Abstract
Stroke is pathologically associated with oxidative stress, protein damage, and neuronal loss. We previously reported that overexpression of a ubiquitin-like protein, ubiquilin-1 (Ubqln), protects neurons against ischemia-caused brain injury, while knockout of the gene exacerbates cerebral ischemia-caused neuronal damage and delays functional recovery. Although these observations indicate that Ubqln is a potential therapeutic target, transgenic manipulation-caused overexpression of Ubqln occurs before the event of ischemic stroke, and it remains unknown whether delayed Ubqln overexpression in post-ischemic brains within a clinically relevant time frame is still beneficial. To address this question, we generated lentiviruses either overexpressing or knocking down mouse Ubqln, and treated post-ischemic stroke mice 6 h following the middle cerebral artery occlusion with the lentiviruses before animal behaviors were evaluated at day 1, 3, 5, and 7. Our data indicate that post-ischemic overexpression of Ubqln significantly promoted functional recovery, whereas post-ischemic downregulation of Ubqln expression delays functional recovery. To further understand the mechanisms underlying how Ubqln functions, we also isolated protein aggregates from the brains of wild-type mice or the mice overexpressing Ubqln following ischemia/reperfusion. Western blot analysis indicates that overexpression of Ubqln significantly reduced the accumulation of protein aggregates. These observations not only suggest that Ubqln is a useful candidate for therapeutic intervention for ischemic stroke but also highlight the significance of proteostasis in functional recovery following stroke.
Keywords: stroke, brain ischemia, reperfusion, neuroprotection, protein aggregates, ubiquilin-1
INTRODUCTION
Maintaining intracellular protein homeostasis (proteostasis) is critical for cell survival and normal functions (Chen et al. 2012). In the condition of ischemic stroke, however, oxidative stress and protein aggregates occur (Ge et al. 2007; Huang et al. 2012), which impair proteostasis. Our previous studies have revealed that ubiquilin1 (Ubqln) interacts with misfolded proteins and selectively enhances their degradation (Wang and Monteiro 2007). Our recent results suggest that Ubqln is a potential therapeutic target for ischemic stroke, as overexpression (OE) of Ubqln protects cells against oxidative stress and ischemia/reperfusion (I/R)-caused brain injury but knockout (KO) of the gene shows the opposite results (Liu et al. 2014b). In these studies, however, alteration of Ubqln expression occurs prior to ischemic insults. It remains unknown whether delayed manipulation of the Ubqln protein level within a clinically relevant time window has a similar effect as what we observed from the transgenic or KO ischemic stroke mice. To address this question, we have generated lentiviruses (LVs) that overexpress either mouse Ubqln or small hairpin (sh) RNAs and tested the effect of OE or knockdown of Ubqln on functional recovery in post-ischemic mice.
MATERIALS AND METHODS
Animals
All animal maintenance and experimental procedures were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of South Dakota.
Middle Cerebral Artery Occlusion (MCAO) and intracerebral injection of LVs
Adult male mice with the C57BL/6 background (2–3 months, 22–30 g, Envigo, Indianapolis, IN, USA) were subjected to 90 min MCAO as previously described (Lu and Wang 2012). After 6 h reperfusion, the mice were intrastriatally injected with 2 µl of concentrated LVs overexpressing mouse Ubqln, the enhanced green fluorescent protein (GFP), Ubqln-shRNAs, or scramble-shRNAs into the ipsilateral brain using previously described method (Wang et al. 2004). Mice were then allowed to survive for 7 days and animal behaviors were tested at day 1, 3, 5, and 7.
Animal behavior tests
We used a previously described modified neurologic severity score (mNSS) to assess neurological deficits after I/R (Chen et al. 2005). With this system, animal neurologic function was graded on a scale of 0 to 14 (normal score 0; maximal deficit score 14) including motor, reflex, and balance tests. One score point is given to a mouse for its failure in performing a specific task or for its lack of a tested reflex; thus, the score received by an animal reflects the severity of the brain injury.
Preparation and verification of lentiviruses
To generate the LV-Ublqn, we replaced the cDNA encoding GFP in the pLenti-CMV-GFP plasmid (Campeau et al. 2009) with a full length mouse Ubqln cDNA sequence (Liu et al. 2014b). The four different mouse LV-Ubqln-shRNAs and the scramble control were purchased from OriGene (Rockville, MD, USA). LV packing and concentration were performed according to previously described methods (Stewart et al. 2003; Park et al. 2008). To verify the LV gene expression, HeLa or mouse striatal cells (CH00097, Coriell, Camden, New Jersey, USA) were transduced with LVs in the presence of polybrene (8 µg/ml) for different times depending on specific experiments, the cells were then subjected to western analysis of either Ubqln or GFP expression using a specific antibody.
