Abstract
Myocardial ischemia/reperfusion and heart failure are the major cardiac conditions in which an imbalance between oxidative stress and anti-oxidant mechanisms is observed. The myocardium has endogenous reducing mechanisms, including the thioredoxin (Trx) and glutathione systems, that act to scavenge reactive oxygen species (ROS) and reduce oxidized proteins. The Trx system consists of Trx, Trx reductase (TrxR), and an electron donor, NADPH, where Trx is maintained in a reduced state in the presence of TrxR and NADPH. Trx1, a major isoform of Trx, is abundantly expressed in the heart and exerts its oxidoreductase activity through conserved Cys32 and Cys35, reducing oxidized proteins through thiol disulfide exchange reactions. In this review, we will focus on molecular targets of Trx1 in the heart, including transcription factors, microRNAs, histone deactylases, and protein kinases. We will then discuss how Trx1 regulates the functions of its targets, thereby affecting the extent of myocardial injury caused by myocardial ischemia/reperfusion and the progression of heart failure.
Keywords: Thioredoxin, disulfide, anti-oxidant, ischemia/reperfusion
1. Introduction
About six million people suffer from heart failure and about five million people are affected by ischemic heart disease in the US. Despite significant advances in medicine, heart failure is a major cause of death in developed countries [1]. Heart failure is often accompanied by mitochondrial dysfunction and increases in oxidative stress [2]. Myocardial ischemia occurs when the oxygen supply to the heart is interrupted due to blockage of a coronary artery. A severe blockage of the coronary vessels leads to myocardial infarction. Although interventions to allow resumption of the blood flow aim to preserve the viability of dying cardiac muscle and prevent expansion of myocardial infarction, reperfusion itself can cause damage as well, due to oxidative stress, calcium overload, and mitochondrial transition pore opening [1, 3]. Ischemia–reperfusion (I/R) is a condition that causes massive leakage of electrons from the mitochondrial electron transport chain and, consequently, a severe redox imbalance in the heart, leading to oxidative damage [3]. Redox stress in cells promotes post-translational oxidative modification of proteins, leading to structural modifications and changes in their function. This, in turn, can disrupt the normal function of the cell, leading to cell death via mechanisms including necrosis, apoptosis, autosis, and necroptosis [4]. Several endogenous reducing mechanisms are present in the cell to counteract this oxidative damage, including the thioredoxin (Trx) and glutathione systems [5]. Of these mechanisms, Trx1, a major isoform of Trx, is a key protein that regulates oxidative stress in the heart. In this review, we will discuss how Trx1 counteracts oxidative stress and regulates signaling mechanisms in the heart.
1.1. Thioredoxin1 (Trx1)
Trx1 is a small (12 kDa) ubiquitously expressed redox protein [6] that is evolutionarily conserved from yeast to mammals [7, 8]. Trx1 contains five cysteine residues of which two, namely Cys32 and Cys35, occur in the vicinity of the catalytic site [9, 10]. These two residues form the evolutionarily conserved redox motif (CXXC) through which Trx1 exerts its oxidoreductase activity [8, 11]. The Trx1 system consists of Trx1, Trx Reductase-1 (TrxR), and an electron donor, NADPH. The latter two are essential for reducing and, thus, recycling the oxidized Trx1 [12].
Through its ability to reduce cysteine residues, Trx1 can alter the structure and function of its target proteins. Trx1 interacts with a wide variety of proteins, including transcription factors and other signaling molecules, and regulates myriad cellular functions [13]. These include defense against oxidative stress, gene transcription, cell growth, cell survival and death and protein quality control. Trx1 is vital for embryo growth, development and survival. Deletion of Trx1 in mice is embryonically lethal since Trx1 is essential for early embryo morphogenesis, DNA replication, normal progression through the cell cycle and differentiation [14].
1.2. Mechanism of action
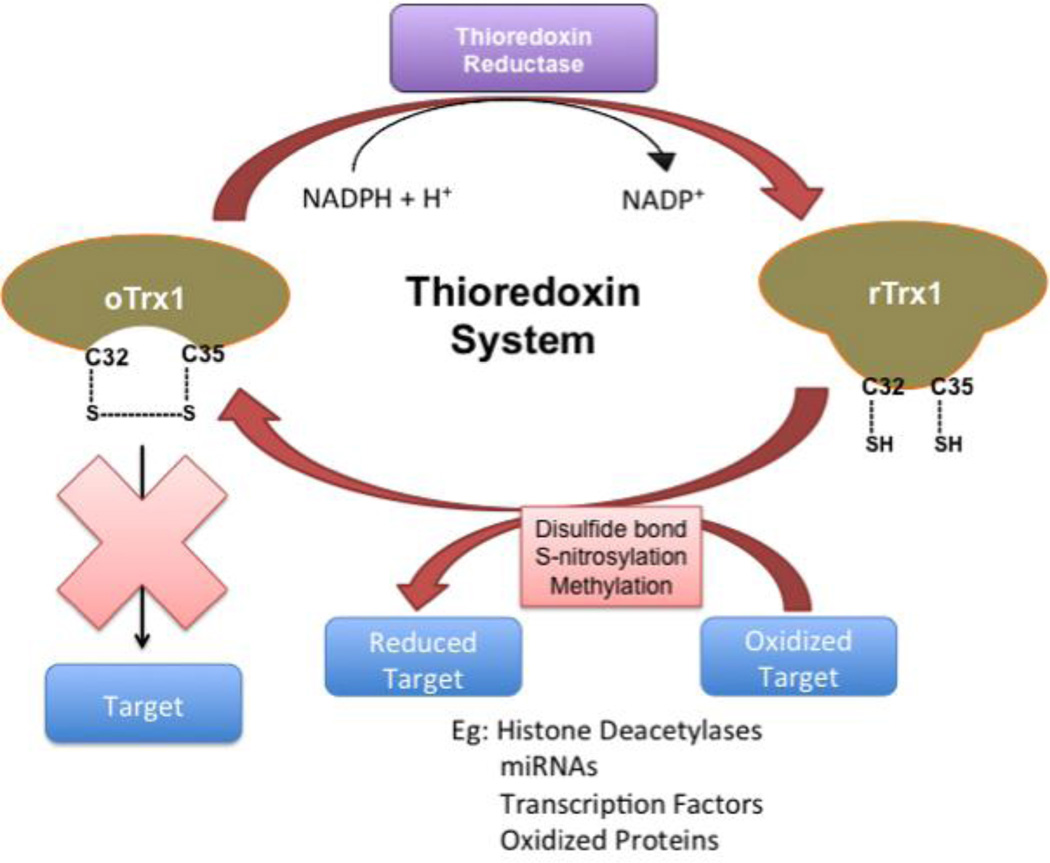
Trx1 primarily interacts with its target proteins to reduce their oxidized cysteine residues or to break disulfide bonds (Figure 1). This reaction is called a thiol disulfide exchange reaction and occurs as a two-step mechanism: First, through a nucleophilic attack, the Cys32 residue binds to the target substrate. Then, the Cys35 residue, almost instantly, reduces this bond and forms an intramolecular disulfide bridge with Cys32. This results in the reduction of the target substrate and oxidation of the Trx1 molecule. The oxidized Trx1 accepts an electron from NADPH and is then reduced by TrxR to continue the cycle [9]. Reduced Trx1 interacts with and reduces peroxiredoxin, which in turn converts H2O2 to water [15]. Thus, peroxiredoxin is a major target of Trx1 and mediates perhaps one of the most important mechanisms of Trx1, namely reduction of H2O2. Nevertheless, Trx1 also interacts with a wide variety of other molecules and regulates multiple cellular functions by directly reducing them.
