Abstract
Purpose
To assess the clinical and pharmacodynamic activity of dovitinib in a treatment resistant, molecularly enriched NMIUC population.
Experimental Design
A multi-site pilot phase 2 trial was conducted. Key eligibility criteria included: BCG unresponsive NMIUC (≥ 2 prior intravesical regimens) with increased phosphorylated FGFR3 (pFGFR3) expression by centrally analyzed immunohistochemistry (IHC+) or FGFR3 mutations (Mut+) assessed in a CLIA-licensed laboratory. Patients received oral dovitinib 500 mg daily (5 days on / 2 days off). The primary endpoint was 6-month TURBT-confirmed complete response (CR) rate.
Results
Between 11/2013 and 10/2014, 13 patients enrolled (10 IHC+ Mut−, 3 IHC+ Mut+). Accrual ended prematurely due to cessation of dovitinib clinical development. Demographics included: median age 70 years; 85% male; CIS (3 pts), Ta/T1 (8 pts), and Ta/T1 + CIS (2 pts); median prior regimens 3. Toxicity was frequent with all patients experiencing at least one grade 3-4 event. 6-month CR rate was 8% (0% in IHC+ Mut−; 33% in IHC+ Mut+). The primary endpoint was not met. Pharmacodynamically active (94-5812 nM) dovitinib concentrations in urothelial tissue were observed in all evaluable patients. Reductions in pFGFR3 IHC staining were observed post-dovitinib treatment.
Conclusions
Dovitinib consistently achieved biologically active concentrations within the urothelium and demonstrated pharmacodynamic pFGFR3 inhibition. These results support systemic administration as a viable approach to clinical trials in NMIUC patients. Long-term dovitinib administration was not feasible due to frequent toxicity. Absent clinical activity suggests that patient selection by pFGFR3 IHC alone does not enrich for response to FGFR3 kinase inhibitors in UC.
Keywords: Urothelial carcinoma, non-muscle invasive, FGFR3 mutation, FGFR3 over-expression, dovitinib
Introduction
Urothelial carcinoma (UC) of the bladder is the fifth most common human cancer diagnosis. In 2016, over 76,000 individuals are expected to be diagnosed with UC, and more than 16,000 patients to die from their disease (1). Most new UC cases (~ 50,000 patients) are non-muscle invasive at diagnosis with disease limited to the mucosal epithelium (Ta/Tis) and immediate connective tissue layer beneath the mucosa (T1) (2). The clinical course of non-muscle invasive UC of the bladder (NMIUC) is dominated by frequent recurrences requiring surveillance (with cystoscopy, bladder biopsy, urine cytology, etc.). The need for long-term invasive monitoring and treatment has significant cost and morbidity for UC patients. Compared to other malignancies, UC ranks highest in lifetime per patient costs with an average cost from diagnosis to death of $96,500 per patient (3).
Standard therapy for high-risk NMIUC patients includes transurethral resection of bladder tumor (TURBT) augmented by intravesical administration of Bacillus Calmette-Guerin (BCG), an attenuated bovine mycoplasma derived agent. Two meta-analyses of randomized trials of TURBT plus BCG versus TURBT alone demonstrated a reduction in 12-month tumor recurrence rate from 56% to 29% (p<0.001) and a reduction in progression to muscle-invasive stages from 13.8% to 9.8% (p=0.001) in association with BCG therapy (4, 5). While BCG therapy is successful at preventing early tumor recurrences, most patients do not maintain sustained remissions. With 5-year follow-up, recurrent bladder tumors requiring repetitive TURBT and further cystoscopic surveillance are observed in 40-66% of patients (6, 7). For post-BCG tumor recurrences, BCG-unresponsive disease is defined by any of the following features: recurrent NMIUC after 2 prior adequate BCG regimens, recurrent T1 disease at the initial 3-month post-treatment TURBT, recurrent NMIUC within 6 months of last BCG administration, and NMIUC involving the prostatic urethra (8). Transient remissions are often observed with additional intravesical therapy approaches, however, only 10-15% of patients remain recurrence-free at 1 year (9, 10). Thus, cystectomy is considered a standard treatment in BCG-unresponsive patients (11). A need clearly exists to explore the clinical efficacy of novel agents in this high-risk NMIUC population.
Across multiple cancer types, the critical role of angiogenesis in tumor migration, proliferation, and metastasis is well established with vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) serving as key mediators (12, 13). In UC, associations between increased tumor VEGF expression and high-grade disease, advanced stage, and poor prognosis have been observed (14-16). Initial phase 2 trials in metastatic UC patients combining chemotherapy with the anti-VEGFR2 monoclonal antibody bevacizumab have demonstrated promising overall survival outcomes compared to historical controls with a definitive phase 3 trial of chemotherapy with or without bevacizumab completed and data maturing (17, 18).
In addition to VEGFR, fibroblast growth factor receptor-3 (FGFR3) has been implicated as a critical facilitator of UC carcinogenesis, particularly in NMIUC (19, 20). FGFR3 mutations or over-expression promote FGFR dimerization and constitutive activation of downstream signaling pathways in the absence of ligand in up to 80% of low-grade NMIUC tumors (21). These mutations result in a hyperplastic phenotype dominated by frequent tumor recurrences with infrequent progression to muscle-invasive stages. While FGFR3 mutations are highly associated with low-grade NMIUC, over-expression of FGFR3 has been observed in up to 42% of high-grade muscle-invasive UC tumors (22). Furthermore, either an FGFR3 mutation or over-expression of the FGFR3 protein in the absence of mutation has been observed in 54% of muscle-invasive UC tumors (22). Thus, while FGFR3 mutations likely are an early event in the tumorigenesis of low-grade non-invasive UC tumors, alterations of FGFR3 may still play a role in the continued proliferation of high-grade UC.
