Abstract
The small GTPase Arf6 is a conserved protein that is expressed in all metazoans. Arf6 remodels cytoskeletal actin and mediates membrane protein trafficking between the plasma membrane in its active form and endosomal compartments in its inactive form. While a rich knowledge exists for the cellular functions of Arf6, relatively little is known about its physiological role in development. This study examines the function of Arf6 in mediating cellular morphogenesis in early development. We dissect the function of Arf6 with a loss-of-function morpholino and constitutively active Arf6-Q67L construct. We focus on the two cell types that undergo active directed migration: the primary mesenchyme cells (PMCs) that give rise to the sea urchin skeleton and endodermal cells that form the gut. Our results indicate that Arf6 plays an important role in skeleton formation and PMC migration, in part due to its ability to remodel actin. We also found that embryos injected with Arf6 morpholino have gastrulation defects and embryos injected with constitutively active Arf6 have endodermal cells detached from the gut epithelium with decreased junctional cadherin staining, indicating that Arf6 may mediate the recycling of cadherin. Thus, Arf6 impacts cells that undergo coordinated movement to form embryonic structures in the developing embryo.
Keywords: primary mesenchyme cells, actin, skeleton, protein trafficking, endoderm, gut
INTRODUCTION
The small guanine nucleotide-binding protein Arf6 is an evolutionarily conserved molecule that has been identified in a myriad of organisms, ranging from yeast to human. Important in vitro studies have revealed that Arf6 is capable of remodeling the actin cytoskeleton and mediating the recycling of cell surface proteins that can impact various signaling pathways and cell movement (Berndt et al., 2014; Blum et al., 2015; D’Souza-Schorey and Chavrier, 2006; Donaldson, 2003; Humphreys et al., 2013; Hunzicker-Dunn et al., 2002; Jacques-Fricke and Gammill, 2014; Powelka et al., 2004; Sabe, 2003; Santy et al., 2001). However, its in vivo functions, particularly during early development, have not been examined extensively.
The Arf family is composed of small guanine-nucleotide-binding proteins that can be separated into three different classes in mammals based on sequence homology: Class I (Arf1, Arf2 and Arf3), Class II (Arf4 and Arf5) and Class III (Arf6) (Gillingham and Munro, 2007). Arf6 activity is regulated by cycling between the plasma membrane in its active GTP bound form and endosomal compartments in its inactive GDP bound form (D’Souza-Schorey et al., 1995; Donaldson, 2002; Donaldson and Radhakrishna, 2001). Arf6 guanine nucleotide exchange factors (GEFs) activate Arf6 by catalyzing the dissociation of GDP to promote the binding of GTP. Conversely, Arf6 GTPase activating proteins (GAPs) bind to the GTP form of Arf6 to catalyze the hydrolysis of GTP to GDP (Bos et al., 2007).
Arf6 regulates a wide variety of cellular processes, including endosomal membrane traffic, phophoinositide metabolism, regulated secretion, phagocytosis, disassembly of adherens junctions, cell motility, and cell signaling (Brown et al., 2001; D’Souza-Schorey et al., 1995; Humphreys et al., 2013; Radhakrishna et al., 1999; Santy et al., 2001; Zhang et al., 1998). The cellular functions of Arf6 are complex, but in general it is thought to act mainly through the activation of lipid-modifying enzymes and modulation of actin structures (Donaldson, 2002). Arf6-GTP is known to interact with several effectors that lead to the regulation of Rac1 activity, thus resulting in actin remodeling at the plasma membrane (Cotton et al., 2007; Humphreys et al., 2013; Kawaguchi et al., 2014; Koo et al., 2007).
In addition to the actin remodeling function, Arf6 mediates cell surface protein recycling and impacts various signaling pathways, such as β-integrin induced receptor tyrosine kinase signaling (Allaire et al., 2013; Heasman et al., 2000; Powelka et al., 2004), EGFR induced MEK-ERK pathway (Tague et al., 2004), the G-protein coupled receptors induced G protein signaling (Claing, 2011; Cotton and Claing, 2009), and cadherin recycling that affects adherens junctions as well as β-catenin abundance downstream of the Wnt signaling pathway (Dikshit et al., 2015; Egami et al., 2015; Grossmann et al., 2013). In the context of a developing embryo, proper regulation and integration of all these signaling pathways are essential for development.
While Arf6 shares conserved residues important for its membrane trafficking and actin remodeling functions, the Arf6 null phenotypes are variable in different organisms in terms of severity and the impact on development. Surprisingly, Arf6 is not essential in Drosophila and is only required during spermatogenesis with no other overt morphological abnormalities (Dyer et al., 2007). Systemic Arf6 loss-of-function in zebrafish caused a defect in epiboly (spreading of the blastoderm over the yolk) by regulating the recycling of syndecan that acts as a co-receptor for adhesion molecules and growth factors, such as FGFs and Wnt (Lambaerts et al., 2009). The arf6 −/− null mice resulted in embryonic lethality at midgestation stage (E13.5), with prominent defect in liver hepatic cord formation (Suzuki et al., 2006). The exact molecular mechanism of Arf6 mediated hepatic defects in the mouse is not known (Suzuki et al., 2006). Arf6 is not required for mouse early development, likely due to multiple endocytic pathways available to the embryo (Doherty and McMahon, 2009). We examined the physiological function of Arf6 in the developing sea urchin embryo as we can perform both loss-of-function and gain-of-function experiments which circumvent embryonic lethality. The cell types of interest in this study are the primary mesenchyme cells (PMCs) that produce the embryonic skeleton spicules and the endodermal cells that form the gut.
The sea urchin PMCs are highly dynamic cells that exhibit many properties of an animal cell (reviewed in (Lyons et al., 2012). During development, the PMCs undergo epithelial to mesenchymal transition (EMT) to ingress into the blastocoel as individual cells (Ettensohn, 2013; Lyons et al., 2012). During gastrulation, they undergo cellular morphogenesis and cell fusion and form a subequatorial ring surrounding the embryonic gut followed by the formation of the two ventrolateral clusters (Ettensohn, 2013; Lyons et al., 2012). At this stage, they are connected by actin-based syncytial cables of filopodial extensions. Once the PMCs position into the subequatorial ring, they migrate anteriorly toward the anterior pole of the embryo guided by sensory filopodia. As they migrate anteriorly, they synthesize calcium carbonate deposits which constitute the skeleton of the embryo that supports the shape, swimming and feeding of the larvae (Hart and Strathmann, 1994; Pennington and Strathmann, 1990).
In contrast to the PMCs that undergo EMT as individual cells and then fuse together via syncytia, the endodermal cells migrate as a coherent sheet of cells during gut invagination (reviewed in (McClay et al., 1992; Wessel and Wikramanayake, 1999). Invagination of the archenteron starts with bending of the thickened vegetal plate of the embryo, followed by the archenteron elongation. Initially elongation is mediated by the rearrangement of the endodermal cells via convergent extension movements, when the endodermal cells locally rearrange (Burke et al., 1991). During gastrulation, these endodermal cells retained junctional cadherin and their cell adhesive properties may be regulated by the interaction of cadherin with cadherin associated proteins that link to the actin cytoskeleton (such as β-catenin) (Miller and McClay, 1997). As the gut elongates, the secondary mesenchyme cells, located at the tip of the archenteron (embryonic gut) extend filopodia to provide additional elongation force through the contact with the specific predefined target site on the ectoderm (Hardin and McClay, 1990). After fusion of the elongated gut with the ectoderm, the gut differentiates into three structurally distinctive domains of foregut, midgut, and hindgut (Burke, 1981; Wessel and Wikramanayake, 1999).
PMCs and endodermal cells exhibit very different behaviors and thus provide a unique opportunity to examine Arf6 function in different cell types. We tested the functions of Arf6 using antisense morpholino oligonucleotide (MASO) and constitutively active form of Arf6 (Arf6-Q67L). Results indicate that Arf6 is critical for the positioning and function of PMCs potentially through regulation of actin remodeling. Morphology of the gut epithelium is also negatively affected by Arf6 perturbations, potentially through Arf6-mediated recycling of cadherin. Overall, our results demonstrated the essential regulatory role of Arf6 in the development and function of the PMCs and morphology of the gut in the sea urchin embryo.
MATERIALS AND METHODS
Animals
Adult Strongylocentrotus purpuratus were received from California (Point Loma Marine Company). Gametes were collected by intracoelmic injection of 0.5 M KCl. All cultures are placed in 15°C incubator.
Constructs
To examine the localization of Arf6 in the sea urchin embryo, the sea urchin Arf6-mcherry was synthesized as a gBlocks gene fragment that contains the 5′UTR and coding sequence of Arf6-WT fused to mCherry with Xenopus β-globin 3′UTR (Idtdna.com) (Fig. S1A). This fragment was subsequently cloned into Zero-Blunt TOPO vector (Thermo Fisher Scientific, Waltham, MA) and sequenced (Genewiz Inc., South Plainfield, NJ). The construct was linearized with XhoI (Thermo Fisher Scientific, Waltham, MA) and in vitro transcribed with the Sp6 mMessage mMachine kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions. Microinjected embryos were collected at gastrula stage (48 hpf), and imaged live on LSM 780 confocal microscope (Carl Zeiss Inc., Thornwood, NY).
To perform Arf6 knockdown (KD) rescue experiment, the sea urchin Arf6 mRNA (SpArf6) was cloned into a plasmid containing Xenopus β-globin 5′ and 3′UTRs that are known to function in the sea urchin embryo, ensuring the translation of Arf6 (Gustafson et al., 2011). To test the function of Arf6, we generated the constitutively active mutant Arf6-Q67L. Mutagenesis primers were designed using a primer design program provided by Stratagene (www.agilent.com/genomics/qcpd) (Arf6_Q67L_F: 5′ ATGGGATGTTGGTGGTCTGGATAAAATTCGGCCTC 3′, Arf6_Q67L_R: 5′ GAGGCCGAATTTTATCCAGACCACCAACATCCCAT 3′). Mutated Arf6 coding sequences were generated using the QuikChange Lightning mutagenesis kit according to manufacturer’s specifications (Stratagene, La Jolla, CA). Positive clones were sequenced to check the fidelity of the mutagenesis (Genewiz Inc., South Plainfield, NJ). The sea urchin SpArf6 and Arf6-Q67L were linearized with XhoI (Thermo Fisher Scientific, Waltham, MA) and in vitro transcribed with the Sp6 mMessage machine kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions.
To test if the human Arf6 mRNA can rescue the Arf6 KD in the sea urchin embryo, we subcloned the human Arf6 (HsArf6) that was received from Dr. Julie Donaldson (NIH) (Macia et al., 2004; Palacios et al., 2001; Peters et al., 1995) into pCR-Blunt vector (Thermo Fisher Scientific, Waltham, MA). The construct was linearized with SpeI (Thermo Fisher Scientific, Waltham, MA) prior to in vitro transcription using the T7 mMessage machine kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions.
GFP-LifeAct plasmid was received as a gift from Dr. Charles Shuster (New Mexico State University). The construct was linearized with NotI (Thermo Fisher Scientific, Waltham, MA) followed by in vitro transcription using the Sp6 mMessage machine kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions.
