Abstract
Background
Chronic hepatitis C is an important public health concern. Recently launched drugs to treat hepatitis C virus (HCV) infection are effective but costly. Uptake of innovative and expensive prescription drugs may not be even across patient groups. We examined racial-ethnic disparities in uptake of new HCV drugs in the first year of their use (year 2014) in Medicare.
Methods
The study population was Medicare beneficiaries who had chronic hepatitis C in 2013 or 2014 and who were continuously enrolled in Part D stand-alone Prescription Drug Plans in 2014. We examined trends in monthly uptake of new HCV drugs and adjusted annual uptake rates by race. We used logistic regressions to obtain adjusted odds ratios and adjusted differences in annual uptake rates.
Results
Monthly uptake of new HCV drugs was lower among Black Medicare patients than Whites or Hispanics in 2014. The racial gap in monthly uptake became narrower toward the end of the year. Adjusted odds of using new HCV drugs were 11% lower for Blacks with cirrhosis than Whites (odds ratio (OR) = 0.89; 95% confidence interval (CI), 0.84–0.95), and 16% lower for Blacks with HCV/HIV coinfection than Whites (OR = 0.81; 95% CI, 0.72–0.92). Annual uptake rates were not significantly different for Whites and Hispanics.
Conclusions
Black Medicare patients with cirrhosis or HCV/HIV coinfection had lower uptake rates than Whites in 2014. As utilization of new HCV drugs increases, continuing efforts will be necessary to ensure equal delivery of the drugs.
Keywords: New hepatitis C drugs, Medicare Part D, Disparities, Prescription drugs, Hepatitis C
Introduction
Chronic hepatitis C is an important public health concern. It is a cause of serious and costly liver diseases such as cirrhosis or liver cancer. Hepatitis C virus (HCV) causes more deaths in the USA than any other infectious disease including HIV/AIDS [1]. More than 3 million Americans are infected with HCV, and its prevalence is concentrated among baby boomers who were born between 1945 and 1965 [2]. Hospitalizations and costs related to chronic hepatitis C and liver diseases have been increasing during the past decade [3].
Recently launched drugs—the second degeneration of direct-acting antivirals—have brought an unprecedented opportunity to treat HCV infection. Those new HCV drugs are highly effective, curing HCV infection in over 90% of patients in randomized clinical trials [4, 5]. Further, their features of easy use (oral administration once daily) and a short therapy period (12 weeks) have improved adherence to the treatment compared with traditional HCV treatments [6]. Prior to the launch of the new HCV drugs, conventional interferon-based HCV treatments caused side effects such as flu-like symptoms. Further, they required 24–48 weeks of treatment, leading to high discontinuation rates [6].
However, the prices of the new HCV drugs are very high. The first new HCV drug, sofosbuvir (Sovaldi), introduced in December 2013, has a list price of $1000 per pill ($84,000 for a course of treatment). The second drug, lediparsir/sofosbuvir (Harvoni), launched in October 2014, costs $94,500 for a course of treatment.
Literature has shown that use of innovative and/or expensive medical technologies is uneven across patient groups [7, 8]. Especially, recent studies of novel but costly cancer drugs reported that racial minority patients were less likely to use those drugs than non-minority patients [9, 10]. Prior work documented racial variation in receipt of traditional interferon-based HCV treatments, with lower rates of utilization for Blacks than for Whites, but medical factors explained some of that difference [10]. Blacks are less responsive to interferon-based HCV therapies [11, 12]; Blacks also have higher rates of comorbidities that make them ineligible for interferon-based therapy [13–15]. Because new HCV drugs do not necessarily require concurrent interferon therapy and they were equally efficacious for Blacks and non-Blacks (Whites, Asians, and others) in clinical trials, it was expected that new HCV drugs would narrow racial gaps in receipt of HCV treatment [11]. However, a recent study reported the presence of racial disparities in receipt of new HCV drugs among patients who received care at Veterans Affairs (VA) facilities nationwide [16].
We compared the uptake of new HCV drugs by race in the Medicare program during the first year the drugs were available (2014). We examined the racial disparities in uptake of new HCV drugs by two risk factors: presence of cirrhosis and HCV/HIV coinfection. Patients with cirrhosis are the highest priority group for new HCV treatments [17, 18]. HCV/HIV coinfected patients face challenges to HCV therapy because of the medical priority of controlling HIV-viral load [19]. Because HCV/HIV coinfection is prevalent in Blacks, it is cited as a reason for low rates of HCV drug use in Blacks [11]. Yet, little is known whether access to HCV drugs is equal across racial groups for patients with cirrhosis or HCV/HIV coinfection.
Looking at this issue in Medicare is important because Medicare paid for about half of new HCV drug “pills” in 2014 and 2015 [20]. This is because many baby boomers—the group with highest prevalence of HCV—are enrolled in Medicare. Spending on HCV drugs in Medicare Part D jumped from $283 million in 2013 to $4.5 billion in 2014, with spending on Sovaldi alone exceeding $3 billion in 2014 [21]. Medicare Part D also covers outpatient prescription drugs for beneficiaries who are dually eligible for Medicare and Medicaid. HCV infection is prevalent among low-income populations, who are likely to qualify for Medicaid [17, 18]. As more baby boomers join Medicare, the demand for new HCV drugs will increase. Thus, identifying racial disparities in the first year of new HCV drug use in Medicare can offer an opportunity to improve equitable access to those drugs.
Study Population and Data
The study population was Medicare Fee-For-Service (FFS) beneficiaries who had chronic hepatitis C in 2013 or 2014 and who were enrolled in Part D for all of 2014. We requested that the Centers for Medicare and Medicaid Services (CMS) identify patients with hepatitis C using 100% of Medicare claims for FFS beneficiaries based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 070.44, 070.54, 070.70, and 070.71.1 A confirmed diagnosis requires having at least one inpatient or skilled nursing facility claim or at least two claims for outpatient visits in a given year.2 This is the standard algorithm used by CMS to identify patients with any condition indicators in the Medicare Chronic Condition Warehouse data [22]. We received Part D data and Beneficiary Summary Files for FFS patients with hepatitis C.
We focused on three racial groups: non-Hispanic Whites, Blacks, and Hispanics.3 Those with unknown race (2.5%) and Asians (<2.5%) were excluded from the study. We excluded beneficiaries who enrolled in Medicare Advantage (MA) plans because claims data are not available for them.
