using the MIG- or MIT-BCLb constructs described above to do site directed mutagenesis with the Quick Change Multi Site Mutagenesis kit (Stratagene/Ambion), as per the manufacturer’s protocol. MYC-UBQLN1 construct was a gift from Annakaisa Haapasalo, Finland. Domain deletion constructs of UBQLN1 were created using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs).
Antibodies
BCLb #ab45412 and GFP #ab6673 (Abcam); VDAC #4866 (Cell Signaling); IGF1R #3018 (Cell Signaling), ESYT2 #HPA002132 (SIGMA), S5a/PSMD4 #12441 (Cell Signaling), BAG6 #A302-039A-T (Bethyl), MYC #F2512 (Santa cruz), Tubulin #B512, FLAG M2 conjugated agarose beads, FLAG poly-clonal #F7425 (Sigma); UBQLN poly-clonal was made by inoculating rabbits with a peptide specific to UBQLN1 (Yenzym Antibodies LLC); HA affinity matrix and HA #3F10 (Roche).
Results
UBQLN1 non-selectively interacts with diverse ubiquitin linkages through its UBA domain
Our previous work suggested that UBQLN1 interacts with mono-ubiquitinated BCLb [Beverly et al., 2012]. However, previous work by Feng et al [Feng et al., 2004] and Zhang et al [Zhang et al., 2008] showed that UBA domain of UBQLN1 is also capable of associating with poly-ubiquitin chains. Therefore, we investigated if UBQLN1 interacts with specific ubiquitination linkages on substrates. We engineered constructs of UBQLN1 missing individual domains (Fig. 1 A). Cells were transfected with empty vector (EV), FLAG-UBQLN1WT or deletion constructs of UBQLN1 (Fig. 1 A) and HA-UbWT(Ubiquitin) (Fig. 1 B,C). FLAG-UBQLN1 was immunoprecipitated and interaction with HA-Ubiquitin was examined. We confirmed that the UBA domain of UBQLN1 interacts with ubiquitin as constructs missing the UBA domain, namely UBQLN1112X and UBQLN1ΔUBA fail to pull down HA-Ubiquitin. Next, we performed a similar experiment using our individual domain deletion constructs (Fig. 1 A) and confirmed that ΔUBA does not interact with ubiquitin. Interestingly, ΔSTI-4 construct also failed to pull down ubiquitin probably because of STI-4 domain’s close proximity to the UBA domain such that when STI-4 is deleted, it directly impacts UBA domain’s ability to interact with ubiquitin. Next, we tested for preference of the UBA domain of UBQLN1 for diverse ubiquitin linkages. We used constructs of HA-UbiqutinWT that have all Lysines (K) mutated to Arginine (R) except one, for example, HA-UBQTNK33 has all K’s mutated to R except K33 and therefore overexpression promotes formation of K33 ubiquitin chains on substrates. K0 indicates all K’s have been mutated to R and this ubiquitin molecule can be conjugated to substrate (mono-ubiquitination), but is not capable of forming ubiquitin chains. Results show that UBQLN1 is capable of interacting with WT, K33-, K48-, K63- poly-ubiquitin chains and mono-ubiquitinated residues. Based on these data and literature [Zhang et al., 2008] we conclude that UBQLN1 non-selectively interacts with diverse ubiquitin linkages on substrates via its UBA domain.
Recognition of the transmembrane domain of BCLb by STI-1/2 domains of UBQLN1 sequesters BCLb in the cytosol
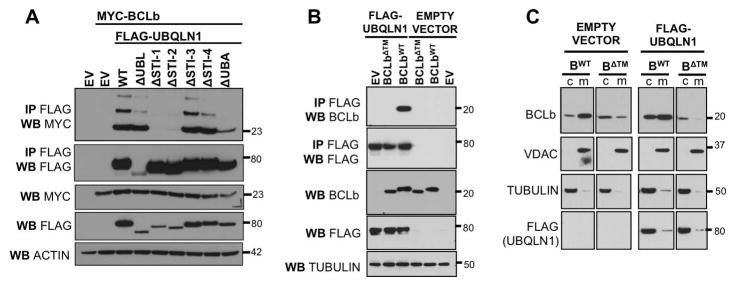
Recently, Itakura et al [Itakura et al., 2016] found that the UBQLN4 recognizes the transmembrane domain of mitochondrial protein OMP25. We have previously shown that UBQLN1 interacts with mitochondrial protein BCLb and stabilizes it in the cytosol. Here, we investigated the domain of UBQLN1 required for interaction with BCLb using our deletion constructs of UBQLN1. We identified STI-1 and STI-2 domains on UBQLN1 as critical for interacting with BCLb. 293T cells were transfected with empty vector (EV), FLAG-UBQLN1WT, FLAG-UBQLN1 domain deletion constructs, MYC-BCLbWT or MYC-BCLbΔTM. Where indicated, immunoprecipitation with anti-FLAG beads was performed and probed for MYC-BCLb by Western Blot analyses. STI-1 and STI-2 domains of UBQLN1 are required for interaction with BCLb (Fig. 2 A). UBQLN1WT and all domain deletion constructs except ΔUBA, interact with multi mono-ubiquitinated BCLb, indicated by the ladder-like pattern at higher molecular weights. Thus, the UBA domain of UBQLN1 is obligatory to interact with ubiquitin on BCLb. Next, we determined that the transmembrane domain (TM) is essential for UBQLN1 to recognize and interact with BCLb. These data suggest a potential 2-fold interaction between UBQLN1 and BCLb such that the STI domains of UBQLN1 associate with the TM domain of BCLb and the UBA domain associates with the ubiquitin on BCLb (Fig. 2 B). Previously, we have shown that UBQLN1 interacts with BCLbWT and sequesters it in the cytosol. As we determined that UBQLN1 needs an intact TM domain of BCLb to interact with it, we tested if UBQLN1 alters the location of the BCLbΔTM. We performed subcellular fractionation by differential centrifugation to determine changes in cellular location of BCLbΔTM. Results show that UBQLN1 does not recognize BCLbΔTM, and therefore, does not interact with it and has no effect on its cellular location (Fig. 2 C). Our data confirm results by Itakura et al [Itakura et al., 2016] that UBQLN proteins are capable of recognizing transmembrane domains of some proteins, like OMP25 and BCLb.