Dovitinib is an oral tyrosine kinase inhibitor of FGFR1-3, VEGFR1-3, PDGFRβ, c-Kit, RET, TrkA, CSF-1R, and FLT3 which has demonstrated a tolerable safety profile in single agent and combination regimens (23). Increasing evidence demonstrates that FGFR1 is a crucial mediator of tumor angiogenesis (24). In preclinical tumor models, blockade of the FGF pathway has proven to be an effective method of overcoming resistance to VEGFR inhibitors (25). Given the previously described importance of VEGF in UC progression and the frequent FGFR3 aberrations in NMIUC, we conducted a multi-site pilot trial in BCG-unresponsive NMIUC patients harboring FGFR3 gene alterations to evaluate the clinical and biological outcomes of oral dovitinib therapy.
Materials and Methods
Study Design
A single-arm, non-randomized, multi-center, phase 2 study (NCT01732107) was conducted between three sites: Indiana University Simon Cancer Center (Indianapolis, IN), Fox Chase Cancer Center (Philadelphia, PA), and Johns Hopkins Sidney Kimmel Comprehensive Cancer Center (Baltimore, MD). Standard of care and correlative biospecimens were collected pre- and post-treatment from all patients. The study was approved by the institutional review boards of each site.
Patients
Key eligibility criteria included: histologically confirmed Ta, T1, or Tis stage NMIUC assessed by TURBT performed within 42 days of registration; somatic tumor mutations in FGFR3 exons 7, 10, or 15 (S373C, G372C, Y375C, G382R, K652E, K652Q, K652T, K652M, A393E, S249C, and R248C) or tumor over-expression of phosphorylated (pFGFR3) by immunohistochemistry (IHC) defined as 1+ or greater tumor pFGFR3 staining; recurrent NMIUC despite at least 2 prior intravesical treatment regimens (no limit), one of which must have been BCG; patients medically unfit for or refusing cystectomy; age ≥ 18 years; ECOG performance status 0-2; adequate hematologic and liver function; creatinine clearance > 30 ml/min by modified Cockcroft-Gault equation; and documented, written informed consent. Major exclusion criteria included: evidence of muscle-invasive or metastatic disease on pre-study screening tests; concurrent upper tract urothelial carcinoma; prior VEGFR or FGFR-targeted therapy.
Treatment
Patients were treated with dovitinib 500 mg by oral administration once per day for five consecutive days followed by two days off each week. A cycle was defined as 4 weeks of therapy. No maximum number of treatment cycles was stipulated. Dose reductions to 400 mg and 300 mg were permitted in the event of treatment related toxicity. Due to drug-drug interactions, full dose anti-coagulation with warfarin was not allowed, however, use of low-molecular weight heparin at full-dose was permitted. Usage of anti-emetic and colony stimulating growth factor medications was at the discretion of the treating physician.
Disease Evaluations
At baseline, the absence of metastatic disease was confirmed by abdomen and pelvis CT scan and chest x-ray or CT scan. Adequate cardiac function was confirmed by echocardiogram and electrocardiogram assessments. History and physical exam findings, vital signs, baseline symptoms, and laboratory assessments were performed within 14 days of registration. Exams, vital signs, toxicity evaluations (per CTCAE v4.0), and laboratory assessments were performed bi-weekly for the first 2 cycles of treatment and every 4 weeks thereafter.
All patients were evaluated with urine cytology and cystoscopy every 3 months during the first year and per the treating physician’s discretion thereafter. TURBT’s were required at 3- and 6-months post-treatment with only for cause TURBT’s thereafter. At each TURBT, biopsy tissue was obtained from all previous and new tumor sites, the bladder dome, anterior bladder wall, left lateral bladder wall, right lateral bladder wall, and the bladder trigone. Patients with any NMIUC at the 6-month evaluation or beyond were considered relapses as were patients with CIS at the 3-month evaluation. Patients with papillary-only disease at the 3-month cystoscopy/TURBT who declined further dovitinib therapy were classified as relapsed. Testing of urine for evidence of relapse by fluorescent in-situ hybridization (FISH) was allowed, but not required. The same was true for the use of blue light cystoscopy. An isolated FISH positive urine finding was not classified as a relapse event. Complete response was defined as no evidence of any remaining urothelial carcinoma tumors of any T-stage (including Tis) as assessed by cystoscopic examination and urine cytology. In addition to these criteria, the 6-month complete response rate required no evidence of tumor within the 6-month post-treatment TURBT biopsies. The 1-year relapse free survival rate was defined as the proportion of patients treated with dovitinib with no evidence of any urothelial carcinoma at 12 months of follow up. Patients with any evidence of muscle-invasive tumors (T2 or above) or metastatic disease in follow up were considered as progressive disease.
At the time of all TURBTs or cystectomy, tumor samples were sent for standard of care diagnostic evaluation and complete pathologic staging information was recorded. Samples from the same blocks were cut and archived for correlative studies. Resolution of any treatment related toxicities were confirmed thirty days after administration of a patient’s last dovitinib dose. Patients were not followed for long-term overall survival outcomes.
FGFR3 Mutation Analysis
At baseline, five individual 5-micron thick slides were cut from the patient’s representative TURBT block with the highest grade tumor and greatest volume of tumor present. In slides with less than 40% tumor cells present, macro-dissection was performed to ensure maximum tumor cell DNA content. Also, at baseline, a 30 ml urine sample was obtained from all patients and centrifuged at 3500 rpm for 10 minutes. The resulting supernatant and cell pellet were transferred into separate cryovials and stored at −70°C until analyzed. Slides and urine cell pellets were shipped to the laboratory for Clinical Genomics and Advanced Technology (CGAT) at the Dartmouth Hitchcock Medical Center. Tumor and urine cell pellet DNA extraction was performed per manufacturer’s specification (Qiagen Puregene (tissue) and Qiagen DNeasy™ (cell pellet), Hilden, Germany). FGFR3 mutational status was determined using a custom designed SNaPshot assay (ThermoFisher Scientific, Waltham, MA) for all common mutations in FGFR3 coding exons including exons 7, 10, and 15. The presence or absence of specific FGFR3 mutations (S373C, G372C, Y375C, G382R, K652E, K652Q, K652T, K652M, A393E, S249C, and R248C) was communicated to HCRN within 14 days of specimen receipt.