The primary data source is the 2014 Medicare Part D Prescription Drug Event (PDE) file, which contains records on each prescription drug fill by Medicare Part D enrollees, including National Drug Code, date of fill, days supplied, and payment. We augmented PDE files with the Part D Plan Characteristics and Formulary Files to obtain information on plan attributes and utilization management tools applied to HCV drugs. Master Beneficiary Summary Files (MBSF) provided each beneficiary’s demographic characteristics, residence (ZIP), and chronic or other potentially disabling condition indicators. HIV infection, a key variable of the study, is one of the disabling conditions identified by CMS and its indicator is included in MBSF.4 Finally, we acquired ZIP-level income and percent college education from the 2010 American Community Survey, and used county-level urban/rural information from Area Health Resource File (AHRF).
Measures and Analysis
We first calculated uptake rates of new HCV drugs in each month to track uptake trends by month in 2014. Two new HCV drugs were available in 2014: sofosbuvir (Sovaldi) and lediparsir/sofosbuvir (Harvoni).5 Monthly uptake is the percent of patients who had their first fill of any new HCV drug in the month among those who had been diagnosed before that month but had not received any new HCV drug until the month. After filling the first prescription for a new HCV drug, patients were excluded from calculating uptake rates in the following months.
Next, we compared annual uptake rates by race separately for patients with and without cirrhosis,6 and patients with and without HIV coinfection. In the sub-group analysis by the presence of cirrhosis, HCV/HIV coinfection was used as a covariate, and vice versa. We constructed an indicator of uptake of new HCV drugs for each individual as having at least one fill of the new drugs in 2014. We used logistic regressions to obtain odds ratios on covariates. All logistic regressions include random intercept effects for each plan to adjust for plan-to-plan variability in uptake of new HCV drugs. This accounts for possible correlation among enrollees within the same plan, and thus standard errors of the regression estimates were adjusted for clustering within plans.
Covariates
The regression analysis controlled for factors affecting receipt of new HCV drugs. We categorized those factors based on variation at patient, plan, and local-area levels. Patient characteristics were age, gender, health risk, and Part D low-income subsidy (LIS) status. Prior literature shows that gender and age are important predictors of receipt of HCV treatments [16]. LIS enrollees receive assistance in paying for Part D cost sharing, which is likely to increase access to new HCV drugs. Patients in poorer health are generally high users of health services; however, they may not initiate a treatment if they face competing demands for services to treat other comorbidities [23–25]. We used indicators for eight health-risk factors (diabetes, hypertension, ischemic heart disease, hyperlipidemia, congestive heart failure, depression, cataract, and COPD) that are common in the study population and usually require prescription drug treatments. We also included the total number of chronic conditions up to 27 included in MBSF [26] to capture patients’ health risk that is not measured by the individual risk factors.
We controlled for two plan attributes: enhanced benefits (more generous than the standard Part D benefits) and coverage for the gap. Both are likely to improve access to new HCV drugs. Being in a plan with a deductible or applying prior authorization to new HCV drugs is a potential deterrent to receiving new HCV drugs.
Finally, we captured socioeconomic status at the local-area (ZIP) level with income and percent college education. We also used urban/rural and census region variables to control for unmeasured differences in regional environments influencing utilization of new HCV drugs.
Adjusted Differences in Annual Uptake
We calculated adjusted differences in annual uptake by race based on the results from the logistic analyses. This allows us to measure the race effect on the probability of receiving a new HCV drug. Specifically, we calculated the predicted probability of using a new HCV drug for each race group as , where X is a vector of covariates and β are the coefficients (logit estimates) on covariates.7 This predicted probability depends on the values of covariates for each individual. We used the mean values of all other covariates except race, and then “turned on” or “turned off” the racial indicators to calculate adjusted differences in uptake rates across race groups.8 We also obtained adjusted differences in uptake across other covariates to assess the effect of each covariate on uptake rates.
Robustness Check
As a sensitivity analysis, we estimated the logit model for LIS enrollees, who make up 80% of HCV patients in Medicare, to assess whether the results are robust after controlling for patients’ ability to pay for new HCV drugs. Due to subsidies from Medicare Part D, LIS enrollees paid on average $22 for a course of new HCV treatment in 2014.
Results
Table 1 describes characteristics of the study population by race. The mean age was 59–60 in all races. Blacks were less likely to have cirrhosis but were more likely to be HCV/HIV coinfected, compared with Whites or Hispanics. Blacks also had more chronic conditions and were more likely to be LIS enrollees than Whites or Hispanics.
Table 1.
Descriptive statistics of all study variables by race
| Variable | Mean (standard deviation) or N (%)
|
||
|---|---|---|---|
| White (N = 81,273) |
Black (N = 32,594) |
Hispanic (N = 13,300) |
|
| Patient characteristics | |||
| Age | 58.8 (11.0) | 60.9 (8.9) | 59.7 (11.2) |
| Male (N, %) | 45,309 (55.7) | 19,525 (59.9) | 8119 (61.0) |
| Cirrhosis (N, %) | 32,150 (39.6) | 10,433 (32.0) | 6680 (50.2) |
| HCV/HIV coinfection (N, %) | 4266 (5.3) | 5407 (16.6) | 1509 (11.3) |
| LIS (low-income subsidy) enrollees (N, %) | 60,581 (74.5) | 29,257 (89.8) | 11,836 (89.0) |
| Having diabetes (N, %) | 25,150 (30.1) | 16,118 (49.5) | 6425 (48.3) |
| Having hypertension (N, %) | 49,646 (61.1) | 27,555 (84.5) | 9122 (68.6) |
| Having ischemic heart disease (%) | 23,562 (28.1) | 12,732 (39.1) | 4184 (31.5) |
| Having hyperlipidemia (N, %) | 27,679 (34.1) | 13,869 (42.6) | 5019 (37.7) |
| Having depression (N, %) | 39,904 (49.1) | 11,408 (35.0) | 5582 (42.0) |
| Having congestive heart failure (N, %) | 14,414 (17.7) | 10,141 (31.1) | 2849 (21.4) |
| Having cataract (N, %) | 9674 (11.9) | 3817 (11.7) | 1643 (12.4) |
| Having chronic obstructive pulmonary disease (N, %) | 25,212 (31.0) | 8149 (25.0) | 2494 (18.8) |
| Number of chronic conditions | 4.3 (2.9) | 5.3 (3.0) | 4.7 (3.0) |
| Local-area characteristics | |||
| Residing in ZIP with below median household income (N, %) | 37,819 (46.5) | 21,200 (65.0) | 7107 (53.4) |
| Percent college educated in beneficiary’s residence | 21.4 (13.1) | 19.2 (12.4) | 18.4 (12.1) |
| Urban (N, %) | 61,636 (75.8) | 29,999 (92.0) | 12,148 (91.3) |
| Plan characteristics | |||
| Applying prior authorization (N, %) | 81,070 (99.8) | 32,540 (99.8) | 13,282 (99.9) |
| Enhanced plan (N, %) | 20,427 (25.1) | 4675 (14.3) | 1765 (13.3) |
| Gap coverage plan (N, %) | 5075 (6.2) | 842 (2.6) | 408 (3.1) |
| Deductible plan (N, %) | 57,012 (70.2) | 25,820 (79.2) | 10,585 (79.6) |
| Census region | |||
| Northeast (N, %) | 15,873 (19.5) | 6701 (20.6) | 948 (7.1) |
| Midwest (N, %) | 16,601 (20.4) | 6455 (19.8) | 3436 (25.8) |
| South (N, %) | 31,026 (38.2) | 16,162 (49.6) | 3955 (29.7) |
| West (N, %) | 17,765 (21.9) | 3276 (10.1) | 4961 (37.3) |
Figure 1 shows monthly uptake of new HCV drugs in 2014 by race among patients with cirrhosis. On average, 2.35–2.40% of Whites or Hispanics initiated a new HCV treatment each month in early 2014, while 1.64% of Black patients did so. This disparity continued but became smaller in the last 2 months of the year, when on average 3.15% of White patients and 2.67% of Black patients used new HCV treatments each month. The racial gap in uptake in patients without cirrhosis was narrower than that in patients with cirrhosis, and it almost closed toward the end of the year. Sub-group analysis by HIV coinfection showed similar patterns (Fig. 2): the racial disparity among patients with HCV/HIV coinfection became much smaller but persisted at the end of year, while it almost disappeared among those with HCV infection only.