Figure 1.
Reduced Trx1 with free Cys32 and Cys35 can interact with and reduce oxidized target molecules having disulfide bonds. Although this reaction produces oxidized Trx1 with an intramolecule disulfide linkage between Cys32 and Cys35, the oxidized Trx1 can be reduced again (recycled) by TrxR in the presence of the electron donor, NADPH. Examples of Trx1 targets include histone deactylase 4 (HDAC4) and AMP activated protein kinase (AMPK).
Several studies have been carried out to identify the substrates of Trx1. Identifying direct substrates of Trx1 has been challenging, however, since the attack by Cys35 on the bond formed between Cys32 and a target protein is virtually instantaneous, resulting in only a rapid, transient interaction between Trx1 and its targets. Various strategies have been employed to overcome this issue. Fu et al. utilized isotope-coded affinity tag (ICAT) - labeling to identify Trx1-targeted protein substrates in transgenic mice that overexpress Trx1 in a cardiac-specific manner (Tg-Trx1) [16]. ICAT reagents can label cysteine residues containing free thiol groups. Thus, proteins with greater ICAT labeling in Tg-Trx1 than in non-transgenic mice may be direct targets of Trx1 in the heart. The group identified 55 proteins containing 78 possible Trx1 redox sites, including proteins involved in the TCA cycle, the mitochondrial permeability transition pore complex and cardiac contractile proteins. However, a caveat to this approach is that ICAT labeling can potentially take place secondarily due to the global reductive environment in Tg-Trx1 rather than due to direct reduction by Trx1.
In order to identify direct targets of Trx1 in the heart in vivo, Shao et al. have developed a trapping mutant of Trx1 in which Cys35 is mutated to Ser [17]. In this mutant, the disulfide bridge between Trx1 Cys32 and the target substrate is relatively stable and, thus, the target protein can be identified through pull-down assays. Using this technique, they have identified AMPK as an oxidoreductase substrate of Trx1. Wu et al. utilized this strategy to identify additional targets of Trx1 in the nucleus [18]. Recently, Booze et al. have modified this trapping mutant transgenic mouse model by including a LoxP–STOP–LoxP cassette between the promoter and Flag-Trx1 C35S to allow temporal and cell-specific transgene expression [19]. In the next section, we will discuss specific targets of Trx1 and how Trx1 affects their function.
2. Specific targets of Trx1
Trx1 is ubiquitously expressed in many cell types and is found in various subcellular localizations. On the other hand, another isoform, Trx2, is primarily localized in mitochondria. In the current review, we will focus on the function of Trx1.
2.1. Nuclear targets
Trx1 interacts with and reduces the cysteine residues of several transcription factors, such as NF-κB, AP1 and p53, thereby promoting their binding with DNA targets and gene expression. For example, in Jurkat T-cells, reduced Trx1 directly interacts with NF-κB to reduce cysteine residues located in its components, namely p50 and p65 [20]. This interaction enhances the DNA binding ability of NF-κB and increases transcription of its target genes. On the other hand, oxidized Trx1 is located in the cytosol and is unable to bind to NF-κB. In macrophages, upon stimulation with lipopolysaccharides, p40phox interacts with oxidized Trx1, preventing its reduction and its ability to bind with NF-κB. On the other hand, inhibition of Nox2 by apocyanin promoted reduction and nuclear translocation of Trx1, thus enhancing its ability to bind to NF-κB and promoting transcription of several inflammatory mediators during sepsis [21]. Cytoplasmic binding of Trx1 to IκB prevents degradation of IκB and inhibits nuclear translocation of NF-κB in HSC-1 cells, thus decreasing its transcriptional activity [22]. Reduced Trx1 binds to Ref1, a DNA repair enzyme, which, in turn, reduces activator protein 1 (AP1), thereby potentiating the activity of AP1 in HeLa cells [23]. Other transcription factors possibly regulated by Trx1 include NRF1, NRF2, and CREB in cardiac myocytes [24]. Some of these transcription factors, such as NF-κB and NRF2, have cysteine residues that are directly reduced by Trx1. However, the mechanism by which IκB, NRF1 and CREB are regulated by Trx1 has not yet been elucidated.