Phosphorylated FGFR3 (pFGFR3) Immunohistochemistry (IHC) Analysis
Simultaneously at baseline, five individual 5-micron thick slides from the patient’s tumor and a single H&E slide from the same block were shipped to the IUSCC Immunohistochemistry Core Laboratory for FGFR3 IHC analysis. Slides were heated to 60°C for 15 minutes. Slides were deparaffinized and rehydrated sequentially with Xylene (5 minutes × 2), 100% ethyl alcohol solution (2 minutes × 2), and 95% ethyl alcohol solution (2 minutes × 2) on a Sakura linear stainer. Antigen retrieval utilized PT Link (PT10030, Dako, Carpineria, CA) in conjunction with EnVision™ FLEX High pH target retrieval solution (K8000, Dako, Carpineria, CA). Cycles began at 85°C and were heated to 100°C for 20 minutes followed by cooling back to 85°C and placement in wash buffer (K8002, Dako, Carpineria, CA). Baseline phosphorylated FGFR3 staining for trial eligibility evaluation was performed on a Dako Autostainer™ platform utilizing the sc-33041 anti-FGFR3 (phospho Y724) antibody (Santa Cruz Biotechnology, Dallas, TX). The sc-33041 pFGFR3 antibody was optimized to a 1:100 dilution for 30 minutes prior to the conduct of this trial utilizing 15 breast cancer cases as positive controls. Following pFGFR3 staining, slides were dehydrated sequentially with 95% ethyl alcohol solution (2 minutes × 1), 100% ethyl alcohol solutions (3 minutes × 2), and xylene (5 minutes × 2) followed by cover slipping. The immunostained slides were evaluated by two different pathologists. Areas within the tumor were scored as follows: 0= negative, 1+ = mild staining, 2+ = moderate staining, 3+ = strong staining. Both positive and negative controls were run in addition to the samples. Since a clinically relevant cutoff for pFGFR3 IHC intensity had not previously been established, tumors with any staining intensity (1+ or greater) were considered pFGFR3 over-expressing. During the conduct of the trial, improved commercial pFGFR3 antibodies became available. The correlative pre- and post-treatment pFGFR3 analyses were, therefore, performed utilizing the ab155960 anti-FGFR3 (phospho Y724) antibody (Abcam, Cambridge, MA). The ab155960 evaluation scheme was validated across 19 different individual cases of bladder cancer for antibody specificity. Triplicate runs of this validation scheme showed that low strainers (1+), moderate strainers (2+), and strong strainers (3+) were replicated across all runs. High, medium, and low staining positive controls were identified and used across all runs. All other antibody optimization procedures mirrored those of the sc-33041 antibody with the exception that the ab155960 antibody was optimized with to a 1:25 dilution for 40 minutes. The sc-33041 antibody continued to be utilized for eligibility determination throughout the entire conduct of the study. Aperio’s ScanScope® CS whole slide digital imaging system (Leica Biosystems, Buffalo Grove, IL) was used for baseline and post-treatment pFGFR3 pathology imaging. The system imaged all slides at 20x. The scan time ranged from 1 ½ minutes to a maximum time of 2 ¼ minutes. The whole images were housed and stored in their Spectrum software system and images were shot from the whole slides. Quantification of pFGFR3 staining was performed on the HALO™ image analysis platform (Indica Labs, Corrales, NM). An algorithm was designed based on pattern recognition that quantified tumor cells within pFGFR3 positive areas (tumor) and pFGFR3 negative areas (invasive margin). HALO’s classifier package performed image analysis based on RGB (red, green, blue) spectra which was used to detect cells positively expressing pFGFR3 against negative expressing counterstained hematoxylin cells. The algorithm calculated the classified area (mm2) and percentage of tumor expression (% positive cells / % of all nucleated cells) using the HALO™ classifier package. The total percent of positive expression in each group was averaged and a standard deviation was calculated. Further analysis was performed on three hotspots on each tissue via HALO’s™ area quantification package. An algorithm was designed to quantify positive pFGFR3 expressing tumor cells in weak, moderate, and high positivity values. An average of hotspots for the tissues collected at Day 1 was calculated along with a standard deviation. This data was compared to an average of hotspots of the tissues collected between cycle 3 days 26 and 30 for their total positivity, and according to weak, moderate, high, and total expression.
Dovitinib Pharmacokinetic Tissue Analysis
At the 3-month post-treatment disease assessment, a bladder biopsy of tumor or normal appearing urothelium was obtained for pharmacokinetic analysis to confirm achievement of biologically active dovitinib tissue concentrations via oral drug administration. The pharmacokinetic biopsy sample was flash frozen, stored in liquid nitrogen, and shipped to the IUSCC Clinical Pharmacology Analytical Core (CPAC) for analyses. Tissue samples were homogenized in phosphate buffered saline, internal standard (sorafenib) was added to each sample, the samples were extracted with ethyl acetate, and injected into a HPLC-MS/MS (API 4000; AB Sciex). Plasma was used for the matrix of the standard samples to estimate tissue concentrations. The lower limit of quantification was 8 ng/sample. For ease of comparison, tissue concentrations (ng/g) were converted to the nanomolar concentrations (assuming 1 g tissue is equivalent to 1 mL water).
