Abstract
INTRODUCTION
We hypothesized that common Alzheimer’s disease (AD)-associated variants within the triggering receptor expressed on myeloid (TREM) gene cluster influence disease through gene expression.
METHODS
Expression microarrays on temporal cortex and cerebellum from ~400 neuropathologically diagnosed subjects, and two independent RNAseq replication cohorts were used for expression quantitative trait locus (eQTL) analysis.
RESULTS
A variant within a DNase hypersensitive site 5′ of TREM2, rs9357347-C, associates with reduced AD-risk and increased TREML1 and TREM2 levels (uncorrected-p=6.3×10-3 and 4.6×10-2, respectively). Meta-analysis on eQTL results from three independent datasets (n=1,006) confirmed these associations (uncorrected-p=3.4×10−2 and 3.5×10−3, Bonferroni-corrected p=6.7×10−2 and 7.1×10−3, respectively).
DISCUSSION
Our findings point to rs9357347 as a functional regulatory variant that contributes to a protective effect observed at the TREM locus in the International Genomics of Alzheimer’s Project (IGAP) GWAS meta-analysis, and suggest concomitant increase of TREML1 and TREM2 brain levels as a potential mechanism for protection from AD.
Keywords: Alzheimer’s disease, eQTL, TREM2, TREML1, regulatory variant
1. Introduction
Whole genome and exome sequencing are used as complementary approaches to uncover novel loci that can be missed by GWAS, and enabled the discovery of rare, missense alleles within TREM2 that have a relatively large effect size on AD-risk [1, 2]. TREM2 is a member of the triggering receptor expressed on myeloid (TREM) family, known to play a key role in modulating inflammation in the innate immune response [3]. This finding provided strong supportive evidence for the importance of inflammation in the etiology of AD, but the specific role played by TREM2 in AD pathophysiology remains unclear [4].
Since the first two reports [1, 2], the risk effect of the most significant TREM2 rare missense variant p.R47H (a.k.a. rs75932628) has been replicated in multiple Caucasian series [5–9], including a large meta-analysis of 24,086 AD cases and 148,993 controls [10]. TREM2 resides within the TREM gene cluster on chromosome 6p21.1 (Fig. 1), which also includes the protein coding genes TREM1, TREML1, TREML2, TREML4 that could be additional plausible AD-risk genes.
Fig. 1. TREM gene cluster on Chr 6p21.1.
The chromosomal positions are based on the human genome assembly from February 2009 (GRCh37/hg19). There are seven RefSeq genes at the TREM locus (TREM1, TREML1, TREM2, TREML2, TREML3P, TREML4 and TREML5P); however, TREML3P and TREML5P are non-coding pseudogenes. The transcript figures are taken from the UCSC Genome Browser.
A missense variant in TREML2, p.S144G (a.k.a. rs3747742), that is not in linkage disequilibrium (LD) with TREM2 p.R47H, was reported to associate with reduced AD-risk [11]. TREML2 p.S144G is in tight LD with the intergenic variant, rs9381040, that demonstrated the most significant association at the TREM locus in the IGAP AD-risk GWAS meta-analysis (rs9381040 p=7.4×10−3 after Bonferroni correction for 11,632 variants tested in the combined IGAP stage 1 and 2 data sets; uncorrected p=6.3×10−7) [12]. The authors concluded that TREML2 p.S144G is the functional variant that accounted for the IGAP TREM locus signal, even though the significance of the AD-risk association with the intergenic rs9381040 is greater than that observed with p.S144G. Further, TREML2 p.S144G does not have a predicted functional consequence (PolyPhen2 score=benign) or demonstrated functional outcome, suggesting that the IGAP signal at the TREM locus may be due to other functional variants.
Some variants at the TREM locus have been reported to show association with AD endophenotypes [11, 13, 14]. Cerebrospinal fluid (CSF) levels of AD biomarkers, tau and ptau, associate with three variants at the TREM locus that are not in LD with each other: TREM2 p.R47H (rs75932628), rs6916710 located in intron 2 of TREML2, and rs6922617 located downstream from NCR2 and outside the TREM cluster. Of these variants, only TREM2 p.R47H was associated with AD-risk [13]. More recently, a variant upstream of TREM2 (rs7759295) and a variant in intron 3 of TREM1 (rs6910730) were reported to be independently associated with increased AD pathology burden and increased rate of cognitive decline [14]. However, neither of these two variants shows association with AD-risk in the IGAP meta-analysis (uncorrected p>0.05) [15]. Thus, other than TREM2 p.R47H, none of the TREM locus variants previously reported to associate with AD endophenotypes show association with AD-risk. Functional AD-risk variants that influence AD endophenotypes are expected to show association both with these endophenotypes and risk of AD. Therefore, it is possible that these latter variants are not the functional variants per se, but merely markers of other un-tested functional variants.
Collectively, these prior findings suggest that besides the TREM2 rare missense variants, there may be common variants at the TREM locus that influence AD-risk and/or its endophenotypes. We hypothesized that some of the common AD-risk variants at the TREM locus confer disease risk via regulation of transcript levels of coding genes at the TREM gene cluster. In this study, we characterized the brain expression levels of the TREM family genes using microarray expression data; validated expression levels by RNA sequencing (RNAseq); performed genetic associations with TREM locus genes reliably detected in cerebellum and temporal cortex with single nucleotide polymorphisms (SNP) that were also tested in the IGAP AD-risk GWAS meta-analysis; and annotated these variants for their effects on TREM gene expression levels and regulatory potential. Further, we obtained results for the top putative regulatory SNP from two other, independent cohorts with brain RNAseq data and performed meta-analysis of all three cohorts.
2. Materials and Methods
2.1 Variant selection
We restricted our analysis to variants located within 100kb of any coding TREM family gene at the chromosome 6p21.1 TREM gene cluster (Fig. 1). Variants were further selected based on the statistical significance of their AD-risk association in the IGAP stage 1 meta-analysis [12] (Supplementary Methods), where only those variants with uncorrected p-values ≤ 0.0015 were kept. This p-value cut-off was arbitrarily chosen to select those variants that existed in both the IGAP stage 1 AD GWAS and our discovery eQTL cohort, Mayo Clinic Whole Genome-DASL dataset, and that could be genotyped, if needed, in the replication eQTL cohorts, using cost-effective medium-throughput assays. Variants were further prioritized by their Regulome score. Regulome scores were obtained from the Regulome database, which annotates variants with regulatory information from 962 different datasets and a variety of sources, including ENCODE [16]. Regulome scores are on a scale from 1 to 6, and these numerical categories are sub-classified with letters based on the number of lines of evidence of functional consequence. A value of 1a is assigned to the variant with the most evidence of regulatory potential, while a score of 6 has the least [16].