Figure 2. The transmembrane domain of BCLb is recognized by STI-1/2 domains of UBQLN1 and stabilizes BCLb in the cytoplasm.
293T cells were transfected with empty vector (EV), FLAG-UBQLN1WT, or FLAG-tagged domain deletion constructs, MYC-BCLbWT or MYC-BCLbΔTM. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed to determine interaction between UBQLN1 and BCLb proteins. (A) STI-1/2 domains of UBQLN1 are required to interact with BCLb. (B) BCLbΔTM does not interact with UBQLN1. A functional transmembrane domain on BCLb is required for UBQLN1 to recognize and interact with it. (C) 293T cells expressing BCLbWT or BCLbΔTM with EV or FLAG-UBQLN1, were subjected to subcellular fractionation, followed by Western Blot analyses. Cells were fractionated into cytosolic (c) and membranes (m). VDAC1, a membrane bound protein, and Tubulin, a cytoplasmic protein were used to document the validity of the fractionations. UBQLN1 does not interact with BCLbΔTM and therefore does not alter the location of BCLbΔTM, as it is unable to recognize it.
Interaction of UBQLN1 with BCLb is independent of ubiquitination of BCLb
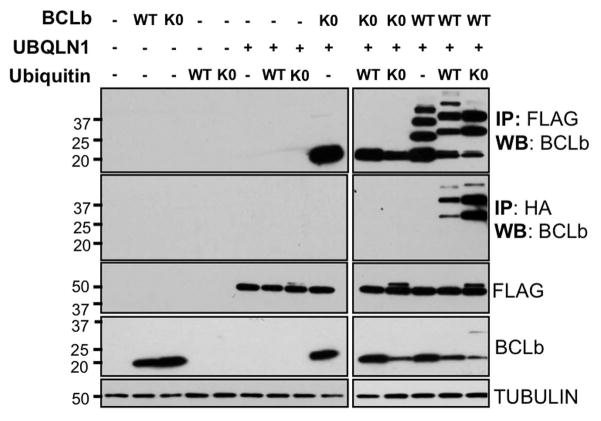
We showed above that the UBA domain which interacts with ubiquitin is dispensable for interaction of UBQLN1 with BCLb. BCLb has 4 lysines that can be potentially ubiquitinated [Beverly et al., 2012] and therefore, we investigated whether ubiquitination of BCLb played a role in its interaction with UBQLN1. We generated a construct of BCLb (BCLbK0) in which all 4 lysines were mutated to arginine. We tested whether BCLbK0 is capable of being ubiquitinated and whether UBQLN1 interacts with non-ubiquitinated BCLb. Cells were transfected with BCLbWT, lysine-less BCLb (BCLbK0), HA-UbiquitinWT, lysine-less Ubiqutin (HA-UbiquitinK0) and FLAG-UBQLN1. Where indicated, immunoprecipitation with either anti-FLAG or anti-HA conjugated beads was performed to determine UBQLN1- or Ubiquitin-interacting complexes, respectively as previously shown (Fig. 3). BCLbWT gets mono-ubiquitinated on several of its lysine residues as observed by the ladder-like pattern of ubiquitination in BCLbWT in the presence of UbiquitinWT. BCLbK0 does not get ubiquitinated. Non-ubiquitinated BCLbK0 also fails to be pulled down with anti HA-Ubiquitin antibody. Immunoprecipitating with anti-FLAG beads showed that UBQLN1 interacts with both BCLbWT and BCLbK0 implying that UBQLN1 interacts with BCLb in its ubiquitinated (BCLbWT) and non-ubiquitinated (BCLbK0) forms. These data indicate interaction of UBQLN1 with BCLb is independent of ubiquitination of BCLb.
Figure 3. Interaction of UBQLN1 with BCLb is independent of ubiquitination of BCLb.
293T cells were transfected with BCLb (MIG-BCLbWT), lysine-less BCLb (MIG-BCLbK0), wild-type Ubiquitin (HA-UbWT), lysine-less Ubiquitin (HA-UbK0) and FLAG-UBQLN1WT. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with either anti-FLAG or anti-HA conjugated agarose beads was performed to determine FLAG-UBQLN1 or HA-Ubiquitin interacting complexes, respectively. BCLb gets ubiquitinated on its lysine residues as seen by failure to pull down BCLbK0 by HA-Ubiquitin. UBQLN1 interacts with both BCLbWT and BCLbK0 implying that UBQLN1 interacts with ubiquitinated (WT) and non-ubiquitinated (K0) BCLb as observed by the ladder-like pattern of ubiquitination in BCLbWT in the presence of HA-Ubiquitin. Note; HA-tag adds ~1kDa to the molecular weight of Ubiquitin.