Statistical Considerations
The primary endpoint of the trial was 6-month complete response (CR) rate. With a 6-month CR rate of clinical interest of ≥ 25%, a sample size of 20 patients provided an 80% power to exclude a lower bound of ≤ 10% utilizing a one-sided 90% confidence interval of Agresti-Couli type. With an estimated FGFR3 mutation or over-expression present in 40% of BCG-unresponsive tumors, screening of 50 patients’ tumors was estimated in order to enroll the required 20 patients on dovitinib therapy. An evaluation of early stopping was planned at the first 10 patients completing 3-month assessment for progression to T2 or greater stages, whose objective was to stop the study if the likelihood of progression rate was over 20%. A rule was chosen that the study should be terminated if 5 or more progressions were observed out of 10 patients, which is the minimal number that leads to a 90% Agresti-Coull confidence interval with a lower bound above 20%. Rates of complete response, progressive disease, and treatment-related toxicity were summarized by 95% confidence intervals. Associations between pre- and post-treatment pFGFR3 IHC staining intensity were compared by paired t-testing with significance set at a p-value of < 0.05.
Results
Patients
Between November 2013 and October 2014, 17 patients were screened and 13 patients were enrolled. Fifteen patients (88%) had sufficient tumor tissue for FGFR3 mutation testing. Two patients with tumors demonstrating no FGFR3 mutations were considered screen-failures after the study amendment capping the enrollment of FGFR3 mutation negative patients was in place. Further accrual was stopped due to cessation of clinical development of dovitinib. Patient demographics are summarized in Table 1 and included: median age 70 years (range 57-78 years), 85% male, and 85% caucasian. Baseline TURBT tumor stages were: CIS – 3 patients, Ta or T1 – 8 patients, and Ta or T1 with concurrent CiS – 2 patients. Patients had received a median of 3 prior intravesical regimens (range 2-6) with all patients having received at least 2 prior BCG induction courses. The median time from last intravesical therapy was 6 months (range 1-33). Tumor FGFR3 mutations were detected in 3 patients (18% of screened patients) with a concordant urine FGFR3 mutation detected in 1 of the 3 patients.
Table 1.
Baseline Patient and Tumor Characteristics
| Patient | Gender | Age (years) | Race | T-stage | Prior Regimens | Time from Last Therapy (months) |
Tumor FGFR3 Mutation |
Urine FGFR3 Mutation |
pFGFR3 IHC Intensity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 77 | C | T1 + CIS | BCG × 2, Gem | 58.3 | G382R | None | 3+ |
| 2 | M | 67 | C | CIS | BCG × 2, MMC | 8.3 | None | None | 3+ |
| 3 | M | 71 | C | Ta | BCG × 2 | 6.3 | None | None | 3+ |
| 4 | M | 64 | C | Ta | BCG × 2, MMC | 23.7 | None | None | 3+ |
| 5 | M | 75 | C | Ta | BCG × 4, MMC, Val | 5.6 | S249C | S249C | 3+ |
| 6 | M | 70 | AA | T1 | BCG × 4, MMC | 6.2 | None | None | 3+ |
| 7 | M | 69 | U | CIS | BCG × 2, Val | 3.7 | None | None | 3+ |
| 8 | M | 78 | C | Ta | BCG × 2 | 5.7 | None | None | 3+ |
| 9 | M | 57 | C | Ta | BCG × 2, MMC | 1.4 | None | None | 3+ |
| 10 | M | 71 | C | T1 | BCG × 2 | 3.7 | None | None | 3+ |
| 11 | F | 67 | C | CIS | BCG × 2, MMC, Val | 15 | None | None | 3+ |
| 12 | M | 77 | C | T1 + CIS | BCG × 3, Val | 24.2 | None | None | 3+ |
| 13 | M | 57 | C | Ta | BCG × 2 | 33.1 | S249C | NE | 2+ |
(pFGFR3 = Phosphorylated FGFR3, IHC = Immunohistochemistry, F = Female, M = Male, C = Caucasian, AA = African-American, U = Unknown, Gem = Gemcitabine, MMC = Mitomycin C, Val = Valrubicin, NE = Not evaluable)
Dovitinib Treatment
Patients received a median of 4 cycles of dovitinib treatment (range 1 – 19). Ten patients (77%) required dovitinib dose reductions. Two patients (15%) discontinued dovitinib treatment prematurely and did not undergo planned 3-month post-treatment disease evaluations. Reasons for discontinuation included: physician discretion discontinuation of treatment due to a traumatic intracranial hemorrhage sustained in a ground-level fall unrelated to study treatment (1 patient) and patient choice to withdraw from study (1 patient). Additionally, dovitinib therapy was discontinued per treating physician’s discretion in a single patient after 19 cycles after the patient revealed a prior history of retinal detachment unknown to the treating team at study enrollment.
Toxicity
Dovitinib therapy was associated with frequent toxicity. All 13 patients (100%) experienced at least 1 grade 3 or 4 event. Treatment related grade 4 hypertriglyceridemia was observed in 1 patient (8%). Treatment related grade 3 events included fatigue, elevated GGT, and elevated lipase in 2 patients (15%) each as well as headache, hypertriglyceridemia, stomatitis, and rash in 1 patient (8%) each. One patient (8%) suffered a subdural intracranial hemorrhage that did not require operative intervention in association with a ground level fall on an ice-covered winter sidewalk that was not deemed treatment related. All grade 3-4 events and other toxicities occurring in over 20% of patients are summarized in Table 2. Complete all grade toxicity is included in Supplementary Table S1.
Table 2.
