Abstract
Objectives
To assess the prevalence and prognostic value of abnormalities in left atrial (LA) phasic volumes and reservoir function in a community cohort.
Background
LA enlargement is associated with adverse cardiovascular outcomes. Real-time three-dimensional (RT3D) echocardiography allows the assessment of LA phasic volumes and reservoir function. However, there is paucity of data regarding normal values, clinical correlates, and prognostic value of RT3D echocardiography-derived LA phasic volumes and reservoir function, especially in the elderly, a subgroup at high risk for cardiovascular events.
Methods
LA maximum volume (LAVimax), minimum volume (LAVimin), and reservoir function assessed as emptying volume (LAEV), emptying fraction (LAEF), and expansion index (LAEI), were measured by RT3D echocardiography in participants from a community-based cohort study. Cut-off values for LA phasic volumes were derived from a healthy subgroup of participants free of cardiovascular disease and risk factors (n=142, mean age 66±9 years, 55% women). Annual follow-up was performed for cardiovascular outcomes (myocardial infarction, ischemic stroke, and vascular death).
Results
The cohort included 706 participants (mean age 71±9 years, 59% women). LAVimax and LAVimin were not associated with age in the healthy subgroup, but progressively increased with age in the entire cohort (both p<0.001). During a median follow-up of 7 years (min 0.06, max 9.5) 78 cardiovascular events occurred. In univariate analysis, LAVimax, LAVimin, and reservoir function parameters were significantly associated with outcome. In multivariate analysis, LAVimin ≥ 20.5 ml/m2 [adjusted hazard ratio (aHR)=1.79, 95% confidence intervals (CI)=1.02-3.16) and LAEV ≤ 5.7 ml/m2 (aHR=1.98, 95% CI=1.02-3.85) remained significantly associated with events. LAVimin and LA reservoir function showed incremental prognostic value over LAVimax.
Conclusions
LA phasic volumes and reservoir function assessed by RT3D echocardiography were strong independent predictors of cardiovascular events in a community-based elderly cohort. LAVimin and reservoir function assessment may improve cardiovascular outcome prediction over LAVimax.
Keywords: Left atrial volume, Reservoir function, Three-dimensional echocardiography, Outcome
INTRODUCTION
Left atrial (LA) enlargement is a strong risk factor for cardiovascular events (1-3). Among different measures of LA size, LA volume showed the strongest association with adverse outcomes (4). Echocardiography is the most frequently used imaging technique to assess LA volume because of its widespread availability and reliable volume assessment. Traditionally, LA volume is measured in end-systole, when the left atrium reaches maximum expansion (LAVimax). The adoption of new technologies, such as real-time three-dimensional (RT3D) echocardiography, has made it possible to measure the change in LA volume throughout the cardiac cycle, and to assess the LA reservoir function. Growing evidence suggests that the analysis of LA volume in different phases of the cardiac cycle (LA phasic volumes) may provide additional, clinically relevant information regarding LA remodeling and dysfunction. In fact, the LA volume measured at end-diastole (LA minimum volume, LAVimin) and the LA reservoir function have been demonstrated to be better correlates of LV diastolic dysfunction and better predictors of incident atrial arrhythmias than LAVimax (5-7). However, it is not clear whether the assessment of LA phasic volumes and reservoir function can provide relevant prognostic information towards cardiovascular outcome, and, if so, whether such information is incremental over the established LAVimax measurement. Furthermore, there is scarce information in the literature regarding normal values of LA phasic volumes and function derived from RT3D echocardiography, especially in the elderly population. Accordingly, the aim of this study was to investigate the prognostic value of LA phasic volumes and reservoir function measured by RT3D echocardiography in a community-based cohort of predominantly elderly composition. Additionally, we assessed the incremental prognostic value of RT3D LA phasic volumes and reservoir function over traditional 2D parameters and risk factors.
METHODS
Study population
The Cardiac Abnormalities and Brain Lesion (CABL) study based its recruitment on the Northern Manhattan Study (NOMAS), a population-based prospective study that enrolled 3,298 participants from the community living in northern Manhattan between 1993 and 2001. The study design and recruitment details of NOMAS have been described previously (8). Beginning in 2003, participants over 50 years of age without contraindications to MRI and without prior stroke were invited to participate in a brain MRI substudy. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo an extensive cardiovascular evaluation including RT3D echocardiography were prospectively enrolled in CABL. Of 836 CABL participants with RT3D echocardiographic data available, 130 were excluded for technical reasons (suboptimal image quality for LA volume analysis) leading to the final study sample of 706. Subjects excluded for suboptimal image quality had significantly higher body mass index (29.5±5.5 vs. 28.0±4.6 kg/m2, p<0.001) and had more frequently diabetes (40% vs. 28%, p=0.004) than the rest of the cohort, but no significant differences were present in terms of age (71.3±9.3 vs. 72.5±9.3 years), sex (60% vs 65% women), hypertension (82% vs. 79%), hypercholesterolemia (72% vs. 66%), and coronary artery disease (6.8% vs. 6.3%; all p>0.05). Written informed consent was obtained from all study participants. The study protocol was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami.
Risk factors and body size assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or self-reported history of hypertension or use of anti-hypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or self-reported history of diabetes or use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-report of hypercholesterolemia, or use of lipid-lowering treatment. Atrial fibrillation was ascertained by ECG tracing at study enrollment or by history from medical records. Coronary artery disease (CAD) was defined as history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. The body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. The race-ethnicity classification was based on self-identification, and modeled after the U.S. Census.
