Abstract
Natural killer (NK) cells are traditionally considered as innate cells but recent studies suggest that NK cells can distinguish antigens, and that memory NK cells expand and protect against viral pathogens. Limited information is available about the mechanisms involved in memory-like NK cell expansion, and their role in bacterial infections and vaccine-induced protective immune responses. In the current study, using a mouse model of tuberculosis (TB) infection, we found that IFN-γ producing CD3-NKp46+CD27+KLRG1+ memory-like NK cells develop during Bacille Calmette-Guerin (BCG) vaccination, expand and provide protection against challenge with Mycobacterium tuberculosis (M. tb). Using antibodies, siRNA and gene-deleted mice, we found that expansion of memory-like NK cells depends on IL-21. NKp46+CD27+KLRG1+ NK cells expanded in healthy individuals with latent TB infection (LTBI) in IL-21 dependent fashion. Our study provides first evidence that memory-like NK cells survive long term, expansion depends on IL-21 and involved in vaccine induced protective immunity against a bacterial pathogen.
INTRODUCTION
Mycobacterium tuberculosis (M. tb) causes almost 1.3 million deaths yearly 1. Of the one-third of household contacts of tuberculosis (TB) patients that develop latent tuberculosis infection (LTBI), most remain healthy, but 10% may develop TB 2. Control of M. tb infection requires cooperation of the innate and adaptive immune systems. Several studies demonstrated the crucial role of T-cells in protective immunity against M. tb 3, mediated in part through production of IFN-γ, which is required for resistance to infection 4. Limited information is available about the role of innate immunity in M. tb infection. Harnessing these innate immune mechanisms is critical to combat the global surge in multidrug-resistant TB, which responds suboptimal to treatment, despite lengthy expensive and toxic regimens.
NK cells are prominent components of the innate immune system that play a central role in resistance to microbial pathogens. NK cells protect against viruses, bacteria, and parasites through destruction of infected cells and by secretion of cytokines that shape the adaptive immune response 5. We found that human NK cells lyse M. tb-infected monocytes and alveolar macrophages, and upregulate CD8+ T-cell responses 6,7. NK cells lyse M. tb-expanded T regulatory cells (Tregs) 8, and eliminating NK cells at the time of Bacille Calmette-Guerin (BCG) vaccination enhances expansion of Tregs and inhibits BCG-induced protection against challenge with M. tb 9. Human NK cells also produce IFN-γ when exposed to BCG 10, and the pleural fluid of TB patients is enriched for CD56brightCD16- NK cells, which are the predominant source of IFN-γ 11.
Recent studies have found antigen-specific memory NK cells 12, homeostatic proliferation of long-lived NK cells 13, and expansion of a unique CD57+NKG2Chi NK cell subset and KLRG1+ memory-like NK cells in viral infections14. Further studies suggest that pro-inflammatory cytokine signaling is required for the generation of NK cell memory 15. In the current study, using a mouse model and cells from persons infected with M. tb, we identified memory-like NK cells and the factors that regulate their expansion. We found that memory-like NK cells contribute to vaccine-induced protective immune responses against M. tb infection and IL-21 mediates development and expansion of memory-like NK cells.
RESULTS
Expansion of memory-like NK cells in BCG-vaccinated mice
To determine if memory-like NK cells expand after vaccination with mycobacteria, we treated wild type C57BL/6 mice with PBS or vaccinated subcutaneously with 106 CFU of BCG. One month after vaccination, spleen and peripheral lymph node cells were isolated, pooled, and cultured, with or without Ag85 or γ-irradiated M. tb H37Rv (γ-M. tb). After 5 days, we determined expansion of CD3-NKp46+ NK cells expressing DNAM1, CD27, NKG2D, KLRG1, and CD62L. In BCG-vaccinated mice, among all the markers tested, only CD3-NKp46+CD27+ cells expanded from 1444 ± 271.9 to 31050 ± 4005 cells per 106 pooled cells (p=0.001, Supplementary Figure. 1A). The above experiment was performed one month after vaccination and it is possible that activated NK cells may be expanding non-specifically in response to antigen.