All Grade 3-4 AEs and Other AEs Occurring in > 20% of Patients
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Constitutional | Fatigue | 5 (39%) | 4 (31%) | 2 (15%) | 0 (0%) |
| Pain | 6 (46%) | 6 (46%) | 0 (0%) | 0 (0% | |
| Fall | 0 (0%) | 0 (0% | 1 (8%) | 0 (0%) | |
| Other Constitutional | 3 (23%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Vascular | Hypertension | 0 (0%) | 2 (15%) | 2 (15%) | 0 (0%) |
| Headache | 5 (39%) | 1 (8%) | 1 (8%) | 0 (0%) | |
| Intracranial Hemorrhage | 0 (0%) | 0 (0% | 1 (8%) | 0 (0%) | |
| Gastrointestinal | GERD | 2 (15%) | 3 (23%) | 1 (8%) | 0 (0%) |
| Constipation | 2 (15%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Diarrhea | 8 (62%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Anorexia | 4 (31%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Weight Loss | 4 (31%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Dysgeusia | 5 (39%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Nausea / Emesis | 6 (46%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Emesis | 4 (31%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other GI | 2 (15%) | 3 (23%) | 0 (0%) | 0 (0%) | |
| Skin | Stomatitis | 0 (0%) | 0 (0%) | 1 (8%) | 0 (0%) |
| Rash | 4 (31%) | 1 (8%) | 1 (8%) | 0 (0%) | |
| Hand Foot Syndrome | 2 (15%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Dry Mouth | 4 (31%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other Skin | 6 (46%) | 2 (15%) | 0 (0%) | 0 (0%) | |
| Genitourinary | Bladder Spasms | 0 (0%) | 3 (23%) | 0 (0%) | 0 (0%) |
| Other Urinary | 7 (54%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Infection | Fever | 4 (31%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Infection | 0 (0%) | 8 (62%) | 0 (0%) | 0 (0%) | |
| Pulmonary | Hoarseness | 3 (23%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other Pulmonary | 4 (31%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Musculoskeletal | Arthralgia / Myalgia | 4 (31%) | 2 (15%) | 0 (0%) | 0 (0%) |
| Metabolic | Hypertriglyceridemia | 1 (8%) | 2 (15%) | 1 (8%) | 1 (8%) |
| Elevated Alkaline Phosphatase | 2 (15%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Elevated GGT | 0 (0%) | 1 (8%) | 2 (15%) | 0 (0%) | |
| Hypoalbuminemia | 2 (15%) | 1 (8%) | 0 (0%) | 0 (0%) | |
| Elevated Lipase | 0 (0%) | 0 (0%) | 2 (15%) | 0 (0%) | |
| Other Metabolic | 6 (46%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hematologic | Anemia | 4 (31%) | 0 (0%) | 0 (0%) | 0 (0%) |
(AE = Adverse Event)
Tumor Response
Anti-tumor responses to dovitinib treatment were infrequent. Of the 13 patients enrolled, a pathologic complete response was observed in 1 patient (8%). Non-response was observed in 11 patients (85%) and progression to muscle-invasive stage occurred in 1 patient (8%). The single complete response patient did harbor an FGFR3 S249C mutation. Thus, the pathologic complete response rate amongst FGFR3 mut+ patients was 33% (1 of 3) as summarized in Table 3. The patient remains in a complete response at 19+ months of follow up. Eight patients (62%) underwent cystectomy per the discretion of their physician at any time point following completion of study therapy with a wide variety of pathologic stages ranging from pT0N0 to pN+ disease (Supplementary Table S2).
Table 3.
Tumor Response to Dovitinib Treatment
| Patient | Baseline T-stage | Tumor FGFR3 Mutations | Duration of Treatment (months) |
Post-Treatment T-stage |
Response Category |
|---|---|---|---|---|---|
| 1 | T1 + CIS | G382R | 0.8 | NE | NR |
| 2 | CIS | None | 2.7 | CIS | NR |
| 3 | Ta | None | 4.4 | Ta | NR |
| 4 | Ta | None | 2.8 | Ta | NR |
| 5 | Ta | S249C | 0.7 | NE | NR |
| 6 | T1 | None | 2.7 | T1 | NR |
| 7 | CIS | None | 5.3 | CIS | NR |
| 8 | Ta | None | 3.3 | T1 | NR |
| 9 | Ta | None | 3 | Ta | NR |
| 10 | T1 | None | 2.7 | T2 | PD |
| 11 | CIS | None | 4.4 | CIS | NR |
| 12 | T1 + CIS | None | 3.2 | T1 | NR |
| 13 | Ta | S249C | 17.5 | T0 | CR |
(NE = not evaluable, NR = non-responder, PD = progressive disease, CR = complete response)
Dovitinib Pharmacokinetic Tissue Analysis
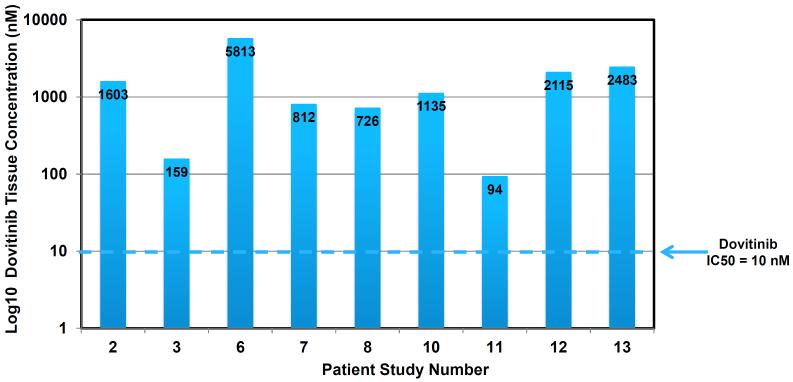
Fresh tumor or adjacent normal urothelium biopsy tissue was available for dovitinib pharmacokinetic analysis from 9 of the 11 patients who underwent post-treatment disease evaluations. As shown in Figure 1, dovitinib was detectable at pharmacologically active levels in all patients examined with tissue concentrations ranging from 94 – 5,813 nM.
Figure 1.