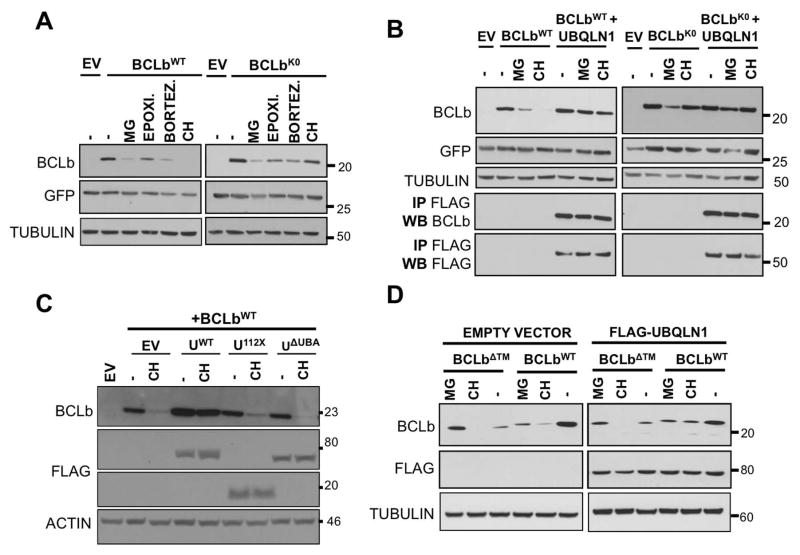
Ubiquilin1 stabilizes BCLbWT after translational and proteasomal inhibition
We have previously shown [Beverly et al., 2012] that BCLb gets completely degraded within 16 hours after blocking new protein synthesis by CH. However, in the presence of UBQLN1WT, it is stabilized and protected from degradation. We wanted to determine whether ubiquitination of BCLb plays a role in its stability. Cells were transfected with BCLbWT or BCLb engineered to lack all its four lysine residues (BCLbK0) or empty vector (EV). Cells were treated with the indicated translational (CH) and proteasomal (MG132, Epoximide, Bortezomib) inhibitors for 16 hours. GFP is used as an expression control since GFP is expressed from the same mRNA as BCLb following an internal ribosomal entry sequence. (Fig. 4 A). BCLbWT was completely degraded in the presence of CH for 16 hours while BCLbK0 expression was completely stable. This may indicate that CH induced degradation of BCLbWT is dependent on its lysines and therefore its ubiquitination, whereas degradation induced by proteasomal inhibition is independent of ubiquitination. UBQLN1 was co-transfected with BCLbWT or BCLbK0 to test its effect on BCLb stability (Fig. 4 B). Presence of UBQLN1 stabilized expression of BCLbWT following both translational inhibition and proteasomal inhibition. Thus, UBQLN1 generally stabilizes BCLbWT. Furthermore, UBQLN1 also stabilizes BCLbK0 from proteasome inhibition-induced degradation. Immunoprecipitating with FLAG antibody demonstrated that UBQLN1 interacted with both BCLbWT and BCLbK0 under conditions of proteasomal inhibition by MG132 or translational inhibition by CH, implying that primary interaction with BCLb is not lysine mediated (Fig. 4 B). We then tested the effect of deletion constructs of UBQLN1 on stability of BCLbWT (Fig.4 C). UBQLN1ΔUBA interacts with BCLb (Fig. 2 A), but fails to prevent loss of BCLbWT when exposed to CH. We conclude that interaction of UBQLN1 with ubiquitin is dispensable for interaction with BCLb, but secondary interaction with ubiquitin on substrates is essential for its stabilization. As the transmembrane domain of BCLb is required for interaction with UBQLN1 (Fig. 3 B), we tested its stability under similar conditions and found that by itself BCLbΔTM is less stable than BCLbWT. However, an interesting observation is that BCLb lacking the transmembrane domain is the only construct that is stabilized following inhibition of the proteasome by MG132. Further, presence of UBQLN1 doesn’t not alter stability of BCLbΔTM (Fig. 4 D), as it is unable to interact with it (Fig. 2 C). Therefore, interaction between UBQLN1 and BCLb is independent of ubiquitination of BCLb but is required for stabilization of BCLb.
Figure 4. UBA domain of UBQLN1 is responsible for stabilization of BCLbWT.
293T cells were transfected with MIG-BCLbWT, MIG-BCLbWT, MIG-BCLbΔTM, FLAG-UBQLN1WT, domain deletion constructs FLAG-UBQLN1112X and FLAG-UBQLN1ΔUBA as indicated. 36 hours post transfection, cells were treated with the indicated inhibitor for 16 hours; vehicle (−), translational elongation inhibitor Cycloheximide (CH) and proteasomal inhibitors MG132 (MG), Epoximicin (Epoxi.) and Bortezomib (Bortez.). Cells were lysed and Western Blot analyses were performed with the indicated antibody. (A) Stability of BCLbWT and BCLbK0 was tested in the presence of UBQLN1, when exposed to the indicated inhibitors for 16 hours. (B) Experiment was performed as in A but in the presence of UBQLN1. As seen in A, expression of BCLbWT is lost upon exposure to CH for 16 hours, but in the presence of UBQLN1, it is stabilized. Loss of BCLbWT seen with proteasomal inhibitors is also rescued by the presence of UBQLN1. UBQLN1 also prevents loss of BCLbK0 seen with MG132. (C) Experiment was performed as in A in the presence of CH and domain deletion constructs of UBQLN1 and we mapped the UBA domain to be responsible for stabilizing BCLbWT. Both UBQLN112X and UBQLN1ΔUBA failed to prevent loss of BCLbWT. (D) Stability of BCLbΔTM was tested similar to BCLbWT and BCLbK0. BCLbΔTM is generally less stable than BCLbWT and presence of UBQLN1 does not alter its expression.