We performed the above experiment three months after BCG vaccination. We measured live BCG in lungs and various lymphoid organs three months after vaccination and were not able to detect any live BCG (data not shown). In BCG-vaccinated mice, CD3-NKp46+CD27+ cells expanded from 1444 ± 271.9 to 27380 ± 3917 cells per 106 pooled cells (p=0.002, Supplementary Figure. 1B). We further characterized the expanding CD3-NKp46+CD27+ cells using various cell surface markers for NK cell receptors. Among various markers tested one month after BCG vaccination only CD3-NKp46+CD27+KLRG1+ cells expanded from 1135 ± 304.1 to 2523 ± 690.2 cells per 106 pooled cells (p=0.04, Supplementary Figure. 1C) upon Ag85 stimulation. In contrast, CD3-NKp46+CD27+KLRG1+ cells did not expand in PBS-treated mice (Supplementary Figure. 1C). Three months after BCG vaccination similar expansion of CD3-NKp46+CD27+KLRG1+ cells was noted upon Ag85 stimulation (Supplementary Figure. 1D).
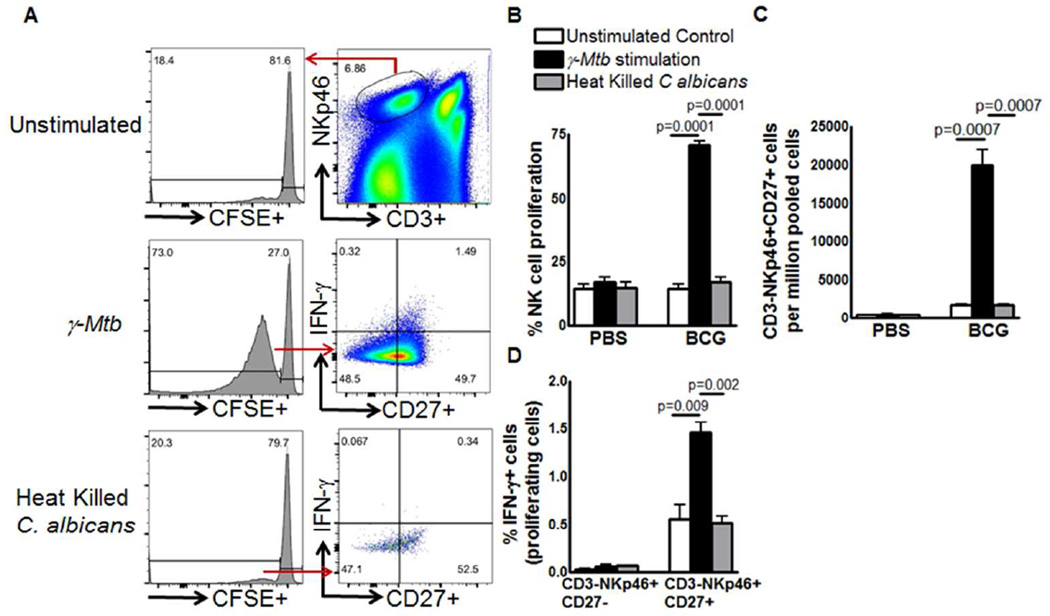
The above results suggest expansion of a subpopulation of NK cells in BCG vaccinated mice upon Ag85 or γ-M. tb stimulation. We determined the antigen specificity and proliferative capacity of expanding memory like CD3-NKp46+CD27+ cells. Six months after BCG vaccination or PBS treatment, spleen and peripheral lymph node cells were isolated, pooled, labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured, with or without γ-M. tb or heat killed Candida albicans and determined the expansion of CD3-NKp46+CD27+NK cells. In BCG-vaccinated mice, upon stimulation with γ-M. tb, the proliferating CD3-NKp46+CD27+ cells expanded from 1777 ± 101.9 to 20050 ± 1918 cells per 106 pooled cells (p=0.0007, Figure. 1C). In contrast, CD3-NKp46+CD27+ cells unable to expand upon heat killed Candida albicans stimulation. In γ-M. tb stimulated cells, IFN-γ+CD3-NKp46+CD27+ cells (gated on proliferating cells) were three fold higher compared to IFN-γ+CD3-NKp46+CD27- cells (p=0.009, Figure. 1D). In PBS-treated mice, γ-M. tb or heat killed Candida albicans unable to expand IFN-γ+CD3-NKp46+CD27+ cells (Figure. 1D).