Fig. 1.
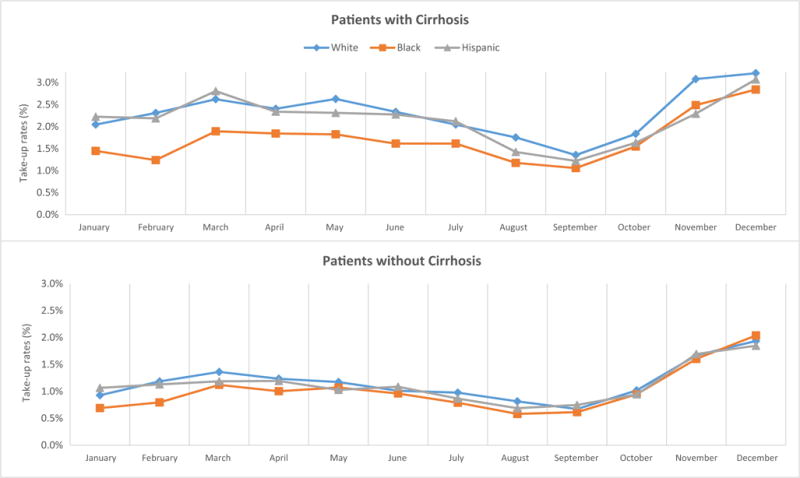
Monthly uptake of new hepatitis C drugs by race by the presence of cirrhosis in Medicare in 2014. Monthly uptake rates were computed as the percent of chronic hepatitis C patients who had the first fill of new hepatitis C virus (HCV) drugs in the month among those who had not received any new HCV drug until that month. After filling the first prescription of a new HCV drug, patients were excluded from calculating uptake rates in the following months
Fig. 2.
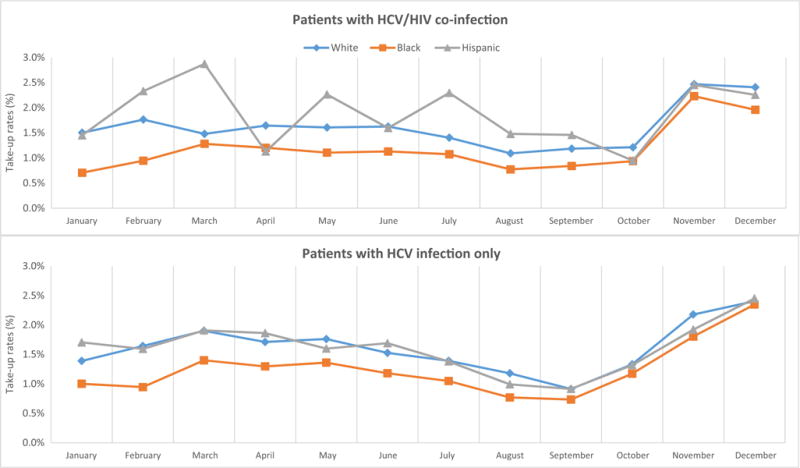
Monthly uptake of new hepatitis C drugs by race by HIV coinfection in Medicare in 2014. Monthly uptake rates were computed as the percent of chronic hepatitis C patients who had the first fill of new hepatitis C virus (HCV) drugs in the month among those who had not received any new HCV drug until that month. After filling the first prescription of a new HCV drug, patients were excluded from calculating uptake rates in the following months
Figure 3 presents unadjusted annual uptake rates of new HCV drugs in 2014 by race. Uptake rates among patients with cirrhosis were 23.4% for Whites, 18.1% for Blacks, and 22.1% for Hispanics. Blacks also had the lowest uptake rates among patients with HCV/HIV coinfection (12.9%), versus 17.3% in Whites and 19.6% in Hispanics. Differences in uptake between White and Black patients without cirrhosis or HCV/HIV coinfection were relatively small.
Fig. 3.