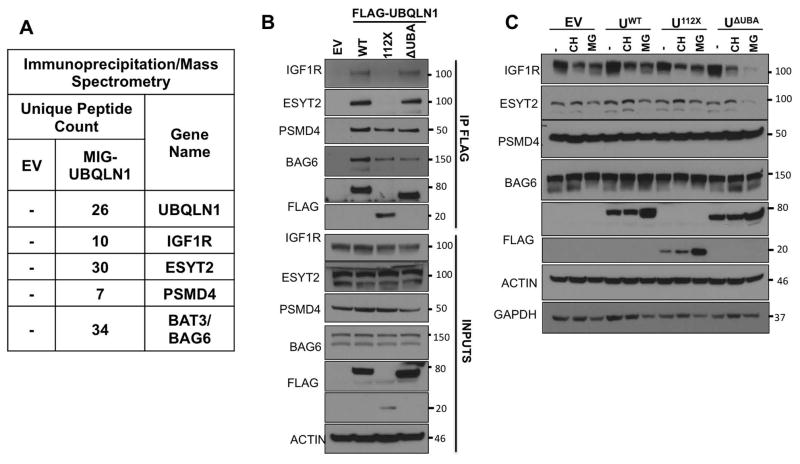
IGF1R, ESYT2 and BAG6 are newly identified substrates of UBQLN1. Interaction of UBQLN1 with IGF1R and ESYT2 is STI-mediated while BAG6 and PSMD4 is UBL-mediated
We characterized the nature of interaction between UBQLN1 and BCLb using BCLb as a model substrate. We wanted to determine if these interaction characteristics could be generalized to other substrates of UBQLN1. Our IP/MS data (Fig. 5 A) identified insulin-like growth factor 1 receptor (IGF1R), a cell surface receptor tyrosine kinase, Extended Synaptogammin-2 (ESYT2), a calcium regulated intrinsic membrane protein and BAG6 (BAT3), a protein involved in proteasomal degradation machinery of misfolded proteins, as potential interacting partners of UBQLN1. We mapped the interaction of UBQLN1 with IGF1R and ESYT2 to the STI domains and BAG6 and PSMD4 (or S5a, 26S proteasomal subunit and known interacting partner of UBQLN proteins) to the UBL domain (Fig. 5 B). Like with BCLb, STI domains of UBQLN1 are required for interaction with IGF1R and ESYT2 as UBQLN1112X missing the STI domains is unable to interact with these 2 substrates. Interaction of UBQLN1 with BAG6 and PSMD4 is purely UBL mediated as detected with all 3 constructs, UBQLN1WT, UBQLN1112X and UBQLN1ΔUBA that have the UBL domain intact.
Figure 5. Fate of substrates targeted by UBQLN1 is dependent on interaction of the substrate with specific domains of UBQLN1.
(A) 293T cells were transfected with FLAG-tagged UBQLN1 (MIG-PLIC1) and an empty vector control (EV) followed by immunoprecipitation and analysis by mass spectrometry. The data from one representative experiment is shown. The number of unique peptides identified for each UBQLN1-interacting protein is shown. (B) 293T cells were transfected with FLAG-tagged deletion constructs of UBQLN1 followed by immunoprecipitation by FLAG antibody and Western Blot analysis. Like BCLbWT, STI domains of UBQLN1 are required to interact with IGF1R and ESYT2 as UBQLN1112X missing the STI and UBA domains do not interact with these 2 substrates. UBQLN1WT, UBQLN1112X and UBQLN1ΔUBA interact with both PSMD4 and BAG6 indicating that this interaction is UBL mediated. (C) Stability of IGF1R, ESYT2, PSMD4 and BAG6 were tested upon CH and MG132 exposure for 48 hours as with BCLb in Fig. 4. In the absence of the UBA domain (UBQLN1ΔUBA), IGF1R and ESYT2 expression are lost upon proteasomal inhibition with MG132. PSMD4 and BAG6 expression are unchanged after 48 hours of CH and MG132 and the presence of UBQLN1WT, UBQLN1112X or UBQLN1ΔUBA does not affect their stability.
Like BCLb, UBQLN1 interacts with IGF1R and ESYT2 via its STI domains but needs the UBA domain to stabilize it after proteasomal inhibition
Once we identified additional substrates that interact with different domains of UBQLN1, we wanted to determine substrate stability with specific interaction domains (Fig. 5 C). We performed stability experiments with IGF1R, ESYT2, PSMD4 and BAG6 as we did with BCLb. Cells were transfected with empty vector (EV), UBQLN1WT, UBQLN1112X or UBQLN1ΔUBA and exposed to CH or MG132 for 48 hours. Our results show that IGF1R expression decreased in the presence of CH, which blocks new protein synthesis. IGF1R expression also decreased in response to proteasomal inhibition by MG132, indicating that it is being degraded through proteasomal-independent pathways. In the presence of UBQLN1ΔUBA, degradation of IGF1R through these non-proteasomal pathways seemed to be accelarated indicating that the presence of UBA domain prevents the loss of expression of IGF1R with MG132 exposure. We found strikingly similar data when we tested stability of another membrane protein ESYT2. UBQLN1ΔUBA failed to prevent loss of IGF1R and ESYT2 under proteasomal blockade suggesting that the UBA domain of UBQLN1 is essential in stabilizing IGF1R and preventing its degradation through non-proteasomal pathways. PSMD4 and BAG6 levels seemed steady and unchanging under both CH and MG132 exposure and the presence of UBQLN1WT, UBQLN1112X and UBQLN1ΔUBA did not alter their expression suggesting that substrate interactions of UBQLN1 that are UBL mediated, are not stabilized as a result of their interaction.