Embryo Injections
The newly fertilized eggs were microinjected with a Arf6 MASO 5′ TCTTTGATAGTACCTTCCCCATCGT 3′, negative control MASO 5′ CCTCTTACCTCAGTTACAATTTATA 3′ (range of 0.5 to 3 mM, with 1–1.5 mM stocks used for all phenotyping experiments) (Genetools, Philomath, OR), or mRNAs of Firefly negative control, constitutively active Arf6-Q67L, GFP-LifeAct, Arf6-mCherry or cytoplasmic mCherry (0.4 μg/μL, based on the empirically determined minimal amount injected to observe a phenotype or expression of the reporter construct). All in vitro transcribed mRNAs were purified using NucleoSpin RNA Clean-up kit (Macherey-Nagel, Bethlehem, PA) according to manufacturer’s specifications and further purified with the Millipore spin columns prior to injections (EMD Millipore Corporation, Billerica, MA). Texas red dextran reporter was used as a marker for injected embryos. Injections were performed as described previously (Stepicheva and Song, 2014). Embryos were cultured at 15°C and collected at various stages of development for further analyses.
Whole mount RNA in situ hybridization
The temporal and spatial distribution of Arf6 were detected with the Arf6 in situ hybridization probe as previously described (Stepicheva et al., 2015; Stepicheva and Song, 2015). Arf6 coding sequence (Fig. S1B) was cloned into a PCRII vector (Thermo Fisher Scientific, Waltham, MA) and used as an in situ probe. The primers used to generate the Arf6 in situ construct are the following: Arf6_insitu_F: 5′ CATGGATCCATGGGGAAGGTACTATCAAA 3′, Arf6_insitu_R: 5′ ACAGTCTCGAGTCAGGGTTTATTATTAGATGTTA 3′. The construct was linearized with BamHI (Promega Corporation, Madison, WI) and labeled with DIG RNA labeling kit (T7 RNA polymerase) (Roche Life Science, Indiannapolis, IN, USA). The negative control was transcribed off plasmid pSPT-18-Neo.
Real time, quantitative PCR (qPCR)
For the Arf6 expression time course, 500 uninjected eggs or embryos were collected at the egg, 32-cell stage, early blastula, mesenchyme blastula, gastrula, and larval stages of development. Total RNA was extracted with the Qiagen microRNeasy kit (Qiagen Inc., Valencia, CA, USA) according to manufacturer’s instructions and reverse transcribed with TaqMan Reverse Transcription Reagents kit (Thermo Fisher Scientific, Waltham, MA). The estimated numbers of Arf6 transcripts are calculated based on the level of ubiquitin in various developmental stages as previously described (Lowe et al., 2006; Materna and Davidson, 2012; Song and Wessel, 2012).
To test the effect of Arf6 perturbations on genes important for biomineralization, fusion, PMC positioning, and endodermal specification, 100 embryos injected with control or Arf6 MASO were collected at 24 and 30 hpf. Total RNA was extracted using the NucleoSpin RNA XS kit (Macherey-Nagel, Bethlehem, PA) and reverse transcribed with iScript (BioRad, Hercules, CA). qPCR primers are listed in Table S1. The relative expression of transcripts in injected samples were analyzed using the Ct−2ΔΔ method.
Arf6 activity assay
Arf6 activity was detected from approximately 50,000 eggs or embryos collected from three different animals at the eggs, early blastula (15 hpf), mesenchyme blastula (24 hpf), early gastrula (30 hpf) and gastrula (48 hpf) stages of development, by using the absorbance-based G-LISA Arf6 activation assay biochem kit (Cytoskeleton Inc. Denver, CO, USA) according to the manufacturer’s instructions. This method is based on preferential recognition of the active GTP bound form of Arf6 by the Arf family effector proteins. During the assay, the active Arf6-GTP binds to the Arf6 effector proteins and detected with an Arf6-specific antibody, followed by the incubation with the secondary antibody bound to HRP. The amount of the active Arf6-GTP is then obtained by measuring absorbance at 490 nm after adding the HRP detection reagent for 10 minutes.
The volume of the lysis buffer used to lyse each sample was 150 μL. The protein concentration (in triplicates) was determined using the Precision Red protein assay at absorbance 600 nm using the Promega Glomax-Multi detection system (Promega, Madison, WI). All the samples were adjusted to the total protein concentration 0.7 mg/mL. The Arf6 activity of each sample (1 technical replicate) was determined for at 490 nm using BioTek Synergy H1 microplate reader (BioTek, Winooski, VT).
Immunolabeling
Immunolabeling with PMC-specific 1D5 antibody (McClay et al., 1983) and endoderm-specific Endo1 antibody (Wessel and McClay, 1985) was performed as previously described (Stepicheva et al. 2015). For the double immunostaining with Endo1 and cadherin (Miller and McClay, 1997), embryos were fixed in 100% ice-cold methanol for 1 h at −20°C followed by four washes in 1X Phosphate Buffered Saline (BioRad, Hercules, CA) containing 0.05% Tween (PBST). Embryos were then blocked in 4% sheep serum (Sigma, St. Louis, MO) in PBST for 1 h at room temperature and then immunolabeled with Endo1 (1:200) and cadherin antibody (1:10) overnight at 4°C in a 72-well mini tray (Thermo Fisher Scientific, Waltham, MA) in a humid chamber. Embryos were washed three times with 1X PBST and incubated with goat anti-mouse Alexa Fluor 647 conjugated antibody at 1:1000 (Thermo Fisher Scientific, Waltham, MA) in blocking buffer (PBST 4% sheep serum) for 1 h at room temperature. Then the embryos were washed three times with 1X PBST and incubated with goat anti-guinea pig Alexa Fluor 488 conjugated antibody at 1:300 (Thermo Fisher Scientific, Waltham, MA) in blocking buffer (PBST 4% sheep serum) for 1 h at room temperature. Embryos were washed four times with PBST and then counterstained with Hoechst dye (Lonza, Walkersville, MD, USA) (1:1000) for 5 min to label DNA, followed by a final three washes with 1X PBST. Immunolabeled embryos were imaged on LSM 780 confocal microscope, or Zeiss Axio Observer Z.1 epifluorescent microscope (Carl Zeiss Inc., Thornwood, NY).
Alkaline phosphatase treatment
Larvae (72 hpf) embryos were fixed in MOPS-paraformaldehyde based fixative (4% paraformaldehyde, 100 mM MOPS pH 7, 2 mM MgSO4, 1 mM EGTA, and 0.8 M NaCl) for 10 min at room temperature as previously described (Stepicheva et al., 2015). Embryos were then washed with alkaline phosphatase buffer 3 times (100 mM Tris pH 9.5, 100 mM NaCl, 50 mM MgCl2, 0.1% Tween-20), followed by staining until the desired color was developed with the staining solution (0.1 M Tris pH 9.5, 50 mM MgCl2, 0.1 M NaCl, 1 mM Levamisole, 10% Dimethylformamide, 45 μl of 75 mg/mL NBT and 35 μl of 50 mg/mL BCIP per 10 ml of solution). Staining was stopped with multiple washes of MOPS buffer (0.1 M MOPS pH 7, 0.5M NaCl, and 0.1% Tween-20). Images were acquired using Nikon D90 digital camera connected to a Zeiss Axio Observer Z.1 microscope.
Phenotyping and statistical analysis
For the measurements of the length of DVC rod and the blastopore width, the Z-stacks (20–40 slices of 1 μm distance apart) of DIC images of gastrulae were acquired with Zeiss Axio Observer Z.1 epifluorescent microscope (Carl Zeiss Inc., Thornwood, NY). The measurements were performed from 2–4 biological replicates using Axiovision 4.8.2 software (Carl Zeiss Inc., Thornwood, NY). The data were analyzed with Student T-test. The average spicule length or blastopore width and s.e.m. were plotted.
PMC positioning defects were assayed based on the 1D5 immunostaining. The gastrulae were considered to be normal if PMCs formed subequatorial ring, connected by syncytial cables, and migrated anteriorly, with no more than two PMCs found at ectopic locations. The gastrulation defects were assayed based on the Endo1 immunostaining. The embryo was considered to be normal if the gut is fully extended anteriorly with no more than two Endo1-positive cells detached from the archenteron. The Z-stacks of 20–50 slices (1–2 μm distance apart) were imaged in DIC and fluorescent channels using Zeiss Axio Observer Z.1 epifluorescent microscope (Carl Zeiss Inc., Thornwood, NY). For gastrulae and larvae, 2–4 biological replicates were performed and analyzed with the Cochran-Mantel-Haenszel test. The average percentage of the normal embryos and s.e.m. were plotted on the graph. N represents the number of individual embryos.
To measure intensity of cadherin immunostaining in the gut, the Z-stacks of the gut (20–25 slices, 1 μm distance apart) were obtained on LSM 780 confocal microscope. Quantification of cadherin levels were performed using Axiovision 4.8.2 software (Carl Zeiss Inc., Thornwood, NY). Maximum intensity projection of cadherin fluorescence was made from 20 digital slices. Total pixel intensity of the gut and blastocoel (used as background) areas of the embryo were measured as the sum of the densitometric values of the selected regions. Measurement of the gut was normalized to the measurement of the blastocoel (background). The data were analyzed with Student T-test. The average sum of the densitometric values and s.e.m. were plotted.
RESULTS
Temporal and spatial expression of Arf6
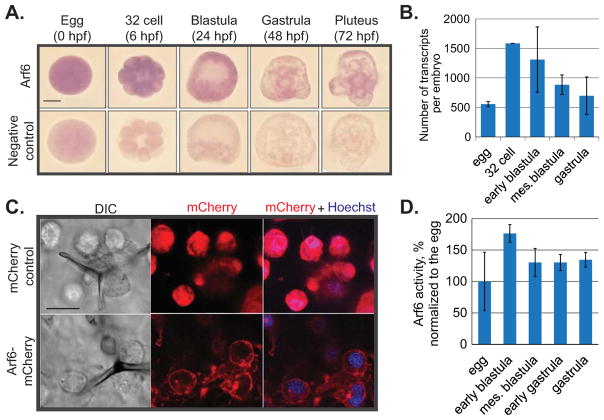
We examined the temporal and spatial expression profile of Arf6 in embryos at various developmental stages. The spatial distribution of Arf6 transcripts was ubiquitous throughout early development (Fig. 1A). The level of accumulated Arf6 transcript was highest in the 32 cell stage (6 hpf) among the developmental stages tested and began to decrease in the mesenchyme blastula stage (24 hpf) (Fig. 1A,B).
Fig. 1. Temporal and spatial expression of Arf6.
(A) RNA in situ hybridization of Arf6 indicated ubiquitous expression. Scale bar is 50 μm. (B) The transcript level of Arf6 peaked at 32 cell stage and decreased later in development as indicated by qPCR. The number of Arf6 transcripts was calculated based on the number of internal control ubiquitin mRNAs in 3 biological replicates. (C) The SpArf6-mCherry or cytoplasmic mCherry control reporters were in vitro transcribed and microinjected into the newly fertilized eggs. Gastrulae (48 hpf) were imaged live using confocal microscopy. Exogenously expressed sea urchin Arf6 protein was enriched at the plasma membrane, in punctate structures that are likely to be endosomes, and in the skeleton spicules. Scale bar is 10 μm. (D) The level of active form of Arf6 was measured in various developmental stages. All Arf6 activity was normalized to the Arf6 activity in the egg stage. Arf6 activity is highest in the early blastula stage.