Echocardiographic assessment
Two-dimensional echocardiography
Transthoracic echocardiography was performed using a commercially available system (iE 33, Philips, Andover, MA) by a trained, registered cardiac sonographer according to a standardized protocol. LV end-diastolic wall thickness and dimension were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography (9), and LV mass was calculated with a validated method (10) and indexed by height2.7 (LV mass index). LA antero-posterior diameter was measured from the parasternal long-axis view and indexed by body surface area. Two-dimensional LA volume was measured using the biplane area-length method and indexed by the body surface area. LV volumes and LVEF were calculated using the biplane modified Simpson's rule. Significant valve disease was defined as mitral or aortic regurgitation or stenosis that were at least moderate in severity. The ratio of the trans-mitral early diastolic flow velocity assessed by pulsed-wave Doppler (E) over the mitral annulus early diastolic velocity assessed by tissue-Doppler (e’) was used as an indicator of LV filling pressure and diastolic function as previously described (7,11).
RT3D echocardiography
RT3D imaging was performed using a commercially available ultrasound machine (iE33, Philips, Andover, MA) equipped with an X3-1 matrix array transducer. A detailed description of LA phasic volumes assessment by RT3D echocardiography has been published previously (7,12). Briefly, a pyramidal full volume was obtained from 4 sub-volumes over four consecutive cardiac cycles. Sector dimensions and depth were set to include the whole left ventricle and the left atrium, allowing volume rates between 15 and 25 per second. Measurement of LA volumes was prospectively performed offline using commercially available software (QLAB Advanced Quantification software, version 8.1, Philips) by a single reader (CR) blinded to the study participants’ baseline clinical characteristics. Five anatomical landmarks (septal, lateral, anterior and inferior mitral annulus, and posterior wall of the LA) were manually identified by the operator, semi-automated border detection was performed by the software, and LA borders were tracked throughout the entire cardiac cycle. Manual correction on all 3D planes was performed in case of inaccurate endocardial detection. LA volume measurements were indexed by the body surface area. LAVimax was measured at end-systole and LAVimin was measured at end-diastole. LA reservoir function was measured as LA emptying volume (LAEV=LAVimax – LAVimin), LA emptying fraction (LAEF=100 × (LAVimax – LAVimin)/LAVimax), and LA expansion index (LAEI=100 × (LAVimax – LAVimin)/LAVimin).
Follow-up and outcome assessment
All subjects were followed-up annually by telephone interviews. Any vascular event or acknowledgment of neurological or cardiac symptoms during the standardized interview triggered an in-person assessment. In addition, active hospital surveillance of admission and discharge ICD-9 codes was performed. Outcomes for this analysis were ischemic stroke, myocardial infarction, and vascular death. Stroke was defined by the first symptomatic occurrence of any type of stroke as defined by TOAST criteria (13). Diagnosis of ischemic stroke was determined by two neurologists independently, and disagreements were adjudicated by the NOMAS principal investigators (RLS/MSVE). Myocardial infarction was defined by criteria adapted from the Cardiac Arrhythmia Suppression Trial (14) and the Lipid Research Clinics Coronary Primary Prevention trial (15) and adjudicated by a study team cardiologist (MDT). Death was classified as either vascular or nonvascular based on information from family, medical records, death certificate, and primary care physicians. Vascular causes of death were stroke, myocardial infarction, heart failure, pulmonary embolus, cardiac arrhythmia, and other vascular causes (8).
Statistical analysis
Data are presented as means ± standard deviation for continuous variables and as percentages for categorical variables. Linear regressions were used to assess relationships between atrial parameters and clinical and demographic variables. Cox proportional hazards models were used to test the association of LA phasic volumes with incident cardiovascular events, and hazard ratios (HR) and 95% confidence intervals (CI) were reported. Multivariate models were built selecting covariates from their univariate association with outcome. The likelihood ratio test was used with a series of nested Cox proportional hazards models to examine the incremental prognostic value of LA volumes and reservoir function, and models’ chi-square was presented. Kaplan-Meier plots were used to assess event-free probability associated with LA volumes and reservoir function abnormalities, and the log-rank test was used to compare the curves. The percentiles used as cut-offs in the survival analysis were selected based on their significant association with cardiovascular events while identifying the largest population at risk. For all statistical analyses, a two-tailed p<0.05 was considered significant. Statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
Reproducibility of LA volumes assessment
Reproducibility of LA volume measurements was assessed in 15 randomly selected subjects. LAVimin and LAVimax were re-measured by the original reader (CR) and by a second reader experienced in 3D echocardiography (AT) in a blinded fashion. Intra-observer ICC were 0.96 for LAVimin [95% confidence intervals (CI): 0.88-0.99] and 0.94 for LAVimax (95% CI: 0.85-0.98). The mean difference between two measurements was 0.13±1.79 ml/m2 for LAVimin (p=0.78) and 0.42±2.29 ml/m2 for LAVimax (p=0.49). Inter-observer ICC were 0.94 for LAVimin (95% CI: 0.85-0.98) and 0.95 for LAVimax (95% CI: 0.86-0.98). The mean difference between two measurements was 0.44±2.27 ml/m2 for LAVimin (p=0.46) and 0.52±2.56 ml/m2 for LAVimax (p=0.45).
RESULTS
The study cohort included 706 participants (mean age 71.2±9.3 years, 59.5% women) with assessment of LA phasic volumes and function by RT3D echocardiography available. The reference subgroup consisted of participants without hypertension, cardiovascular disease, cardiac arrhythmias, significant valve disease, and in sinus rhythm at the time of enrollment (n=142, mean age: 66±9 years, 55% women). Clinical and echocardiographic characteristics of the study cohort are shown in Table 1.
Table 1.