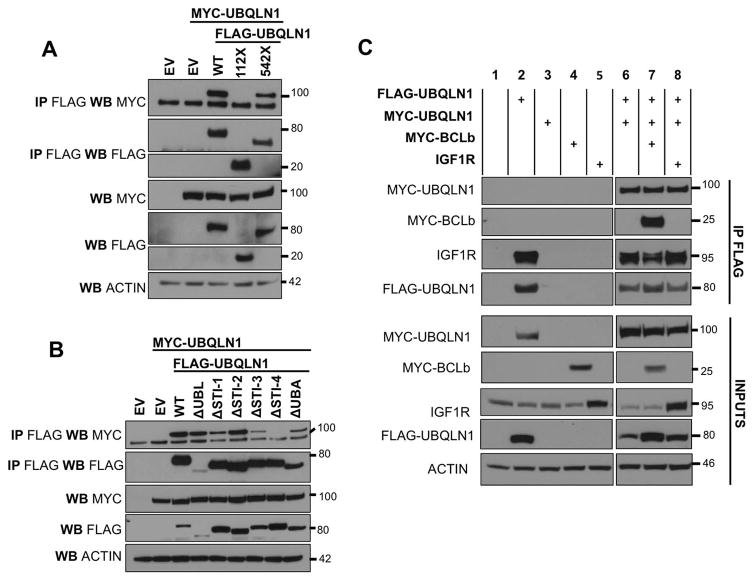
UBQLN1 dimerizes via its STI-4 domains
Several studies have reported that UBQLN1 is capable of homodimerizing [Ford and Monteiro, 2006] and UBQLN2 heterodimerizes with UBQLN4 [Hjerpe et al., 2016]. Feng [Feng et al., 2004] and Ford [Ford and Monteiro, 2006] showed that the middle region with STI domains is responsible for dimerization while other studies suggest a UBL-UBA dependent interaction. We investigated the presence of UBQLN1 dimers and determined if it is capable of interacting with substrates in its dimerized state. Cells were transfected with empty vector (EV), FLAG-UBQLN1WT or deletion constructs of FLAG-UBQLN1WT and MYC-UBQLN1WT (Fig. 6 A). Results show MYC-UBQLN1 was pulled down when we immunoprecipitated FLAG-UBQLN1 and the STI domains located between amino acid 112 and 542 in UBQLN1 are required for this interaction to take place (Fig. 6 A). We mapped this interaction specifically to the STI-4 domain (Fig. 6 B). Our data do not indicate a UBL-UBA interaction, as recently suggested [Itakura et al., 2016]. Next, we investigated if interaction with substrate disrupted UBQLN1 dimers (Fig. 6 C). Results show that endogenous substrates of UBQLN1 (IGF1R; lanes 2,6,7) or introducing exogenous substrates (BCLb and IGF1R; lanes 7 and 8 respectively) do not alter the amount of dimerized UBQLN1 protein as witnessed by a consistent amount of MYC-UBQLN1 being pulled down with FLAG-UBQLN1. This suggests that the dimers do not need to be disrupted for interaction with substrates.
Figure 6. UBQLN1 dimerizes via its STI-4 domains.
293T cells were transfected with empty vector (EV), MYC-UBQLN1WT, FLAG-UBQLN1WT, domain deletion constructs of UBQLN1 MYC-UBQLN1WTas indicated. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed to determine interaction. (A) FLAG-UBQLN1WT interacts with MYC-UBQLN1 and the STI domains located between amino acid 112 and 542 are required for this interaction to take place. (B) Domain deletion constructs map the dimerization to the STI-4 domain. (C) The level of dimerization of UBQLN1 proteins remain unchanged in the presence of endogenous substrates (IGF1R; lanes 2,6,7) and introducing exogenous substrates (BCLb and IGF1R; lanes 7 and 8 respectively).
Discussion
In this study we aimed to identify characteristics of UBQLN1-substrate interaction that lead to proteostasis of the substrate. Using BCLb as a model substrate, our data challenge the dogma that the primary function of UBQLN1 is to facilitate degradation of misfolded proteins. We undertook a systematic approach to investigate indicators of stabilization of BCLb as a substrate of UBQLN1.
First, we investigated whether diverse ubiquitin linkages on proteins that interact with UBQLN1 play a role in its proteostasis. We used domain deletion constructs of UBQLN1 and confirmed that its UBA domain associates with ubiquitin (Fig. 1 B,C). Further, we found no difference in the type of ubiquitin linkage recognized by the UBA domain when we overexpressed HA-Ubiquitin constructs capable of forming K33, K48, K63 poly-ubiquitin chains as well as mono-ubiquitinating substrates (Fig. 1 D). Our data complement findings by Zhang et al [Zhang et al., 2008] that the UBA domain of UBQLN1 binds to both monomeric ubiquitin as well as poly-ubiquitin chains. Feng et al [Feng et al., 2004] also reported that the UBA domain is capable of interacting with poly-ubiquitin chains but did not test for interaction with monomeric ubiquitin. We have previously shown that BCLb lysine residues get mono-ubiquitinated and van de Kooji et al [van de Kooij et al., 2013] showed that one out of its 4 lysines, K128 residue on BCLb is capable of forming K48 poly-ubiquitin chains which signals BCLb for proteasomal degradation. Our data indicate that the UBA domain non-selectively interacts with ubiquitin on BCLb, prevents binding of additional ubiquitin molecules that signal BCLb for proteasomal degradation (Fig. 2 C, 3, 4 C). Thus, unlike K48 ubiquitination, which specifically signals for degradation or K63 ubiquitination, which signals for trafficking, the ubiquitin code on UBQLN1’s substrates does not decide its fate.