To examine the protein localization of the sea urchin Arf6, we constructed an Arf6-mCherry chimera reporter (Figs. 1C, S2). We observed that the exogenously expressed sea urchin Arf6 had subcellular enrichment in the plasma membrane and presumptive endosomal compartments in all cells (Fig. 1C). In contrast, the control mCherry protein without fusion with Arf6, had a ubiquitous localization.
To examine the dynamics of Arf6 activity throughout early development of the sea urchin, we performed G-LISA Arf6 activation assay (Fig. 1D). We observed that Arf6 activity peaked at early blastula stage of development, at a time of active cellular morphogenesis. However, this increase of Arf6 activity at early blastula stage was not significantly higher compared to the egg stage (Student T-test; p=0.23). Consistent with the Arf6 mRNA expression detected by qPCR and in situ hybridization, Arf6 activity decreased after blastula stage.
Arf6 KD led to defects in PMC development and function
To test the function of Arf6 in the early embryo, we injected Arf6 MASO into newly fertilized eggs to inhibit newly translated Arf6, leading to a knockdown of the Arf6 protein. 87% of control MASO injected embryos survived at 24 hpf, whereas 66% of the Arf6 MASO injected embryos survived at 24 hpf. We assayed for percentage of healthy or dead embryos over time and found that overall the percentage of healthy embryos in control embryos is much higher than in the Arf6 KD embryos by 72 hpf (69% vs. 12%), indicating that Arf6 KD embryos had lower survival rate (Fig. S4A).
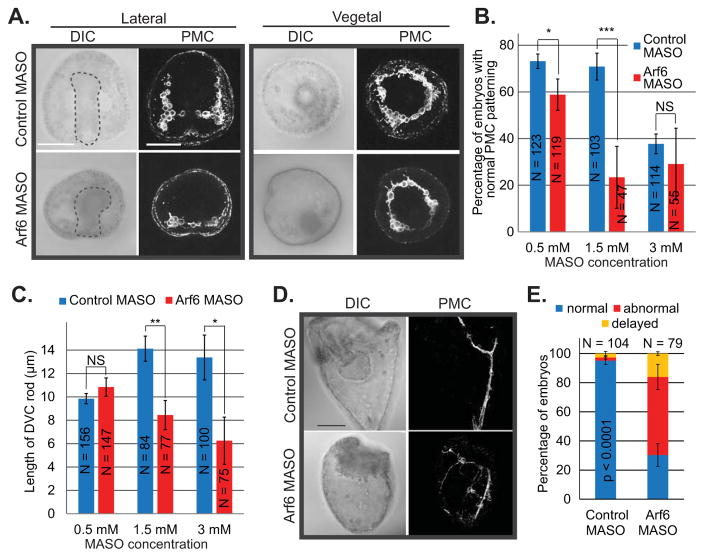
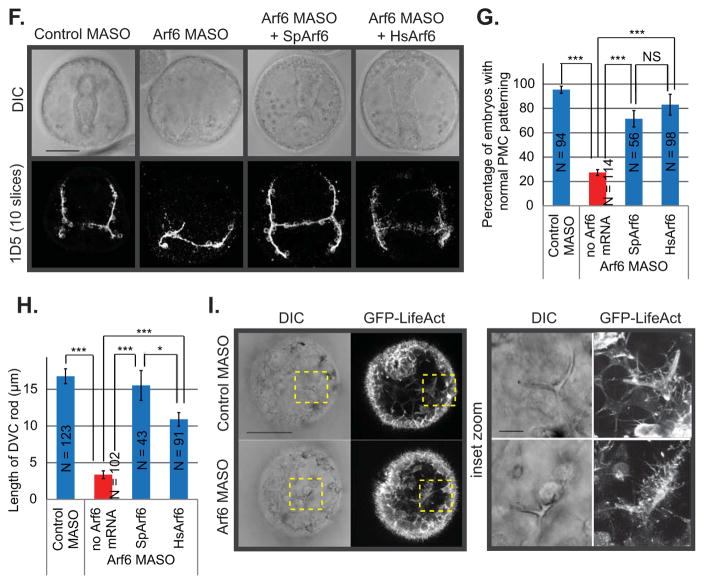
We did not detect any significant defects in the PMC positioning or archenteron invagination in the Arf6 KD embryos at blastula (24 hpf) or early gastrula (30 hpf) stages of development (Fig. S3); however, by gastrula stage (48 hpf), the morphological defects become apparent. We assessed PMC function by measuring the length of the dorsoventral connecting (DVC) rods at the gastrula stage, PMC positioning with an 1D5 antibody against the membrane protein present on PMCs, and their actin-based filopodial connections (Fig. 2A). We observed that Arf6 KD gastrulae had a dose-dependent severity of defects in both the length of the DVC rods and the positioning of the PMCs (Fig. 2B,C). The aberrant PMC patterning for both control and Arf6 MASO at 3mM is likely a result of non-specific morpholino toxicity, since the control embryos have only 40% of embryos having normal PMC patterning (Fig. 2B). At the same 3mM concentration, we observed that control MASO embryos had normal DVC rod length and a significantly shorter DVC rod length with the Arf6 MASO (Fig. 2C). This discrepancy is likely due to the different batches of embryos assayed for PMC patterning and for spicule measurement. PMCs in Arf6 KD gastrulae were able to form a subequatorial ring around the gut, but consistently failed to migrate anteriorly (Fig. 2A). Further, KD of Arf6 has persistent effect on PMC development in that the Arf6 KD larvae (plutei at 72 hpf) were smaller and developmentally delayed with mispositioned PMCs compared to the control (Fig. 2D,E). Importantly, both the PMC skeleton and positioning defects were significantly rescued by the co-injection of Arf6 MASO with either the sea urchin (SpArf6) or the human (HsArf6) Arf6 mRNA, indicating that Arf6 KD phenotypes were specifically induced by Arf6 MASO treatment (Fig. 2F–H). The injected Arf6 mRNA would be recalcitrant to the Arf6 MASO, since it lacks the Arf6 5′UTR that is complementary to the Arf6 MASO sequence. The sea urchin Arf6 shares 91.4% protein identity with the human Arf6, so they are highly conserved at the protein sequence level (Fig. S1C). The fact that the HsArf6 mRNA can rescue the sea urchin Arf6 KD phenotypes indicated that Arf6 is also functionally conserved between the sea urchin and the human.
Fig. 2. Arf6 knockdown resulted in skeletogenesis and PMC positioning defects.
(A) Control or Arf6 MASO was microinjected into the newly fertilized eggs. Gastrulae were immunolabeled using 1D5 antibody against PMCs. The gut is outlined in the DIC image. The maximum intensity projection of 4 digital slices is shown for the PMC immunostaining. Scale bar is 50 μm. (B) The percentage of embryos with normal PMC positioning in Arf6 MASO injected embryos is significantly lower than in control MASO injected embryos in a dose-dependent manner (Cochran-Mantel-Haenszel test). (C) The lengths of DVC rod were significantly decreased in the Arf6 KD embryos compared to the control in a dose-dependent manner (Student’s T-test). (D) Skeletogenic defects caused by Arf6 KD (1–1.5 mM MASO) persisted to the larval stage (72 hpf). The maximum intensity projection of 10 digital slices is shown. Scale bar is 50 μm. (E) The percentage of larvae at 72 hpf with normal PMC positioning was significantly reduced upon Arf6 KD (1 mM MASO) (Cochran-Mantel-Haenszel test). The delayed category is defined as embryos that were developmentally delayed with normal PMC positioning. (F) 1 mM Arf6 MASO was microinjected into the newly fertilized eggs alone or co-injected with either sea urchin Arf6 (SpArf6) or human Arf6 (HsArf6) mRNA (0.4 μg/μL), which significantly rescued the Arf6 KD phenotype. Gastrulae were immunolabeled using 1D5 antibody against PMCs. Scale bar is 50 μm. (G) PMC positioning defects and (H) shortened DVC rods induced by Arf6 KD (1 mM MASO) were rescued with either co-injection with SpArf6 or HsArf6 mRNA. (I) The GFP-LifeAct was co-injected with either Arf6 MASO or control MASO (1 mM MASO). Dashed areas indicate the enlarged area of the embryo. Arf6 KD embryos had less abundant and shorter filopodia extending from the cells surrounding the skeleton compared to the control. The maximum intensity projection of 5 digital slices is shown, scale bar is 50 μm for the whole embryos and 10 μm for the inset. *** p < 0.0001; * 0.01 < p < 0.05. NS indicates not significant (p > 0.05).
To identify the molecular mechanism that underlies the Arf6 KD induced phenotypes, we tested the transcript levels of genes involved in biomineralization, fusion or PMC positioning in Arf6 KD embryos at mesenchyme blastula (24 hpf) and early gastrula (30 hpf) stages. None of the tested genes had more than a 2-fold change at the mRNA level in the Arf6 KD embryos compared to the control (Fig. S6A,B), indicating that Arf6 is likely to regulate PMC development at the post-transcriptional or post-translational levels.
Arf6 is known to play an important role in actin remodeling (Cotton et al., 2007; Humphreys et al., 2013; Kawaguchi et al., 2014; Koo et al., 2007). We observed that the Arf6 KD gastrulae have less filopodia extending from the PMCs that form a subequatorial ring compared to the control (Fig. 2A). To test how actin remodeling is impacted by Arf6 KD, we microinjected the newly fertilized eggs with the GFP-LifeAct reporter mRNA (Riedl et al., 2008) along with the control or Arf6 MASO to examine actin distribution in living embryos (Fig. 2I). We observed that Arf6 KD gastrulae had shorter and less abundant actin-based protrusions in cells surrounding the skeleton spicule in comparison to the control gastrulae (Fig. 2I, insets), indicating that Arf6 plays an important role in actin remodeling in the developing embryo. The shortened actin protrusions observed in the Arf6 KD embryos seem to be specific to the PMCs, since we did not observe obvious differences in the structure of actin-based apical microvilli of the ectoderm with GFP-LifeAct (Fig. 2I).
Arf6 activity is important for skeletogenesis and PMC positioning
The sea urchin Arf6 contains conserved residues for activation with GTP binding and inactivation with GTP hydrolysis (Fig. S1B, C). Thus, to test the effect of Arf6 activity on PMC function and development, we microinjected newly fertilized eggs with constitutively active Arf6-Q67L mRNA that encodes Arf6 that is unable to hydrolyze GTP (Macia et al., 2004; Palacios et al., 2001; Peters et al., 1995). 92% of Firefly mRNA-injected embryos survived at 24 hpf, whereas 67% of the Arf6-Q67L mRNA-injected embryos survived at 24 hpf (Fig. S4B). By 72 hpf, 73% and 25% of the Firefly mRNA and Arf6-Q67L mRNA-injected embryos remained healthy, respectively.