Post-Treatment Dovitinib Tissue Concentration
Immunohistochemical Analysis of Dovitinib Treatment on pFGFR3
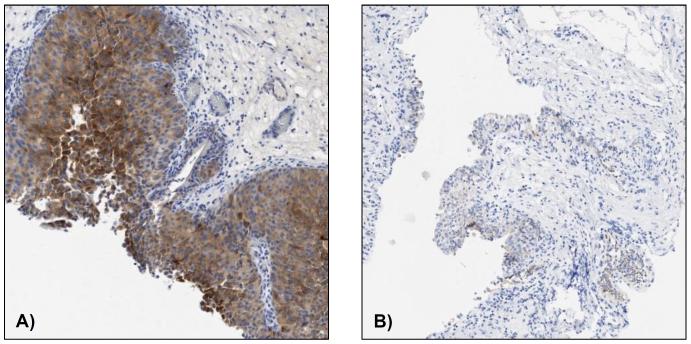
All baseline slides for eligibility determination demonstrated positive pFGFR3 staining as assessed by the sc-33041 pFGFR3 antibody. Staining intensities according to the use of the sc-33041 and ab155960 pFGFR3 antibodies showed significant heterogeneity (Supplemental Table S3). Pre- and post-dovitinib treatment slides were available from 9 patients including 8 tumor pairs. Utilizing the quantitative Halo Classifier imaging platform, reductions in averaged pFGFR3 staining area from 41.2 mm2 to 31.3 mm2 were observed following dovitinib treatment. This post-treatment reduction in mean pFGFR3 staining area showed a strong trend, but did not reach statistical significance (p=0.08). Marked reductions in pFGFR3 staining were observed in 4 of 9 patients, of which one reduction is demonstrated in Figure 3.
Figure 3. Dovitinib pFGFR3 IHC Pathology Samples. A) Patient 3 – Baseline. B) Patient 3 – Post-treatment cycle 3 day 26.
(pFGFR3 = Phosphorylated FGFR3, IHC = Immunohistochemistry)
Discussion
Until the recent FDA approval of the immunotherapy agent atezolizumab in metastatic urothelial carcinoma patients, nearly a quarter century had passed without any significant advances in systemic therapy for urothelial carcinoma (26). While the approval of atezolizumab is encouraging, it is important to note that only a small subset of patients derive benefit. Thus, additional novel approaches to treat UC are clearly needed. In particular, innovative strategies for the two-thirds of UC patients initially presenting with NMIUC are paramount. Given the established relevance of VEGFR in UC cancer invasion and metastases and the striking frequency of FGFR3 aberrations in low-grade NMIUC, we postulated that an FGFR3/VEGFR2 directed approach with dovitinib would prove both feasible and beneficial in BCG-unresponsive NMIUC patients with tumors harboring FGFR3 alterations.
Our study failed to demonstrate significant clinical activity with dovitinib therapy in the enrolled study population. Limitations in the enrollment criteria for the study population likely played a major factor in the absent anti-tumor activity observed. At the time the study was designed, the relative importance of FGFR3 mutations vs. gene fusions vs. over-expression was unknown. Furthermore, clinically relevant cutoffs for pFGFR3 IHC staining had not been established and available commercial pFGFR3 antibodies were limited. Therefore, even though robust methodology was developed prior to study initiation to optimize pFGFR3 antibody procedures, our trial allowed patients with any degree of pFGFR3 IHC staining at baseline to enroll. This allowed for an early influx of IHC+ Mut− patients. As demonstrated by the frequent heterogeneity that was observed in baseline IHC intensities according to the pFGFR3 antibody utilized, this likely resulted in a less biologically enriched population than intended. An amendment to cap the number of IHC+ Mut− patient enrollment at 10 patients was instituted, however, the trial was closed after enrolling only 3 Mut+ patients. In recent trial reports of other FGFR3 inhibitors (JNJ-42756493, BGJ398, AZD4547) in metastatic UC patients, it now appears clear that activating FGFR3 mutations or fusions are required for tumor responses (27-29). In trials of these agents mandating either FGFR3 mutation or fusions, reduction of tumor size was observed in 50-60% of metastatic UC patients (27, 29). Interestingly, in a prior report of dovitinib in metastatic UC patients, no responses were observed amongst 12 patients with FGFR3 mutations (30). It is not clear whether differences in FGFR3 mutation testing methodology or individual drug FGFR3 binding site properties explain discordant clinical activity. An observed complete response 1 of the 3 Mut+ patients treated with dovitinib in our trial is consistent with the more recent FGFR3 inhibitor results. With only 3 Mut+ patients enrolled, it is impossible for our study to provide any meaningful confidence intervals around the true complete response rate. However, it is encouraging that the single complete response patient has demonstrated a sustained remission out to 19+ months. Furthermore, a strong trend in decreased post-treatment pFGFR3 staining was observed regardless of FGFR3 mutation status.
In addition to patient selection limitations, the high rate of treatment related toxicity led to frequent dose reductions including 2 of the 3 FGFR3 Mut+ patients discontinuing dovitinib early. These dose modifications led to reduced dovitinib dose intensity in most patients and may have compromised anti-tumor effects. For future trials, particularly in the NMIUC population, our study provides a good example of the need to have a drug that is not only effective but tolerable at therapeutic doses to impart true benefit. Specifically, as in the case of dovitinib, the acceptance of relatively high rates of chronic toxicity in heavily pretreated metastatic solid tumor phase I trials may be greater than in NMIUC, given that NMIUC can be cured with cystectomy (31). In future design of NMIUC trials, particular attention to high rates of acute or chronic grade 1-2 toxicities is warranted particularly if a drug will require chronic or lifelong administration to prevent tumor recurrence. In addition, perioperative complication rates from patients who proceed to post-treatment cystectomies are of critical importance in NMIUC trials, particularly when agents with known effects on bleeding and wound-healing such as FGFR or VEGFR inhibitors are studied. While no life-threatening perioperative complications were observed in our trial, our sample size is insufficient to discount the possibility of such risks.