Figure 1. BCG vaccination induces expansion of memory-like NK cells.
(A, B) C57BL/6 mice (5 mice per group) were given 100 µl of PBS (unimmunized) or immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS. Six months after vaccination, spleen, and peripheral lymph node cells were isolated, pooled, labeled with carboxyfluorescein succinimidyl ester (CFSE) and cultured, with or without γ-M. tb or heat killed Candida albicans. After 5 days, expanding CD3-NKp46+CD27+ NK cells and IFN-γ producing cells were measured by flow cytometry. (A) A representative flow cytometry plot is shown. NK cells were identified by sequentially gating on singlet population and then on CD3− NKp46+ NK cells. The events within the gated CD3-NKp46+ NK cells were analyzed for CFSE+ cells and plotted in the histograms. Total lung CFSE+CD3-NKp46+CD27+ NK cell numbers are shown. (B) Percent proliferating NK cells (C) Absolute number of CD3-NKp46+CD27+ cells (D) CD3-NKp46+CD27-IFN-γ+ and CD3-NKp46+CD27+IFN-γ+ cells. Mean values and SEs are shown. Data are representative of two independent experiments.
Memory-like NK cells expand during M. tb Infection
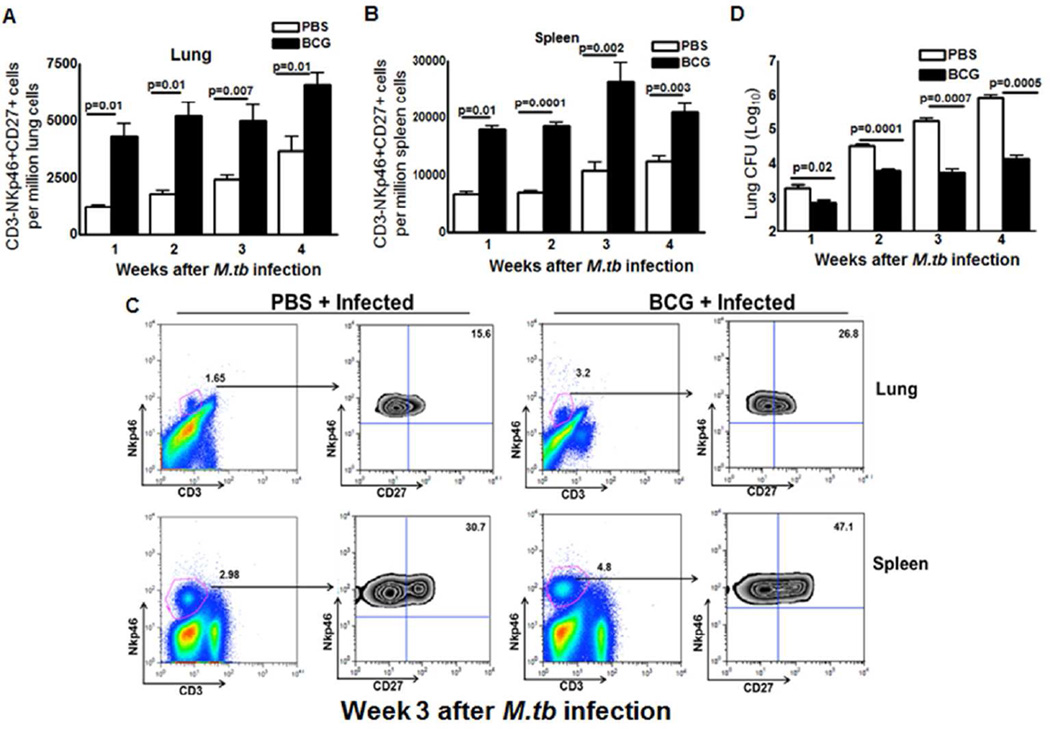
To determine whether expansion of CD3-NKp46+CD27+ cells correlates with decreased bacterial burden in M. tb-infected mice, we treated mice subcutaneously with PBS or immunized them with BCG. After one month, mice were infected by aerosol challenge with M. tb H37Rv., We measured CD3-NKp46+CD27+ cells in lungs and spleens, as well as bacterial burden in lungs every 7 day until 1 month. As shown in Figure. 2A and B, one week after challenge with M. tb H37Rv, there is a significant difference in the proportion of CD3-NKp46+CD27+ cells in lungs and spleen of BCG-vaccinated, compared to PBS-treated mice. These differences persisted in the lungs at least four weeks after M. tb infection (Figure. 2B) and fold changes were shown in Supplementary Figure. 2. The bacterial burden was significantly higher in the lungs of PBS-treated than BCG-vaccinated mice one week after infection, and these differences widened to a 2-log by four weeks after infection (Figure. 2D).