Clinical and echocardiographic characteristics of the study cohort.
| n=706 | |
|---|---|
| Clinical characteristics | |
| Age, years | 71.2±9.3 |
| Women, n (%) | 420 (59.5) |
| BMI, kg/m2 | 27.8±4.6 |
| SBP, mmHg | 135.8±17.4 |
| DBP, mmHg | 78.3±9.6 |
| Hypertension, n (%) | 556 (78.8) |
| Anti-hypertensive medications, n (%) | 507 (71.8) |
| Diabetes, n (%) | 200 (28.3) |
| Hypercholesterolemia, n (%) | 462 (65.4) |
| CAD, n (%) | 45 (6.4) |
| Atrial fibrillation, n (%) | 40 (5.7) |
| 2D Echocardiography | |
| LV end-diastolic volume, ml/m2 | 54.4±15.7 |
| LV end-systolic volume, ml/m2 | 20.5±10.4 |
| LV mass index, g/m2.7 | 50.7±13.8 |
| LVEF, % | 63.2±7.3 |
| LA antero-posterior diameter, mm/m2 | 22.6±3.1 |
| LA volume (2D biplane), ml/m2 | 27.2±8.8 |
| Significant valve disease,* n (%) | 79 (11.2) |
| E/e’ | 10.3±3.31 |
| RT3D Echocardiography | |
| LAVimax, ml/m2 | 24.9±7.5 |
| LAVimin, ml/m2 | 14.2±6.7 |
| LAEV, ml/m2 | 10.7±3.8 |
| LAEF, % | 44.1±12.5 |
| LAEI, % | 88.1±43.3 |
Moderate or more valve regurgitation/stenosis. BMI: Body mass index. SBP: Systolic blood pressure. DBP: Diastolic blood pressure. CAD: Coronary artery disease. LV: Left ventricular. LVEF: LV ejection fraction. LA: Left atrial. LAVimax: LA maximum volume. LAVimin: LA minimum volume. LAEV: LA emptying volume. LAEF: LA emptying fraction. LAEI: LA expansion index.
Correlates of LA phasic volumes in the healthy subgroup and in the overall cohort
In the healthy subgroup, age did not show significant associations with LA volumes and reservoir function parameters (all p>0.10). Both LAVimax (β=0.22) and LAVimin (β=0.13) were significantly greater in men than women with a mean difference of 2.2 ml/m2 and 1.3 ml/m2 respectively (both p<0.05), whereas LAEV, LAEF, and LAEI were not significantly associated with sex. LAVimax and LAVimin were not correlated with BMI, whereas LAEV, LAEF, and LAEI showed inverse correlation with BMI (all p<0.05). Diabetes and hypercholesterolemia did not show any correlation with LA volumes and function.
Table 2 shows the clinical correlates of LA phasic volumes and reservoir function in the entire study cohort. LA volumes increased with age whereas LA function worsened with age (all p<0.01). LAVimax, LAVimin, LAEF, and LAEI were significantly associated with SBP, hypertension, atrial fibrillation, valve disease, and E/e’ (all p<0.05). LA volumes were larger in presence of CAD (both p<0.05). LAEV was inversely associated with BMI (p=0.02) and atrial fibrillation (p<0.001). LAEI was inversely correlated with DBP (p=0.04). None of the LA parameters were significantly associated with sex.
Table 2.
Association of LA phasic volumes with cardiovascular risk factors in the entire study cohort.
| N=706 | LAVimax | LAVimin | LAEV | LAEF | LAEI |
|---|---|---|---|---|---|
| B (SE), p value | B (SE), p value | B (SE), p value | B (SE), p value | B (SE), p value | |
| Age | 0.23 (0.03), <0.001 | 0.27 (0.03), <0.001 | −0.04(0.02), 0.007 | −0.48 (0.05), <0.001 | −1.58 (0.16), <0.001 |
| Male sex | 0.90 (0.57), 0.118 | 0.70 (0.52), 0.172 | 0.19 (0.29), 0.505 | −1.01 (0.96), 0.295 | −2.03 (3.32), 0.541 |
| BMI | −0.11 (0.06), 0.068 | −0.04(0.05), 0.493 | −0.07 (0.03), 0.016 | −0.13 (0.10), 0.203 | −0.46 (0.35), 0.189 |
| SBP | 0.04 (0.02), 0.012 | 0.05 (0.01), 0.001 | −0.01 (0.01), 0.252 | −0.11 (0.03), <0.001 | −0.40(0.09), <0.001 |
| DBP | −0.03 (0.03), 0.244 | −0.01 (0.03), 0.795 | −0.03 (0.01), 0.064 | −0.09 (0.05), 0.072 | −0.35 (0.17), 0.037 |
| Hypertension | 2.76 (0.68), <0.001 | 3.10 (0.61), <0.001 | −0.34 (0.35), 0.329 | −5.45(1.13), <0.001 | −15.5 (3.94), <0.001 |
| Diabetes | −0.68 (0.62), 0.278 | −0.14 (0.56), 0.807 | −0.54(0.31), 0.086 | −1.01(1.05), 0.332 | −4.15 (3.61), 0.251 |
| Hypercholesterolemia | −0.76 (0.59), 0.201 | −0.52 (0.53), 0.332 | −0.24 (0.30), 0.418 | 0.35 (0.99), 0.727 | 0.26 (3.43), 0.940 |
| CAD | 2.49 (1.14), 0.031 | 2.32 (1.03), 0.025 | 0.17 (0.58), 0.773 | −3.57 (1.92), 0.064 | −12.1 (6.65), 0.069 |
| Atrial fibrillation | 6.46 (1.19), <0.001 | 8.94 (1.04), <0.001 | −2.48(0.61), <0.001 | −13.5 (1.97), <0.001 | −33.9 (6.93), <0.001 |
| Significant valve disease | 7.37 (0.85), <0.001 | 6.62 (0.76), <0.001 | 0.75(0.45), 0.095 | −6.28 (1.48), <0.001 | −16.3 (5.13), 0.002 |
| E/e’ | 0.61 (0.08), <0.001 | 0.70 (0.07), <0.001 | −0.08 (0.04), 0.055 | −1.16 (0.14), <0.001 | −3.45 (0.48), <0.001 |
Values in table are parameter estimates (B), standard error in parentheses, and p values.