Isolation of Triton-X100 insoluble protein aggregates
Isolation of Triton-X100 insoluble protein aggregates was based on a previously described method (Ge et al. 2007).
Western blot analysis
Cell lysates or isolated protein aggregates were sonicated and then separated in 12% SDS PAGE before transferred onto a nitrocellulose membrane for immunoblotting analysis as previously described (Dong et al. 2012). The primary and secondary antibodies were previously described (Liu et al. 2014a).
Statistical analysis
Two group comparisons were statistically analyzed by the two-tailed Student’s t-test. P < 0.05 was regarded as statistically significant.
RESULTS AND DISCUSSION
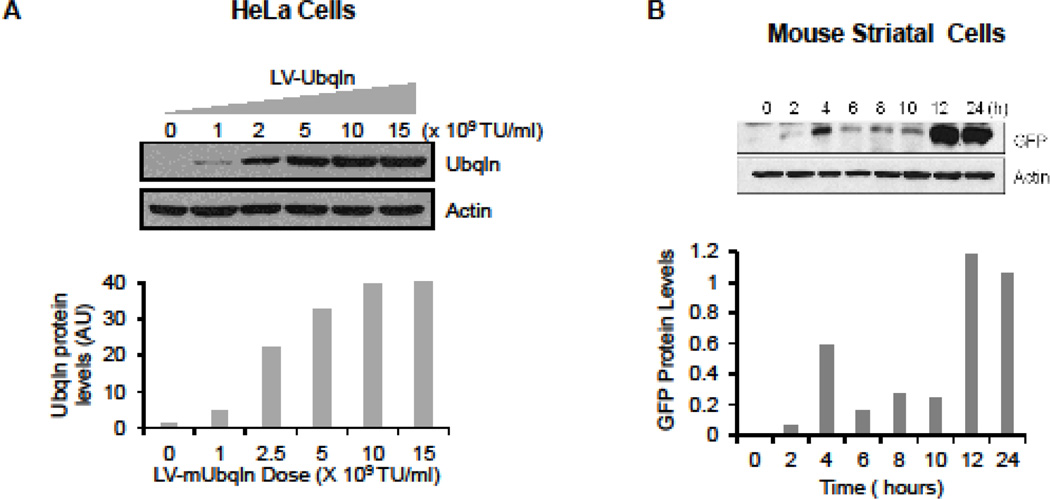
LVs are a class of retrovirus that are highly efficient in infecting both dividing and non-dividing cells, making the recombinant lentiviral vectors an ideal tool for manipulation of gene expression in post-mitotic neurons (Deglon and Aebischer 2002). To test whether upregulation of Ubqln in the post-ischemic mouse brain exerts a beneficial effect on animal functional recovery, we generated pseudo-LVs expressing either Ubqln or GFP, and validated the LVs in HeLa cells. Our results indicate that 24 h after transduction, the cells showed remarkable OE of Ubqln in a dose-dependent manner (Fig. 1A). To further determine the temporal expression pattern of the LV vector in neurons following transduction, we transduced LV-GFP into cultured mouse striatal cells before the cells were collected every 2 h for assessing GFP levels. This allowed us to conveniently determine how long for the LV gene to express, as the striatal cells did not express endogenous GFP. As shown in Fig. 1B, expression of LV-GFP in the mouse neurons was detectible at 2 h but reached a peak at 12 h following transduction. These data suggest that the LV gene shows rapid expression following transduction. We then tested whether delivering the LVs into the I/R-injured brains alters animal functional outcomes. Accordingly, we performed MCAO to the animals and after 6 h following reperfusion, mice were intrastriatally injected with concentrated LVs either overexpressing Ubqln or GFP into the injury side (Fig. 1C). Treatment of the stroke mice with LV-Ubqln significantly enhanced the animal functional recovery compared to the control LV-GFP treatment (Fig.1D).
Figure 1. Treatment of ischemic stroke mice with LV-Ubqln enhances animal functional recovery.
A. Verification of LV-Ubqln expression. Upper panel, western blot analysis of Ubqln protein levels 24 h following transduction. Lower panel, quantitation of Ubqln expression using actin as a loading control. Data are shown as mean ± SD; n = 3.