2.2 Mayo Clinic Whole Genome-DASL dataset (Discovery eQTL cohort)
We utilized Illumina (Whole Genome-DASL=WG-DASL, Illumina, San Diego, CA) microarray gene expression data from our published human brain expression genome-wide association study (Mayo Clinic eGWAS) [17] conducted on brain tissue from autopsied AD patients (197 cerebellum, 202 temporal cortex) and non-AD subjects (177 cerebellum, 197 temporal cortex) (Table 1). All AD subjects had neuropathologic diagnosis of definite AD [2]. The non-AD subjects did not fulfill neuropathologic criteria for definite AD, but many had other unrelated pathologies. Expression measures were generated as described previously [17]. A description of this cohort and generation of expression measures is provided in the Supplemetary Methods.
Table 1.
Description of samples included in the discovery and replication cohorts utilized for eQTL analysis.
| Mayo Clinic WG-DASL | Mayo Clinic RNAseq | ROS/MAP RNAseq | ||||||
|---|---|---|---|---|---|---|---|---|
| CER | TCX | TCX | PFCX | |||||
| AD | Non-AD | AD | Non-AD | AD | non-AD | AD | non-AD | |
| N | 197 | 177 | 202 | 197 | 84 | 48 | 288 | 206 |
| Mean age +/− SD | 73.6 ± 5.6 | 71.7 ± 5.5 | 73.6 ± 5.5 | 71.6 ± 5.6 | 83.2 ± 8.7 | 85.7 ± 8.3 | 89.8 ± 5.8 | 86.5 ± 7.2 |
| Female, N (%) | 101 (51%) | 63 (36%) | 108 (53%) | 78 (40%) | 48 (57%) | 26 (54%) | 186 (65%) | 121 (59%) |
| % APOE ε4+ | 64% | 25% | 61% | 25% | 51% | 17% | 34% | 12% |
Samples included in the Mayo Clinic eGWAS (discovery cohort), with cerebellar (CER) and temporal cortex (TCX) gene expression measurements from Illumina WG-DASL arrays have been previously described [17]. Samples in the Mayo Clinic RNAseq cohort (replication cohort #1) had temporal cortex gene expression measurements, and did not overlap with the Mayo eGWAS (WG-DASL) cohort. The ROS/MAP RNAseq cohort (replication cohort #2) had dorsolateral prefrontal cortex (PFCX) gene expression measurements, and did not overlap with the Mayo eGWAS (WG-DASL), or with the Mayo Clinic RNAseq cohort. The RNAseq data for these two cohorts is available at the Sage Synapse, AMP AD Knowledge Portal (https://www.synapse.org/#!Synapse:syn2580853/wiki/66722), under synapse IDs syn3388564 (ROS/MAP RNAseq) and syn3163039 (Mayo RNAseq).
2.3 RNAseq datasets (Replication eQTL cohorts)
Temporal cortex RNAseq data from two RNAseq cohorts: “Mayo Clinic RNASeq” and “ROS/MAP RNAseq” were employed for replication of the associations that were detected with the WG-DASL gene expression measurements. The Mayo Clinic RNASeq dataset is comprised of 84 LOAD and 48 non-AD brains from the Mayo Clinic Brain Bank that were not part of the Mayo Clinic WG-DASL cohort but whose neuropathological diagnosis followed the same criteria. The ROS/MAP RNAseq dataset is comprised of RNAseq data from 288 AD and 206 non-AD samples that are part of the ROS/MAP cohort (Table 1) previously described [18, 19]. Methodological details for the RNAseq data generation are provided in the Supplementary Methods.
2.4. Statistical Analysis
Normalized transcript expression levels, on a log2 scale, were tested for associations with TREM locus genotypes in each of the three datasets (Mayo WG-DASL, Mayo Clinic RNAseq and ROS/MAP RNAseq) via multivariable linear regression analyses implemented in PLINK [20]. An additive model was applied adjusting for age-at-death, sex, diagnosis, RNA Integrity Number (RIN) and adjusted RIN squared (RIN-RINmean)2 in all expression analyses, and APOE ε4 dosage and PCR plate in Mayo WG-DASL only, and flowcell in the Mayo Clinic RNAseq dataset only. The eQTL analysis in the discovery, WG-DASL dataset, included APOE ε4 dose as a covariate given the strong effect of this allele on AD. However, since a significant association was not detected with this covariate in the rs9357347 eQTL analyses in the discovery set, APOE ε4 dose was not included in the eQTL analyses implemented on the replication cohorts. For comparison, we have performed the eQTL analyses in all three datasets with and without adjustment for APOE ε4 dose and do not observe a substantial difference in the association results between these two models. Given the tight LD between variants at this locus, and the potential for co-regulation of TREM genes at this cluster across tissues, a correction for multiple testing was not applied, except for the final meta-analysis results, for which both the uncorrected and Bonferroni corrected p-value (adjusted for the two genes tested) are provided.
Meta-analyses were performed on eQTL results from the three independent datasets. For these analyses, METAL [21] was implemented using weighted average of z-scores from the individual study p-values, weighted according their sample size.
To assess if diagnosis is associated with TREML1 and/or TREM2 gene expression levels, linear regression analyses were performed in R in each of the three datasets, adjusting for all other covariates included in the eQTL analyses, as well as rs9357347 minor allele dose.
3. Results
In the WG-DASL gene expression data from the temporal cortex (n=399) and cerebellum (n=374) of neuropathologically diagnosed AD and non-AD subjects (Table 1), we observed that of the 5 TREM locus coding genes, only TREML1 and TREM2 were reliably detected (Table S1 and Fig. 2). TREML1 was detected in both the temporal cortex and cerebellum, while TREM2 was reliably detected only in the temporal cortex. We validated TREML1 and TREM2 WG-DASL temporal cortex gene expression measurements, using RNAseq data generated from a subset of 93 autopsied AD subjects who also had microarray data. There was highly significant correlation between WG-DASL and RNAseq measurements for both TREML1 (rs=0.65, p<10−40) and TREM2 (rs=0.80, p<10−40) (Fig. S1).