Trx1 negatively regulates cardiac hypertrophy [25]. In order to elucidate the mechanism through which Trx1 regulates hypertrophy in the heart, Ago et al. performed a DNA microarray analysis to identify cardiac genes regulated by Trx1 [24]. Tg-Trx1 mouse hearts were compared with non-transgenic hearts and more than 3800 genes were found to be upregulated or downregulated in Tg-Trx1. Based on gene ontology grouping, genes involved in mitochondrial oxidative phosphorylation and the TCA cycle were found to be upregulated, including PGC-1α, complex-III, complex-IV, cytochrome C, Cox IV, Cox VB and TFAM. Furthermore, the ratio of mitochondrial DNA to genomic DNA was significantly increased and mitochondrial ATP content was increased in Tg-Trx1 hearts, suggesting that Trx1 is important for regulation of proteins involved in mitochondrial biogenesis and ATP production. Using transcription factor binding site (TFBS) analysis, they found stress-inducible transcription factors, like CREB and HIF-1, to be upregulated. These results suggest that Trx1 may alleviate heart failure during cardiac hypertrophy or I/R by preventing mitochondrial dysfunction. The molecular mechanism by which Trx1 affects the activity of these transcription factors in the heart remains to be elucidated.
Trx1 also regulates nucleo-cytoplasmic shuttling of class II histone deacetylases (HDACs) to attenuate cardiac hypertrophy [26]. Using transverse aortic constriction (TAC), a mouse model of pressure overload, cardiac hypertrophy and heart failure, HDAC4 was found to be oxidized at Cys667 and Cys669 and localized in the cytosol during cardiac hypertrophy. Hypertrophic stimuli upregulated Nox4 in the nucleus and HDAC4 oxidation was inhibited in cardiac-specific Nox4 knock-out mice, suggesting that reactive oxygen species (ROS) produced by nuclear Nox4 play an important role in mediating HDAC oxidation [27]. Cysteine oxidation of HDAC4 affects its three-dimensional structure and interferes with interaction between exportin and the nuclear export signal located in the C-terminus of HDAC4, thereby inducing nuclear localization of HDAC4. In Tg-Trx1 mice, however, oxidation of HDAC4 was decreased. Upon reduction of the oxidized residues, HDAC4 was transported back into the nucleus. In addition, DnaJb5 mRNA and protein expression levels were upregulated in Tg-Trx1 mice subjected to TAC surgery compared to in wild type mice. Trx1 forms a complex with DnaJb5 and thioredoxin-binding protein (TBP) to interact with and reduce HDAC4 at Cys667 and Cys669. This, in turn, affects HDAC4 localization and, thus, its ability to bind to its nuclear targets. HDAC4, when localized in the nucleus, interacts with transcription factors that mediate cardiac hypertrophy, such as NFAT, and suppresses their activity. Thus, Trx1 affects cardiac muscle growth during cardiac hypertrophy by regulating the redox milieu in the nucleus.
2.2. microRNAs
Trx1 also mediates its anti-hypertrophic activity through regulation of microRNA levels in the heart. Yang et al. have demonstrated that Trx1 inhibits angiotensin-II (Ang-II)-induced cardiac hypertrophy through upregulation of miR-98 [28]. Several members of the let-7 family of miRNAs were upregulated in Tg-Trx1 mice, including miR-98 and let a, b, c, e and f. In Tg-Trx1 mouse hearts, miR-98 was upregulated, which, in turn, reduced cyclin D2 expression. Cyclin D2 has three miR-98 binding sites in its 3’UTR. Cyclin D2 is essential for cell size regulation by Ang-II and downregulation of its expression by miR-98 reduced Ang-II-induced cardiac hypertrophy. However, reversal of this miR-98-mediated cyclin D2 downregulation with adenovirus expressing cyclin D2 abolished the suppression of Ang II-induced hypertrophy by miR-98. The molecular mechanism by which Trx1 upregulates miR-98 in cardiomyocytes remains to be elucidated.
2.3. Proteins kinases
Various studies have shown that Trx1 inhibits apoptosis, a cell death mechanism that plays an important role in the development of heart failure. One of the mechanisms by which Trx1 inhibits apoptosis is via its interaction with Apoptosis signal-regulating kinase 1 (ASK1). Oxidative stress activates the ASK1-JNK-p38 pathway, which can, in turn, activate caspase 3 and thus promote apoptosis. Using the yeast two-hybrid system, reduced Trx1 has been shown to bind to the N-terminal region of ASK1 to inhibit the kinase activity of ASK1 and thus prevent apoptosis [29]. Trx1 binding to ASK1 promoted ASK1 ubiquitination and degradation [30]. This interaction is dependent upon the redox status of Trx1 and the redox inactive (DN-Trx1) mutant of Trx1 fails to bind to ASK1, although binding is not disrupted if either one of the cysteines (Cys32 or Cys35) is mutated [30]. During oxidative stress, the disulfide bond formed between Cys32 and Cys35 of Trx1 prevents intermolecular disulfide bond formation between Trx1 and ASK1 at Cys200 and Trx1 dissociates from ASK1 [31]. This not only stabilizes ASK1 but also allows ASK1 to be oxidized at Cys250, which in turn stimulates activation of JNK and induction of apoptosis [32]. Although dissociation of Trx1 and stabilization of ASK1 are critical for activation of ASK1 by oxidative stress, the additional post-translational modification, namely oxidation of Cys250, appears to be critical for further activation of JNK and apoptosis.
In type 2 diabetes cardiac injury models, cardiac dysfunction is enhanced, Trx1-ASK1 interaction is significantly reduced and apoptosis is stimulated [33]. In addition, Ang II activates ASK1 to promote hypertrophy in cardiomyocytes [34]. Thus, Trx1 interaction with ASK1 could regulate both hypertrophy and apoptosis. However, in patients with dilated cardiomyopathy, Trx1 levels were comparable with those of healthy persons while Trx2 expression was decreased and ASK1 activity was increased. Trx2 also directly interacts with ASK1 and Trx2-cKO mice exhibited increases in ASK1 activity, mitochondrial ROS and apoptosis, leading to mitochondrial dysfunction and development of dilated cardiomyopathy. Attenuation of ASK1 activity in Trx2-cKO mice prevented them from developing dilated cardiomyopathy, suggesting that, like Trx1, Trx2 regulates cellular apoptosis, possibly through regulation of ASK1 [35]. These studies indicate that cytosolic Trx1 and mitochondrial Trx2 have unique mechanisms to protect the heart - Trx1 against cardiac hypertrophy and Trx2 against dilated cardiomyopathy.