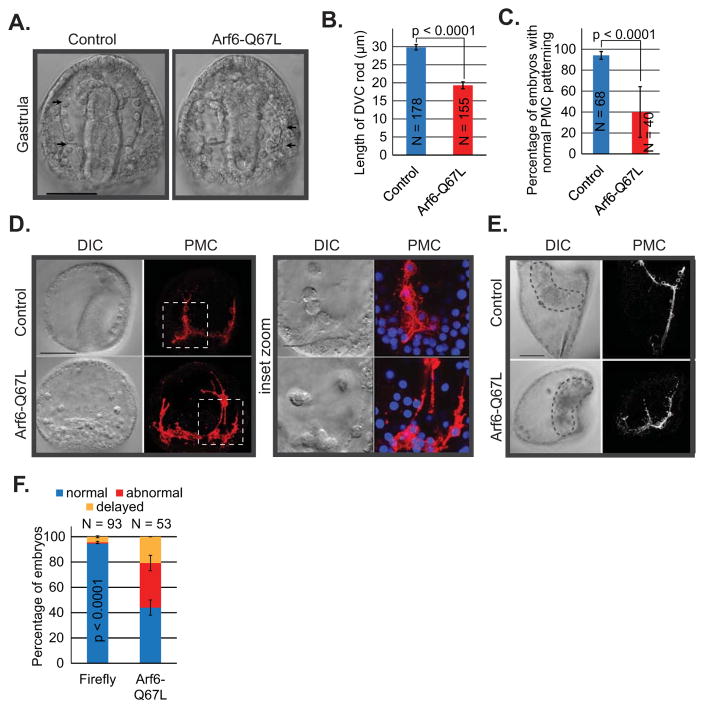
Similar to the Arf6 KD embryos, no significant defects in the PMC positioning or archenteron invagination were observed in Arf6-Q67L mRNA-microinjected embryos at blastula (24 hpf) or early gastrula (30 hpf) stages of development (Fig. S5). By gastrula stage (48 hpf), embryos injected with the constitutively active Arf6-Q67L mRNA had significantly shorter DVC rod and mispositioned PMCs compared to the control (Fig. 3A–D). The PMCs of Arf6-Q67L mRNA-injected embryos were connected by thick, extensive filopodial cables (Fig. 3D), consistent with its role in actin remodeling observed in the case of Arf6 KD (Fig. 2I). By larval stage (72 hpf), the embryos injected with the constitutively active Arf6-Q67L mRNA were developmentally delayed and displayed abnormal PMC positioning and skeletal development (Fig. 3E,F). PMC defects induced by the microinjection of Arf6-Q67L mRNA were not due to the nonspecific overexpression of Arf6 protein, but to the specific blockade of Arf6 inactivation. This is supported by the lack of defects in SpArf6 mRNA-injected embryos compared to the Firefly mRNA-injected embryos (Fig. S7). Thus, the active form of Arf6 impacts skeleton spicule formation, PMC positioning, and actin-based filopodia formation.
Fig. 3. Embryos injected with constitutively active Arf6-Q67L mRNA resulted in PMC positioning and actin remodeling defects.
(A, B) The skeleton spicule length was significantly shorter in the Arf6-Q67L mRNA-injected (0.4 μg/μL) embryos compared to the control (Student’s T-test). Arrows indicate the length of DVC rod measured. Scale bar is 50 μm (C) The percentage of the embryos with the normal PMC positioning was significantly reduced in the Arf6-Q67L injected embryos compared to the control (Cochran-Mantel-Haenszel test). (D) Embryos injected with control or Arf6Q67L mRNA were immunolabeled using PMC-specific antibody 1D5 (red) and counterstained with Hoechst dye for DNA (blue). The Arf6-Q67L mRNA injected embryo contains a normal gut; this is not seen because the gut is in a different plane as the PMCs. In addition to PMC positioning defects, exogenous Arf6Q67L mRNA in embryos resulted in thick and extensive filopodial structures. Dashed areas indicate the enlarged area of the embryo. The maximum intensity projections of 10 digital slices and 5 digital slices are shown for the whole embryos and insets, respectively. Scale bars are 50 μm for the whole embryo images and 10 μm for the insets. (E,F) Defects in PMC positioning persisted to the larval stage (72 hpf) (Cochran-Mantel-Haenszel test). The maximum intensity projection of 10 digital slices is shown, scale bar is 50 μm. The delayed category indicated that embryos had developmental delay with normal PMC positioning.
Arf6 perturbation results in endodermal defects
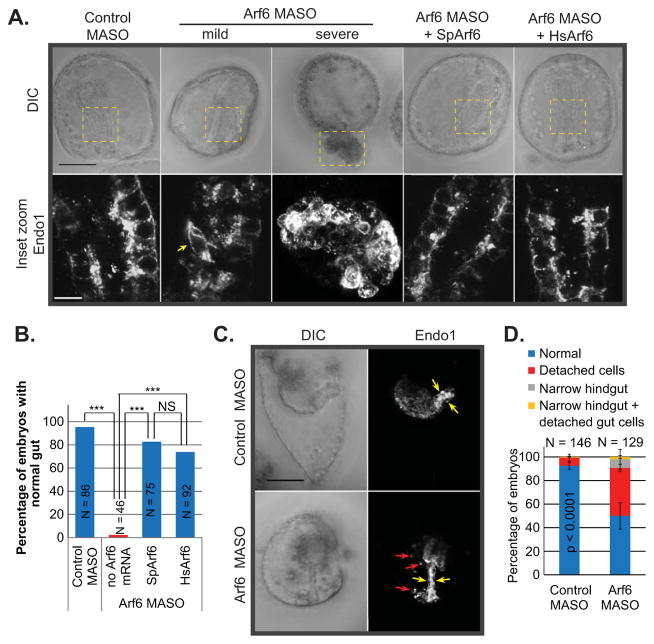
In contrast to the PMCs that undergo EMT and migrate as individual cells, endodermal cells migrate as a coherent sheet of cells, enabling us to study the regulatory role of Arf6 in a different cell type. At gastrula stage, Arf6 KD embryos exhibited a range of defects in gastrulation, including morphological disorganization of the gut epithelium, lack of gut formation, and exogastrulation (protruding gut) (Fig. 4A,B). By larval stage, Arf6 KD embryos displayed phenotypes such as delayed development, defective gut that clearly lacked distinct foregut, midgut, and hindgut structures, and disconnected endodermal cells (Fig. 4C,D). The defective gut phenotype in Arf6 KD embryos was significantly rescued with co-injection with SpArf6 or HsArf6 mRNA, indicating that the gastrulation defect is specifically induced by the Arf6 MASO and that Arf6 is functionally conserved between the sea urchin and the human (Fig. 4A,B).
Fig. 4. Arf6 KD embryos have gastrulation and endodermal defects.
(A) Gastrulae (48 hpf) were collected and immunolabeled with Endo1 antibody against the midgut and hindgut. Arf6 KD (1 mM MASO) resulted in a range of defects, including mild phenotype of disorganized endodermal cells (shown by arrow) that line the gut or severe phenotype of exogastrulated (protruding gut) embryos. Embryos co-injected with 1 mM Arf6 MASO with 0.6 μg/μL of either SpArf6 or HsArf6 had normal gut, indicating that the KD phenotype is specific to the Arf6 KD. Dashed regions indicate the enlarged area of the gut. The maximum intensity projection of 10 digital slices is shown for the enlarged image of the exogastrulated embryo to visualize all the disorganized endodermal cells. Scale bar is 50 μm for the whole embryos and 10 μm for the enlarged gut. (B) SpArf6 or HsArf6 significantly rescued the Arf6 KD phenotype. *** p < 0.0001. NS indicates not significant (p > 0.05). (C,D) Arf6 KD larvae exhibited lack of the proper gut structures including detached endodermal cells (red arrows) and narrow hindgut (hindgut is indicated by yellow arrows). Scale bar is 50 μm.
To test if endodermal specification is affected by Arf6 KD, we measured transcript level changes in transcription factors and signaling molecules known to be critical for endodermal specification (Fig. S6C). We performed qPCR at mesenchyme blastula (24 hpf) and early gastrula (30 hpf) stages. All the genes tested were not altered more than 2-fold in the Arf6 KD embryos compared to the control. Thus, Arf6 is likely to regulate gut development at the post-transcriptional or post-translational levels.
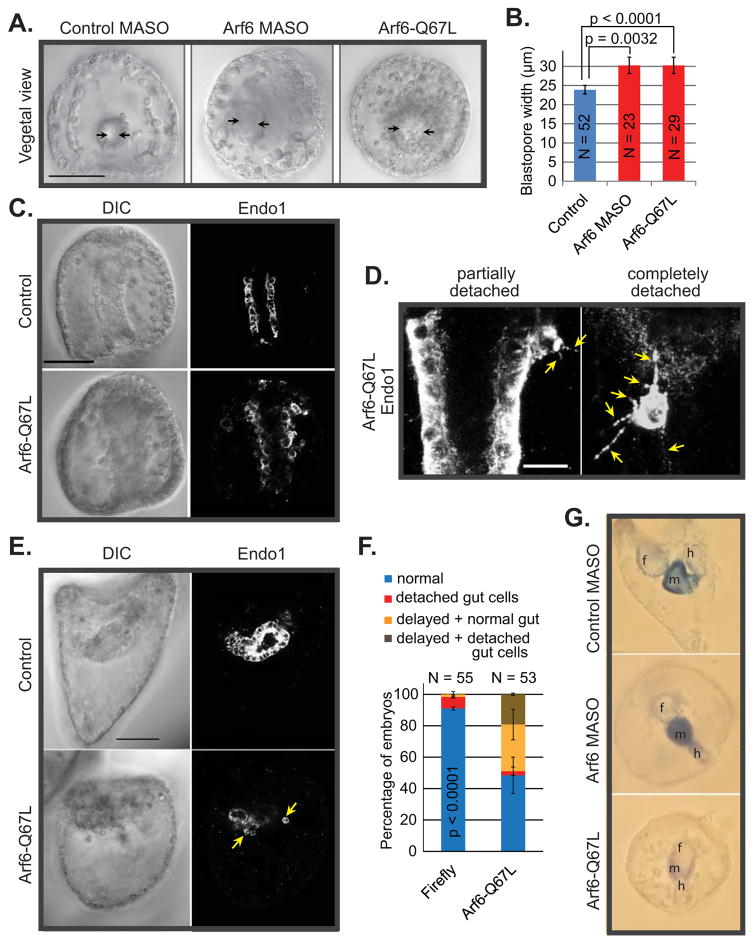
In addition, we observed that Arf6 KD gastrulae had significantly wider blastopore compared to the control (Fig. 5A,B). Similarly, embryos with exogenous constitutively active Arf6-Q67L mRNA also displayed a similar phenotype, suggesting that both the levels and activity of Arf6 are important for blastopore formation and gut elongation process (Fig. 5A,B). In addition to the widening of blastopore, microinjection of constitutively active Arf6-Q67L mRNA into zygotes resulted in detached Endo1-positive cells from the gut epithelium (Fig. 5C). The detached endodermal cells had aberrant filopodial projections, in comparison to cells that are part of the gut epithelium with no filopodial projections (Fig. 5D). The defects of the gut persisted to the larval stage, where Arf6-Q67L mRNA-injected embryos were developmentally delayed and exhibited disorganized and defective gut morphology (Fig. 5E,F).
Fig. 5. Embryos overexpressing constitutively active Arf6-Q67L have endodermal defects.