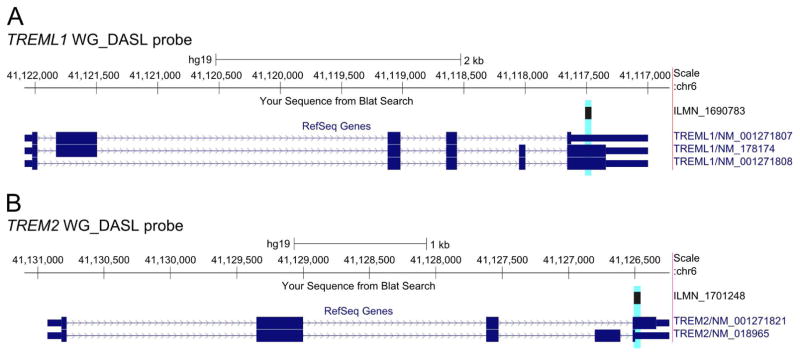
Fig. 2. Location of TREML1 and TREM2 WG-DASL probes.
The location of the (A) TREML1 and (B) TREM2 WG-DASL probes (highlighted in light blue) are shown relative to their Refseq transcripts. The chromosomal positions are based on the human genome assembly from February 2009 (GRCh37/hg19). As shown, both of these probes are complementary to all RefSeq transcripts for the respective gene. The transcript figures are taken from the UCSC Genome Browser.
Variants located within 100kb of the 5′ or 3′end of any TREM coding gene that demonstrated association with AD-risk in the IGAP stage 1 meta-analysis (17,800 AD vs. 37,154 controls, p≤0.0015), were evaluated for their association with TREML1 expression in the temporal cortex and cerebellum, and with TREM2 expression in the temporal cortex. Of the 1,002 variants tested at this locus in the IGAP stage 1 meta-analysis, 28 had p-values ≤ 0.0015, and 16 of these have been genotyped in the autopsied samples in the Mayo Clinic brain expression genome-wide association study (Mayo eGWAS). We also assessed 5 other variants at this locus previously reported to be associated with either reduced AD-risk (rs3747742) [11], increased AD pathology burden and cognitive decline (rs6910730, rs7759295) [14], or decreased CSF tau levels (rs6916710, rs6922617) [13]. Table 2 shows the association of TREML1 and TREM2 gene expression with these 21 variants. In 399 combined AD and non-AD temporal cortex samples tested for the 16 IGAP variants, 5 SNPs showed association (uncorrected p<0.05) with increased levels of both TREML1 and TREM2 (rs9381040, rs2093395, rs9357347, rs9394778, rs9296359), and a sixth variant (rs9394767) was significantly associated with increased TREML1 levels only. As shown in Fig. 3, four of the six variants that associate with increased levels of TREML1 and TREM2 are in a single LD block (block 2: rs9357347, rs9381040, rs2093395 and rs9394767) and in tight linkage disequilibrium with each other (r2≥0.90). Of these variants, rs9381040 has the most significant AD-risk association in the IGAP stage 1 meta-analysis (Table 2). This IGAP “hit” is located 5.5kb downstream from TREML2 and 23.7kb upstream from TREM2 and is associated with TREML1 and TREM2 expression (uncorrected p=0.0083, beta=0.086 and uncorrected p=0.048, beta=0.091, respectively). Given that the expression measures were on a log2 scale, these changes in expression are equivalent to TREML1 and TREM2 fold-changes of 1.06 and 1.07, for each copy of the minor allele, respectively. Notably, the minor allele of the IGAP “hit” rs9381040 is associated with both decreased AD-risk and increased TREML1 and TREM2 levels. However, based on data from the Roadmap Epigenomics Consortium [22], rs9381040 lacks evidence of regulatory potential in brain regions relevant to AD.
Table 2.
Association of variants at the TREM locus with AD-risk and TREM WG-DASL brain gene expression levels.
| Chr | SNP | Position hg19 | AD-Risk (IGAP Stage1 Meta-analysis) | Brain eQTL (Mayo Clinic eGWAS) | Regulome Score | HapMap CEU MAF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Allele | Non Effect Allele | OR (95% CI) | P-value | TREML1 CER BETA | TREML1 CER P | TREML1 TCX BETA | TREML1 TCX P | TREM2 TCX BETA | TREM2 TCX P | |||||
| 6 | rs9381040 | 41,154,650 | T | C | 0.94 (0.91 – 0.98) | 5.97E-04 | 0.021 | 2.30E-01 | 0.086 | 8.30E-03 | 0.091 | 4.80E-02 | NA | 26.70% |
| 6 | rs2093395 | 41,155,026 | C | G | 0.94 (0.91 – 0.98) | 6.40E-04 | 0.021 | 2.30E-01 | 0.086 | 8.30E-03 | 0.091 | 4.80E-02 | 6 | 27.90% |
| 6 | rs2038568 | 41,158,132 | C | G | 1.14 (1.05 – 1.23) | 7.93E-04 | 0.018 | 7.80E-01 | −0.08 | 3.90E-01 | −0.186 | 1.60E-01 | 5 | 8.30% |
| 6 | rs12194214 | 41,028,574 | C | A | 1.16 (1.06 – 1.26) | 8.36E-04 | −0.081 | 9.40E-02 | −0.104 | 2.70E-01 | −0.129 | 3.20E-01 | 6 | 4.20% |