A recent work by Shao et al. further investigated the mechanism by which Trx1 protects the heart from cell death during energy starvation [17]. The authors used a Flag-Trx1 C35S-HA-Tg mutant mouse model that can trap the reaction between Trx1 and its target proteins. Using this mouse model, the authors found that Trx1 interacts with AMPK, an energy sensor in cardiomyocytes. This interaction is further enhanced during oxidative stress conditions, such as upon treatment with H2O2 or during prolonged ischemia in the heart. The Trx1-AMPK interaction occurs via a disulfide linkage, as evidenced by the fact that the enhanced interaction was abolished when the protein lysis buffer contained DTT, a reducing agent. During myocardial ischemia, AMPK is oxidized at Cys130 and Cys174 to form intermolecular disulfide bonds. Oxidized AMPK forms protein aggregates, preventing phosphorylation of AMPK by AMPK kinases. Hence, oxidized AMPK is kinase inactive and cannot function as an energy sensor. Trx1 can reduce oxidized AMPK to prevent its inactivation and reduce cardiac infarct size following 3 hours of ischemia. However, under high fat feeding conditions, Trx1 is downregulated, resulting in increased AMPK oxidation levels. These mice are more prone to myocardial infarction during ischemia than those fed a normal diet. Taken together, these results suggest that Trx1 acts as a co-factor for AMPK during energy stress. Since cellular conditions in which AMPK is activated are often accompanied by oxidative stress, the presence of reduced Trx1 in cardiomyocytes is quite important to maintain the function of AMPK during times when activation of AMPK is really needed.
3. Post-translational modifications other than oxidation
3.1. S-nitrosylation
Reactive cysteines in Trx1 can undergo other forms of post-translational modification as well. Trx1 is S-nitrosylated and can also trans/denitrosylate its target proteins to mediate its functions [36–39]. S-nitrosylation of Trx1 may occur at Cys62, Cys69 or Cys73, depending on the pH of the cellular milieu and redox status of Trx1 [36, 37, 40, 41]. During ischemic preconditioning, cellular protein S-nitrosylation was increased, and it has been shown to be protective during I/R [42, 43]. Trx1 has been shown to trans/denitrosylate proteins, such as ASK1, caspase 3, caspase 8, caspase 9 and NF-κB, to regulate apoptosis and, thus, regulate cell survival in various cell types, including endothelial cells, Jurkat cells, HeLa cells, RAW macrophages, and HepG2 cells [39, 44–51]. Extracellular delivery of S-nitrosylated Trx1 increases the abundance of S-nitrosylated proteins in the heart, which is accompanied by cardioprotection [52]. Although direct trans/denitrosylation of these proteins by Trx1 has not been demonstrated in the heart in an unequivocal manner, modulation of these proteins has been shown to regulate apoptosis and cardiomyocyte survival during cardiac hypertrophy and I/R. A possible hypothesis is that regulation of cardiac protein S-nitrosylation by Trx1 may serve as a redox modification to protect the target protein molecules from irreversible oxidative damage. More studies are required to understand the functional role of Trx1-mediated regulation of S-nitrosylation in the heart.
3.2. H2S
Nicholson et al. investigated whether Trx1 contributes to H2S-mediated cardioprotection [53]. H2S increases Trx1 expression and activity in the heart and has been shown to be cytoprotective. In addition, mice treated with H2S during I/R show better cardiac function with increased ejection fraction and fractional shortening. However, the H2S treatment did not exert its protective effect in Tg-DN-Trx1 mice. Furthermore, H2S treatment Trx1-dependently reduced activation of the ASK1-JNK pathway and nuclear export of HDAC4. This demonstrates that Trx1 is essential for the cardioprotection mediated by H2S. It is possible that Trx1, in conjunction with H2S, may also induce some other types of post-translational modification in proteins, such as S-sulfhydration.
3.3. Methylation
In addition to its oxidoreductase-mediated regulation of target proteins, Trx1 participates in other post-translational modifications such as methylation. Recently, Liu et al. found that Trx1 regulates lysine methylation, a non-redox protein modification [54]. They used iTRAQ to evaluate alterations in protein expression levels after TAC surgery in Tg-Trx1 mice. The expression level was altered for a few proteins, including SMYD1, a lysine methyltransferase that plays an important role in cardiac development, which was accompanied by changes in the level of protein methylation of some proteins in the heart. Thus, it would appear that Trx1 may regulate gene expression and protein function during cardiac hypertrophy through modification of methylation.
4. Role of Trx1 in the heart in vivo
4.1. Cardiac hypertrophy and heart failure
Trx1 negatively regulates Ang II-induced myocardial hypertrophy [28, 55]. Overexpressed DN-Trx1 (Trx1 C32S/C35S lacking Trx1 redox activity) acts as a dominant negative, thereby decreasing the activity of endogenous Trx1. The DN-Trx1 mouse model showed increased oxidative stress, as well as increases in cardiomyocyte size and, consequently, enhanced cardiac hypertrophy [25]. These results suggest that Trx1 is a negative regulator of cardiac hypertrophy.
In a rat model of type I diabetes subjected to MI, rats that received intramyocardial administration of adenovirus harboring Trx1 (Ad-Trx1) exhibited reduced fibrosis and apoptosis and improved cardiac function compared to rats that were administered adenovirus harboring β-galactosidase (Ad-LacZ), suggesting that Trx1 gene therapy confers cardioprotection during heart failure [56]. Overexpression of Trx1 also inhibits mitochondrial dysfunction, promotes mitochondrial turnover and prevents heart failure in septic mice [57], and reduces adriamycin-induced oxidative stress, thus preventing cardiotoxicity [55].
Sumanth et al. administered mesenchymal stem cells (MSCs) transduced with either Ad-Trx1 or Ad-LacZ into the cells in the peri-infarct area during MI. Increasing Trx1 expression in the MSCs increased their proliferation and pluripotency in the heart, and the MSCs more readily differentiated into cardiomyocytes, smooth muscle cells and endothelial cells than MSCs without Trx1 overexpression. Furthermore, fibrosis was decreased and intercellular connections were increased, suggesting that engineering MSCs to express Trx1 could be a potential therapeutic measure to treat heart failure [58].