Figure 2. Memory-like NK cells expand after BCG vaccination and challenge with M. tb H37Rv.
C57BL/6 mice (20 mice per group) were given 100 µl of PBS or immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS. After thirty days, mice were challenged with 75–100 CFU of M. tb H37Rv by aerosol. At weekly intervals up to 4 weeks, five mice in each group were sacrificed, and the lung bacterial burden and percentages of CD3-NKp46+ cells in lungs and spleen that were CD27+ were determined. (A) CD3-NKp46+CD27+ cells in lungs. (B) CD3-NKp46+CD27+ cells in spleens. (C) A representative flow cytometry plot is shown. Gating strategy to identify NK cells was similar to Figure 1. (D) Bacterial burden in lungs. Mean values and SEs are shown. Data are representative of two independent experiments.
Memory-like NK cells proliferate and produce IFN-γ in M.tb infected mice
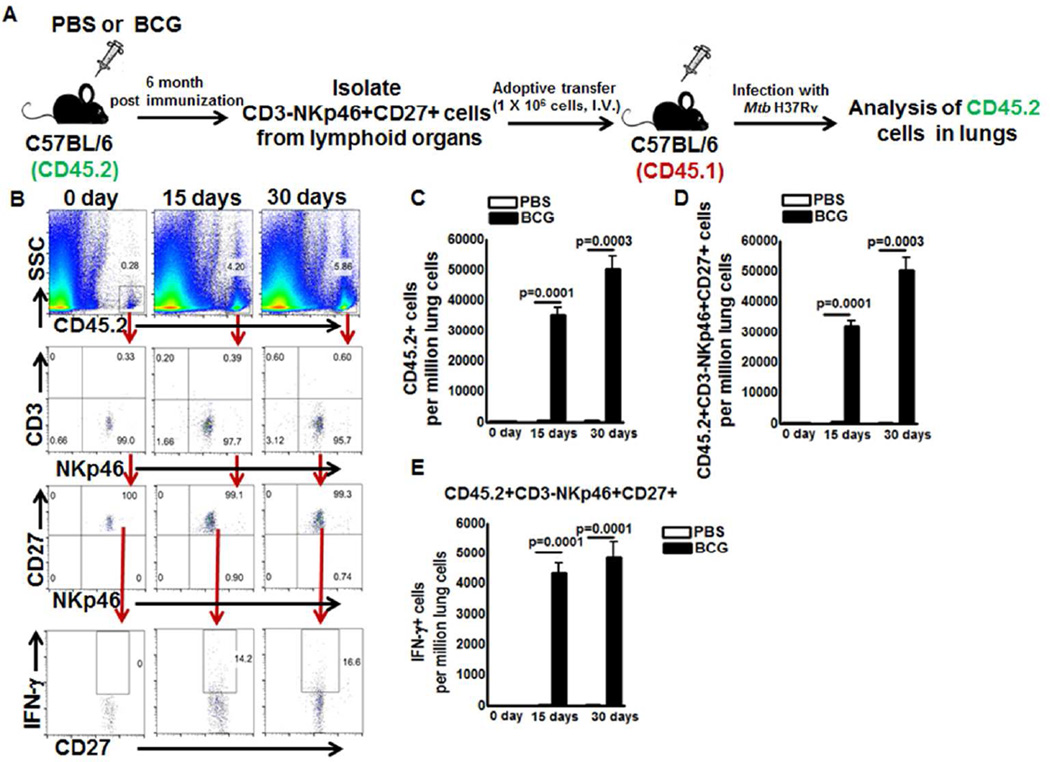
We determined whether memory-like NK cells (CD3-NKp46+CD27+ and CD3-NKp46+CD27+KLRG1+) proliferate and produce IFN-γ upon adoptive transfer to M. tb infected recipient mice. CD57BL/6 (CD45.2 congenic) mice were vaccinated with BCG or treated with PBS. After six month, cells were pooled from spleen and lymph nodes, and CD3-NKp46+CD27+ NK cells were isolated and adoptively transferred to naive C57BL/6 mice expressing congenic marker CD45.1. As shown in Figure 3B and C, 10 days after adoptive transfer, 192.3 ± 80.76 cells per million lung cells were CD45.2 NK cells (CD3-NKp46+CD27+). Ten days after adoptive transfer, recipient mice were infected with M. tb H37Rv. Fifteen days after infection, the numbers of CD45.2 NK cells (adoptively transferred NKp46+CD3-CD27+ cells from BCG vaccinated mice) were 3.7 ± 0.6 percent (32000 ± 1799 vs. 2590 ± 236.3 cells per million lung cells, 12 fold increase compared to CD45.2 NK cells from PBS treated mice, p=0.0001, Figure. 