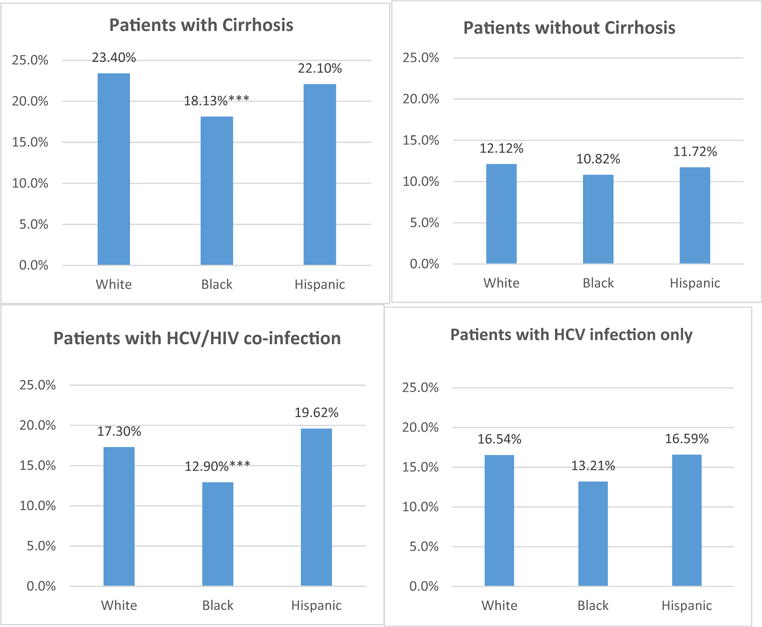
Unadjusted annual uptake of new hepatitis C drugs by race in Medicare in 2014. Uptake of new hepatitis C virus (HCV) drugs was defined as having at least one fill of new HCV drugs in the year; ***p < 0.01
Table 2 presents estimates of racial differences in annual uptake. After adjusting for covariates, the odds of using a new HCV drug were 11% lower for Black patients with cirrhosis than Whites (odds ratio (OR) = 0.89; 95% confidence interval (CI), 0.84–0.95), and 19% lower for Blacks with HCV/HIV coinfection than Whites (OR = 0.81; 95% CI, 0.72–0.92).
Table 2.
Results from logistic regression of uptake of new hepatitis C drugs
| Variable | Odds ratio (95% confidence interval)
|
|||
|---|---|---|---|---|
| With cirrhosis (N = 49,263) |
Without cirrhosis (N = 77,904) |
With HCV/HIV coinfection (N = 11,182) |
With HCV infection only (N = 115,985) |
|
| Race (ref: non-Hispanic White) | ||||
| Black | 0.89 (0.84–0.95)*** | 1.04 (0.99–1.11) | 0.81 (0.72–0.92)*** | 1.00 (0.95–1.05)*** |
| Hispanic | 0.98 (0.91–1.06) | 1.04 (0.95–1.14) | 1.04 (0.90–1.21) | 1.00 (0.94–1.06) |
| HCV/HIV coinfection | 1.06 (0.96–1.17) | 0.98 (0.91–1.06) | ||
| Cirrhosis | 2.37 (2.13–2.65)*** | 2.44 (2.34–2.54)*** | ||
| LIS (low-income subsidy) enrollees | 0.92 (0.86–0.98)*** | 0.95 (0.88–1.02) | 1.08 (0.88–1.32) | 0.92 (0.87–0.97)*** |
| Age | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.01 (1.00–1.02)** | 1.00 (1.00–1.00) |
| Male | 0.95 (0.9–1.00)* | 0.98 (0.93–1.03) | 1.12 (1.00–1.27)* | 0.96 (0.92–1.00)** |
| Number of chronic conditions | 0.87 (0.86–0.88)*** | 0.88 (0.86–0.90)*** | 0.86 (0.82–0.90)*** | 0.88 (0.86–0.89)*** |
| Having diabetes | 1.23 (1.17–1.29)*** | 1.05 (0.99–1.12) | 1.36 (1.20–1.54)*** | 1.14 (1.09–1.18)*** |
| Having hypertension | 0.94 (0.89–0.99)** | 0.94 (0.88–1.01)* | 0.95 (0.82–1.09) | 0.94 (0.90–0.98)*** |
| Having ischemic heart disease | 1 (0.96–1.06) | 0.97 (0.93–1.03) | 1.23 (1.04–1.46)** | 0.97 (0.94–1.01) |
| Having hyperlipidemia | 0.98 (0.92–1.04) | 1.02 (0.97–1.08) | 1.23 (1.07–1.42)*** | 0.98 (0.94–1.02) |
| Having depression | 1.03 (0.97–1.10) | 1.00 (0.95–1.05) | 0.99 (0.88–1.12) | 1.02 (0.98–1.06) |
| Having congestive heart failure | 0.71 (0.66–0.77)*** | 0.65 (0.59–0.72)*** | 0.63 (0.52–0.75)*** | 0.69 (0.65–0.74)*** |
| Having cataract | 1.29 (1.21–1.37)*** | 1.28 (1.20–1.38)*** | 1.38 (1.17–1.63)*** | 1.28 (1.21–1.34)*** |
| Having chronic obstructive pulmonary disease | 0.80 (0.75–0.85)*** | 0.82 (0.77–0.88)*** | 0.78 (0.67–0.91)*** | 0.81 (0.78–0.85)*** |
| Below median household income | 0.89 (0.85–0.94)*** | 0.93 (0.87–0.99)** | 1.03 (0.92–1.14) | 0.9 (0.85–0.94)*** |
| Percent college educated | 1.00 (1.00–1.01)*** | 1.00 (1.00–1.01)*** | 1.00 (1.00–1.01) | 1.00 (1.00–1.01)*** |
| Urban area | 1.07 (1.00–1.16)* | 1.02 (0.95–1.09) | 1.32 (1.05–1.66)** | 1.03 (0.97–1.09) |
| Applying prior authorization | 1.39 (0.98–1.97)* | 0.83 (0.52–1.33) | 1.01 (0.32–3.23) | 1.08 (0.83–1.42) |
| Enhanced plan | 1.24 (1.13–1.35)*** | 1.22 (1.11–1.34)*** | 1.17 (0.89–1.53) | 1.23 (1.15–1.32)*** |
| Gap coverage plan | 1.04 (0.93–1.16) | 0.94 (0.80–1.09) | 0.91 (0.63–1.30) | 1.00 (0.90–1.12) |
| Deduct | 1.07 (0.99–1.16)* | 1.06 (0.96–1.16) | 0.91 (0.71–1.17) | 1.08 (1.01–1.16)** |
| Region (ref: Northeast) | ||||
| Midwest | 1.08 (0.97–1.20) | 1.04 (0.96–1.12) | 1.02 (0.82–1.27) | 1.05 (0.97–1.14) |
| South | 0.90 (0.83–0.98)** | 0.95 (0.87–1.02) | 0.76 (0.62–0.92)*** | 0.94 (0.88–1.01)* |
| West | 0.89 (0.81–0.98)** | 0.76 (0.70–0.83)*** | 0.78 (0.60–1.02)* | 0.83 (0.77–0.90)*** |
p < 0.01,
p < 0.05, and
p < 0.10; standard errors are clustered within a plan
The adjusted difference in annual uptake (based on the mean values of all other covariates) between Blacks and Whites was 1.8 percentage points for patients with cirrhosis, and 2.6 percentage points for patients with HCV/HIV coinfection (Appendix Tables 4 and 5). We found no significant differences in adjusted uptake rates between Whites and Blacks for patients without cirrhosis or HCV infection only. The adjusted differences in uptake between Whites and Hispanics were all insignificant.