| 6 | rs9462675 | 41,153,238 | A | G | 1.15 (1.06 – 1.25) | 9.54E-04 | −0.015 | 7.50E-01 | −0.114 | 1.60E-01 | −0.207 | 6.50E-02 | 5 | 3.60% |
| 6 | rs6933067 | 41,133,522 | C | T | 1.15 (1.06 – 1.25) | 1.07E-03 | −0.013 | 7.60E-01 | −0.098 | 2.10E-01 | −0.134 | 2.10E-01 | 7 | 3.50% |
| 6 | rs9357347 | 41,150,591 | C | A | 0.95 (0.91 – 0.98) | 1.10E-03 | 0.013 | 4.60E-01 | 0.088 | 6.30E-03 | 0.09 | 4.60E-02 | 2b | 28.10% |
| 6 | rs9394767 | 41,159,905 | G | A | 0.95 (0.91 – 0.98) | 1.14E-03 | 0.011 | 5.70E-01 | 0.096 | 6.50E-03 | 0.083 | 1.00E-01 | 5 | 28.80% |
| 6 | rs1542638 | 41,286,604 | G | A | 1.06 (1.02 – 1.09) | 1.14E-03 | −0.022 | 2.20E-01 | −0.035 | 2.90E-01 | −0.064 | 1.60E-01 | 4 | 28.30% |
| 6 | rs9471491 | 41,153,622 | A | C | 1.15 (1.05 – 1.26) | 1.31E-03 | −0.015 | 7.50E-01 | −0.114 | 1.60E-01 | −0.207 | 6.50E-02 | 7 | 3.50% |
| 6 | rs9471495 | 41,157,372 | A | C | 1.15 (1.05 – 1.25) | 1.40E-03 | 0.014 | 8.30E-01 | −0.099 | 2.90E-01 | −0.235 | 7.20E-02 | 7 | 3.50% |
| 6 | rs9462677 | 41,158,856 | A | T | 1.15 (1.05 – 1.25) | 1.41E-03 | 0.016 | 8.10E-01 | −0.099 | 2.90E-01 | −0.235 | 7.30E-02 | 7 | 4.30% |
| 6 | rs9394778 | 41,215,058 | A | G | 0.95 (0.92 – 0.98) | 1.44E-03 | 0.015 | 3.30E-01 | 0.065 | 2.70E-02 | 0.099 | 1.50E-02 | 6 | 39.80% |
| 6 | rs9471494 | 41,157,344 | G | C | 1.15 (1.05 – 1.25) | 1.46E-03 | 0.01 | 8.70E-01 | −0.102 | 2.60E-01 | −0.221 | 8.20E-02 | 6 | 4.50% |
| 6 | rs6912013 | 41,061,593 | C | T | 1.15 (1.05 – 1.25) | 1.48E-03 | −0.076 | 1.20E-01 | −0.104 | 2.70E-01 | −0.124 | 3.40E-01 | 5 | 2.70% |
| 6 | rs9296359 | 41,205,690 | A | G | 0.95 (0.92 – 0.98) | 1.48E-03 | 0.017 | 2.80E-01 | 0.066 | 2.40E-02 | 0.116 | 4.60E-03 | 6 | 27.40% |
| 6 | rs3747742* | 41,162,518 | C | T | 0.96 (0.92 – 0.99) | 8.56E-03 | 0.018 | 2.90E-01 | 0.072 | 2.30E-02 | 0.064 | 1.50E-01 | 6 | 28.30% |
| 6 | rs6916710* | 41,164,788 | T | C | 0.97 (0.94 – 1.01) | 1.03E-01 | 0.013 | 4.30E-01 | 0.054 | 7.70E-02 | 0.072 | 9.20E-02 | 7 | 38.40% |
| 6 | rs7759295* | 41,135,850 | T | C | 0.98 (0.93 – 1.03) | 3.66E-01 | −0.023 | 3.50E-01 | 0.094 | 4.00E-02 | −0.008 | 9.00E-01 | 6 | 13.30% |
| 6 | rs6910730* | 41,246,633 | G | A | 0.99 (0.94 – 1.04) | 6.86E-01 | −0.046 | 8.50E-02 | −0.079 | 1.20E-01 | −0.032 | 6.50E-01 | 4 | 8.40% |
| 6 | rs6922617* | 41,336,101 | A | G | 0.99 (0.93 – 1.05) | 6.98E-01 | −0.033 | 2.60E-01 | −0.098 | 7.40E-02 | 0.011 | 8.90E-01 | 7 | 8.50% |
Shown are variants located within 100kb of a TREM gene that had an AD-risk p ≤ 0.0015 in the IGAP stage 1 meta-analysis (top 16 rows), as well as 5 common TREM locus variants with previous reports of AD-risk or endophenotype association (bottom 5 rows, SNP marked with an *). AD-risk association results are from the publicly available IGAP meta-analysis stage 1. Brain gene expression associations are from the Mayo Clinic eGWAS and based on cerebellar (CER) and temporal cortex (TCX) gene expression measurements with Illumina WG-DASL arrays with TREML1 probe ILMN_1690783 and TREM2 probe ILMN_1701248. All p-values shown on this table are uncorrected, given that many of the variants tested at this locus are correlated due to LD, and the genes tested are likely co-regulated. Variants showing association with gene expression (uncorrected p<0.05) are underlined and in italic font. The variant with the most significant AD-risk association in the IGAP meta-analysis (rs9381040), and the variant with the most significant gene expression association and best Regulome score (rs9357347) are in bold font. OR (95% CI): odds ratio and 95% confidence interval. Given that the eGWAS expression measures were on a log2 scale, fold-change for the Mayo eGWAS beta coefficients = 2beta.
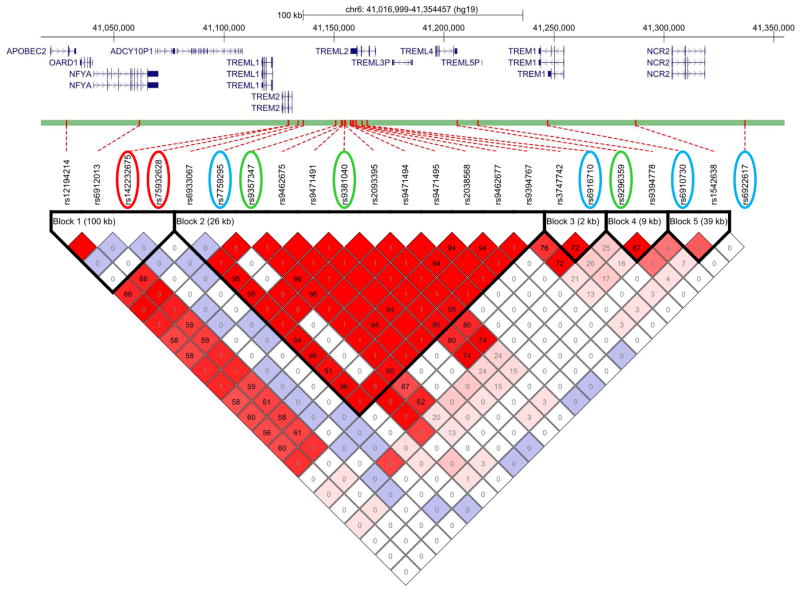
Fig. 3. LD Plot of TREM locus variants.