(A,B) The blastopore opening (shown by arrows) is significantly larger in gastrulae injected with the 1–1.5 mM Arf6 MASO or 0.4 μg/μL Arf6-Q67L mRNA in comparison to the control (Student’s T-test). Scale bar is 50 μm. (C) Injection of Arf6-Q67L mRNA into the newly fertilized eggs resulted in endodermal cells detached from the gut. Scale bar is 50 μm. (D) The endodermal cells detached from the gut epithelium in the Arf6-Q67L mRNA injected gastrulae had multiple filopodial projections (shown by the arrows). The endodermal cells that are part of the gut do not have such filopodial projections. The maximum intensity projection of 5 digital slices is shown. Scale bar is 10 μm. (E,F) By larval stage, embryos injected with Arf6-Q67L mRNA exhibited a clear lack of proper gut structures with multiple detached endodermal cells (arrows) (Cochran-Mantel-Haenszel test). (G) Arf6 perturbation results in endodermal differentiation defects, as detected with alkaline phosphatase stain. Embryos injected with active Arf6-Q67L mRNA have significant developmental delay as well as reduced endogenous alkaline phosphatase. Although the Arf6 KD embryos stained for alkaline phosphatase, these embryos are significantly delayed in development. f=foregut, m=midgut, and h=hindgut.
We also examined Arf6 perturbed embryos with tissue-specific endodermal differentiation marker alkaline phosphatase, which in sea urchin larvae is expressed only in the gut epithelium (Drawbridge, 2003; Kumano and Nishida, 1998; Nishida and Kumano, 1997; Whittaker, 1990). Although the Arf6 KD larvae stained for alkaline phosphatase (Fig. 5G), these embryos were significantly delayed in development and had aberrant larval gut structures. The gastrulae injected with Arf6-Q67L mRNA had much less alkaline phosphatase staining, indicating that their gut potentially did not differentiate properly. Thus, Arf6 regulates various aspects of gut development.
Arf6 may regulate junctional cadherin
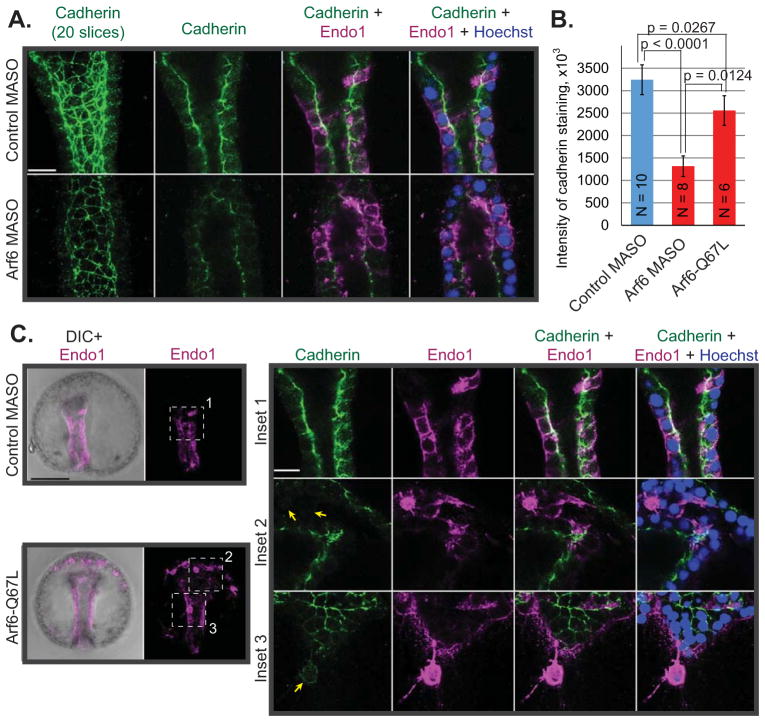
Arf6 KD and Arf6-Q67L mRNA injected embryos had disorganized endodermal epithelial cells that sometimes detached from the main gut epithelium, suggesting potential defects in cell-to-cell adhesion. To test this possibility, we examined cadherin in Arf6 perturbed embryos (Fig. 6). In wild type embryos, cadherin in the gut epithelium localizes to the apical and lateral membranes (Miller and McClay, 1997). We observed that Arf6 perturbations resulted in significant decrease (2.5 fold for Arf6 KD and 1.3 fold for Arf6-Q67L) in the levels of cadherin staining in the gut compared to the control (Fig. 6B). Further, endodermal cells that were detached from the basal lamina of the gut in the Arf6-Q67L injected embryos lacked polarized stain of cadherin and had much lower level of cadherin staining at their cell membrane compared to the endodermal cells of the gut epithelium in either control or Arf6-Q67L injected embryos (Fig. 6C). Interestingly, Arf6 perturbations do not seem to affect cadherin expression that would affect cell-to-cell adhesion of ectodermal cells (Fig. S8). These results suggest that Arf6 may directly or indirectly mediate cadherin levels at the plasma membrane of endodermal cells that potentially impacts gastrulation and the morphology of the embryonic gut.
Fig. 6. Arf6 perturbations resulted in defective cadherin levels and localization.
(A) Control or Arf6 MASO were microinjected into newly fertilized eggs. Gastrulae were collected and immunostained against cadherin (green) and Endo1 (magenta) and counterstained with Hoechst dye for DNA (blue). Scale bar is 10 μm. (B) Embryos injected with Arf6 KD or Arf6-Q67L had significant decrease in the cadherin staining at the apical and lateral membranes in the gut epithelium (Student’s T-test). (C) Detached endodermal cells in the Arf6-Q67L injected embryos lacked membrane cadherin staining (arrows). Dashed areas are enlarged with a single digital slice shown. The maximum intensity projection of 5 digital slices is shown for the whole embryos. Scale bars are 50 μm for the whole embryos and 10 μm for the insets.
DISCUSSION
Arf6 is a key small GTPase that regulates endocytosis, cortical actin rearrangements, cytokinesis, and recycling of endosomal membrane proteins (Donaldson, 2003). Even though the cellular functions of Arf6 are well understood, the physiological role and regulatory mechanism of Arf6 in development has not been examined extensively in the context of a developing embryo. To examine the physiological function of Arf6, we took advantage of the different morphogenic behavior of the two cell types in the sea urchin embryos: PMCs that undergo EMT as they ingress into the blastocoel as individual cells to form the skeleton and endodermal cells that invaginate as a sheet of cells to form the embryonic gut. Results from this study indicate that Arf6 may mediate proper skeleton formation via remodeling actin and maintain the gut epithelium through regulating junctional cadherin.
In the sea urchin embryo, both Arf6 KD and overexpression of constitutively active Arf6-Q67L resulted in the failure of PMCs to migrate anteriorly and shortened DVC skeletal rods (Figs 2, 3). This may be due to defects in the ability of PMCs to receive chemotactic cues, to form a syncytium, or to migrate in general. To address these questions, we first assayed for the transcript level changes of VegfR10, Vegf3 and Prestin that had previously been shown to be important for directed migration of PMCs (Adomako-Ankomah and Ettensohn, 2013; Duloquin et al., 2007; Lapraz et al., 2006; Piacentino et al., 2016; Sodergren et al., 2006), as well as genes Kirrell and Hypp1164 that may regulate PMC fusion (Rafiq et al., 2014) (Fig. S6A). The transcripts of these genes in Arf6 KD embryos were not altered greater than 2-fold compared to the control, suggesting that depletion of Arf6 did not impact transcript levels of these genes. Arf6 may regulate PMC development via its recycling of target proteins, such as the receptors on PMCs that would be important for receiving migratory cues. However, we do not have direct evidence for this possibility.
In addition to the PMC positioning defects, we observed that Arf6 perturbed embryos had defects in actin remodeling. Arf6 KD gastrulae had shorter and less abundant actin protrusions compared to the control (Fig. 2I), indicating that the level of Arf6 protein is important for the proper actin remodeling. The shortened actin-based extensions may directly contribute to the PMC positioning defects. During PMC migration, sensory filopodia make contact with the ectoderm to provide directional cues for PMC migration (Lyons et al., 2012; Malinda et al., 1995; Miller et al., 1995). Thus, lack of proper sensory filopodia may impact defective PMC migration in the Arf6 KD embryos.
In contrast to the shortened filopodia in Arf6 KD PMCs, we observed the formation of aberrantly thick and long actin-based filopodial extensions in PMCs of the embryos microinjected with constitutively active Arf6-Q67L mRNA (Fig. 3D). This result indicates that exogenously overexpressed Arf6-Q67L may remodel and recruit more actin to promote the formation of these actin-based filopodia, possibly through interactions with a number of effectors that promote Rac1 activity, thus resulting in actin remodeling at the plasma membrane (Cotton et al., 2007; Humphreys et al., 2013; Kawaguchi et al., 2014; Koo et al., 2007). Changes in the filopodial structures upon perturbation of Arf6 activity are reminiscent of Arf6-Q67L phenotypes in the rat hippocampal neurons, where Arf6 regulated the branching of dendrites and axons (Choi et al., 2006; Hernandez-Deviez et al., 2004). The rat hippocampal neurons that were transfected with the Arf6-Q67L promoted the formation of thick dendritic spines (Choi et al., 2006; Hernandez-Deviez et al., 2004). Thus, Arf6 seems to play an evolutionarily conserved role in mediating actin-based structures. Further, these results indicate that it is likely the overall balance of Arf6-GDP and Arf6-ATP forms in the embryo that mediate proper actin-based filopodial structures to enable proper PMC anterior migration.
In addition to PMC positioning defects and formation of abnormally thick filopodial structures, Arf6-perturbed gastrulae exhibited a significant reduction in the length of DVC skeletal rods. The transcripts of biomineralization genes in Arf6 KD embryos were not altered greater than 2-fold compared to the control embryos, indicating that Arf6 does not regulate these genes at the transcriptional level (Fig. S6A). However, Arf6 may contribute to biomineralization defects by mediating endocytosis of calcium carbonate granules that are necessary to form the single calcite crystal that make up the skeleton (Beniash et al., 1999; Vidavsky et al., 2014). Since we observed Arf6-mCherry localized in PMCs and along the skeleton spicules (Fig. 1C) and GFP-LifeAct localized to the skeleton spicules (Fig. 2I), Arf6 may also be involved in assembling the actin-containing PMC filopodial cables. Because the PMCs use the syncytium as a mold to deposit calcium carbonate to synthesize the skeleton (Wilt, 2005), defects in Arf6-mediated formation of syncytium may indirectly result in the reduction of DVC length and defective larval skeletal structures.
In addition to the skeletogenic defects, Arf6 perturbation also resulted in gastrulation and gut defects (Figs. 4,5). one of the observed defects was widening of the blastopore (Fig. 5A,B). One possible explanation for the widened blastopore phenotype is the role of Arf6 in actin rearrangements in the cells that form the lip of the blastopore. In the sea urchin species L. pictus, blastopore formation is mediated by cells that are constricted apically (Hardin, 1989; Kimberly and Hardin, 1998). It is proposed that the morphological changes of these cells drive the forces required for invagination (Nakajima and Burke, 1996). Thus, defects in actin remodeling in the Arf6 perturbed embryos may hinder the morphogenic movement of cells of the blastopore during gastrulation.
We also observed that embryos with overexpressed Arf6-Q67L had endodermal cells detached from the elongated archenteron (Fig. 5). Interestingly, similar to the PMCs, the detached endodermal cells in the Arf6-Q67L injected gastrulae exhibited unusual thick extensive filopodia (Figs. 3D and 5B) which may serve as an indication of the abnormal actin remodeling in the Arf6-Q67L injected embryos. Reorganization of actin cytoskeleton has been a well-known mediator of EMT (Shankar and Nabi, 2015; Wu and McClay, 2007), a process which directly correlates with the detachment of the endodermal cells. However, it is not clear if the detached Endo-positive cells acquired migratory ability due to these aberrant filopodia or that these filopodial extensions resulted from their ability to move.