Cirrhosis had a large positive association with receiving new HCV drugs (OR = 2.37; 95% CI, 2.13–2.65 among patients with HCV/HIV coinfection, and OR = 2.44; 95% CI, 2.34–2.54 among patients with HCV infection only). Chronic conditions were negatively related to receipt of new HCV drugs in all analyses, but the effects of individual chronic conditions were mixed depending on condition and analysis. Residing in urban areas had a positive association with new HCV drug use for patients with cirrhosis or HCV/HIV coinfection. Being in plans with generous drug benefits was positively associated with use of new HCV drugs.
Next, we calculated adjusted differences in annual uptake across covariate groups (Appendix Tables 4 and 5). Patients with cirrhosis had a higher uptake rate than those without cirrhosis by 11 percentage points–a large difference that would lead to a clinically significant impact. The gender effect was significant but small–a 0.6 percentage point difference between male and female patients with HCV infection only. This effect is unlikely to be clinically meaningful. Being in a plan with generous benefits (enhanced plan) increased the probability of using a new HCV drug by 2–3 percentage points. These results imply that certain factors, such as cirrhosis or plan benefits, had clinically meaningful effects on new HCV drug use.
Table 3 reports results on racial-ethnic variables from logistic regressions of LIS enrollees only. Patterns of racial/ethnic disparities in uptake of new HCV drugs for LIS enrollees are very similar to the entire population (Appendix Tables 6 and 7 report full regression results).
Table 3.
Results from sensitivity analysis of low-income subsidy (LIS) enrollees
| Variable | Odds ratio (95% confidence interval)
|
|||
|---|---|---|---|---|
| With cirrhosis (N = 38,457) |
Without cirrhosis (N = 63,217) |
With HCV/HIV coinfection (N = 10,169) |
With HCV infection only (N = 91,505) |
|
| Race (ref: non-Hispanic White) | ||||
| Black | 0.88 (0.81–0.96)*** | 1.03 (0.97–1.10) | 0.80 (0.71–0.92)*** | 0.99 (0.93–1.04) |
| Hispanic | 0.97 (0.89–1.06) | 1.04 (0.96–1.14) | 1.01 (0.86–1.18) | 1.00 (0.94–1.07) |
p < 0.01; standard errors are clustered within a plan
Discussions
Black Medicare Part D enrollees with cirrhosis or HCV/HIV coinfection were less likely to use new HCV drugs than White enrollees. Racial-ethnic disparities in use of medical care are not new. However, our finding raises a new concern that Blacks face larger access barriers to innovative new HCV drugs than others, particularly among patients with cirrhosis or HCV/HIV coinfection in Medicare. Medical factors, such as high prevalence of HCV/HIV coinfection, do not fully explain racial differences in use of HCV treatments. Finally, similar results from the analysis of LIS enrollees imply that financial affordability does not explain the difference in new HCV drug uptake between Blacks and Whites.
A potential explanation for our results is that Black patients may not be as active in seeking new treatments or that providers may not be as active in recommending new treatments to Black patients because of their poor experience with earlier HCV treatments. Another possible explanation is that payers use prior authorization for new HCV drugs due to the high prices of the drugs [17, 18, 27]. These tools—such as requiring that the drug be prescribed by a specialist and having control of HIV viral load when coinfected with HIV—may have disproportionally affected Black patients, who may be less engaged with the health care system [28]. Although restrictive coverage for new HCV drugs has been an ongoing concern, its potential impacts on disparities in uptake of the new drugs have not been assessed. Examining that possibility or exploring reasons for differences in uptake of new HCV drugs across races was not part of our study aims, but it is an important topic to pursue in future research.
We analyzed uptake of new HCV drugs during the first year of use (2014), when sofosbuvir (Sovaldi) was the dominant HCV drug. Sofosbuvir (Sovaldi) was used in combination with other drugs. Our monthly uptake data indicated that racial disparities became smaller during the year in 2014. This is probably due to the launch of ledipasvir/sofosbuvir (Harvoni), which does not require other concurrent therapy, in late 2014. Complexity of treatments is cited as a potential contributor to variation in HCV drug use across races [11]. The availability of newer HCV drugs that can be used alone could potentially reduce racial/ethnic gaps in receipt of HCV treatments. While our finding of a trend in the first year of new HCV drug use is only suggestive, it shows that racial gaps in HCV drug use can be closed. As data for more recent years become available, future work should assess how disparities in use of new HCV drugs change over the years.
Recently, the VA and a few state governments have expanded coverage for new HCV drugs to all infected people with no restrictions [29, 30]. This decision is encouraging and important. It will be critical, as coverage expands, to assess changes in patterns of utilization across patient groups, identify reasons for any access barriers, and develop policies to ensure equitable access and uptake of innovative drugs.
Our study has a few limitations. Medicare claims data lack detailed clinical information. Thus, we could not identify viral responses, which would determine cure rates and genotype. We were also unable to control for patients’ substance use status, a predictor of receiving HCV treatments. However, use of a large claims data set allowed us to identify patterns of racial-ethnic disparities by patients’ health-risk factors, such as HCV/HIV coinfection. Finally, we used 2014 data only from Medicare fee-for-service enrollees with Part D coverage. The findings may not generalize to more recent years or to enrollees in Medicare Advantage (MA) plans.
Despite these limitations, we are the first to report racial disparities in uptake of novel but costly new HCV drugs in Medicare. Black patients with cirrhosis or HCV/HIV coinfection had lower uptake rates than Whites in 2014, although the gap in monthly uptake became narrower toward the end of the year. As utilization of new HCV drugs increases, continuing efforts are necessary to ensure equal delivery of the drugs across all patient groups.
Acknowledgments
Funding This work was supported by NIH/NIA grant number 1R01AG047934-01 and NIH grant number R24 HD041025.
Appendix
Table 4.