B. Determination of the temporal expression pattern of the lentiviral vector. Upper panel, western blot analysis of GFP expression levels in mouse striatal cells at indicated time points after transduction. Lower panel, graph showing measured GFP protein levels using actin as a loading control. Data are shown as mean ± SD; n = 3.
C. Diagram illustrating experimental procedures.
D. Treating stroke mice with LV-Ubqln enhances animal functional recovery. Data are shown as mean ± SD; n = 5–8 for each group; *p < 0.05.
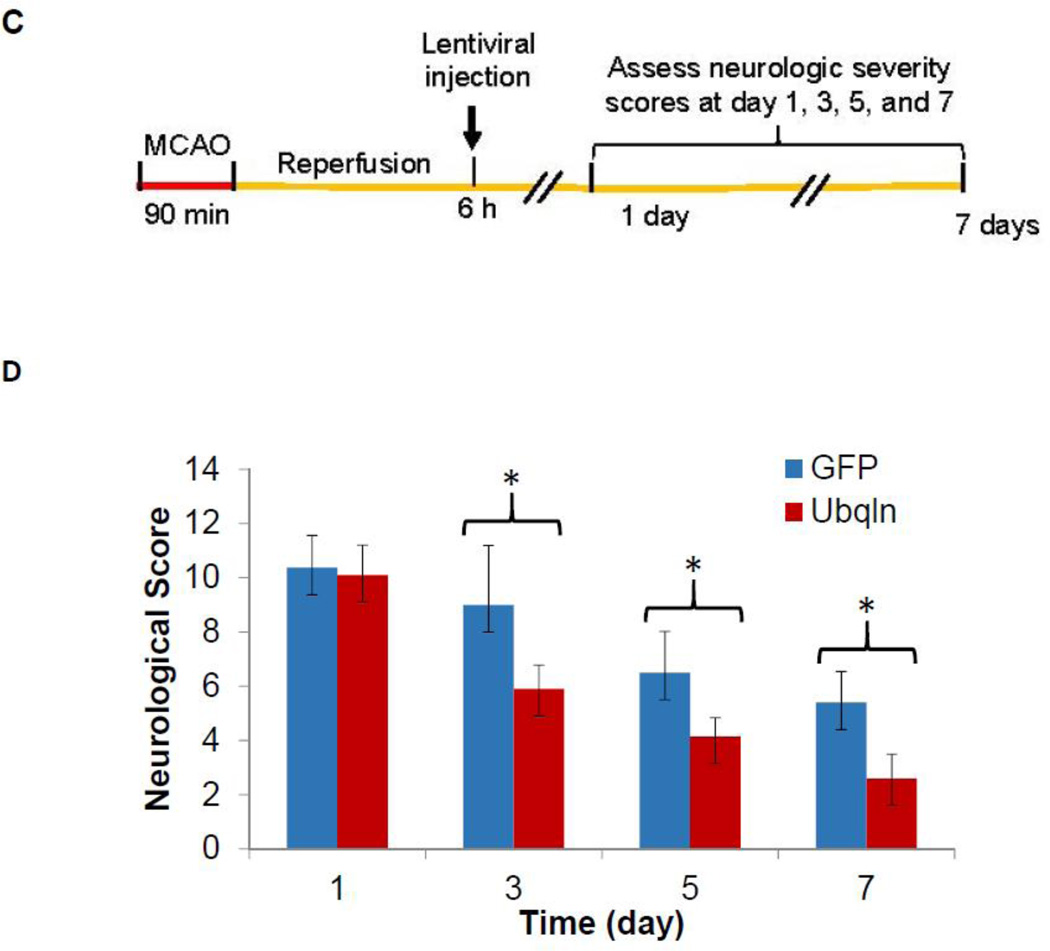
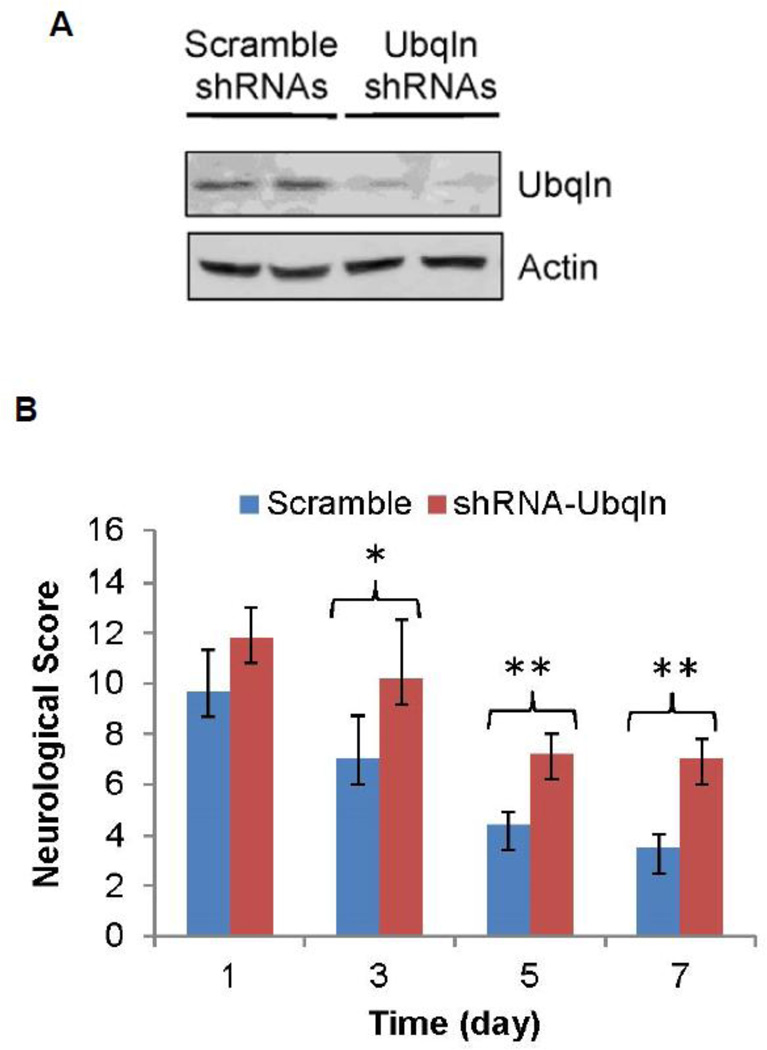
To confirm above observation and further define whether appropriate Ubqln expression is required for neuroprotection after stroke occurs, we also generated lentiviral particles expressing Ubqln shRNAs to knockdown Ubqln expression or scramble RNAs to function as a control, and then verified these lentiviruses in cultured striatal cells. As shown in Fig. 2A, transduction of the LV-Ubqln-shRNAs reduced Ubqln protein levels compared to the scramble shRNA transductions thus indicating that the lentiviruses can effectively downregulate Ubqln expression in neuronal cells. We then treated ischemic stroke mice with either lentiviral Ubqln shRNAs or scramble shRNAs by intrastriatally injecting them into the injured brain sides. Our results showed that knockdown of Ubqln in mice after ischemic stroke exacerbated animal neurological deficits (Fig. 2B), as reflected by increased mNSSs compared to the scramble treatment. These data suggest that downregulation of Ubqln expression in ischemic stroke mice delays functional recovery.