BMI: Body mass index. SBP: Systolic blood pressure. DBP: Diastolic blood pressure. CAD: Coronary artery disease. LAVimax: LA maximum volume. LAVimin: LA minimum volume. LAEV: LA emptying volume. LAEF: LA emptying fraction. LAEI: LA expansion index.
Prevalence and prognostic value of LA phasic volume abnormalities
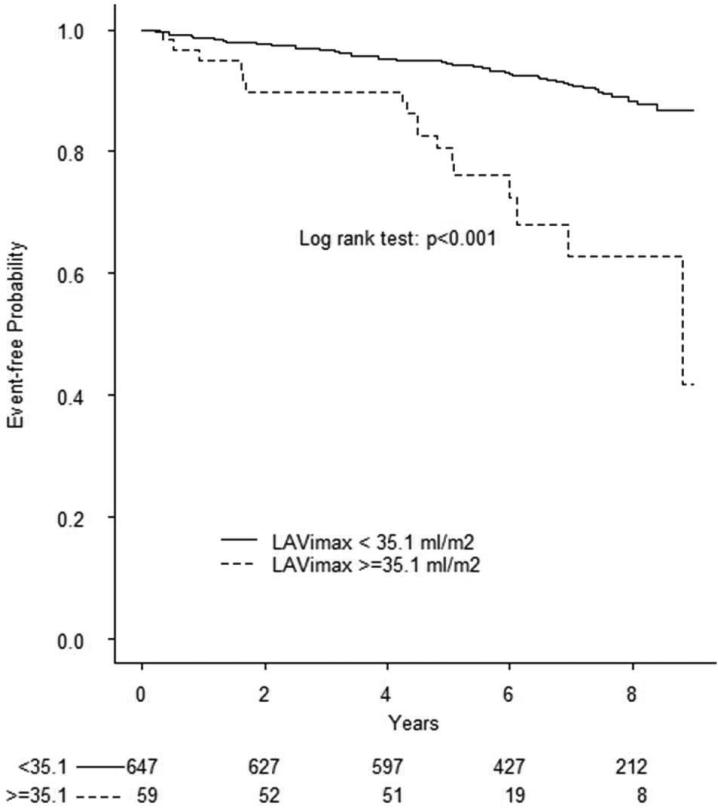
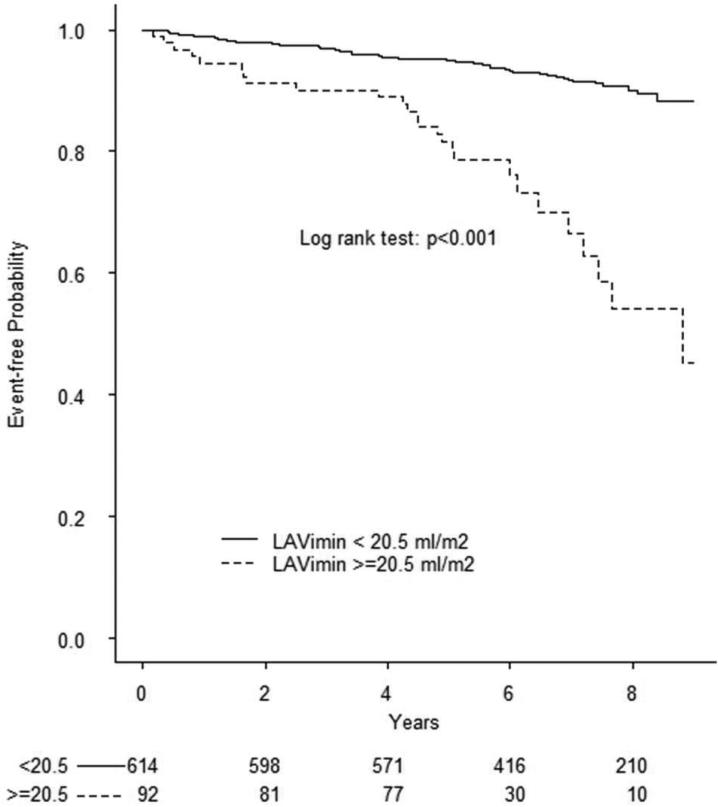
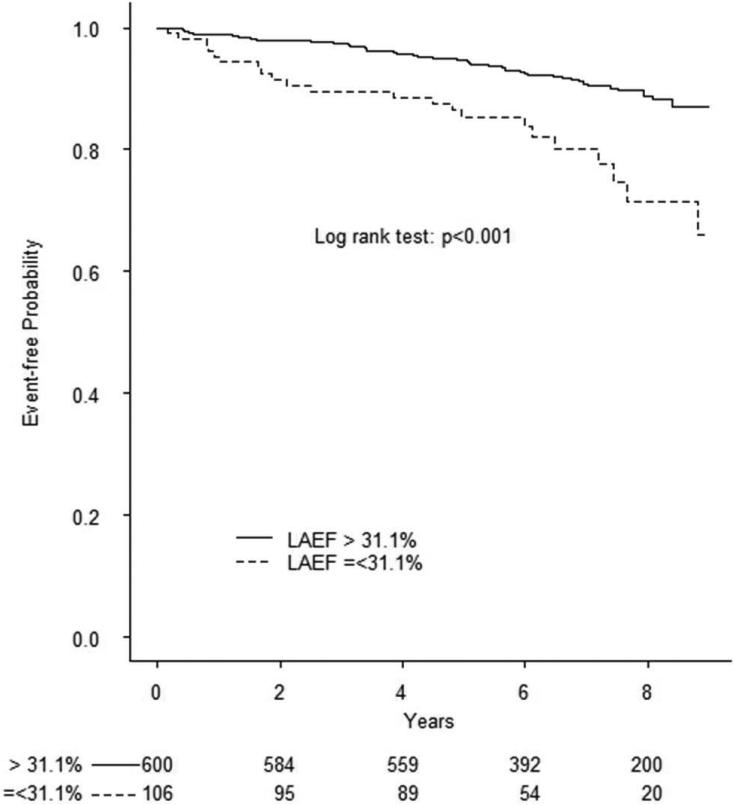
During a mean follow-up of 6.5 years (median=7, min=0.06, max=9.5), 78 cardiovascular events occurred, including 28 ischemic strokes, 13 myocardial infarctions, and 37 vascular deaths. Causes of death were: 1 fatal stroke, 1 fatal myocardial infarction, 2 acute heart failure, 1 pulmonary embolus, 25 sudden death/cardiac arrhythmia, and 7 were scored as other vascular causes. In Table 3 are shown the univariate and multivariate associations of demographics, risk factors, and echocardiography parameters with cardiovascular events. To assess the prognostic value of LA phasic volume parameters, we identified in the healthy reference group cut-offs corresponding to the 97.5th and 99th percentiles of LAVimax and LAVimin and the 2.5th and 1st percentile of LAEV, LAEF, and LAEI distributions. Kaplan-Meier event-free survival plots for LA abnormalities are shown in Figure 1. All RT3D LA volumes and reservoir function abnormalities were significantly associated with outcome (all log-rank test p values <0.001). The prevalence of LA abnormalities in the overall cohort by using the different cut-offs and their ability to predict cardiovascular events in multivariate analyses is shown in Table 4. Abnormal LA parameters derived from two-dimensional echocardiography (LA diameter and 2D LAVimax) were not associated with events after adjusting for covariates, whereas RT3D-derived LAVImin ≥ 20.5 ml/m2 (adjusted HR=1.79, 95% CI 1.02-3.16) and LAEV ≤5.7 ml/m2 (adjusted HR=1.98, 95% CI 1.02-3.85) remained significantly associated with outcome.