Effects on the probability of using new HCV drugs by the presence of cirrhosis
| Variable | Marginal effect (robust standard errors)
|
|
|---|---|---|
| Patients with cirrhosis (N = 49,263) |
Patients without cirrhosis (N = 77,904) |
|
| Race (reference: White) | ||
| Black | −0.018 (0.005)*** | 0.004 (0.003) |
| Hispanic | −0.003 (0.006) | |
| HCV/HIV coinfection (low-income subsidy) enrollees |
0.009 (0.008) −0.014 (0.005)*** |
−0.002 (0.004) −0.006 (0.004) |
| Age | 0.000 (0.000) | 0.000 (0.000) |
| Male | −0.008 (0.005)* | −0.002 (0.003) |
| Number of chronic conditions | −0.023 (0.001)*** | −0.013 (0.001)*** |
| Having diabetes | 0.034 (0.004)*** | 0.005 (0.003) |
| Having hypertension | −0.010 (0.004)** | −0.006 (0.003)* |
| Having ischemic heart disease | 0.001 (0.004) | −0.003 (0.003) |
| Having hyperlipidemia | −0.004 (0.005) | 0.002 (0.003) |
| Having depression | 0.005 (0.005) | 0.000 (0.003) |
| Having congestive heart failure | −0.056 (0.006)*** | −0.043 (0.005)*** |
| Having cataract | 0.041 (0.005)*** | 0.025 (0.004)*** |
| Having chronic obstructive pulmonary disease | −0.037 (0.005)*** | −0.020 (0.003)*** |
| Below median household income | −0.018 (0.004)*** | −0.007 (0.003)** |
| Percent college educated | 0.001 (0.000)*** | 0.000 (0.000)*** |
| Urban | 0.012 (0.006)* | 0.002 (0.004) |
| Applying prior authorization | 0.054 (0.029)* | −0.018 (0.024) |
| Enhanced plan | 0.035 (0.008)*** | 0.020 (0.005)*** |
| Gap coverage plan | 0.006 (0.009) | −0.007 (0.008) |
| Deduct | 0.012 (0.007)* | 0.006 (0.005) |
| Region (reference: Northeast) | ||
| Midwest | 0.013 (0.009) | 0.004 (0.004) |
| South | −0.017 (0.007)** | −0.006 (0.004) |
| West | −0.019 (0.008)** | −0.028 (0.004)*** |
| Pseudo R2 | 0.047 | 0.0314 |
| Log pseudolikelihood | −24,800.999 | −27,253.904 |
Data source: 2014 Medicare Part D Drug Event File
p < 0.01,
p < 0.05, and
p < 0.10; standard errors are clustered within a plan
Table 5.
Effects on the probability of using new HCV drugs by HIV coinfection
| Variable | Marginal effect (robust standard errors)
|
|
|---|---|---|
| Patients with HCV/HIV coinfection (N = 11,182) |
Patients with HCV infection only (N = 115,985) |
|
| Race (reference: White) | ||
| Black | −0.026 (0.007)*** | 0.000 (0.003) |
| Hispanic | 0.005 (0.010) | 0.000 (0.004) |
| Cirrhosis | 0.107 (0.007)*** | 0.112 (0.003)*** |
| LIS (low-income subsidy) enrollees | 0.009 (0.013) | −0.011 (0.003)*** |
| Age | 0.001 (0.000)** | 0.000 (0.000) |
| Male | 0.015 (0.008)* | −0.006 (0.003)** |
| Number of chronic conditions | −0.019 (0.003)*** | −0.017 (0.001)*** |
| Having diabetes | 0.038 (0.008)*** | 0.016 (0.003)*** |
| Having hypertension | −0.007 (0.009) | −0.007 (0.003)*** |
| Having ischemic heart disease | 0.026 (0.011)* | −0.003 (0.002) |
| Having hyperlipidemia | 0.026 (0.009)*** | −0.003 (0.003) |
| Having depression | −0.001 (0.008) | 0.002 (0.003) |
| Having congestive heart failure | −0.058 (0.012)*** | −0.047 (0.004)*** |
| Having cataract | 0.040 (0.011)*** | 0.031 (0.003)*** |
| Having chronic obstructive pulmonary disease | −0.031 (0.010)*** | −0.026 (0.003)*** |
| Below median household income | 0.003 (0.007) | −0.014 (0.003)*** |
| Percent college educated | 0.000 (0.000) | 0.000 (0.000)*** |
| Urban | 0.035 (0.015)** | 0.004 (0.004) |
| Applying prior authorization | 0.001 (0.074) | 0.010 (0.017) |
| Enhanced plan | 0.019 (0.017) | 0.026 (0.004)*** |
| Gap coverage plan | −0.012 (0.023) | 0.000 (0.007) |
| Deduct | −0.012 (0.016) | 0.010 (0.004)** |
| Region (reference: Northeast) | ||
| Midwest | 0.002 (0.014) | 0.007 (0.005) |
| South | −0.035 (0.013)*** | −0.008 (0.004)* |
| West | −0.031 (0.017)* | −0.024 (0.005)*** |
| Pseudo R2 | 0.0551 | 0.0603 |
| Log pseudolikelihood | −4556.4864 | −47,492.815 |
Data source: 2014 Medicare Part D Drug Event File
p < 0.01,
p < 0.05, and
p < 0.10; standard errors are clustered within a plan
Table 6.