Figure 2. Treating stroke mice with LV-Ubqln-shRNAs delays animal functional recovery.
A. Verification of LV-Ubqln-shRNAs. Western blot analysis of Ubqln protein levels in a mouse striatal cell line 48 h following transduction of LV-Ubqln-shRNAs.
B. LV-Ubqln-shRNA treatment exacerbates neurological deficits and delays mouse functional recovery. Data are shown as mean ± SD; n = 5 for each group; *p < 0.05, **p < 0.01.
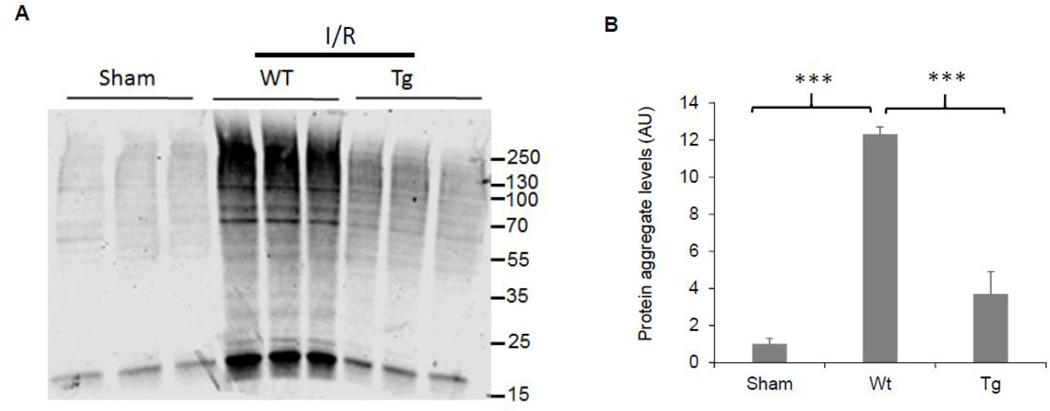
Ischemic stroke causes oxidative stress, protein damages and accumulation of ubiquitinated protein aggregates (Ge et al. 2007). To further understand how Ubqln funcitons in the condition of ischemic stroke, we isolated protein aggregates from the wild-type or Ubqln transgenic (Tg) mouse brains 24 h following I/R since the Tg mice exhibted significant tolerance to I/R-caused brain injury and marked Ubqln overexpression in different brain regions (Liu et al. 2014a). Western blot analysis of the protein aggregates indicated that, compared to the sham procedure, I/R induced pronounced accumulation of ubiquitinated protein aggregates (Figs. 3A & 3B). In contrast, the transgenic mouse brains showed significantly reduced protein aggregates (Figs. 3A & 3B). Thus, OE of Ubqln attenuates damaged protein accumulation.