To explore if cadherin was regulated by Arf6, we examined the localization and levels of cadherin in Arf6 perturbed embryos (Fig. 6). Endodermal cells have intact junctional cadherin that does not seem to alter during gastrulation (Miller and McClay, 1997). Thus, Arf6 KD embryos with decreased cadherin staining may indicate that these cells did not adhere to each other properly, leading to severe gastrulation defects with higher dosage of Arf6 MASO (Fig. 4A and 6A).
Overexpression of the constitutively active Arf6-Q67L in embryos, in turn, had a less severe impact on the levels of the cadherin on the cell surface of the endodermal cells of the gut epithelium (2.5 fold decrease in the Arf6 KD embryos vs. 1.3 fold decrease in the Arf6-Q67L injected embryos, compared to the control) (Fig. 6B). However, detached Endo1-positive cells from these embryos had relatively little membrane cadherin (Fig. 6C), indicating that Arf6 is likely to mediate cadherin dynamics at the cell surface. These results are consistent with previous studies that demonstrated Arf6 to mediate the recycling of E-cadherin of mammalian epithelial cells (Palacios et al., 2001; Paterson et al., 2003). Together with the Arf6-mediated changes in actin remodeling in the Arf6 perturbed embryos, internalization of cadherin may contribute to the EMT of endodermal cells and their migration to ectopic locations.
These phenotypes caused by Arf6 perturbation that were observed in our study are reminiscent to those caused by Par6 knockdown in the sea urchin embryos, including impaired skeletogenesis and gut differentiation (Shiomi et al., 2012). In sea urchin embryos, Par6-aPKC complex mediates cell polarity in the early embryo which impact later developmental stages (Alford et al., 2009; Moorhouse et al., 2015). Arf6-dependent vesicular trafficking has been shown to control Cdc42-mediated polarity signaling pathway via recruitment of Par6 and aPKC to the leading edge of the migrating astrocytes (Osmani et al., 2010). Thus, the membrane trafficking function of Arf6 may regulate Cdc42 to recruit Par6 and aPKC complexes to mediate PMC migration and skeletogenesis.
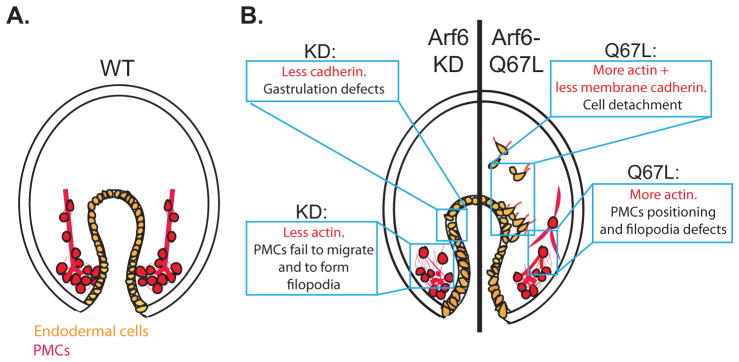
Taken together, this research has demonstrated that Arf6 is essential for early development and plays a critical role in regulating cellular morphogenesis of PMCs and endodermal cells (Fig. 7). The results from this study revealed important regulatory mechanism of Arf6 in remodeling actin in PMCs and their filopodial connections, and Arf6 in regulating cadherin to impact gut morphology and potentially its function. For the future studies, it would be important to identify cargos of Arf6 that contributes to the directed migration of PMCs.
Fig. 7. Model of Arf6 functions in the embryo.
(A) The gut of the WT gastrulae consists of a single layer of endodermally-derived epithelial cells that adhere to each other in an organized pattern (orange). The PMCs (red) migrated anteriorly and produced skeleton rods (red line) within their filopodial cables. (B) Arf6 perturbed embryos result in endodermal and skeletogenesis defects. Arf6 KD gastrula (left side of the embryo) has gastrulation defects, possibly due to the decrease of the junctional cadherin of the endodermal cells. Arf6 KD gastrula also has clumped PMCs that fail to migrate anteriorly, potentially due to decreased Arf6 that remodel actin. Gastrula injected with Arf6-Q67L mRNA (right side of the embryo) has detached endodermal cells, potentially due to the increase in actin remodeling and decrease in the levels of membrane cadherin, which may promote EMT of the endodermal cells. PMCs in the gastrula injected with Arf6-Q67L mRNA are connected by extensive thick filopodia that may interfere with the proper skeletogenesis, potentially due to the excessive actin remodeling.
Supplementary Material
(A) The Arf6-mCherry reporter contained the Arf6 5′UTR and coding region (highlighted in yellow) fused with mCherry (red font) and β-globin 3′ UTR (underlined). (B) The mRNA sequence of SpArf6. The position of the amino acid important for the GTP hydrolysis (Q67) is highlighted in light blue. (C) The SpArf6 protein is 91.4% identical to the HsArf6 protein using LALIGN program. Amino acid differences between SpArf6 and HsArf6 are highlighted in yellow. The conserved amino acid important for the GTP hydrolysis (Q67) is highlighted in light blue.
The SpArf6-mCherry or cytoplasmic mCherry control reporters were in vitro transcribed and microinjected into the newly fertilized eggs. Living gastrula stage embryos were imaged using confocal microscopy. Exogenously expressed mCherry fused to the sea urchin Arf6 was enriched at the plasma membrane in all cells. Scale bar is 50 μm.
1 mM of control MASO or Arf6 MASO were microinjected into the newly fertilized eggs. The blastula (24 hpf) and early gastrula (30 hpf) stage embryos were collected to assess PMC and archenteron development. (A) Blastulae were fixed and immunostained with PMC-specific marker 1D5. About 20% of the Arf6 KD embryos exhibited mislocalized PMCs; however, this percentage was not significantly different than the control embryos (Cochran-Mantel-Haenszel test, 2 biological replicates). (B) 15% of the Arf6 KD blastulae exhibited delay in development; however, this delay was not statistically significant compared to the control (Cochran-Mantel-Haenszel test, 2 biological replicates). (C) PMC patterning was not disrupted by Arf6 KD assessed at the early gastrula stage (30 hpf) (Cochran-Mantel-Haenszel test, 3 biological replicates). Scale bar is 50 μm. (D) Archenteron invagination was evaluated by ratios of the gut length and the embryo height. To account for the differences in the development of embryos from different biological replicates, the gut/height ratios in the Arf6 KD embryos were normalized to the ratios obtained for the control embryos. Archenteron formation was not affected by Arf6 KD (Student’s T-test, 3 biological replicates). N is the number of embryos evaluated.
The newly fertilized eggs were microinjected with either (A) 1 mM control or Arf6 MASO or (B) 0.4 μg/μL Arf6-Q67L or Firefly control mRNA. The same embryos were tracked throughout development and evaluated for survival (solid lines). The embryos were assessed based on their morphology and ability to swim (dashed lines) at three developmental stages: blastula (24 hpf), gastrula (48 hpf) and pluteus (72 hpf). Both perturbations in Arf6 levels and activity resulted in almost 40% drop in survival after the blastula stage. About 40–50% of Arf6 perturbed embryos were still alive by pluteus stage, of which approximately half of the survived embryos were healthy.
0.4 μg/μL of Arf6-Q67L or control Firefly mRNA were microinjected into the newly fertilized eggs and the embryos were collected at blastula stage (24 hpf) and early gastrula stage (30 hpf) of development. (A,B) Blastulae were fixed and immunostained with PMC-specific marker 1D5. About 40% of the Arf6 KD embryos exhibited mislocalized PMCs; however, this percentage was not significantly different from the control embryos (Fisher’s exact test of independence). (C, D) PMC patterning was also not disrupted by Arf6 KD by early gastrula stage (30 hpf) (Fisher’s exact test of independence). Scale bar is 50 μm. (E) Archenteron invagination was evaluated by measuring the gut length and the embryo height and obtaining the ratios between them. Archenteron formation was not affected by Arf6 KD (Student’s T-test, 1 biological replicate). N is the number of embryos evaluated.
100 embryos injected with either control MASO or Arf6 MASO were collected at various time points. (A) Embryos at early gastrula stage (30 hpf) were assayed for the transcript levels of biomineralization genes (brown) and fusion genes (green). (B) Embryos at mesenchyme blastula (24 hpf) or early gastrula stage (30 hpf) were collected and assayed for their transcript levels of genes that had been shown to be important for PMC positioning and migration. (C) Embryos at mesenchyme blastula and early gastrula stages were collected and assayed for genes expressed in the ectoderm (blue) and endoderm (orange).
This result indicates that skeletogenesis defects induced by the microinjection of Arf6-Q67L mRNA were not due to the nonspecific overexpression of Arf6 protein, but to the specific blockade of Arf6 activation or inactivation.
Control vs Arf6 MASO (1 mM) or Arf6-Q67L vs Firefly control mRNA (0.4 μg/μL) were microinjected into newly fertilized eggs. Gastrulae were collected and immunostained against cadherin. No significant changes in cadherin localization or the organization of ectodermal cells were detected in Arf6 perturbed embryos compared to the control. The gut failed to invaginate in the Arf6 KD embryo. The maximum intensity projection of 10–20 digital slices is shown. Scale bar is 50 μm.
Acknowledgments
We are grateful for Dr. David McClay’s generous gift of 1D5 and cadherin antibodies. We thank Dr. Charles Shuster for the GFP-LifeAct construct and Dr. Julie Donaldson for the HsArf6 construct. We also thank Michael Moore and Santiago Suarez for some of the confocal imaging. We thank the three anonymous reviewers for their critical feedback. This work is funded by National Science Foundation [#1553338] and National Institutes of Health [5P20GM10365301A].