Results from the logit analysis of new hepatitis C drug use by the presence of cirrhosis among LIS (low-income subsidy) enrollees
| Variable | Patients with cirrhosis (N = 38,457) Odds ratio (95% CI) |
Patients without cirrhosis (N = 63,217) Odds ratio (95% CI) |
|---|---|---|
| Race (ref: non-Hispanic white) | ||
| Black | 0.88 (0.81–0.96)*** | 1.03 (0.97–1.10) |
| Hispanic | 0.97 (0.89–1.06) | 1.04 (0.96–1.14) |
| HCV/HIV coinfection | 1.09 (0.99–1.21)* | 1.01 (0.93–1.09) |
| Age | 1.00 (1.00–1.01)*** | 1.00 (1.00–1.00) |
| Male | 0.93 (0.87–0.99)** | 0.95 (0.90–1.01)* |
| Number of chronic conditions | 0.86 (0.84–0.87)*** | 0.88 (0.86–0.90)*** |
| Having diabetes | 1.25 (1.18–1.32)*** | 1.06 (0.99–1.13) |
| Having hypertension | 0.97 (0.92–1.03) | 0.97 (0.90–1.04) |
| Having ischemic heart disease | 0.99 (0.93–1.05) | 0.97 (0.92–1.03) |
| Having hyperlipidemia | 1.01 (0.94–1.08) | 1.05 (0.99–1.12) |
| Having depression | 1.05 (0.98–1.13) | 1.00 (0.94–1.06) |
| Having congestive heart failure | 0.73 (0.67–0.80)*** | 0.66 (0.59–0.73)*** |
| Having cataract | 1.3 (1.21–1.41)*** | 1.27 (1.17–1.38)*** |
| Having chronic obstructive pulmonary disease | 0.80 (0.75–0.86)*** | 0.83 (0.77–0.89)*** |
| Below median household income | 0.87 (0.82–0.92)*** | 0.89 (0.83–0.95)*** |
| Percent college educated | 1.00 (1.00–1.00)** | 1.00 (1.00–1.00) |
| Urban area | 1.07 (0.99–1.17)* | 0.99 (0.91–1.07) |
| Applying prior authorization | 1.31 (0.67–2.56) | 1.02 (0.47–2.25) |
| Enhanced plan | 1.24 (1.07–1.44)*** | 1.17 (1.06–1.30)*** |
| Gap coverage plan | 0.90 (0.73–1.11) | 0.96 (0.78–1.19) |
| Deduct | 1.04 (0.95–1.14) | 1.10 (1.01–1.21)** |
| Region (ref: Northeast) | ||
| Midwest | 1.08 (0.95–1.22) | 1.01 (0.93–1.09) |
| South | 0.88 (0.80–0.97)** | 0.92 (0.85–1.00)** |
| West | 0.82 (0.74–0.91)*** | 0.70 (0.64–0.77)*** |
| Pseudo R2 | 0.0456 | 0.0289 |
| Log pseudolikelihood | −18,772.967 | −21,728.44 |
p < 0.01,
p < 0.05, and
p < 0.10; standard errors are clustered within a plan
Table 7.
Results from the logit analysis of new hepatitis C drug use by HIV coinfection among LIS (low-income subsidy) enrollees
| Variable | Patients with HCV/HIV coinfection (N = 10,169) Odds ratio (95% CI) |
Patients with HCV infection only (N = 91,505) Odds ratio (95% CI) |
|---|---|---|
| Race (ref: non-Hispanic white) | ||
| Black | 0.80 (0.71–0.92)*** | 0.99 (0.93–1.04) |
| Hispanic | 1.01 (0.86–1.18) | 1.00 (0.94–1.07) |
| Cirrhosis | 2.37 (2.11–2.66)*** | 2.37 (2.26–2.49)*** |
| Age | 1.01 (1.00–1.02)*** | 1.00 (1.00–1.00)* |
| Male | 1.13 (1.01–1.27)** | 0.92 (0.88–0.97)*** |
| Number of chronic conditions | 0.85 (0.81–0.90)*** | 0.87 (0.86–0.88)*** |
| Having diabetes | 1.36 (1.18–1.56)*** | 1.14 (1.09–1.19)*** |
| Having hypertension | 0.99 (0.85–1.15) | 0.97 (0.93–1.02) |
| Having ischemic heart disease | 1.22 (1.03–1.45)** | 0.96 (0.91–1.00)* |
| Having hyperlipidemia | 1.21 (1.04–1.40)** | 1.01 (0.96–1.06) |
| Having depression | 1.01 (0.89–1.15) | 1.02 (0.98–1.07) |
| Having congestive heart failure | 0.63 (0.51–0.76)*** | 0.71 (0.66–0.76)*** |
| Having cataract | 1.40 (1.18–1.67)*** | 1.28 (1.20–1.35)*** |
| Having chronic obstructive pulmonary disease | 0.78 (0.66–0.93)*** | 0.82 (0.78–0.86)*** |
| Below median household income | 0.97 (0.86–1.09) | 0.86 (0.82–0.91)*** |
| Percent college educated | 1.00 (0.99–1.00) | 1.00 (1.00–1.00)* |
| Urban area | 1.37 (1.07–1.76)** | 1.01 (0.95–1.08) |
| Applying prior authorization | 1.29 (0.16–10.81) | 1.15 (0.65–2.04) |
| Enhanced plan | 1.09 (0.84–1.41) | 1.22 (1.12–1.33)*** |
| Gap coverage plan | 0.95 (0.63–1.43) | 0.92 (0.79–1.08) |
| Deduct | 0.86 (0.67–1.09) | 1.10 (1.03–1.17)*** |
| Region (ref: Northeast) | ||
| Midwest | 1.04 (0.85–1.27) | 1.03 (0.94–1.12) |
| South | 0.77 (0.64–0.93)*** | 0.92 (0.85–0.99)** |
| West | 0.80 (0.63–1.01)** | 0.76 (0.70–0.82)*** |
| Pseudo R2 | 0.0547 | 0.0553 |
| Log pseudolikelihood | −4101.8783 | −36,392.668 |
p < 0.01,
p < 0.05, and
p < 0.10; standard errors are clustered within a plan
Footnotes
We did not have access to the 100% files. CMS provides researchers with the minimum data sets that are necessary for a proposed study. Specific data files searched by CMS are Inpatient Standard Analytic File (SAF), Skilled Nursing Facility SAF, Hospital Outpatient (OP) file, and Carrier file (that includes claims on non-institutional provider care such as physician office visits).
Counting cases with one outpatient claim might include patients who used only a diagnostic service and did not have hepatitis infection.
We focused on Black and Hispanic racial/ethnic minority groups because HCV treatments are particularly important to these two groups. As described earlier, Blacks have higher rates of HCV/HIV coinfection, which creates challenges to HCV treatment, than other races. They also have had lower rates of HCV treatments than others. Hispanics have higher rates of cirrhosis than other races. Patients with cirrhosis, the most advanced form of fibrosis, have high priority for HCV treatment.
ICD-9 codes used by CMS to create the HIV indicator are 042, 042.0, 042.1, 042.2, 042.9, 043, 043.1, 043.2, 043.3, 043.9, 044, 044.0, 044.9, 079.53, 795.71, and V08 [22].
There is no difference in indications or contraindications between these two drugs. Sovaldi is used with a combination of peginferon plus ribavirin, ribavirin only, or simeprevir (Olysio). Harvoni is newer than Sovaldi and improves on Sovaldi by eliminating the need for a combination therapy.
Cirrhosis may be caused by other reasons than HCV infection. However, in the study population of HCV patients, cirrhosis is likely due to HCV infection.
Logit model fits , where P(Y=1) is the probability of using a new HCV drug. Thus, , and .