Figure 3. OE of Ubqln reduces protein aggregates following I/R.
A. Western blot analysis of protein aggregates isolated from either wild-type sham-treated mice, or I/R-treated wild-type or Ubqln transgenic (Tg) mice.
B. Quantitation of ubiquitinated protein levels of isolated protein aggregates. Data are shown as mean ± SD; n = 3; ***p < 0.001
Cerebral I/R is associated with oxidative stress and causes accumulation of protein aggregates in the brain cells (Ge et al. 2007; Huang et al. 2012). To develop effective treatment for ischemic stroke, it is important to determine whether enhanced removal of these damaged proteins is beneficial. Here we demonstrated that that post-ischemic OE of Ubqln, a protein that selectively enhances degradation of misfolded proteins (Wang and Monteiro 2007), enhances animal functional recovery, whereas knockdown of Ubqln delays functional recvoery following I/R. More importantly, OE of Ubqln reduces accumulation of protein aggregates in I/R damaged brains. These data highlight the significance of protein homeostasis in neuroprotection following I/R.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke under research grants R03NS084340 and R01NS088084 (HW).
Abbreviations
- MCAO
middle cerebral artery occlusion
- I/R
ischemia/reperfusion
- Ub
ubiquitin
- UPS
ubiquitin-proteasome system
- Ubqln
ubiquilin-1
- OE
overexpression
- KO
knockout
- shRNA
small hairpin ribosomal nucleic acid
- LV
lentivirus
- GFP
green fluorescence protein
- mNSS
modified neurologic severity score
- TX
Triton-X100
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD. A versatile viral system for expression and depletion of proteins in mammalian cells. PloS one. 2009;4(8):e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural regeneration research. 2012;7(5):376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglon N, Aebischer P. Lentiviruses as vectors for CNS diseases. Current topics in microbiology and immunology. 2002;261:191–209. doi: 10.1007/978-3-642-56114-6_10. [DOI] [PubMed] [Google Scholar]
- Dong G, Callegari EA, Gloeckner CJ, Ueffing M, Wang H. Prothymosin-alpha interacts with mutant huntingtin and suppresses its cytotoxicity in cell culture. J Biol Chem. 2012;287(2):1279–1289. doi: 10.1074/jbc.M111.294280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Luo Y, Liu CL, Hu B. Protein aggregation and proteasome dysfunction after brain ischemia. Stroke; a journal of cerebral circulation. 2007;38(12):3230–3236. doi: 10.1161/STROKEAHA.107.487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HF, Guo F, Cao YZ, Shi W, Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS neuroscience & therapeutics. 2012;18(10):811–818. doi: 10.1111/j.1755-5949.2012.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hettinger CL, Zhang D, Rezvani K, Wang X, Wang H. The proteasome function reporter GFPu accumulates in young brains of the APPswe/PS1dE9 Alzheimer's disease mouse model. Cellular and molecular neurobiology. 2014a;34(3):315–322. doi: 10.1007/s10571-013-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci. 2014b;34(8):2813–2821. doi: 10.1523/JNEUROSCI.3541-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang H. Transient focal cerebral ischemia upregulates immunoproteasomal subunits. Cellular and molecular neurobiology. 2012;32(6):965–970. doi: 10.1007/s10571-012-9854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Monteiro MJ. Ubiquilin interacts and enhances the degradation of expanded-polyglutamine proteins. Biochemical and biophysical research communications. 2007;360(2):423–427. doi: 10.1016/j.bbrc.2007.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci. 2004;24(48):10963–10973. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]