4.2. Myocardial ischemia and I/R
Acute myocardial ischemia upregulates endogenous Trx1 in the heart. Myocardial infarct size was increased after 3-hour prolonged ischemia in Tg-Trx1 C35S and Tg-DN-Trx1 mice, demonstrating that the redox activity of Trx1 is vital for cardiac protection during myocardial ischemia [17]. These results suggest that Trx1 represents an endogenous mechanism to protect the heart against myocardial ischemia. Exogenous application of hTrx1 after 30 minutes of ischemia and 10 minutes before reperfusion decreased infarct size following I/R compared to in the vehicle-treated model. Interestingly, addition of S-nitrosylated Trx1 enhanced this protective effect of Trx1, possibly by stimulating uptake of Trx1 into cardiomyocytes or increasing the abundance of S-nitrosylated proteins in the heart [52]. Thus, enhancing the action of Trx1 appears to be beneficial during I/R injury.
4.3. Preconditioning
Earlier studies have demonstrated that Trx1 is upregulated during ischemic preconditioning [59] and that Tg-Trx1 mice subjected to I/R show a significantly decreased infarct size compared to non-Tg mice [17]. Thus, it is likely that Trx1 mediates the effect of ischemic preconditioning, an endogenous mechanism of protection against myocardial ischemia. Interestingly, however, this protective effect of Trx1 appears to be age-dependent: Although the infarct size was reduced following I/R in young Tg-Trx1 mice (3-month old) compared to in non-Tg mice, the protective effect was not observed in middle-aged (12-month old) Tg-Trx1 mice [60]. Both the protein level and the activity of Trx1 were decreased after 15 minutes of reperfusion in young mice, but they were decreased sooner in middle-aged mice. Inactivation of Trx1 could be due to an increase in protein nitration in middle-aged Trx1 mice compared to in young Trx1 mice. Other studies have reported that during reperfusion after ischemia, there is an increase in peroxynitrite concentration, which could in turn lead to depletion of antioxidants such as Trx1 [61]. It is proposed that Trx1-mediated cardioprotection occurs via activation of the Akt-GSK-3β pathway, possibly through an interaction between Trx1 and Akt [60].
Remote ischemic preconditioning (RIPC) in cardiac surgery patients led to increased levels of Trx1 in cardiac tissue, suggesting that Trx1 is an RIPC-induced factor that could potentially mediate RIPC-induced cardioprotection [62].
4.4. Postconditioning
Postconditioning is another endogenous protective mechanism that reduces infarct size after I/R. In Tg-Trx1 mice, the infarct size following I/R was reduced compared to in WT mice, but postconditioning did not show an additive effect. In contrast, in Tg-DN-Trx1, the protective effect of postconditioning was not observed [63, 64]. Furthermore, in young mice, ischemic postconditioning conferred a protective phenotype after I/R by preventing Trx1 degradation and, thus, increasing Akt and GSK-3β phosphorylation. However, the postconditioning effect was abolished in middle-aged and older mice, as Trx1 was degraded rapidly [64, 65]. This suggests that Trx1 plays an important role in mediating the cardioprotection afforded by ischemic postconditioning.
4.5. Protein quality control
Trx1 is also protective in the R120G αβ-crystallin mouse, a mouse model of desmin-related cardiomyopathy, where accumulation of protein aggregates leads to cardiac hypertrophy and heart failure. In these mice, both reduced Trx1 and TrxR1 are upregulated through Nrf2 activation and prevent accumulation of cytoplasmic protein aggregates. This finding is of interest because it has been suggested that protein aggregates are formed due to reductive stress in desmin-related myopathy [66]. The fact that an antioxidant mechanism can reduce protein aggregates even in the presence of reductive stress conditions indicates that the protective effect of the Trx1 system may be compartmentalized.
Although Trx1 functions to protect the heart in many instances, some exceptions seem to exist. In a recent study, Korge et al. proposed that activation of the Trx system can cause excessive production of mitochondrial ROS, thereby playing a detrimental role in the heart. They demonstrated that TrxR and glutathione reductase can leak electrons and add them to O2 to generate ROS in the absence of the appropriate electron acceptors, such as oxidized Trx, during reductive stress in the heart, leading to oxidative damage [67]. These results suggest that, depending on the redox atmosphere in the cell, Trx can be either beneficial or detrimental. Hence, therapeutic upregulation of Trx1 must be carefully regulated based on the cellular redox milieu in the heart. More studies are essential to identify the appropriate cellular conditions under which Trx1 can be administered to a patient for it to have a beneficial effect rather than a detrimental one.
4.6. Autophagy
Recent studies propose a novel function for Trx1 in the regulation of macroautophagy, a major mechanism of degradation by which proteins and organelles are removed through lysosomes. Increasing lines of evidence suggest that Trx1 is involved in regulation of autophagy by interacting with the molecules regulating autophagy. For example, Trx1-mediated autophagy regulation has been implicated in neurodegenerative disorders. In amyotrophic lateral sclerosis, Trx1 binds to Mst1, rendering it inactive and, thus, stimulating autophagosome formation. Dissociation of this binding occurs via an ALS-associated G93A mutant of human superoxide dismutase 1, leading to an autophagic flux imbalance in lumbar spinal cord motor neurons that is responsible for the severity of the disease [68]. Trx1 also regulates autophagy through Atg4 (Autophagy related 4). Trx1 reduces the disulfide bond between Cys338 and Cys394 of Atg4 to activate it, thus regulating autophagy in S. Cerevisiae [69]. During physiological stresses such as hypoxia, exercise or starvation, Trx–interacting protein (TXNIP), a negative regulator of Trx1, binds to REDD1 to increase ROS. ROS can inhibit Atg4B and promote stress-induced autophagy during energy deprivation [70].