In spite of the absent clinical activity, our study establishes several innovative principles in the design of NMIUC trials that should facilitate improved future clinical trial designs in this population. First, our study demonstrated the feasibility of tumor genomic testing as an eligibility requirement in the NMIUC population in a multi-site setting. In fact, of the 17 patients screened, 15 (88%) had sufficient tumor available for FGFR3 mutation testing. While investigation of oral kinase inhibitors in NMIUC patients has been pursued by other investigators, to our knowledge, our trial is the first to be undertaken in a molecularly enriched NMIUC population (32). With the establishment of intrinsic basal and luminal tumor subtypes from analysis of The Cancer Genome Atlas (TCGA) UC samples, we expect an increased need for future UC clinical trials to target specific genomically-defined patient subsets (33). Our study demonstrates that, despite the small tumor samples obtained from standard of care TURBT specimens, enrichment of NMIUC patient subsets based on molecular testing is possible and should be pursued if scientific hypotheses warrant it.
In addition, our results establish the frequency of FGFR3 mutations in the BCG-unresponsive NMIUC population at 18% (3 of 17 patients), a previously unknown benchmark. Our a priori design assumption that the FGFR3 mutation rate in BCG-unresponsive NMIUC patients would fall somewhere between the reported rates in low-grade NMIUC (65%) and muscle-invasive UC (15%) patients proved incorrect. Our results suggest that BCG-unresponsive NMIUC more closely resembles muscle-invasive and metastatic UC than a low-grade NMIUC predecessor tumor. The lower rate of FGFR3 mutations observed in the BCG-unresponsive NMIUC population has implications on future sample size considerations of FGFR3 targeting trials in this population.
Importantly, our trial showed that oral administration of dovitinib unanimously achieved pharmacologically active urothelial tissue concentrations. This finding suggests that lack of clinical activity was related to drug toxicity and study population design issues rather than drug delivery failure. These results support further investigation of systemically administered agents in the NMIUC population. A caveat, however, is the fact that the urothelial tissue bioavailability is not usually investigated or provided in pre-clinical testing data provided in investigator brochures of most novel cancer drugs. A high intact urinary excretion of drug can be reassuring of adequate urothelial tumor drug concentration exposure. However, if urothelial tissue concentrations are critical in the decision process to assess the effectiveness of systemic versus intravesical routes of drug administration, development of clinical pharmacology assays to measure urothelial tissue drug concentrations are strongly recommended.
Lastly, our study demonstrates the importance of multi-specialty investigator engagement in the conduct of early stage UC trials. At each participating center, a urologist, medical oncologist, and pathologist were identified to serve as local champions for the trial. While multi-disciplinary teams in varying forms are often utilized in the administration of neoadjuvant cisplatin-based chemotherapy for muscle-invasive UC, our study highlights the importance of also developing highly functional cross-discipline research collaborations. The need for UC multi-specialty research infrastructure is increasing in parallel with the rapid expansion of clinical trials being conducted in the muscle-invasive adjuvant, neoadjuvant, and BCG-unresponsive NMIUC populations.
In summary, our study firmly establishes that pFGFR3 IHC alone should not be used as a solitary qualifying criteria for enrollment in future UC trials of FGFR3 kinase inhibitors. In addition, the unfavorable toxicity profile of dovitinib precludes further development in the NMIUC population. However, anti-tumor activity consistent with other reports in FGFR3 Mut+ patients was observed further implying FGFR3 as a viable therapeutic target in UC across all stages including NMIUC. The demonstration that genomic testing as an eligibility requirement in NMIUC patients is feasible and the detection of pharmacologically active dovitinib urothelial tissue concentrations by oral drug administration are novel findings with implications for future NMIUC trial designs.
Supplementary Material
Figure 2. Pre- and Post-Dovitinib pFGFR3 IHC Results.
(pFGFR3 = Phosphorylated FGFR3, IHC = Immunohistochemistry)
Translational Relevance.
This trial reports the toxicity, pharmacodynamics, and clinical efficacy profiles of the oral FGFR1-3 and VEGFR1-3 multi-targeted tyrosine kinase inhibitor, dovitinib, in a pilot phase II investigation in patients with BCG-unresponsive non-muscle invasive urothelial carcinoma of the bladder (NMIUC) with tumors harboring FGFR3 alterations. In addition to demonstrating reductions in post-treatment pFGFR3, confirmed biologically active dovitinib concentrations were observed in the bladder urothelium. This trial is the first NMIUC study to require genomic testing as an eligibility requirement and to demonstrate successful achievement of therapeutic urothelial tissue concentrations of systemically administered targeted therapies. Thus, it greatly expands the potential therapeutic approaches to treat this high-risk population. Lack of clinical efficacy was hampered by frequent drug toxicity and a paucity of patients harboring FGFR3 mutations. Additional FGFR3 targeting approaches in molecularly enriched urothelial carcinoma populations are ongoing and clearly worthy of further study, including in NMIUC patients.