To further understand the characteristics of UBQLN1-BCLb interaction, we mapped the location of their interaction on both proteins. We identified the STI-1/2 domains on UBQLN1 and transmembrane domain (TM) of BCLb as essential for this association to take place (Fig. 2 A,B). UBQLN1 most likely recognizes the TM domain as BCLb is being translated and binds to it, stabilizing BCLb in the cytoplasm. We performed several stability experiments of BCLb in the presence of translational and proteasomal inhibitors (Fig. 3). BCLbWT is degraded completely following 16 hours of cycloheximide (CH) treatment. UBQLN1 prevents loss of BCLb implying that their physical interaction protects BCLb from degradation. In the presence of CH, UBQLN1ΔUBA interacts with BCLb via its STI-1/2 domains (Fig. 2 A) but fails to stabilize it (Fig. 4 C) indicating that the UBA domain is required to stabilize BCLb. Lysine-less BCLb (BCLbK0) is more stable than BCLbWT at 16 hours post cycloheximide treatment as our group (2009) and van de Kooji et al in 2013 have previously shown. Added stability of BCLbK0 over BCLbWT suggests that degradation is dependent on its lysines and therefore, likely its ubiquitination. van de Kooji et al showed that BCLb gets K48 poly-ubiquitinated on its K128 residue, which signals BCLb for proteasomal degradation. We hypothesize that when UBQLN1 is overexpressed, its UBA domain acts as a ubiquitin receptor and blocks chain elongation past mono-ubiquitination, thus preventing formation of K48 ubiquitin chains and degradation.
We tested whether UBQLN1-induced stability observed with BCLb could also be a phenomenon for other substrates of UBQLN1. Our IP/MS data (Fig. 5 A) and IP/WB data (Fig. 5 B) indicate that UBQLN1 interacts with insulin-like growth factor 1 receptor (IGF1R) and Extended Synaptogammin-2 (ESYT2) and like BCLb, it interacts with these 2 membrane proteins through its STI domains. We tested stability of IGF1R and ESYT2 (Fig. 5 C) under similar conditions as with BCLb (Fig. 4). Upon blocking the proteasome with MG132, IGF1R and ESYT2 expression is lost and most likely due to degradation via proteasomal-independent pathways. However, in the presence of UBQLN1, its loss is prevented. UBQLN1112X and UBQLN1ΔUBA, both missing the UBA domain, fail to protect IGF1R and ESYT2 from degradation. We found that as with BCLb, the UBA domain of UBQLN1 is responsible for stabilizing IGF1R and ESYT2.
Recently, Suzuki et al [Suzuki and Kawahara, 2016], showed that UBQLN4 is a component of the multi-protein proteasomal machinery complex involving BAG6 and is responsible for recognition of the exposed transmembrane domains of newly synthesized defective polypeptides, eventually leading to their proteasomal degradation. We found that BAG6 interacts with the UBL domain of UBQLN1 and also confirmed PSMD4 to be an interacting partner of UBQLN1 (Fig. 5 B). As UBL-interacting substrates of UBQLN1, we examined whether UBQLN1 binding affects stability of PSMD4 and BAG6 (Fig. 5 C). We tested this under similar conditions as with BCLb, IGF1R and ESYT2 and found that UBQLN1 does not regulate proteostasis of PSMD4 and BAG6 (Fig. 5 C). We detected no differences in the stability of PSMD4 and BAG6 in the presence UBQLN1WT, UBQLN1112X and UBQLN1ΔUBA when treated with translational or proteasomal inhibitors.
Several studies have addressed the role of UBA domain of UBQLN1 in stabilizing proteins. Mah et al [Mah et al., 2000], showed that the UBA domain of UBQLN1 binds Presenilin1 (PSEN1) and Presenilin2 (PSEN2) proteins. Massey et al [Massey et al., 2004] confirmed that the UBA domain binds PSEN1/2 and stabilizes these proteins by preventing its poly-ubiquitination and proteasomal degradation. Haapaslo et al [Haapasalo et al., 2011] found that overexpression of UBQLN1 stabilized higher molecular weight PSEN1, preventing its proteasomal enodproteolysis into smaller N-terminal fragment (NTF) and C-terminal fragment (CTF). Other substrates that have been reported to be stabilized by the UBA domain of UBQLN1 are p53 and IκBα. Overexpression of UBQLN1/2 prevented degradation of p53 by E6AP ubiquitin ligases and IκBα by SCFβTRCP ubiquitin ligase complex by blocking additional ubiquitination [Kleijnen et al., 2000].
Currently, there is little consensus on the working model of UBQLN1. Our data suggest that UBQLN1 is capable of forming dimers via its STI-4 domain (Fig. 6 A,B) and is active in its dimerized state as it continues to interact with exogenous BCLb as well as endogenous and exogenous IGF1R (Fig. 6 C). Our dimerization data confirm the mapped location of dimerization shown by Feng [Feng et al., 2004] (region 441-589) and Ford [Ford and Monteiro, 2006] referring to the STI-4 domain (Fig. 7 B) as middle regions, i.e. not the N-terminal UBL or C-terminal UBA. Hjerpe et al [Hjerpe et al., 2016] suggest that when not associated with substrates, UBQLN2 exists in a UBL-UBA conformation or in an inactive resting state. They hypothesize that UBQLN1 opens up and adopts a monomer form to execute its function. The UBL-UBA dimer hypothesis subsists because the UBL domain is essentially like the ubiquitin molecule, but this domain shares only 4 lysines (K6, K11, K27, K48) out of 7 (other three – K29, K33, K63) found in a ubiquitin molecule which may contribute to its decreased affinity for the UBA domain. The UBL domain of UBQLN1 has been shown to interact with proteins involved in the proteasomal machinery and not just UBA containing proteins. hHR23a is a UBL-UBA protein in this class that adopts a closed conformation due to a UBL-UBA interaction When UBL of hHR23a binds to the proteasomal subunit S5a, the closed conformation is disrupted [Walters et al., 2003]. However, hHR23a has a UBL domain with 11 lysines and 2 UBA domains and an XPC region for DNA excision repair. Functionally and location wise, UBQLN1 is different from hHR23a. UBQLN1 is a largely cytosolic protein, its UBL domain has fewer lysines and one UBA domain and it is probably ill advised to compare functional dynamics of UBQLN1 to another UBL-UBA protein.