This adjusted difference is the marginal effect of a covariate on the probability of receiving a new HCV drug. Marginal effects are readily obtained using statistical software. In STATA, the command, “margins, dydx,” produces marginal effects of covariates on the outcome variable in a regression analysis.
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
References
- 1.Centers for Disease Control and Prevention. Hepatitis C kills more Americans than any other infectious disease. 2016 http://www.cdc.gov/media/releases/2016/p0504-hepc-mortality.html.
- 2.Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, Lu M, Nerenz DR, Nakasato CC, Boscarino JA, Henkle EM, Oja-Tebbe NJ, Xing J, Ward JW, Holmberg SD, Chronic hepatitis cohort study investigators Baseline characteristics and mortality among people in care for chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis. 2013;56(1):40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 3.Xu F, Tong X, Leidner AJ. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health Aff (Millwood) 2014;33(10):1728–35. doi: 10.1377/hlthaff.2014.0096. [DOI] [PubMed] [Google Scholar]
- 4.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Bräu N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P, ION-1 Investigators Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 5.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P, ION-2 Investigators Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 6.Tice JA, Ollendorf DA, Pearson SD. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic hepatitis c infection: institute for clinical & economic review. 2014 doi: 10.1001/jamainternmed.2014.2151. https://icer-review.org/wp-content/uploads/2016/02/CTAF_Hep_C_Apr14_final.pdf. Accessed September 25, 2016. [DOI] [PubMed]
- 7.Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–91. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 8.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271:1175–80. [PubMed] [Google Scholar]
- 9.Check DK, Samuel CA, Rosenstein DL, Dusetzina SB. Investigation of racial disparities in early supportive medication use and end-of-life care among Medicare beneficiaries with stage IV breast cancer. J Clin Oncol. 2016;34 doi: 10.1200/JCO.2015.64.8162. Published Ahead of Print, http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2015.64.8162. Accessed September 25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeder-Hayes K, Peacock Hinton S, Meng K, Carey LA, Dusetzina SB. Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol. 2016;34(17):2003–9. doi: 10.1200/JCO.2015.65.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaeffer S, Khalili M. Reasons for HCV non-treatment in under-served African Americans: implications for treatment with new therapeutics. Ann Hepatol. 2015;14(2):234–42. [PMC free article] [PubMed] [Google Scholar]
- 12.Melia MT, Muir AJ, Mc Cone J, Shiffman ML, King JW, Herrine SK, Galler GW, Bloomer JR, Nunes FA, Brown KA, Mullen KD, Ravendhran N, Ghalib RH, Boparai N, Jiang R, Noviello S, Brass CA, Albrecht JK, Mc Hutchison JG, Sulkowski MS, IDEAL Study Team Racial differences in hepatitis C treatment eligibility. Hepatology. 2011;54:70–8. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ja Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S, ADVANCE Study Team Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 14.Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007;52:1168–76. doi: 10.1007/s10620-006-9579-1. [DOI] [PubMed] [Google Scholar]
- 15.Borum ML, Igiehon E, Shafa S. African Americans may differ in their reasons for declining hepatitis C therapy compared to non-African Americans. Dig Dis Sci. 2009;54:1604. doi: 10.1007/s10620-009-0806-4. author reply 1604–05. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, Kramer JR, El-Serag HB, Frayne S, Clark J, Cao Y, Taylor T, Smith D, White D, Asch SM. Race and gender differences in the use of direct acting antiviral agents for hepatitis C virus. Clin Infect Dis. 2016;63(3):291–9. doi: 10.1093/cid/ciw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med. 2015;163(3):226–8. doi: 10.7326/M15-0320. [DOI] [PubMed] [Google Scholar]
- 18.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215–23. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 19.Winnock M, Bani-Sadr F, Pambrun E, Loko MA, Carrieri P, Neau D, Morlat P, Marchou B, Dabis F, Salmon D, French National Agency for Research on AIDS and Viral Hepatitis (ANRS) CO13 HEPAVIH Study Group Factors associated with guideline-based hepatitis C virus (HCV) treatment initiation in HIV/HCV-coinfected patients: role of comorbidities and physicians’ perceptions. HIV Med. 2013;14(7):430–6. doi: 10.1111/hiv.12023. [DOI] [PubMed] [Google Scholar]
- 20.IMS Health Institute for Healthcare Informatics. Medicines use and spending in the US—a review of 2015 and outlook to 2020. IMS Health Incorporate; 2016. http://www.imshealth.com/en/thought-leadership/ims-institute/reports/medicines-use-and-spending-in-the-us-a-review-of-2015-and-outlook-to-2020. Accessed September 25, 2016. [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. Medicare drug spending dashboard [Internet] CMS.gov. 2016 https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-12-21.html. Accessed September 25, 2016.
- 22.Chronic conditions data warehouse. other chronic or potentially disabling conditions. Condition Algorithm. https://www.ccwdata.org/web/guest/condition-categories. Accessed September 25, 2016.
- 23.Jaen C, Stange K, Nutting P. Competing demands of primary-care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38(2):166–71. [PubMed] [Google Scholar]
- 24.Chernof BA, Sherman SE, Lanto AB, Lee ML, Yano EM, Rubenstein LV. Health habit counseling amidst competing demands: effects of patient health habits and visit characteristics. Med Care. 1999;37(8):738–47. doi: 10.1097/00005650-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Nutting PA, Rost K, Smith J, Werner JJ, Elliot C. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Fam Med. 2000;9(10):1059. doi: 10.1001/archfami.9.10.1059. [DOI] [PubMed] [Google Scholar]
- 26.Chronic conditions data warehouse, chronic condition indicators. https://www.ccwdata.org/web/guest/condition-categories. Accessed November 11, 2016.
- 27.Jung JK, Feldman R, Cheong C, Du P, Leslie D. Coverage for hepatitis C drugs in Medicare part D. Am J Manag Care. 2016;22(6 Spec):SP220–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau CM, Ioannou GN, Todd-Stenberg JA, Sloan KL, Larson MF, Forsberg CW, Dominitz JA. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Public Health. 2008;98(5):846–52. doi: 10.2105/AJPH.2007.113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Veterans Affairs. VA expands hepatitis C drug treatment. 2016 http://www.va.gov/opa/pressrel/pressrelease.cfm?id=2762. Accessed September 25, 2016.
- 30.Executive Office of Health and Human Services. Mass health implements new drug rebate program, expands access to hepatitis C treatment. 2016 http://www.mass.gov/eohhs/gov/newsroom/press-releases/eohhs/masshealth-implements-new-drug-rebate-program.html. Accessed September 25, 2016.