Abbreviations
- PMCs
primary mesenchyme cells
- DVC rod
dorsoventral connecting rod
- EMT
epithelial to mesenchymal transition
- MASO
antisense morpholino oligonucleotide
- GEF
guanine nucleotide exchange factors
- GAPs
GTPase activating proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adomako-Ankomah A, Ettensohn CA. Growth factor-mediated mesodermal cell guidance and skeletogenesis during sea urchin gastrulation. Development. 2013;140:4214–4225. doi: 10.1242/dev.100479. [DOI] [PubMed] [Google Scholar]
- Alford LM, Ng MM, Burgess DR. Cell polarity emerges at first cleavage in sea urchin embryos. Dev Biol. 2009;330:12–20. doi: 10.1016/j.ydbio.2009.02.039. [DOI] [PubMed] [Google Scholar]
- Allaire PD, Seyed Sadr M, Chaineau M, Seyed Sadr E, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. Journal of Cell Science. 2013;126:722–731. doi: 10.1242/jcs.112375. [DOI] [PubMed] [Google Scholar]
- Beniash E, Addadi L, Weiner S. Cellular control over spicule formation in sea urchin embryos: A structural approach. J Struct Biol. 1999;125:50–62. doi: 10.1006/jsbi.1998.4081. [DOI] [PubMed] [Google Scholar]
- Berndt C, Poschmann G, Stuhler K, Holmgren A, Brautigam L. Zebrafish heart development is regulated via glutaredoxin 2 dependent migration and survival of neural crest cells. Redox Biol. 2014;2:673–678. doi: 10.1016/j.redox.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, De Robertis EM, Wallingford JB, Niehrs C. Morpholinos: Antisense and Sensibility. Dev Cell. 2015;35:145–149. doi: 10.1016/j.devcel.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. Structure of the digestive tract of the pluteus larva of Dendraster Excentricus (Echinodermata:Echinoida) Zoomorphology. 1981;98:209–225. [Google Scholar]
- Burke RD, Myers RL, Sexton TL, Jackson C. Cell movements during the initial phase of gastrulation in the sea urchin embryo. Dev Biol. 1991;146:542–557. doi: 10.1016/0012-1606(91)90255-2. [DOI] [PubMed] [Google Scholar]
- Choi S, Ko J, Lee JR, Lee HW, Kim K, Chung HS, Kim H, Kim E. ARF6 and EFA6A regulate the development and maintenance of dendritic spines. Journal of Neuroscience. 2006;26:4811–4819. doi: 10.1523/JNEUROSCI.4182-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing AC, Miller Wei, Vitale William E, Moss Nicolas, Premont Joel, Lefkowitz Richard T, Robert J. β-Arrestin-mediated ADP-ribosylation Factor 6 Activation and β2-Adrenergic Receptor Endocytosis. Journal of Biological Chemistry. 2011;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- Cotton M, Boulay PL, Houndolo T, Vitale N, Pitcher JA, Claing A. Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration. Mol Biol Cell. 2007;18:501–511. doi: 10.1091/mbc.E06-06-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cellular Signalling. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Dikshit N, Bist P, Fenlon SN, Pulloor NK, Chua CE, Scidmore MA, Carlyon JA, Tang BL, Chen SL, Sukumaran B. Intracellular Uropathogenic E. coli Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells. PLoS Pathog. 2015;11:e1005083. doi: 10.1371/journal.ppat.1005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Donaldson JG. Arf6 and its role in cytoskeletal modulation. Methods Mol Biol. 2002;189:191–198. doi: 10.1385/1-59259-281-3:191. [DOI] [PubMed] [Google Scholar]
- Donaldson JG. Multiple roles for Arf6: Sorting, structuring, and signaling at the plasma membrane. Journal of Biological Chemistry. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Radhakrishna H. Expression and properties of ADP-ribosylation factor (ARF6) in endocytic pathways. Methods Enzymol. 2001;329:247–256. doi: 10.1016/s0076-6879(01)29085-5. [DOI] [PubMed] [Google Scholar]
- Drawbridge J. The color purple: analyzing alkaline phosphatase expression in experimentally manipulated sea urchin embryos in an undergraduate developmental biology course. Int J Dev Biol. 2003;47:161–164. [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Duloquin L, Lhomond G, Gache C. Localized VEGF signaling from ectoderm to mesenchyme cells controls morphogenesis of the sea urchin embryo skeleton. Development. 2007;134:2293–2302. doi: 10.1242/dev.005108. [DOI] [PubMed] [Google Scholar]
- Dyer N, Rebollo E, Dominguez P, Elkhatib N, Chavrier P, Daviet L, Gonzalez C, Gonzalez-Gaitan M. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development. 2007;134:4437–4447. doi: 10.1242/dev.010983. [DOI] [PubMed] [Google Scholar]
- Egami Y, Fujii M, Kawai K, Ishikawa Y, Fukuda M, Araki N. Activation-Inactivation Cycling of Rab35 and ARF6 Is Required for Phagocytosis of Zymosan in RAW264 Macrophages. J Immunol Res. 2015;2015:429439. doi: 10.1155/2015/429439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn CA. Encoding anatomy: developmental gene regulatory networks and morphogenesis. Genesis. 2013;51:383–409. doi: 10.1002/dvg.22380. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The small G proteins of the arf family and their regulators. Annual Review of Cell and Developmental Biology. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Grossmann AH, Yoo JH, Clancy J, Sorensen LK, Sedgwick A, Tong Z, Ostanin K, Rogers A, Grossmann KF, Tripp SR, Thomas KR, D’Souza-Schorey C, Odelberg SJ, Li DY. The small GTPase ARF6 stimulates beta-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal. 2013;6:ra14. doi: 10.1126/scisignal.2003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EA, Yajima M, Juliano CE, Wessel GM. Post-translational regulation by gustavus contributes to selective Vasa protein accumulation in multipotent cells during embryogenesis. Developmental Biology. 2011;349:440–450. doi: 10.1016/j.ydbio.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. Local shifts in position and polarized motility drive cell rearrangement during sea urchin gastrulation. Dev Biol. 1989;136:430–445. doi: 10.1016/0012-1606(89)90268-6. [DOI] [PubMed] [Google Scholar]
- Hardin J, McClay DR. Target recognition by the archenteron during sea urchin gastrulation. Dev Biol. 1990;142:86–102. doi: 10.1016/0012-1606(90)90153-a. [DOI] [PubMed] [Google Scholar]
- Hart MW, Strathmann RR. Functional consequences of phenotypic plasticity in echinoid larvae. Biological Bulletin. 1994;186:291–299. doi: 10.2307/1542275. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hernandez-Deviez DJ, Roth MG, Casanova JE, Wilson JM. ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase alpha. Molecular Biology of the Cell. 2004;15:111–120. doi: 10.1091/mbc.E03-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys D, Davidson AC, Hume PJ, Makin LE, Koronakis V. Arf6 coordinates actin assembly through the WAVE complex, a mechanism usurped by Salmonella to invade host cells. Proc Natl Acad Sci U S A. 2013;110:16880–16885. doi: 10.1073/pnas.1311680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Gurevich VV, Casanova JE, Mukherjee S. ARF6: a newly appreciated player in G protein-coupled receptor desensitization. FEBS Lett. 2002;521:3–8. doi: 10.1016/s0014-5793(02)02822-3. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Gammill LS. Neural crest specification and migration independently require NSD3-related lysine methyltransferase activity. Mol Biol Cell. 2014;25:4174–4186. doi: 10.1091/mbc.E13-12-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Saito K, Asami H, Ohta Y. ADP ribosylation factor 6 (Arf6) acts through FilGAP protein to down-regulate Rac protein and regulates plasma membrane blebbing. J Biol Chem. 2014;289:9675–9682. doi: 10.1074/jbc.M113.546051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly EL, Hardin J. Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev Biol. 1998;204:235–250. doi: 10.1006/dbio.1998.9075. [DOI] [PubMed] [Google Scholar]
- Koo TH, Eipper BA, Donaldson JG. Arf6 recruits the Rac GEF Kalirin to the plasma membrane facilitating Rac activation. BMC Cell Biol. 2007;8:29. doi: 10.1186/1471-2121-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano G, Nishida H. Maternal and zygotic expression of the endoderm-specific alkaline phosphatase gene in embryos of the ascidian, Halocynthia roretzi. Dev Biol. 1998;198:245–252. [PubMed] [Google Scholar]
- Lambaerts K, Wilcox-Adelman SA, Zimmermann P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Current Opinion in Cell Biology. 2009;21:662–669. doi: 10.1016/j.ceb.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapraz F, Rottinger E, Duboc V, Range R, Duloquin L, Walton K, Wu SY, Bradham C, Loza MA, Hibino T, Wilson K, Poustka A, McClay D, Angerer L, Gache C, Lepage T. RTK and TGF-beta signaling pathways genes in the sea urchin genome. Dev Biol. 2006;300:132–152. doi: 10.1016/j.ydbio.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Lowe CJ, Terasaki M, Wu M, Freeman RM, Jr, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, Smith M, Kirschner M, Gerhart J. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DC, Kaltenbach SL, McClay DR. Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states. Wiley Interdiscip Rev Dev Biol. 2012;1:231–252. doi: 10.1002/wdev.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Luton F, Partisani M, Cherfils J, Chardin P, Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Fisher GW, Ettensohn CA. Four-dimensional microscopic analysis of the filopodial behavior of primary mesenchyme cells during gastrulation in the sea urchin embryo. Dev Biol. 1995;172:552–566. doi: 10.1006/dbio.1995.8044. [DOI] [PubMed] [Google Scholar]
- Materna SC, Davidson EH. A comprehensive analysis of Delta signaling in pre-gastrular sea urchin embryos. Dev Biol. 2012;364:77–87. doi: 10.1016/j.ydbio.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay DR, Armstrong NA, Hardin J. Pattern formation during gastrulation in the sea urchin embryo. Dev Suppl. 1992:33–41. [PubMed] [Google Scholar]
- Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- Miller JR, McClay DR. Characterization of the role of cadherin in regulating cell adhesion during sea urchin development. Dev Biol. 1997;192:323–339. doi: 10.1006/dbio.1997.8740. [DOI] [PubMed] [Google Scholar]
- Moorhouse KS, Gudejko HF, McDougall A, Burgess DR. Influence of cell polarity on early development of the sea urchin embryo. Dev Dyn. 2015;244:1469–1484. doi: 10.1002/dvdy.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Burke RD. The initial phase of gastrulation in sea urchins is accompanied by the formation of bottle cells. Dev Biol. 1996;179:436–446. doi: 10.1006/dbio.1996.0273. [DOI] [PubMed] [Google Scholar]
- Nishida H, Kumano G. Analysis of the temporal expression of endoderm-specific alkaline phosphatase during development of the ascidian Halocynthia roretzi. Dev Growth Differ. 1997;39:199–205. doi: 10.1046/j.1440-169x.1997.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]
- Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–1269. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. The EMBO journal. 2001;20:4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- Pennington JT, Strathmann RR. Consequences of the calcite skeletons of planktonic echinoderm larvae for orientation, swimming, and shape. Biological Bulletin. 1990;179:121–133. doi: 10.2307/1541746. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino ML, Zuch DT, Fishman J, Rose S, Speranza EE, Li C, Yu J, Chung O, Ramachandran J, Ferrell P, Patel V, Reyna A, Hameeduddin H, Chaves J, Hewitt FB, Bardot E, Lee D, Core AB, Hogan JD, Keenan JL, Luo L, Coulombe-Huntington J, Blute TA, Oleinik E, Ibn-Salem J, Poustka AJ, Bradham CA. RNA-Seq identifies SPGs as a ventral skeletal patterning cue in sea urchins. Development. 2016;143:703–714. doi: 10.1242/dev.129312. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Sun JL, Li J, Gao MG, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta 1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112( Pt 6):855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Rafiq K, Shashikant T, McManus CJ, Ettensohn CA. Genome-wide analysis of the skeletogenic gene regulatory network of sea urchins. Development. 2014;141:950–961. doi: 10.1242/dev.105585. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. Journal of Biochemistry. 2003;134:485–489. doi: 10.1093/jb/mvg181. [DOI] [PubMed] [Google Scholar]