LD plot of TREM locus variants where haplotype blocks were determined with the solid spine definition; square colors correspond to D′ (tight LD=warmer colors, weak LD=cooler colors) and r2 values are shown within the squares (Supplementary Methods). Red circles: The rare TREM2 AD-risk missense variants rs142232675 (p.D87N) and rs75932628 (p.R47H) [1]. Blue circles: Variants that associate with increased AD pathology burden and cognitive decline (rs7759295 and rs6910730) [14], or with lower CSF ptau (rs6922617 and rs6916710) [13]. Green circles: The variant with the most significant AD-risk association in the IGAP meta-analysis (rs9381040); rs9357347, which has the most significantTREML1 gene expression association, also shows association withTREM2 gene expression, IGAP AD-risk association and the best Regulome score within all tested SNPs; and rs9296359 which has the most significant association with TREM2 expression. RefSeq gene transcripts are shown above the LD plot relative to the variant position according to the February 2009 human genome assembly (GRCh37hg19) across the targeted genomic region (TREM gene +/−100 kb: chr6:41016999–41354457).
The variant with the most significant association with brain TREML1 expression, which also associates with TREM2 levels, is rs9357347 in block 2 (Fig. 3). This SNP is located 6.9kb downstream from TREML2 and 19.6kb upstream from TREM2 and is in tight LD with the IGAP “hit” rs9381040 (D′=0.99, r2=0.96). As expected, the minor allele of rs9357347 is associated with reduced AD-risk (OR=0.95, 95% CI=0.91–0.98, uncorrected p=0.001) in the IGAP meta-analysis [12] and with increased TREML1 and TREM2 expression in the temporal cortex in the Mayo Clinic WG-DASL eQTL analysis (uncorrected p=0.0063, beta=0.088 and uncorrected p=0.046, beta=0.090, respectively) (Table 2 and Fig. S2). These beta coefficients can be interpreted as an estimated 1.06-fold change of both TREML1 and TREM2, per rs9357347 minor allele, in this temporal cortex dataset. Given the tight LD between variants at this locus, and the potential for co-regulation of TREM genes at this cluster across tissues, correcting for the 21 variants and two genes tested is unwarranted. It should be noted however, that although the association of rs9357347 with gene expression would not survive a strict Bonferroni correction for all 21 variants evaluated, its association with TREML1 gene expression in temporal cortex remains significant (Bonferroni corrected p=0.019) when applying a Bonferroni correction for the tissues and genes tested (TREML1 in cerebellum, TREML1 in temporal cortex and TREM2 in temporal cortex). Unlike the IGAP “hit” (rs9381040), rs9357347 lies within sequence subject to histone modifications and within a DNAse hypersensitive site detected by the Roadmap Epigenomics Consortium [22] in brain regions relevant to AD pathology such as the hippocampus. Furthermore, this variant is predicted to affect transcription factor binding (SP1 and PPAR) as catalogued in HaploReg (http://www.broadinstitute.org/mammals/haploreg/haploreg.php) [23]. Consequently, it has a compelling Regulome score of 2b (http://www.regulomedb.org/) due to the evidence of its regulatory potential [16] (Table 2). Indeed, of all the variants with an AD-risk p-value<0.0015 in the IGAP meta-analysis, and p-values<0.05 in our WG-DASL eQTL analysis of temporal cortex TREML1 and TREM2 gene expression levels, rs9357347 had the greatest regulatory potential as determined by their Regulome scores (Fig. S3 and Fig. S4).
The other two variants with gene expression associations in the temporal cortex are in a different LD block (block 4: rs9394778 and rs9296359) and in tight LD with each other (r2 = 0.67). These SNPs are more significantly associated with TREM2 than with TREML1 expression; however, neither has compelling evidence of regulatory potential as both have Regulome scores of 6 (Table 2). In the 374 AD and non-AD subjects with cerebellum expression measures, none of the 16 IGAP AD-risk associated variants that were tested, associate with TREML1 gene expression (p>0.05).
We determined the extent of linkage disequilibrium (LD) between the likely regulatory variant rs9357347, the IGAP “hit” rs9381040 and the significant TREM2 rare missense AD-risk variants p.D87N (rs142232675) and p.R47H (rs75932628) [1]. As shown in Fig. 3, these two TREM2 rare missense AD-risk variants are not in LD with either rs9357347 or rs9381040. This suggests that the protective effect of the regulatory rs9357347 and the IGAP “hit” are independent of the rare, missense TREM2 variants.
We next evaluated LD amongst variants tested at this locus, including common TREM locus variants previously reported to have associations with AD-risk (rs3747742) [11], increased AD pathology burden and cognitive decline (rs7759295 and rs6910730) [14], or with lower CSF ptau (rs6922617 and rs6916710) [13]. The missense TREML2 variant rs3747742 (p.S144G) is in LD with the regulatory variant implicated in our study, rs9357347. As reported, rs3747742 is also in LD with rs9381040 (IGAP hit); and as expected associates with reduced AD-risk (uncorrected p=0.009), however with slightly lesser significance than the AD-risk association of the regulatory rs9357347 (uncorrected p=0.001) or the IGAP “hit” rs9381040 (uncorrected p=0.0006). Further, the association of rs3747742 with TREML1 expression is not as significant as that of rs9357347. In addition, rs3747742 has no association with brain TREM2 levels, and has a weak Regulome score of 6 (Table 2).
Of the four common TREM locus variants that associate with AD endophenotypes, only rs6916710 is in tight LD with the regulatory rs9357347 (D′=0.91, r2=0.62). However, rs6916710, does not show significant association with AD-risk in the IGAP meta-analysis (uncorrected p=0.103) nor with TREML1 or TREM2 gene expression levels (Table 2).