Table 3.
Univariate predictors of outcome.
| HR* (95% CI) | P value | |
|---|---|---|
| Risk factors | ||
| Age | 1.09 (1.06-1.12) | <0.001 |
| Male | 1.59 (1.02-2.47) | 0.042 |
| BMI | 0.97 (0.92-1.02) | 0.184 |
| SBP | 1.01 (0.99-1.02) | 0.136 |
| DBP | 0.99 (0.97-1.01) | 0.318 |
| Hypertension | 2.01 (1.03-3.89) | 0.040 |
| Diabetes | 1.52 (0.96-2.42) | 0.075 |
| Hypercholesterolemia | 1.22 (0.76-1.98) | 0.411 |
| CAD | 1.38 (0.60-3.19) | 0.445 |
| AF | 4.95 (2.82-8.71) | <0.001 |
| RT3D Echocardiography | ||
| LAVimax | 1.52 (1.28-1.81) | <0.001 |
| LAVimin | 1.59 (1.40-1.81) | <0.001 |
| LAEV | 1.49 (1.16-1.91) | <0.001 |
| LAEF | 1.89 (1.54-2.31) | <0.001 |
| LAEI | 2.14 (1.61-2.84) | <0.001 |
| 2D Echocardiography | ||
| LV mass index | 1.03 (1.02-1.05) | <0.001 |
| LVEF | 0.97 (0.95-0.99) | 0.008 |
| LA diameter | 1.46 (1.19-1.79) | <0.001 |
| LAVImax | 1.49 (1.20-1.85) | <0.001 |
| Valvular disease | 2.60 (1.52-4.46) | <0.001 |
| E/e’ | 1.12 (1.06-1.19) | <0.001 |
HR for continuous variables are for 1 standard deviation. LAVimax: LA maximum volume. LAVimin: LA minimum volume. LAEV: LA emptying volume. LAEF: LA emptying fraction. LAEI: LA expansion index.
Figure 1. Prognostic value of abnormal LA volumes and reservoir function in the entire cohort.
Kaplan-Meier plots showing event-free survival associated with abnormal LAVimax (A), LAVimin (B), LAEV (C), LEAF (D), and LAEI (E). The log-rank test was significant for all parameters.
Table 4.