In the heart, Tg-Trx1 mice showed increased LC3-II/LC3-I, indicating upregulation of autophagy, compared to in WT mice [57]. To our knowledge, no studies have been carried out to study the effect of Trx1 on autophagy in the heart during stress. Autophagy is an important prosurvival mechanism during myocardial ischemia. More studies are necessary to better understand how Trx1 can be utilized therapeutically to control autophagy in the myocardium.
5. Conclusions
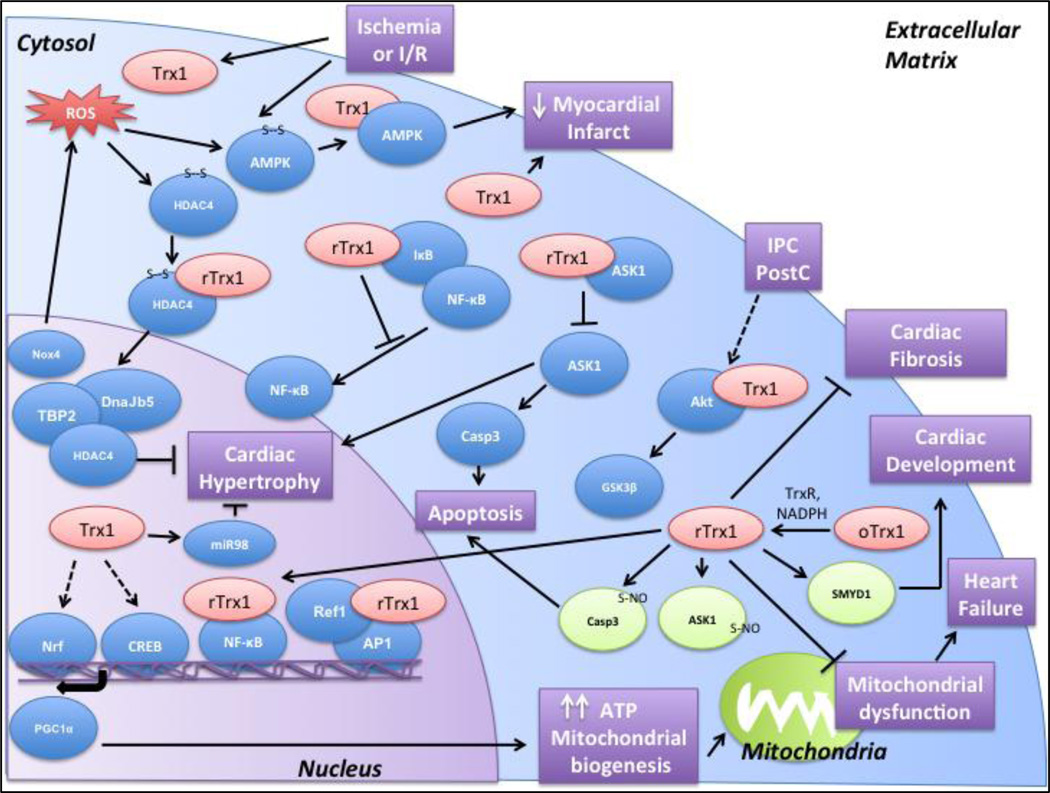
Trx1 regulates various signaling pathways via its redox activity to exert cardiac protection (Figure 2). In addition to the above targets discussed in this review, several other targets have been identified in various global profiling studies. Studying these protein targets will shed light on several other mechanisms regulated by Trx1. In addition, Trx1 has been implicated in other forms of post-translational modification besides oxidation, namely protein S-nitrosylation and methylation of targets. Elucidating the mechanisms by which Trx1 performs its functions and the signaling mechanisms it regulates will help in developing novel therapeutic approaches to treating ischemic heart disease and heart failure.
Figure 2.
Trx1 binds to its targets and exerts cardioprotection by preventing mitochondrial dysfunction, increasing ATP production and mitochondrial biogenesis, inhibiting apoptosis, and preventing cardiac hypertrophy and cardiac fibrosis. It mainly interacts with and reduces disulfide bonds in its target molecules. It can also participate in other post-translational modifications such as S-nitrosylation and methylation (indicated in green). The functional significance of Trx1 in the heart is highlighted in purple. (Abbreviations: ROS- Reactive Oxygen Species, I/R- Ischemia/Reperfusion, IPC- Ischemic Preconditioning, Post-Postconditioning)
Highlights.
Trx1 is an important antioxidant that is cardioprotective during myocardial ischemia/reperfusion and heart failure.
Trx1 reduces oxidized proteins through a thiol disulfide exchange reaction.
Trx1 may also modify the nitrosylation and methylation status of proteins.
Acknowledgments
We thank Daniela Zablocki (Rutgers New Jersey Medical School) for assistance with the manuscript.
Sources of Funding
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330 and AG23039 (J.S.), and by the Leducq Foundation Transatlantic Network of Excellence (J.S.). N.N. has been supported by a Predoctoral Fellowship from the American Heart Association, Founders Affiliate.