None of the other three common TREM locus variants with reported AD-endophenotype associations are in tight LD with the regulatory rs9357347 or any of the other TREM locus variants that are associated with AD-risk. Only rs7759295 showed association with TREML1 gene expression (uncorrected p=0.04), but neither this nor any of the other AD-endophenotype-associated SNPs have evidence of AD-risk association or Regulome scores that are indicative of likely regulatory function (Fig. 3 and Table 2).
Utilizing publicly available RNAseq data from two independent cohorts (Table 1) that do not overlap with the samples included in the WG-DASL eQTL analysis, we sought replication of the rs9357347 association with TREML1 and TREM2. Although in the ROS/MAP RNAseq dataset a significant association was only detected with the levels of TREM2; and the Mayo Clinic RNAseq dataset showed no evidence of association with either TREM2 or TREML1 (Table 3), meta-analysis from the three independent study p-values (Mayo WG-DASL, Mayo RNAseq and ROS/MAP RNAseq) yielded significant results (TREML1 uncorrected p=3.4×10−2; TREM2 uncorrected p=3.5×10−3), confirming the association of the rs9357347 minor allele with increased TREML1 and TREM2 gene expression. The evidence of association with TREM2 expression was greater upon meta-analysis compared to the association observed in our discovery dataset; whereas the evidence of association with TREML1 expression was slightly greater in our discovery dataset compared to the meta-analysis.
Table 3.
Meta-analysis of rs9357347 eQTL results from three independent datasets.
| Dataset | Sample size | MAF |
TREML1
|
TREM2
|
||||
|---|---|---|---|---|---|---|---|---|
| beta | SE | P | beta | SE | P* | |||
| Mayo WG-DASL | 380 | 0.307 | 0.088 | 0.032 | 6.28E-03 | 0.090 | 0.045 | 4.61E-02 |
| Mayo Clinic RNAseq | 132 | 0.311 | −0.030 | 0.108 | 7.82E-01 | 0.084 | 0.128 | 5.13E-01 |
| ROS/MAP RNAseq | 494 | 0.281 | 0.089 | 0.114 | 4.36E-01 | 0.124 | 0.060 | 3.77E-02 |
|
| ||||||||
| Meta-analysis | 1006 | +−+ | 3.36E-02 (6.72E-02)* | +++ | 3.54E-03 (7.08-03)* | |||
Meta-analysis of rs9357347 eQTL results from temporal cortex (Mayo WG-DASL and Mayo Clinic RNAseq) and dorsolateral prefrontal cortex samples (ROS/MAP). MAF = minor allele frequency. SE = standard error. Since in all three datasets the expression measures analyzed were on a log2 scale, fold-change for the beta coefficients = 2beta. The meta-analysis was performed using METAL, with weighted average of z-scores from the individual study p-values, weighted according their sample size.
All p-values shown on this table are uncorrected, except for a strict Bonferroni correction applied to the p-value shown in parenthesis, which accounts for the two different genes that were tested.
The association of TREML1 and/or TREM2 gene expression with diagnosis was also tested. The box plots in Fig. S5 show the difference in expression between AD and nonAD subjects, and indicate the significance of the association for each comparison (uncorrected p-values). We observe a consistent trend of higher TREML1 and TREM2 expression in AD versus nonADs, although some of these associations do not reach statistical significance (Mayo WG-DASL: TREML1 p=1.9×10−6, TREM2 p=1.1×10−1; Mayo Clinic RNAseq: TREML1 p=1.4×10−2, TREM2 p=8.5×10−7; ROS/MAP RNAseq: TREML1 p=6.6×10−1, TREM2 p=5.2×10−2).
4. Discussion
In this study, we first sought to characterize the brain expression of TREM locus genes based on the premise that those TREM cluster genes that are expressed in the brain are likely to be candidate AD-risk genes. We determined that besides TREM2, only TREML1 has reliable expression in the brain regions we studied. Whereas TREML1 is expressed in both cerebellum and temporal cortex of all subjects, TREM2 is expressed in 98% of temporal cortex and 41% of cerebellum samples. This suggests that cerebellar levels of TREM2 are lower than those for temporal cortex, consistent with previous reports showing higher gene levels in the temporal cortex than cerebellum [24] and higher protein levels correlating with AD neuropathology [25]. In contrast, TREM1, TREML2 and TREML4 are expressed in only 0%–17% of the subjects. While lack of reliable brain expression of these genes does not definitively rule them out as plausible AD-risk genes, our findings provide the strongest evidence for TREML1, besides TREM2, as most likely TREM locus genes for further studies in AD.
Consequently, we focused our studies on TREML1 and TREM2, and utilized their brain expression levels as endophenotypes to identify putative regulatory variants that modify risk for AD. Focusing on brain TREML1 and TREM2 expression associations with variants at the TREM locus that also show evidence of AD-risk association in the publicly available IGAP meta-analysis, we identified a putative regulatory variant, rs9357347, located between TREM2 and TREML2. The minor allele of this variant is associated with both decreased AD-risk and with increased TREML1 and TREM2 brain expression in the temporal cortex, although not reaching genome-wide or transcriptome-wide significance criteria. The direction of effect of this variant on AD-risk and brain expression levels of these genes appears to be biologically congruent based on the known functions of these genes.
TREML1, which is also known as TREM-like transcript 1 (TLT-1), is a myeloid receptor expressed exclusively in the α-granules of platelets and megakaryocytes [26]. Identification of higher levels of soluble TREML1 (sTLT-1) in septic patients vs. controls and development of hemorrhage in mice lacking Treml1 when exposed to inflammatory injury led to the conclusion that TREML1 functions to maintain vascular integrity during inflammation [27]. Further, TREML1 was shown to dampen leukocyte activation during sepsis, and inhibited pro-inflammatory activation of TREM1 by competing with its ligand [28]. These studies strongly support a role for TREML1 in promoting vascular homeostasis and limiting inflammation.