Prevalence and prognostic value of LA abnormalities in multivariate analysis (cut-offs derived from the reference group).
| Percentile | Cut-off* | Population at risk | HR† (95% CI) | P value | |
|---|---|---|---|---|---|
| 2D Echocardiography | |||||
| LA diameter | 97.5th | ≥2.6 cm/m2 | 11.3% | 0.91 (0.46-1.83) | 0.800 |
| 99th | ≥2.8 cm/m2 | 4.4% | 0.63 (0.24-1.68) | 0.356 | |
| LAVimax | 97.5th | ≥36.4 ml/m2 | 11.6% | 1.06 (0.53-2.09) | 0.875 |
| 99th | ≥40.4 ml/m2 | 6.4% | 0.86 (0.37-1.99) | 0.722 | |
| RT3D Echocardiography | |||||
| LAVimax | 97.5th | ≥33.1 ml/m2 | 12.5% | 1.35 (0.76-2.42) | 0.310 |
| 99th | ≥35.1 ml/m2 | 8.4% | 1.83 (0.98-3.40) | 0.058 | |
| LAVimin | 97.5th | ≥20.5 ml/m2 | 13.0% | 1.79 (1.02-3.16) | 0.044 |
| 99th | ≥22.0 ml/m2 | 10.5% | 1.77 (0.98-3.21) | 0.059 | |
| LAEV | 2.5th | ≤6.0 ml/m2 | 8.1% | 1.72 (0.94-3.17) | 0.080 |
| 1st | ≤5.7 ml/m2 | 6.2% | 1.98(1.02-3.85) | 0.043 | |
| LAEF | 2.5th | ≤31.1 % | 15.0% | 1.11 (0.63-1.96) | 0.727 |
| 1st | ≤29.2% | 11.8% | 1.24 (0.68-2.26) | 0.480 | |
| LAEI | 2.5th | ≤45.0 % | 14.9% | 1.12 (0.63-1.98) | 0.703 |
| 1st | ≤41.3 % | 11.8% | 1.24(0.68-2.26) | 0.480 | |
Cut-offs derived from the reference group. aHR: Adjusted hazard ratio. LAVimax: LA maximum volume. LAVimin: LA minimum volume. LAEV: LA emptying volume. LAEF: LA emptying fraction. LAEI: LA expansion index.
Covariates: Age, sex, hypertension, atrial fibrillation, significant valve disease, LV mass index, LVEF, E/e’.
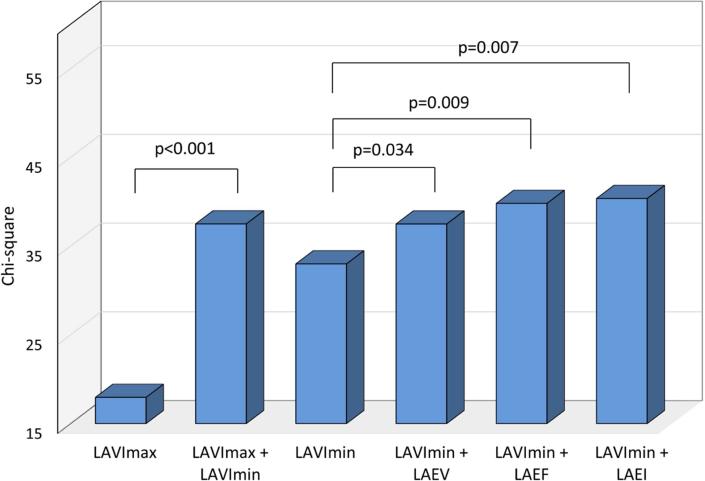
Incremental prognostic value of 3D LA phasic volumes
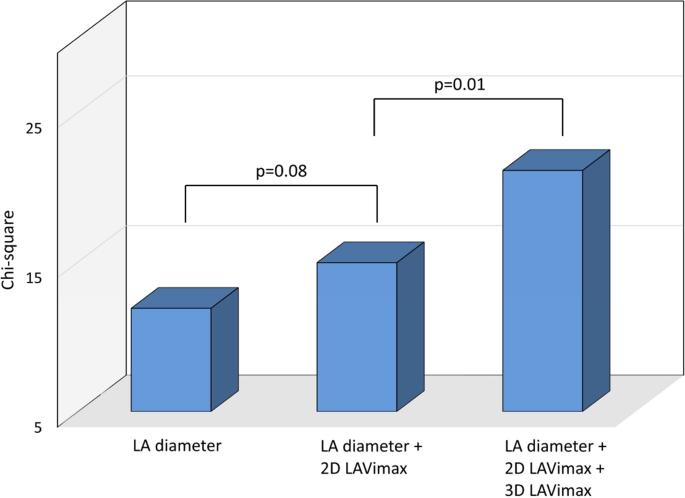
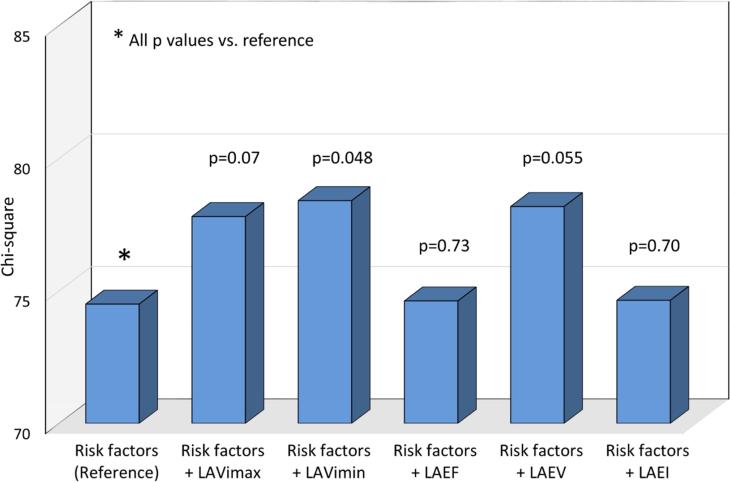
The incremental prognostic value of LA parameters was assessed in progressive nested Cox proportional hazards regressions, and chi-square values are shown for each step in Figure 2. Two-dimensional LAVimax was not significantly incremental to LA diameter in predicting events (p=0.08, Figure 2A), whereas 3D LAVimax was incremental when added to the model (p=0.01). Figure 2B shows that 3D LAVImin was incremental to LAVimax in predicting outcome (p<0.001), and that LA reservoir function added further incremental prognostic value over LAVImin (p=0.034 for LAEV, p=0.009 for LAEF, and p=0.007 for LAEI respectively). Figure 2C shows the incremental prognostic value of 3D LA parameters over risk factors. When added to a model including demographics and risk factors, LAVimin showed incremental prognostic value (p=0.048), whereas LAVimax did not (p=0.07). Among LA reservoir function parameters, LAEV showed a borderline trend in incrementing prognostic information over LAVimin (p=0.055).