Acknowledgments
Supported by grant funding from investigator-initiated funds from Novartis (Basel, Switzerland) and NCI Cancer Center support grant funding (P30CA006973)
Footnotes
Presented in part as a poster at the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL
Study ClinicalTrials.gov Identifier: NCT01732107
Conflicts of Interest: Noah Hahn discloses consulting compensation from Bristol Myers-Squibb, Merck, OncoGeneX, AstraZeneca / MedImmune, Genentech / Roche, Pieris Pharmaceuticals, and Inovio Pharmaceuticals; research support to the institution from Novartis, Bristol Myers-Squibb, Merck, OncoGenex, Mirati Pharmaceuticals, AstraZeneca / MedImmune, Genentech / Roche, and Acerta Pharma. Daniel Geynisman discloses consulting compensation from Pfizer; research support to the institution from Millennium Pharmaceuticals, Genentech/Roche, Merck, and Pfizer
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ries L, Harkins D, Krapcho M, Mariotto A, Miller B, Feuer E, et al. SEER Cancer Statistics Review, 1975-2003. National Cancer Institute; 2003. [Google Scholar]
- 3.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 4.Shelley MD, Court JB, Kynaston H, Wilt TJ, Fish RG, Mason M. Intravesical Bacillus Calmette-Guerin in Ta and T1 Bladder Cancer. Cochrane Database of Systematic Reviews. 2000 doi: 10.1002/14651858.CD001986. CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 6.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. MAINTENANCE BACILLUS CALMETTE-GUERIN IMMUNOTHERAPY FOR RECURRENT TA, T1 AND CARCINOMA IN SITU TRANSITIONAL CELL CARCINOMA OF THE BLADDER: A RANDOMIZED SOUTHWEST ONCOLOGY GROUP STUDY. The Journal of Urology. 2000;163:1124–9. [PubMed] [Google Scholar]
- 7.Herr HW. How to Manage Patients who Fail Intravesical BCG Therapy. 2009 Genitourinary Cancers Symposium; Orlando, FL. 2009 February 27; 2009. 2009. [Google Scholar]
- 8.Lerner SP, Dinney C, Kamat A, Bivalacqua TJ, Nielsen M, O'Donnell M, et al. Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bladder cancer. 2015;1:29–30. doi: 10.3233/BLC-159002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalbagni G, Russo P, Bochner B, Ben-Porat L, Sheinfeld J, Sogani P, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin-refractory transitional cell carcinoma of the bladder. Journal of Clinical Oncology. 2006;24:2729–34. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group.[Erratum appears in J Urol. 2008 Jan;179(1):386] Journal of Urology. 2000;163:761–7. [PubMed] [Google Scholar]
- 11.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. European urology. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 13.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature Reviews. 2002;2:727–39. doi: 10.1038/nrc905. Cancer. [DOI] [PubMed] [Google Scholar]
- 14.Fauconnet S, Bernardini S, Lascombe I, Boiteux G, Clairotte A, Monnien F, et al. Expression analysis of VEGF-A and VEGF-B: relationship with clinicopathological parameters in bladder cancer. Oncology Reports. 2009;21:1495–504. doi: 10.3892/or_00000380. [DOI] [PubMed] [Google Scholar]
- 15.Swellam M, El-Aal AAA. Correlation between Tissue and Released VEGF Levels in Urine of Bladder Cancer Patients. American Journal of Biochemistry and Biotechnology. 2005;1:37–42. [Google Scholar]
- 16.Yang C-C, Chu K-C, Yeh W-M. The expression of vascular endothelial growth factor in transitional cell carcinoma of urinary bladder is correlated with cancer progression. Urologic Oncology. 2004;22:1–6. doi: 10.1016/S1078-1439(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 17.Balar AV, Apolo AB, Ostrovnaya I, Mironov S, Iasonos A, Trout A, et al. Phase II Study of Gemcitabine, Carboplatin, and Bevacizumab in Patients With Advanced Unresectable or Metastatic Urothelial Cancer. Journal of Clinical Oncology. 2013;31:724–30. doi: 10.1200/JCO.2012.42.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn NM, Stadler WM, Zon R, Waterhouse DM, Picus J, Nattam SR, et al. Mature results from Hoosier Oncology Group GU04-75 phase II trial of cisplatin (C), gemcitabine (G), and bevacizumab (B) as first-line chemotherapy for metastatic urothelial carcinoma (UC). 2010 ASCO Annual Meeting; Chicago, Illinois. 2010; 2010. [Google Scholar]
- 19.Mitra AP, Datar RH, Cote RJ. Molecular Pathways in Invasive Bladder Cancer: New Insights Into Mechanisms, Progression, and Target Identification. J Clin Oncol. 2006;24:5552–64. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 20.Wu X-R. Urothelial Tumorigenesis: A Tale of Divergent Pathways. Nature Reviews Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 21.Knowles MA. Novel therapeutic targets in bladder cancer: mutation and expression of FGF receptors.(fibroblast growth factor)(Report) Future Oncology. 2008;4:71. doi: 10.2217/14796694.4.1.71. (13) [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. Journal of Pathology. 2007;213:91–8. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porta C, Giglione P, Liguigli W, Paglino C. Dovitinib (CHIR258, TKI258): structure, development and preclinical and clinical activity. Future oncology (London, England) 2015;11:39–50. doi: 10.2217/fon.14.208. [DOI] [PubMed] [Google Scholar]
- 24.Powers C, McLeskey S, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 25.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal SK, Rosenberg JE, Keam B, Wolf J, Berger R, Dittrich C, et al. Efficacy of BGJ398, a fibroblast growth factor receptor (FGFR) 1-3 inhibitor, in patients (pts) with previously treated advanced/metastatic urothelial carcinoma (mUC) with FGFR3 alterations. J Clin Oncol. 2016;34 doi: 10.1158/2159-8290.CD-18-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Vida A, Saggese M, Hughes S, Rudman S, Chowdhury S, Smith NR, et al. Complexity of FGFR signalling in metastatic urothelial cancer. Journal of Hematology & Oncology. 2015;8:119. doi: 10.1186/s13045-015-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan–Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- 30.Milowsky MI, Dittrich C, Durán I, Jagdev S, Millard FE, Sweeney CJ, et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. European Journal of Cancer. 2014;50:3145–52. doi: 10.1016/j.ejca.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Schäfer N, Gielen GH, Kebir S, Wieland A, Till A, Mack F, et al. Phase I trial of dovitinib (TKI258) in recurrent glioblastoma. Journal of Cancer Research and Clinical Oncology. 2016;142:1581–9. doi: 10.1007/s00432-016-2161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helfand AM, Lee CT, Hafez K, Hussain M, Liebert M, Daignault S, et al. Phase II clinical trial of intravesical bacillus Calmette-Guerin (BCG) followed by sunitinib for the treatment of high-risk nonmuscle-invasive bladder cancer (NMIBC) J Clin Oncol. 2015;33 [Google Scholar]
- 33.The Cancer Genome Atlas Research N Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.