Abbreviations
- Akt
protein kinase B
- AMPK
Adenosine Monophosphate-activated Protein Kinase
- Ang-II
Angiotensin-II
- AP1
Activator protein 1 (AP-1)
- ASK1
Apoptosis Signal-regulating kinase
- Atg4
Autophagy related-4
- ATP
Adenosine Triphosphate
- CDDP
cis-diammine-dichloroplatinum
- CREB
cAMP response element binding protein
- Cyp
Cyclophilin
- Cys
Cysteine
- DN
Dominant Negative
- DTT
Dithiothreitol
- GSK
Glycogen Synthase Kinase
- H2S
Hydrogen Sulfide
- HDAC
Histone deacetylase
- HIF-1
Hypoxia-inducible factor-1
- ICAT
Isotope-coded affinity tag
- iTRAQ
Isobaric tags for relative and absolute quantitation
- IκB
Inhibitor-κB
- MI
Myocardial Infarction
- MSC
Mesenchymal Stem Cells
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NF-κB
Nuclear Factor-κB
- NO
Nitric Oxide
- Nox
NADPH oxidase
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator-1α
- Prx
Peroxiredoxin
- REDD1
Regulated in development and DNA damage responses 1
- RIPC
Remote Ischemic Preconditioning
- ROS
Reactive Oxygen Species
- SMYD1
SET And MYND Domain Containing 1
- SOD1
Superoxide Dismutase 1
- TAC
Transverse Aortic Constriction
- TCA
Tricarboxylic acid
- TFBS
Transcription Factor Binding Site
- Tg
Transgenic
- Trx
Thioredoxin
- TrxR
Thioredoxin Reductase
- TXNDC17
Trx1 domain containing 17
- TXNIP
Trx-interacting protein
- UTR
Untranslated region
- ZDF
Zucker Diabetic Fatty rats
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8(3):132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65(14):1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292(3):H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 6.Tagaya Y, Maeda Y, Mitsui A, Kondo N, Matsui H, Hamuro J, Brown N, Arai K, Yokota T, Wakasugi H, et al. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiolreduction in the IL-2 receptor induction. The EMBO journal. 1989;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powis G, Mustacich D, Coon A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic Biol Med. 2000;29(3–4):312–322. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 8.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 10.Yamawaki H, Berk BC. Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens. 2005;14(2):149–153. doi: 10.1097/00041552-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396(1):120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 13.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 14.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178(1):179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 15.Watson WH, Yang X, Choi YE, Jones DP, Kehrer JP. Thioredoxin and its role in toxicology. Toxicol Sci. 2004;78(1):3–14. doi: 10.1093/toxsci/kfh050. [DOI] [PubMed] [Google Scholar]
- 16.Fu C, Wu C, Liu T, Ago T, Zhai P, Sadoshima J, Li H. Elucidation of thioredoxin target protein networks in mouse. Mol Cell Proteomics. 2009;8(7):1674–1687. doi: 10.1074/mcp.M800580-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao D, Oka S, Liu T, Zhai P, Ago T, Sciarretta S, Li H, Sadoshima J. A Redox-Dependent Mechanism for Regulation of AMPK Activation by Thioredoxin1 during Energy Starvation. Cell Metab. 2014;19(2):232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Jain MR, Li Q, Oka S, Li W, Kong AN, Nagarajan N, Sadoshima J, Simmons WJ, Li H. Identification of novel nuclear targets of human thioredoxin 1. Mol Cell Proteomics. 2014;13(12):3507–3518. doi: 10.1074/mcp.M114.040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booze ML, Hansen JM, Vitiello PF. A novel mouse model for the identification of thioredoxin-1 protein interactions. Free Radic Biol Med. 2016;99:533–543. doi: 10.1016/j.freeradbiomed.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20(15):3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trevelin SC, Dos Santos CX, Ferreira RG, de Sa Lima L, Silva RL, Scavone C, Curi R, Alves-Filho JC, Cunha TM, Roxo-Junior P, Cervi MC, Laurindo FR, Hothersall JS, Cobb AM, Zhang M, Ivetic A, Shah AM, Lopes LR, Cunha FQ. Apocynin and Nox2 regulate NF-kappaB by modifying thioredoxin-1 redox-state. Scientific reports. 2016;6:34581. doi: 10.1038/srep34581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274(39):27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 23.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ago T, Yeh I, Yamamoto M, Schinke-Braun M, Brown JA, Tian B, Sadoshima J. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid Redox Signal. 2006;8(9–10):1635–1650. doi: 10.1089/ars.2006.8.1635. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112(9):1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133(6):978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima S, Kuroda J, Ago T, Zhai P, Park JY, Xie LH, Tian B, Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ Res. 2013;112(4):651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Ago T, Zhai P, Abdellatif M, Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res. 2011;108(3):305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO journal. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90(12):1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 31.Kylarova S, Kosek D, Petrvalska O, Psenakova K, Man P, Vecer J, Herman P, Obsilova V, Obsil T. Cysteine residues mediate high-affinity binding of thioredoxin to ASK1. Febs J. 2016;283(20):3821–3838. doi: 10.1111/febs.13893. [DOI] [PubMed] [Google Scholar]
- 32.Nadeau PJ, Charette SJ, Landry J. REDOX reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20(16):3628–3637. doi: 10.1091/mbc.E09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Zhang Y, Li X, Wang R, Jiao X. Variations of thioredoxin system contributes to increased susceptibility to apoptosis in cardiomyocytes of type 2 diabetic rats. Acta Biochim Biophys Sin (Shanghai) 2014;46(4):318–329. doi: 10.1093/abbs/gmu006. [DOI] [PubMed] [Google Scholar]
- 34.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93(9):874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Zhou HJ, Zhang H, Huang Y, Hinojosa-Kirschenbaum F, Fan P, Yao L, Belardinelli L, Tellides G, Giordano FJ, Budas GR, Min W. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation. 2015;131(12):1082–1097. doi: 10.1161/CIRCULATIONAHA.114.012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barglow KT, Knutson CG, Wishnok JS, Tannenbaum SR, Marletta MA. Site-specific and redox-controlled S-nitrosation of thioredoxin. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):E600–E606. doi: 10.1073/pnas.1110736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Liu T, Chen W, Oka S, Fu C, Jain MR, Parrott AM, Baykal AT, Sadoshima J, Li H. Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cell Proteomics. 2010;9(10):2262–2275. doi: 10.1074/mcp.M110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta R, Holmgren A. Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid Redox Signal. 2013;18(3):259–269. doi: 10.1089/ars.2012.4716. [DOI] [PubMed] [Google Scholar]