Figure 2. Incremental prognostic value analysis.
In Figure 2A, 2D LAVimax was not incremental over the LA antero-posterior diameter, however 3D LAVimax was significantly incremental over 2D measurements in predicting outcome. Figure 2B shows the incremental prognostic value of 3D LAVImin and reservoir function over 3D LAVImax. 3D LAVImin showed significant incremental prognostic value over 3D LAVimax. LA reservoir function showed incremental prognostic value over LAVimin. In Figure 2C, 3D LAVimin, but not 3D LAVimax, showed incremental prognostic value over risk factors (age, sex, hypertension, atrial fibrillation, significant valve disease, LV mass index, LVEF, E/e’), while LAEV showed a borderline trend in its incremental prognostic value over risk factors + LAVimin.
DISCUSSION
In this study, we assessed the correlates and the prognostic value of LA phasic volumes and reservoir function assessed by RT3D echocardiography in an elderly community-based cohort. We found that increased LA phasic volumes and reduced reservoir function were strong predictors of cardiovascular events in univariate analysis. In multivariate analyses adjusted for confounders and risk factors associated with outcome, RT3D-derived LAVimin and LAEV remained associated with events. In multivariate analysis, 2-dimensional echocardiographic parameters (LA antero-posterior diameter and 2D LAVimax) lost their significant association with events. We also demonstrated that LAVImin and LA reservoir function provided incremental prognostic value over LAVImax. Furthermore, our study is the first to provide, and validate prognostically, reference values for abnormal RT3D echocardiography-derived LA volumes and reservoir function parameters in the elderly.
LA enlargement is an established predictor of cardiovascular events, and its powerful predictive value reflects the effect on LA pressure of several diseases or conditions that in turn carry poor prognosis (16). Some of these conditions, such as hypertension, diabetes, and arterial stiffening, result in LV hypertrophy and LV remodeling, leading to the development of LV diastolic dysfunction, LV wall stiffening, and increased LV filling pressure, which in turn are the cause of the LA enlargement over time (7,17-21). Higher BMI was also associated with lower LA reservoir function, likely due to the established effects of obesity on LV structure and function such as increased LV mass, and subclinical systolic and diastolic dysfunction (11,22). Although most data on the prognostic value of LA enlargement derive from end-systolic LA assessment, considerable evidence is accumulating in favor of LAVimin as a better correlate of LV diastolic dysfunction and outcome (6,7). LAVimin is measured at end-diastole, when the LA is directly exposed to the LV pressure, and when the LV is in a relaxed state. In a previous study, we demonstrated the LAVimin is a better correlate of LV diastolic dysfunction than LAVimax, and that the latter is strongly impacted by the longitudinal LV systolic function (7). While the prognostic value of LA enlargement has been investigated in previous studies, we are reporting for the first time on the prognostic value of LA reservoir function. A reduced LA reservoir function is associated with hypertension, LV hypertrophy, LV diastolic dysfunction, and other cardiovascular risk factors, and therefore could be a surrogate marker of cardiovascular risk (6,7,23). Because the LV longitudinal systolic function is a major determinant of the LA reservoir function (7), it can be hypothesized that a reduction in reservoir function might indicate LV longitudinal systolic dysfunction, a known predictor of cardiovascular events (24-26). Besides being possibly explained by the same underlying disease, such as small vessel disease and atherosclerotic changes in various arterial territories, the link between LA reservoir function and cardiovascular events could also involve cardioembolism as another possible cause in some cases. In fact, in a previous study we demonstrated that a reduced LA reservoir function is associated with silent brain infarctions and cerebral white matter disease detected by magnetic resonance imaging (27). Furthermore, LA reservoir function has been also associated with future incidence of atrial arrhythmias, which might in part mediate the association with cardiovascular events (5).
The prognostic value of LA enlargement has been investigated in several previous studies. Increased M-mode antero-posterior linear dimension has been linked to adverse cardiovascular outcome (1-3), although LA volume has generally shown better correlation with outcome than linear dimensions (4). Very few studies, however, assessed the prognostic value of LA phasic volumes so far, and no study so far investigated the prognostic value of LA reservoir function. Recently, Wu et al. found that, in Japanese patients referred to clinical echocardiography for assessment of underlying cardiac disease, LAVimax and LAVimin were not associated with cardiac death in multivariate analysis, but were associated with a composite of cardiovascular events including heart failure admissions during a ≈2.5 years follow-up (28). In another study in 178 patients referred for clinical echocardiography, Caselli et al. showed that LAVimax and LAVimin were associated with cardiovascular events with similar HRs (1.06 and 1.05 respectively) over a 45 months follow-up (29).
Measurement of LA phasic volumes is not currently part of a routine echocardiographic exam. However, the present and previous studies show that measuring LA phasic volumes can significantly improve cardiovascular risk stratification. RT3D echocardiography is gaining popularity in clinical setting, and modern software is becoming faster and more user-friendly. The feasibility of measuring LAVImin by RT3D echocardiography, together with the large body of evidence showing its superiority compared to LAVImax as an indicator of LA remodeling and its strong prognostic value, support the case for LAVimin to replace LAVimax for cardiovascular risk prognostication in routine echocardiographic exams.