3C and 3D), and out of these, 14.4 ± 1.2 percent of CD45.2+NKp46+CD3-CD27+ cells were IFN-γ producing cells (Figure. 3E) in the lungs of M. tb infected CD45.1 recipient mice. After 30 days the number of CD45.2 cells were 6.3 ± 0.7 percent (35 fold increase compared to CD45.2 cells from PBS treated mice, 63520 ± 3164 vs. 378.7 ± 87.61 cells per million lung cells, p=0.0001, Figure. 3C and 3D) and out of these 15.7 ± 2.6 percent CD45.2+NKp46+CD3-CD27+ cells were IFN-γ producing cells (Figure. 3E) in the lungs of M. tb infected CD45.1 recipient mice. Our findings suggest CD3-NKp46+CD27+ NK cells from BCG vaccinated mice proliferate and produce IFN-γ upon challenge with M. tb.
Figure 3. Memory-like NK cells proliferate and produce IFN-γ in M. tb infected mice.
C57BL/6 (CD45.2+ congenic) mice were given 100µl PBS or immunized subcutaneously with 106 CFU of BCG in 100µl PBS. Six months after vaccination, CD3-NKp46+CD27+ NK cells were isolated from pooled spleens and peripheral lymph node cells. 1 × 106 cells were adoptively transferred to CD45.1 mice (5 mice per group) through tail vein injection 10 days prior to infection with M. tb H37Rv. (A) Schematic representation of the adoptive transfer experiment (B) A representative flow cytometry plot is shown. Gating strategy to identify NK cells was similar to Figure 1. (C) Absolute number of adoptively transferred CD45.2+ NK cells in lungs were determined at day 0, 15 and 30 after infection (D) Absolute number of adoptively transferred CD45.2+NKp46+CD27+ NK cells in lungs were determined at day 0, 15 and 30 after infection and (E) Absolute number of adoptively transferred CD45.2+NKp46+CD27+IFN-γ+ NK cells in lungs were determined at day 0, 15 and 30 after infection. Mean values and SEs are shown. Data are representative of two independent experiments.