Figure 7.
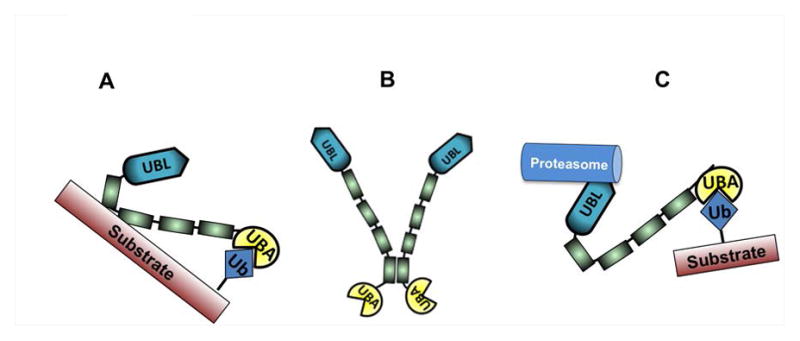
(A) Proposed substrate stabilization model of UBQLN1. (B) UBQLN1 dimerization via STI-4 domains. (C) Current working model of UBQLN1. Refer to discussion for detailed description of models.
Current model of UBQLN1 suggests that UBA domain of UBQLN1 binds to poly-ubiquitin chains of substrate and shuttles it to the proteasome via its UBL domain for degradation (Fig. 7 C). Our data indicate that UBQLN1 is capable of interacting with substrates via STI domains (BCLb, IGF1R, ESYT2), UBL domain (PSMD4, BAG6), and UBA domain (ubiquitin). Kleijnen et al [Kleijnen et al., 2003] showed that not only the UBL domain but also UBA domain on UBQLN2 is capable of directly binding to the S5a cap of the proteasome. The ability of the UBA domain to bind the proteasome is unique to UBQLN proteins as UBA domain of c-CBL is unable to bind to the proteasome [Kleijnen et al., 2003]. Therefore, it is possible that the role of UBQLN1’s UBA domain is to bind to ubiquitin on substrates and shield them from being recognized for degradation. The UBA domain of UBQLN1 is a highly conserved region across different species and across different isoforms of UBQLN1 protein (UBQLN1-4, UBQLNL). For over a decade, UBQLN1 has been shown to assist in degradation of proteins but our data suggest that UBQLN1 also stabilizes some proteins that it binds to, like BCLb, IGF1R and ESYT2. We propose a substrate-stabilization model of UBQLN1 (Fig. 7 A) such that primary association of UBQLN1 with substrate occurs through the STI domains and a secondary association through its UBA domain, which leads to stabilization of the substrate. We do not know whether primary associations through the UBL domain result in degradation of substrate but it does not appear to stabilize the substrates it binds to. We conclude that fate of substrates that UBQLN1 associates with, is interaction-domain specific. All 3 interacting partners of UBQLN1 stabilized by the UBA domain are membrane proteins; BCLb – mitochondrial membrane protein, IGF1R – plasma membrane protein, ESYT2 – endoplasmic reticulum/plasma membrane protein [Min et al., 2007]. We identified the transmembrane domain of BCLb to be essential to be recognized by UBQLN1 (Fig. 2 B). Thus, it is a plausible hypothesis that UBQLN1 recognizes proteins with transmembrane domains via its STI domains and stabilizes them via its UBA domain.
UBQLN1 is a known stress response protein and protects cells from heat shock and hypoxic stress [Hjerpe et al., 2016] and it seems reasonable that UBQLN1 stabilizes crucial survival proteins like BCLb, IGF1R and p53 over others. Future work will involve characterizing the mechanisms involved in regulation of IGF1R expression and activity by UBQLN1 and investigating whether UBQLN1 can be targeted in disorders resulting from IGF1R dysregulation.
Acknowledgments
Grant Information (to Levi Beverly)
This work was supported by an NCI RO1 R01CA193220 to LJB, funds from James Graham Brown Cancer Center, University of Louisville and Kosair Pediatric Cancer Program and Molecular Targets COBRE 8P20GM103482-10 from NIH, an award from the Lung Cancer Research Foundation to LJB, an award from the Rounsavall Foundation to LJB
We thank the members of the Beverly and Siskind labs for helpful comments and technical assistance. We thank Dr. Annakasia Haapasalo for the gift of the MYC-RFP-TV1 (UBQLN1) plasmid.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest with the contents of this article.