Strengths and limitations
The main strengths of our study are the prospective design, the long follow-up, the large number of subjects studied with RT3D echocardiography, the wide range of cardiovascular risk profiles present in our population, the multi-ethnic composition of our study sample, and the confirmation of our findings after adjustment for multiple covariates. However, our study also has limitations. Our study population included subjects over 50 years of age and with high mean BMI and high prevalence of risk factors, therefore the results might not apply to younger, healthier populations. Furthermore, because of the imbalance in race-ethnic distribution in our cohort, an analysis in different race-ethnic groups was not performed. In 15% of the population, suboptimal image quality prevented 3D assessment of LA parameters. Although similar rates have been described in other studies, this is a common limitation of ultrasound assessment, especially in population-based studies. Finally, outcome analysis for separate type of events was not performed due low number of events in each category.
Conclusions
LA phasic volumes and reservoir function were strong predictors of future cardiovascular events in a predominantly elderly community-based cohort. The prognostic value of LA volumes and reservoir function was independent of confounders and cardiovascular risk factors. Among LA volume parameters, LAVimin was a stronger predictor of events than LAVimax, and was incremental to it. LA reservoir function was also incremental to LA volumes in the prediction of cardiovascular events. The assessment of LA phasic volumes by RT3D echocardiography may improve cardiovascular risk stratification in the elderly.
PERSPECTIVES.
Clinical competencies in medical knowledge
Assessment of the left atrial (LA) cavity volume is traditionally performed at the end-systolic phase of the cardiac cycle (LAVimax). Recent evidence suggest that LA end-diastolic volume (LAVimin) provides a better correlate of LA remodeling and is more closely correlated with LV diastolic dysfunction. In this study, we provided normal reference values for RT3D-derived LA volumes and reservoir function in subjects over 50 years of age. Furthermore, we provided prognostic validation of those reference values in a community setting, and demonstrated that LAVmin and LA reservoir function were stronger predictors of cardiovascular outcome and showed incremental prognostic value over LAVmax.
Clinical competencies in patient care and procedural skills
RT3D echocardiography is a feasible method that allows fast and precise measurement of LAVmax, LAVmin, and LA reservoir function without geometric assumptions. A widespread use of this technique will make 3D assessment of cardiac chambers a standard procedure with the advantage over 2D assessment of improving cardiovascular risk stratification.
Translational Outlook
The assessment of LA phasic volumes has the potential of improving cardiovascular risk stratification. LAVimin and LA reservoir function have shown superior ability than LAVimax to predict cardiovascular events and incidence of atrial fibrillation. We provide, for the first time, normal reference values for LA phasic volumes by RT3D echocardiography in subjects from a community cohort study. These reference values will contribute to an improved cardiovascular risk stratification in subjects from the general population.
Acknowledgments
The authors wish to thank Janet De Rosa, MPH (project manager), Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (collection and management of the data).
Grant support: This work was supported by the National Institute of Neurological Disorders and Stroke [grant number R01 NS36286 to MDT and R37 NS29993 to RLS/MSE].
Funding sources
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [grant numbers R01 NS36286 to M.D.T., and R37 NS29993 to R.L.S./M.S.V.E.]. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040.
ABBREVIATION LIST
- LA
Left atrial
- LAVimax
LA maximum volume
- LAVimin
LA minimum volume
- LAEV
LA emptying volume
- LAEF
LA emptying fraction
- LAEI
LA expansion index
- RT3D
Real-time three-dimensional
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, Janssen Pharmaceuticals and Sanofi-Regeneron; serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UpToDate for chapters related to cryptogenic stroke.
References
- 1.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 2.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 3.Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 5.Abhayaratna WP, Fatema K, Barnes ME, et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626–1629. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 6.Fatema K, Barnes ME, Bailey KR, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10:282–286. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 7.Russo C, Jin Z, Homma S, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–20. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco RL, Khatri M, Rundek T, et al. Improving Global Vascular Risk Prediction With Behavioral and Anthropometric Factors The Multiethnic NOMAS (Northern Manhattan Cohort Study). J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 11.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo C, Hahn RT, Jin Z, Homma S, Sacco RL, Di Tullio MR. Comparison of echocardiographic single-plane versus biplane method in the assessment of left atrial volume and validation by real time three-dimensional echocardiography. J Am Soc Echocardiogr. 2010;23:954–60. doi: 10.1016/j.echo.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HP, Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Greene HL, Richardson DW, Barker AH, et al. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study). Am J Cardiol. 1989;63:1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer EJ, Lamon-Fava S, Jenner JL, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 16.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–39. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 19.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 20.Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circulation Heart failure. 2013;6:944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo C, Sera F, Jin Z, et al. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail. 2016;18:537–44. doi: 10.1002/ejhf.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–23. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo C, Jin Z, Elkind MS, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo C, Jin Z, Sera F, et al. Left Ventricular Systolic Dysfunction by Longitudinal Strain Is an Independent Predictor of Incident Atrial Fibrillation: A Community-Based Cohort Study. Circ Cardiovasc Imaging. 2015;8:e003520. doi: 10.1161/CIRCIMAGING.115.003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ersboll M, Valeur N, Andersen MJ, et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc Imaging. 2013;6:851–860. doi: 10.1016/j.jcmg.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu VC, Takeuchi M, Kuwaki H, et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6:1025–1035. doi: 10.1016/j.jcmg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Caselli S, Canali E, Foschi ML, et al. Long-term prognostic significance of three-dimensional echocardiographic parameters of the left ventricle and left atrium. Eur J Echocardiogr. 2010;11:250–256. doi: 10.1093/ejechocard/jep198. [DOI] [PubMed] [Google Scholar]