- Santy LC, Frank SR, Casanova JE. Expression and analysis of ARNO and ARNO mutants and their effects on ADP-ribosylation factor (ARF)-mediated actin cytoskeletal rearrangements. Methods Enzymol. 2001;329:256–264. doi: 10.1016/s0076-6879(01)29086-7. [DOI] [PubMed] [Google Scholar]
- Shankar J, Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS One. 2015;10:e0119954. doi: 10.1371/journal.pone.0119954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi K, Yamazaki A, Kagawa M, Kiyomoto M, Yamaguchi M. Par6 regulates skeletogenesis and gut differentiation in sea urchin larvae. Dev Genes Evol. 2012;222:269–278. doi: 10.1007/s00427-012-0409-5. [DOI] [PubMed] [Google Scholar]
- Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, Angerer LM, Arnone MI, Burgess DR, Burke RD, Coffman JA, Dean M, Elphick MR, Ettensohn CA, Foltz KR, Hamdoun A, Hynes RO, Klein WH, Marzluff W, McClay DR, Morris RL, Mushegian A, Rast JP, Smith LC, Thorndyke MC, Vacquier VD, Wessel GM, Wray G, Zhang L, Elsik CG, Ermolaeva O, Hlavina W, Hofmann G, Kitts P, Landrum MJ, Mackey AJ, Maglott D, Panopoulou G, Poustka AJ, Pruitt K, Sapojnikov V, Song X, Souvorov A, Solovyev V, Wei Z, Whittaker CA, Worley K, Durbin KJ, Shen Y, Fedrigo O, Garfield D, Haygood R, Primus A, Satija R, Severson T, Gonzalez-Garay ML, Jackson AR, Milosavljevic A, Tong M, Killian CE, Livingston BT, Wilt FH, Adams N, Bellé R, Carbonneau S, Cheung R, Cormier P, Cosson B, Croce J, Fernandez-Guerra A, Genevière AM, Goel M, Kelkar H, Morales J, Mulner-Lorillon O, Robertson AJ, Goldstone JV, Cole B, Epel D, Gold B, Hahn ME, Howard-Ashby M, Scally M, Stegeman JJ, Allgood EL, Cool J, Judkins KM, McCafferty SS, Musante AM, Obar RA, Rawson AP, Rossetti BJ, Gibbons IR, Hoffman MP, Leone A, Istrail S, Materna SC, Samanta MP, Stolc V, Tongprasit W, Tu Q, Bergeron KF, Brandhorst BP, Whittle J, Berney K, Bottjer DJ, Calestani C, Peterson K, Chow E, Yuan QA, Elhaik E, Graur D, Reese JT, Bosdet I, Heesun S, Marra MA, Schein J, Anderson MK, Brockton V, Buckley KM, Cohen AH, Fugmann SD, Hibino T, Loza-Coll M, Majeske AJ, Messier C, Nair SV, Pancer Z, Terwilliger DP, Agca C, Arboleda E, Chen N, Churcher AM, Hallböök F, Humphrey GW, Idris MM, Kiyama T, Liang S, Mellott D, Mu X, Murray G, Olinski RP, Raible F, Rowe M, Taylor JS, Tessmar-Raible K, Wang D, Wilson KH, Yaguchi S, Gaasterland T, Galindo BE, Gunaratne HJ, Juliano C, Kinukawa M, Moy GW, Neill AT, Nomura M, Raisch M, Reade A, Roux MM, Song JL, Su YH, Townley IK, Voronina E, Wong JL, Amore G, Branno M, Brown ER, Cavalieri V, Duboc V, Duloquin L, Flytzanis C, Gache C, Lapraz F, Lepage T, Locascio A, Martinez P, Matassi G, Matranga V, Range R, Rizzo F, Röttinger E, Beane W, Bradham C, Byrum C, Glenn T, Hussain S, Manning G, Miranda E, Thomason R, Walton K, Wikramanayke A, Wu SY, Xu R, Brown CT, Chen L, Gray RF, Lee PY, Nam J, Oliveri P, Smith J, Muzny D, Bell S, Chacko J, Cree A, Curry S, Davis C, Dinh H, Dugan-Rocha S, Fowler J, Gill R, Hamilton C, Hernandez J, Hines S, Hume J, Jackson L, Jolivet A, Kovar C, Lee S, Lewis L, Miner G, Morgan M, Nazareth LV, Okwuonu G, Parker D, Pu LL, Thorn R, Wright R Consortium SUGS. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JL, Wessel GM. The forkhead transcription factor FoxY regulates Nanos. Mol Reprod Dev. 2012 doi: 10.1002/mrd.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepicheva N, Nigam PA, Siddam AD, Peng CF, Song JL. microRNAs regulate beta-catenin of the Wnt signaling pathway in early sea urchin development. Dev Biol. 2015;402:127–141. doi: 10.1016/j.ydbio.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepicheva NA, Song JL. High throughput microinjections of sea urchin zygotes. J Vis Exp. 2014:e50841. doi: 10.3791/50841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepicheva NA, Song JL. microRNA-31 modulates skeletal patterning in the sea urchin embryos. Development. 2015 doi: 10.1242/dev.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kanai Y, Hara T, Sasaki J, Sasaki T, Kohara M, Maehama T, Taya C, Shitara H, Yonekawa H, Frohman MA, Yokozeki T, Kanaho Y. Crucial role of the small GTPase ARF6 in hepatic cord formation during liver development. Molecular and Cellular Biology. 2006;26:6149–6156. doi: 10.1128/MCB.00298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague SE, Muralidharan V, D’Souza-Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci U S A. 2004;101:9671–9676. doi: 10.1073/pnas.0403531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidavsky N, Addadi S, Mahamid J, Shimoni E, Ben-Ezra D, Shpigel M, Weiner S, Addadi L. Initial stages of calcium uptake and mineral deposition in sea urchin embryos. Proc Natl Acad Sci U S A. 2014;111:39–44. doi: 10.1073/pnas.1312833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel GM, McClay DR. Sequential expression of germ-layer specific molecules in the sea urchin embryo. Dev Biol. 1985;111:451–463. doi: 10.1016/0012-1606(85)90497-x. [DOI] [PubMed] [Google Scholar]
- Wessel GM, Wikramanayake A. How to grow a gut: ontogeny of the endoderm in the sea urchin embryo. Bioessays. 1999;21:459–471. doi: 10.1002/(SICI)1521-1878(199906)21:6<459::AID-BIES3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Whittaker JR. Determination of Alkaline Phosphatase Expression in Endodermal Cell Lineages of an Ascidian Embryo. Biol Bulletin. 1990;178:222–230. doi: 10.2307/1541823. [DOI] [PubMed] [Google Scholar]
- Wilt FH. Developmental biology meets materials science: Morphogenesis of biomineralized structures. Dev Biol. 2005;280:15–25. doi: 10.1016/j.ydbio.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Wu SY, McClay DR. The Snail repressor is required for PMC ingression in the sea urchin embryo. Development. 2007;134:1061–1070. doi: 10.1242/dev.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cox D, Tseng CC, Donaldson JG, Greenberg S. A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages. J Biol Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The Arf6-mCherry reporter contained the Arf6 5′UTR and coding region (highlighted in yellow) fused with mCherry (red font) and β-globin 3′ UTR (underlined). (B) The mRNA sequence of SpArf6. The position of the amino acid important for the GTP hydrolysis (Q67) is highlighted in light blue. (C) The SpArf6 protein is 91.4% identical to the HsArf6 protein using LALIGN program. Amino acid differences between SpArf6 and HsArf6 are highlighted in yellow. The conserved amino acid important for the GTP hydrolysis (Q67) is highlighted in light blue.
The SpArf6-mCherry or cytoplasmic mCherry control reporters were in vitro transcribed and microinjected into the newly fertilized eggs. Living gastrula stage embryos were imaged using confocal microscopy. Exogenously expressed mCherry fused to the sea urchin Arf6 was enriched at the plasma membrane in all cells. Scale bar is 50 μm.
1 mM of control MASO or Arf6 MASO were microinjected into the newly fertilized eggs. The blastula (24 hpf) and early gastrula (30 hpf) stage embryos were collected to assess PMC and archenteron development. (A) Blastulae were fixed and immunostained with PMC-specific marker 1D5. About 20% of the Arf6 KD embryos exhibited mislocalized PMCs; however, this percentage was not significantly different than the control embryos (Cochran-Mantel-Haenszel test, 2 biological replicates). (B) 15% of the Arf6 KD blastulae exhibited delay in development; however, this delay was not statistically significant compared to the control (Cochran-Mantel-Haenszel test, 2 biological replicates). (C) PMC patterning was not disrupted by Arf6 KD assessed at the early gastrula stage (30 hpf) (Cochran-Mantel-Haenszel test, 3 biological replicates). Scale bar is 50 μm. (D) Archenteron invagination was evaluated by ratios of the gut length and the embryo height. To account for the differences in the development of embryos from different biological replicates, the gut/height ratios in the Arf6 KD embryos were normalized to the ratios obtained for the control embryos. Archenteron formation was not affected by Arf6 KD (Student’s T-test, 3 biological replicates). N is the number of embryos evaluated.
The newly fertilized eggs were microinjected with either (A) 1 mM control or Arf6 MASO or (B) 0.4 μg/μL Arf6-Q67L or Firefly control mRNA. The same embryos were tracked throughout development and evaluated for survival (solid lines). The embryos were assessed based on their morphology and ability to swim (dashed lines) at three developmental stages: blastula (24 hpf), gastrula (48 hpf) and pluteus (72 hpf). Both perturbations in Arf6 levels and activity resulted in almost 40% drop in survival after the blastula stage. About 40–50% of Arf6 perturbed embryos were still alive by pluteus stage, of which approximately half of the survived embryos were healthy.
0.4 μg/μL of Arf6-Q67L or control Firefly mRNA were microinjected into the newly fertilized eggs and the embryos were collected at blastula stage (24 hpf) and early gastrula stage (30 hpf) of development. (A,B) Blastulae were fixed and immunostained with PMC-specific marker 1D5. About 40% of the Arf6 KD embryos exhibited mislocalized PMCs; however, this percentage was not significantly different from the control embryos (Fisher’s exact test of independence). (C, D) PMC patterning was also not disrupted by Arf6 KD by early gastrula stage (30 hpf) (Fisher’s exact test of independence). Scale bar is 50 μm. (E) Archenteron invagination was evaluated by measuring the gut length and the embryo height and obtaining the ratios between them. Archenteron formation was not affected by Arf6 KD (Student’s T-test, 1 biological replicate). N is the number of embryos evaluated.
100 embryos injected with either control MASO or Arf6 MASO were collected at various time points. (A) Embryos at early gastrula stage (30 hpf) were assayed for the transcript levels of biomineralization genes (brown) and fusion genes (green). (B) Embryos at mesenchyme blastula (24 hpf) or early gastrula stage (30 hpf) were collected and assayed for their transcript levels of genes that had been shown to be important for PMC positioning and migration. (C) Embryos at mesenchyme blastula and early gastrula stages were collected and assayed for genes expressed in the ectoderm (blue) and endoderm (orange).
This result indicates that skeletogenesis defects induced by the microinjection of Arf6-Q67L mRNA were not due to the nonspecific overexpression of Arf6 protein, but to the specific blockade of Arf6 activation or inactivation.
Control vs Arf6 MASO (1 mM) or Arf6-Q67L vs Firefly control mRNA (0.4 μg/μL) were microinjected into newly fertilized eggs. Gastrulae were collected and immunostained against cadherin. No significant changes in cadherin localization or the organization of ectodermal cells were detected in Arf6 perturbed embryos compared to the control. The gut failed to invaginate in the Arf6 KD embryo. The maximum intensity projection of 10–20 digital slices is shown. Scale bar is 50 μm.