Functional, in-vitro studies of TREM2 rare, missense mutations revealed reduced TREM2 function as a consequence of decreased maturation and ectodomain shedding, also supported by findings of decreased soluble TREM2 levels in the cerebrospinal (CSF) levels of patients with these mutations [13, 29]. TREM2 deficiency also led to increased amyloid pathology and neuronal loss in the 5XFAD mouse model of AD [30]. Interestingly, TREM2 deficiency in an ischemic mouse model resulted in reduced phagocytosis and resorption of infarcted brain tissue, and worse neurological recovery [31]. Collectively, these findings support a neuroprotective role for TREM2 in various neuronal injury models. There are, however, studies with contradictory results for TREM2. In a different mouse model of AD (APP/PS1), knock-out of Trem2, resulted in reduction of macrophages infiltrating from the periphery, along with less brain inflammation and reduced amyloid and tau pathology [32]. These opposite findings of Trem2 knock-out could be due to differences in the mouse models of Alzheimer’s disease tested, different Trem2 knockout mouse lines, and analyses performed at different time points (early stages versus later stages of Alzheimer’s disease).
Given these collective data, a regulatory variant that enhances levels of TREML1 in platelets, and levels of TREM2 in brain resident microglia could conceivably promote vascular homeostasis and limit inflammatory damage to neurons in AD and potentially other nervous system diseases. Indeed, rs9357347 has compelling evidence of regulatory potential as it is located in a known DNase hypersensitive site and affects histone modification in the hippocampus and transcription factor binding, according to the evidence compiled in the Regulome database and HaploReg [16, 23]. Interestingly, rs9357347 is predicted to affect transcription factor binding (SP1 and PPAR) as catalogued in HaploReg. These two transcription factors are known be important in regulating key players in the inflammatory response and lipid metabolism [33, 34]. Further, rs9357347 shows the most significant association with TREML1 gene expression amongst variants at the TREM locus with IGAP meta-analysis AD-risk p-values≤0.0015, in addition to its association with brain TREM2 levels.
The regulatory rs9357347 SNP is in the same haplotype block as the variant with the most significant AD-risk association at the TREM locus in the IGAP meta-analysis, rs9381040, which is an intergenic variant downstream of TREML2. Though this IGAP TREM locus “hit” SNP has greater evidence of AD-risk association than rs9357347, there is no evidence of regulatory potential for rs9381040 in brain regions relevant to AD.
While the fold change estimates in gene expression associated with rs9357347-C are modest at 6–7%, the biological impact of the increase attributed to each copy of the minor allele, can be significant and may provide sufficient protection from disease in some individuals, particularly when considered over a lifetime. Furthermore, these estimates are based on RNA isolated from tissue samples and not microglial cells where both TREM2 and TREML1 are predominantly expressed [35], and where expression levels of these genes may be impacted to a greater extent by regulatory variants. Indeed, it is worth noting that we observed a trend toward higher TREML1 and TREM2 expression in AD subjects, which could be a reflection of microglial activation and/or proliferation known to occur in AD brains. Future gene expression studies from isolated microglia of AD subjects and controls would provide additional valuable insights as to the impact of these expression changes on the biology of microglial cell function.
The TREML2 p.S144G variant [11], which associates with reduced AD-risk, is also in LD with both rs9357347 and rs9381040. Though proposed to be the functional variant that accounts for the IGAP signal at this locus, TREML2 p.S144G is not predicted to have a functional consequence based on PolyPhen2 nor does it have evidence of regulatory potential. Further, TREML2 expression is too low to be reliably measured in brain tissue (TCX and CER). This raises the possibility that the association with TREML2 p.S144G is due to its LD with a functional variant(s) that influences the function or level of a nearby TREM gene(s), such as TREML1 or TREM2. Alternatively, the protective effect of p.S144G could be mediated directly through the function of TREML2 in a cell with abundant expression, such as macrophages, in which TREML2 is known to be upregulated in response to inflammation, [36]. It is also possible that significant rs9357347 eQTL associations would be detected with TREML2 or other TREM locus transcripts in tissues were these genes are more abundantly expressed.
Our findings therefore challenge the conclusion that p.S144G is the only functional variant accounting for the protective effect detected in the IGAP meta-analysis at this locus, and propose rs9357347 as an alternative functional variant with regulatory effects. In reality, both variants could have functional consequences and contribute to the IGAP signal. It should be emphasized that, as demonstrated in our LD analysis, TREM2 p.R47H is not in LD with these two variants, and thus affects AD-risk independently. Both rs9357347 and p.S144G should be tested for their functional potential and influence on outcomes of inflammation and neuroprotection. It remains possible that rs9357347 is in LD with an untested true functional variant with effects on transcription and AD-risk. It is likewise possible that while rs9357347 is associated with both AD-risk and gene expression levels, these joint effects are coincidental due to LD, rather than being related. These possibilities need to be explored through sequencing of the entire TREM locus, or via targeted sequencing of LD block 2 where rs9357347 resides. Thus, our findings provide a testable hypothesis for a strong candidate functional variant, specific transcription factors and their effects on TREML1 and TREM2 levels.
Furthermore, our investigation of variants previously shown to associate with AD-related endophenotypes [13–15] suggests that these are unlikely to be functional AD-risk variants per se, though it remains possible that they are markers of functional variants at the TREM locus.
In summary, we characterized expression of TREM genes in cerebellum and temporal cortex and determined TREML1 and TREM2 to be the only reliably expressed TREM genes in these brain regions. We identified rs9357347 as a putative regulatory variant that is associated with protection from AD and with increased TREML1 and TREM2 brain levels, and nominate rs9357347 as one of the functional variants that accounts for the IGAP AD-risk signal. Additional studies are needed to validate the function of this variant, and to explore the possibility of the presence of other variants at this locus that could contribute to associations observed with rs9357347. Importantly, these findings suggest a potential link between TREML1 and TREM2, as well as vascular homeostasis and neuroinflammation as related mediators of neuronal protection and injury in AD and possibly other central nervous system diseases.
Supplementary Material
Research in Context.
Systematic review
We performed a comprehensive review of existing literature investigating the role of the TREM locus in AD. Although the involvement of TREM genes in AD pathophysiology and the underlying variants modifying AD-risk remain unclear, there have been several studies demonstrating association with AD risk and its endophenotypes.
Interpretation
We hypothesized that some variants at the TREM locus may modify AD-risk via regulation of TREM gene expression. We found a variant in a regulatory region (rs9357347-C) at the TREM locus that associates with reduced AD risk and higher TREML1 and TREM2 brain gene expression.
Future directions
Our findings nominate regulation of brain TREML1 and TREM2 as a potential mechanism for AD risk modification by TREM locus variants. In-depth sequencing of the TREM locus is needed to fully characterize regulatory variants at this locus that may modify AD-risk.