References
- Beverly LJ, Lockwood WW, Shah PP, Erdjument-Bromage H, Varmus H. Ubiquitination, localization, and stability of an anti-apoptotic BCL2-like protein, BCL2L10/BCLb, are regulated by Ubiquilin1. Proc Natl Acad Sci U S A. 2012;109:E119–26. doi: 10.1073/pnas.1119167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Scott CW, Cho NH, Nakamura H, Chung YH, Monteiro MJ, Jung JU. Kaposi’s sarcoma-associated herpesvirus K7 protein targets a ubiquitin-like/ubiquitin-associated domain-containing protein to promote protein degradation. Mol Cell Biol. 2004;24:3938–48. doi: 10.1128/MCB.24.9.3938-3948.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DL, Monteiro MJ. Dimerization of ubiquilin is dependent upon the central region of the protein: evidence that the monomer, but not the dimer, is involved in binding presenilins. Biochemical Journal. 2006;399:397–404. doi: 10.1042/BJ20060441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Tu H, Shi ST, Lee KJ, Asanaka M, Hwang SB, Lai MM. Interaction with a ubiquitin-like protein enhances the ubiquitination and degradation of hepatitis C virus RNA-dependent RNA polymerase. J Virol. 2003;77:4149–59. doi: 10.1128/JVI.77.7.4149-4159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo A, Viswanathan J, Kurkinen KM, Bertram L, Soininen H, Dantuma NP, Tanzi RE, Hiltunen M. Involvement of ubiquilin-1 transcript variants in protein degradation and accumulation. Communicative & integrative biology. 2011;4:428–432. doi: 10.4161/cib.4.4.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heir R, Ablasou C, Dumontier E, Elliott M, Fagotto-Kaufmann C, Bedford FK. The UBL domain of PLIC-1 regulates aggresome formation. EMBO Rep. 2006;7:1252–8. doi: 10.1038/sj.embor.7400823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerpe R, Bett JS, Keuss MJ, Solovyova A, McWilliams TG, Johnson C, Sahu I, Varghese J, Wood N, Wightman M. UBQLN2 Mediates Autophagy-Independent Protein Aggregate Clearance by the Proteasome. Cell. 2016;166:935–949. doi: 10.1016/j.cell.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Zavodszky E, Shao S, Wohlever ML, Keenan RJ, Hegde RS. Ubiquilins Chaperone and Triage mitochondrial membrane proteins for degradation. Molecular Cell. 2016;63:21–33. doi: 10.1016/j.molcel.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen MF, Alarcón RM, Howley PM. The ubiquitin-associated domain of hPLIC-2 interacts with the proteasome. Molecular biology of the cell. 2003;14:3868–3875. doi: 10.1091/mbc.E02-11-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, Gill G, Howley PM. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Molecular cell. 2000;6:409–419. doi: 10.1016/s1097-2765(00)00040-x. [DOI] [PubMed] [Google Scholar]
- Lee DY, Arnott D, Brown EJ. Ubiquilin4 is an adaptor protein that recruits Ubiquilin1 to the autophagy machinery. EMBO reports. 2013;14:373–381. doi: 10.1038/embor.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Hiltunen M, Romano DM, Soininen H, Hyman BT, Bertram L, Tanzi RE. Effects of ubiquilin 1 on the unfolded protein response. J Mol Neurosci. 2009;38:19–30. doi: 10.1007/s12031-008-9155-6. [DOI] [PubMed] [Google Scholar]
- Mah AL, Perry G, Smith MA, Monteiro MJ. Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation. J Cell Biol. 2000;151:847–62. doi: 10.1083/jcb.151.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey LK, Mah AL, Ford DL, Miller J, Liang J, Doong H, Monteiro MJ. Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins. J Alzheimers Dis. 2004;6:79–92. doi: 10.3233/jad-2004-6109. [DOI] [PubMed] [Google Scholar]
- Medicherla B, Kostova Z, Schaefer A, Wolf DH. A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep. 2004;5:692–7. doi: 10.1038/sj.embor.7400164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S-W, Chang W-P, Südhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proceedings of the National Academy of Sciences. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Kamionka M, Feigon J. Specificity of the interaction between ubiquitin-associated domains and ubiquitin. J Biol Chem. 2004;279:11926–36. doi: 10.1074/jbc.M312865200. [DOI] [PubMed] [Google Scholar]
- N’Diaye EN, Kajihara KK, Hsieh I, Morisaki H, Debnath J, Brown EJ. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO reports. 2009;10:173–179. doi: 10.1038/embor.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PP, Lockwood WW, Saurabh K, Kurlawala Z, Shannon SP, Waigel S, Zacharias W, Beverly LJ. Ubiquilin1 represses migration and epithelial-to-mesenchymal transition of human non-small cell lung cancer cells. Oncogene. 2015;34:1709–17. doi: 10.1038/onc.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Kawahara H. UBQLN4 recognizes mislocalized transmembrane domain proteins and targets these to proteasomal degradation. EMBO reports. 2016;17:842–857. doi: 10.15252/embr.201541402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kooij B, Rooswinkel RW, Kok F, Herrebout M, de Vries E, Paauwe M, Janssen GM, van Veelen PA, Borst J. Polyubiquitination and proteasomal turnover controls the anti-apoptotic activity of Bcl-B. Oncogene. 2013;32:5439–5448. doi: 10.1038/onc.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KJ, Lech PJ, Goh AM, Wang Q, Howley PM. DNA-repair protein hHR23a alters its protein structure upon binding proteasomal subunit S5a. Proceedings of the National Academy of Sciences. 2003;100:12694–12699. doi: 10.1073/pnas.1634989100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Wang J, Zheleznyak A, Brown EJ. Ubiquitin-related proteins regulate interaction of vimentin intermediate filaments with the plasma membrane. Mol Cell. 1999;4:619–25. doi: 10.1016/s1097-2765(00)80212-9. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raasi S, Fushman D. Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains. J Mol Biol. 2008;377:162–80. doi: 10.1016/j.jmb.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Z, Gu J, Zhang Y, Wang W, Shen H, Chen G, Wang X. Plic-1, a new target in repressing epileptic seizure by regulation of GABAAR function in patients and a rat model of epilepsy. Clinical Science. 2015;129:1207–1223. doi: 10.1042/CS20150202. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–52. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zheng C, Geetha T, Babu JR. Failure of ubiquitin proteasome system: risk for neurodegenerative diseases. Neurodegenerative Diseases. 2014;14:161–175. doi: 10.1159/000367694. [DOI] [PubMed] [Google Scholar]