- 39.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320(5879):1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weichsel A, Brailey JL, Montfort WR. Buried S-nitrosocysteine revealed in crystal structures of human thioredoxin. Biochemistry. 2007;46(5):1219–1227. doi: 10.1021/bi061878r. [DOI] [PubMed] [Google Scholar]
- 41.Weichsel A, Kem M, Montfort WR. Crystal structure of human thioredoxin revealing an unraveled helix and exposed S-nitrosation site. Protein Sci. 2010;19(9):1801–1806. doi: 10.1002/pro.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong G, Aponte AM, Kohr MJ, Steenbergen C, Murphy E, Sun J. Postconditioning leads to an increase in protein S-nitrosylation. Am J Physiol Heart Circ Physiol. 2014;306(6):H825–H832. doi: 10.1152/ajpheart.00660.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101(11):1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 44.Sengupta R, Billiar TR, Kagan VE, Stoyanovsky DA. Nitric oxide and thioredoxin type 1 modulate the activity of caspase 8 in HepG2 cells. Biochem Biophys Res Commun. 2010;391(1):1127–1130. doi: 10.1016/j.bbrc.2009.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sengupta R, Holmgren A. The role of thioredoxin in the regulation of cellular processes by S-nitrosylation. Biochim Biophys Acta. 2012;6:689–700. doi: 10.1016/j.bbagen.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Sumbayev VV. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2003;415(1):133–136. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 47.Kelleher ZT, Sha Y, Foster MW, Foster WM, Forrester MT, Marshall HE. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor kappaB (NF-kappaB) activation. J Biol Chem. 2014;289(5):3066–3072. doi: 10.1074/jbc.M113.503938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell DA, Morton SU, Fernhoff NB, Marletta MA. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1(3):154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 50.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Wan A, Xu G, Ye D. Small changes huge impact: the role of thioredoxin 1 in the regulation of apoptosis by S-nitrosylation. Acta Biochim Biophys Sin (Shanghai) 2013;45(3):153–161. doi: 10.1093/abbs/gms103. [DOI] [PubMed] [Google Scholar]
- 52.Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, Feelisch M, Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected] . Proc Natl Acad Sci U S A. 2004;101(31):11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicholson CK, Lambert JP, Molkentin JD, Sadoshima J, Calvert JW. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler Thromb Vasc Biol. 2013;33(4):744–751. doi: 10.1161/ATVBAHA.112.300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T, Wu C, Jain MR, Nagarajan N, Yan L, Dai H, Cui C, Baykal A, Pan S, Ago T, Sadoshima J, Li H. Master redox regulator Trx1 upregulates SMYD1 & modulates lysine methylation. Biochim Biophys Acta. 2015;1854(12):1816–1822. doi: 10.1016/j.bbapap.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S, Yodoi J. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation. 2002;106(11):1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 56.Samuel SM, Thirunavukkarasu M, Penumathsa SV, Koneru S, Zhan L, Maulik G, Sudhakaran PR, Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121(10):1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Villamil JP, D'Annunzio V, Finocchietto P, Holod S, Rebagliati I, Perez H, Peralta JG, Gelpi RJ, Poderoso JJ, Carreras MC. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. Int J Biochem Cell Biol. 2016 doi: 10.1016/j.biocel.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 58.Suresh SC, Selvaraju V, Thirunavukkarasu M, Goldman JW, Husain A, Alexander Palesty J, Sanchez JA, McFadden DW, Maulik N. Thioredoxin-1 (Trx1) engineered mesenchymal stem cell therapy increased pro-angiogenic factors, reduced fibrosis and improved heart function in the infarcted rat myocardium. Int J Cardiol. 2015;201:517–528. doi: 10.1016/j.ijcard.2015.08.117. [DOI] [PubMed] [Google Scholar]
- 59.Das DK. Thioredoxin regulation of ischemic preconditioning. Antioxid Redox Signal. 2004;6(2):405–412. doi: 10.1089/152308604322899477. [DOI] [PubMed] [Google Scholar]
- 60.V DA, Perez V, Mazo T, Munoz MC, Dominici FP, Carreras MC, Poderoso JJ, Sadoshima J, Gelpi RJ. Loss of myocardial protection against myocardial infarction in middle-aged transgenic mice overexpressing cardiac thioredoxin-1. Oncotarget. 2016;7(11):11889–11898. doi: 10.18632/oncotarget.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Przyklenk K. Efficacy of cardioprotective 'conditioning' strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28(5):331–343. doi: 10.2165/11587190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Zitta K, Meybohm P, Gruenewald M, Cremer J, Zacharowski KD, Scholz J, Steinfath M, Albrecht M. Profiling of cell stress protein expression in cardiac tissue of cardiosurgical patients undergoing remote ischemic preconditioning: implications for thioredoxin in cardioprotection. J Transl Med. 2015;13:34. doi: 10.1186/s12967-015-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez V, V DA, Mazo T, Marchini T, Caceres L, Evelson P, Gelpi RJ. Inhibition of endogenous thioredoxin-1 in the heart of transgenic mice does not confer cardioprotection in ischemic postconditioning. Int J Biochem Cell Biol. 2016 doi: 10.1016/j.biocel.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 64.D'Annunzio V, Perez V, Boveris A, Gelpi RJ, Poderoso JJ. Role of thioredoxin-1 in ischemic preconditioning, postconditioning and aged ischemic hearts. Pharmacol Res. 2016;109:24–31. doi: 10.1016/j.phrs.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Perez V, V DA, Mazo T, Marchini T, Caceres L, Evelson P, Gelpi RJ. Ischemic postconditioning confers cardioprotection and prevents reduction of Trx-1 in young mice, but not in middle-aged and old mice. Mol Cell Biochem. 2016;415(1–2):67–76. doi: 10.1007/s11010-016-2677-2. [DOI] [PubMed] [Google Scholar]
- 66.Banerjee Mustafi S, Grose JH, Zhang H, Pratt GW, Sadoshima J, Christians ES, Benjamin IJ. Aggregate-prone R120GCRYAB triggers multifaceted modifications of the thioredoxin system. Antioxid Redox Signal. 2014;20(18):2891–2906. doi: 10.1089/ars.2013.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korge P, Calmettes G, Weiss JN. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim Biophys Acta. 2015;1847(6–7):514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JK, Shin JH, Hwang SG, Gwag BJ, McKee AC, Lee J, Kowall NW, Ryu H, Lim DS, Choi EJ. MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(29):12066–12071. doi: 10.1073/pnas.1300894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Perez ME, Zaffagnini M, Marchand CH, Crespo JL, Lemaire SD. The yeast autophagy protease Atg4 is regulated by thioredoxin. Autophagy. 2014;10(11):1953–1964. doi: 10.4161/auto.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun. 2015;6:7014. doi: 10.1038/ncomms8014. [DOI] [PMC free article] [PubMed] [Google Scholar]