Highlights.
rs9357347-C, 5′ TREM2, is associated with reduced AD-risk (puncorrected=1×10−03).
TREM2 and TREML1 are the only TREM cluster genes with reliable brain expression.
Higher brain levels of TREM2 and TREML1 associate with rs9357347-C.
rs9357347 is predicted to affect transcription factor binding (SP1 and PPAR).
Increased gene expression of TREML1 and TREM2 may reduce AD-risk.
Acknowledgments
We thank the patients and their families for their participation, without whom these studies would not have been possible, and the clinicians, technicians, and administrative staff who helped in the implementation of this study.
This work was supported by the Alzheimer’s Association [MNIRGD 2013 award to M.M.C]; Mayo Alzheimer’s Disease Research Center [P50 AG0016574 to D.W.D, N.E.T, N.R.G.-R., R.C.P. and S.G.Y.]; National Institute on Aging [R01 AG025711, AG017216, AG003949 to D.W.D.; R01 AG032990 to N.E.T.; R01 AG018023 to N.R.G.-R. and S.G.Y.; and U01 AG046139 to N.E.T., T.E.G, N.P. and S.G.Y.]; National Institute of Neurological Disorders and Stroke [R01 NS080820 to N.E.T].
Footnotes
Conflict of Interest Statement
Dr. Petersen has been a consultant to Genentech, Inc. Merck, Inc. and Roche, Inc. and has served on a data safety monitoring committee for Pfizer and Janssen Alzheimer Immunotherapy. Dr. Graff-Radford has multicenter treatment study grants from Lilly, TauRx and consulted for Cytox. Dr. Ertekin-Taner consulted for Cytox.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer’s disease. The New England journal of medicine. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. The New England journal of medicine. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Current opinion in immunology. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanzi RE. TREM2 and Risk of Alzheimer’s Disease--Friend or Foe? The New England journal of medicine. 2015;372:2564–2565. doi: 10.1056/NEJMcibr1503954. [DOI] [PubMed] [Google Scholar]
- 5.Pottier C, Wallon D, Rousseau S, Rovelet-Lecrux A, Richard AC, Rollin-Sillaire A, et al. TREM2 R47H variant as a risk factor for early-onset Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2013;35:45–49. doi: 10.3233/JAD-122311. [DOI] [PubMed] [Google Scholar]
- 6.Benitez BA, Cooper B, Pastor P, Jin SC, Lorenzo E, Cervantes S, et al. TREM2 is associated with the risk of Alzheimer’s disease in Spanish population. Neurobiology of aging. 2013;34:1711 e1715–1717. doi: 10.1016/j.neurobiolaging.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez Murcia JD, Schmutz C, Munger C, Perkes A, Gustin A, Peterson M, et al. Assessment of TREM2 rs75932628 association with Alzheimer’s disease in a population-based sample: the Cache County Study. Neurobiology of aging. 2013;34:2889 e2811–2883. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz A, Dols-Icardo O, Bullido MJ, Pastor P, Rodriguez-Rodriguez E, Lopez de Munain A, et al. Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiology of aging. 2014;35:444 e441–444. doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Hooli BV, Parrado AR, Mullin K, Yip WK, Liu T, Roehr JT, et al. The rare TREM2 R47H variant exerts only a modest effect on Alzheimer disease risk. Neurology. 2014;83:1353–1358. doi: 10.1212/WNL.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lill CM, Rengmark A, Pihlstrom L, Fogh I, Shatunov A, Sleiman PM, et al. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 2015; doi: 10.1016/j.jalz.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benitez BA, Jin SC, Guerreiro R, Graham R, Lord J, Harold D, et al. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiology of aging. 2014;35:1510 e1519–1526. doi: 10.1016/j.neurobiolaging.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature genetics. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Replogle JM, Chan G, White CC, Raj T, Winn PA, Evans DA, et al. A TREM1 variant alters the accumulation of Alzheimer-related amyloid pathology. Annals of neurology. 2015;77:469–477. doi: 10.1002/ana.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G. No association of TREM1 rs6910730 and TREM2 rs7759295 with Alzheimer’s disease. Annals of Neurology. 2015 doi: 10.1002/ana.24458. epub ahead opf print. [DOI] [PubMed] [Google Scholar]
- 16.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome research. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou F, Chai HS, Younkin CS, Allen M, Crook J, Pankratz VS, et al. Brain expression genome-wide association study (eGWAS) identifies human disease-associated variants. PLoS genetics. 2012;8:e1002707. doi: 10.1371/journal.pgen.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, et al. The NIH Roadmap Epigenomics Mapping Consortium. Nature biotechnology. 2010;28:1045–1048. doi: 10.1038/nbt1010-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic acids research. 2016;44:D877–881. doi: 10.1093/nar/gkv1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forabosco P, Ramasamy A, Trabzuni D, Walker R, Smith C, Bras J, et al. Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiology of aging. 2013;34:2699–2714. doi: 10.1016/j.neurobiolaging.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lue LF, Schmitz CT, Serrano G, Sue LI, Beach TG, Walker DG. TREM2 Protein Expression Changes Correlate with Alzheimer’s Disease Neurodegenerative Pathologies in Post-Mortem Temporal Cortices. Brain pathology. 2015;25:469–480. doi: 10.1111/bpa.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washington AV, Schubert RL, Quigley L, Disipio T, Feltz R, Cho EH, et al. A TREM family member, TLT-1, is found exclusively in the alpha-granules of megakaryocytes and platelets. Blood. 2004;104:1042–1047. doi: 10.1182/blood-2004-01-0315. [DOI] [PubMed] [Google Scholar]
- 27.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. The Journal of clinical investigation. 2009;119:1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, Washington V, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. Journal of immunology. 2012;188:5585–5592. doi: 10.4049/jimmunol.1102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinberger G, Yamanishi Y, Suarez-Calvet M, Czirr E, Lohmann E, Cuyvers E, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Science translational medicine. 2014;6:243ra286. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:3384–3396. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. The Journal of experimental medicine. 2015;212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. Journal of immunology. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. Journal of immunology. 2006;176:6012–6021. doi: 10.4049/jimmunol.176.10.6012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.