Memory-like NK cells protect mice from M. tb infection
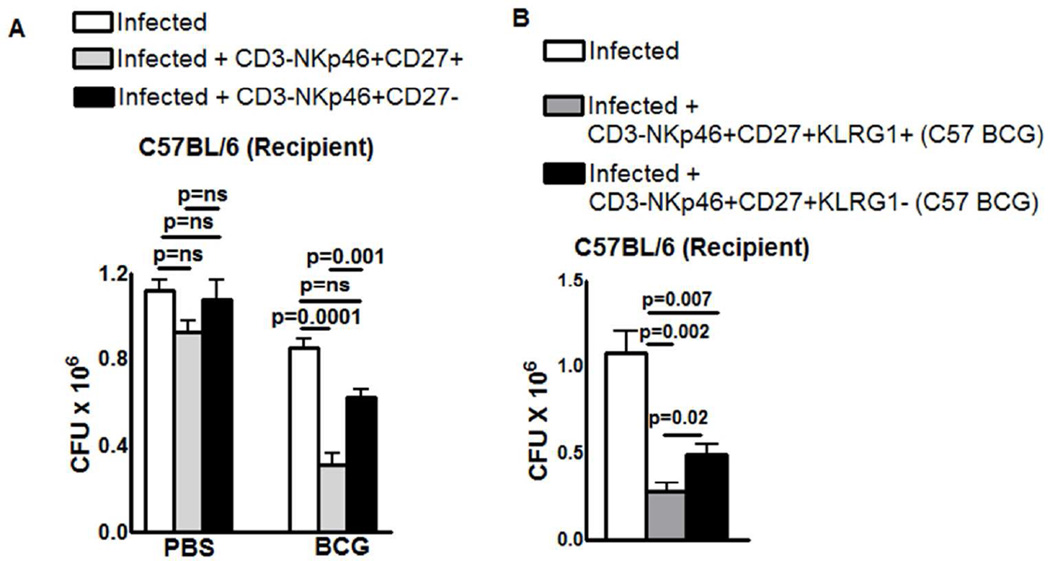
Next we determined whether adoptively transferred CD3-NKp46+CD27+ cells protect mice against M. tb infection. C57BL/6 mice were vaccinated with BCG or treated with PBS. After thirty days, cells were pooled from spleen and lymph nodes, and CD3-NKp46+CD27+ and CD3-NKp46+CD27- cells were isolated and adoptively transferred to naive C57BL/6 mice at the time of infection with M. tb H37Rv. Adoptive transfer of CD3-NKp46+CD27+ cells from BCG-vaccinated mice reduced the bacterial burden in lungs by approximately 60% (0.8 ± 0.04 × 106 to 0.3 ± 0.05 × 106, p=0.0001, Figure. 4A). In contrast adoptive transfer of CD3-NKp46+CD27-cells from BCG-vaccinated mice or CD3-NKp46+CD27+ cells from PBS-treated mice had no effect on lung CFU (Figure. 4A).
Figure 4. CD3-NKp46+CD27+ and CD3-NKp46+CD27+KLRG1+ NK cells protects mice from M. tb infection.
(A) Wild type C57BL/6 mice (5 mice per group) were immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS or treated with PBS. After one month, CD3-NKp46+CD27+ or CD3-NKp46+CD27- NK cells from pooled spleens and peripheral lymph node cells were isolated and adoptively transferred (1 × 106 cells once on day 0 of infection) to M. tb H37Rv-infected C57BL/6 mice. (B) The same experiment was performed as in panel A, except that M. tb-infected wild type mice received CD3-NKp46+CD27+KLRG1+ or CD3-NKp46+CD27+KLRG1- NK cells from BCG vaccinated C57BL/6 mice. Infected mice in both panels were sacrificed thirty days post-infection, lung bacterial burden was measured. Mean values and SEs are shown. Data are representative of two independent experiments.
In Supplementary Figure. 1D, three months after BCG vaccination, we found expansion of CD3-NKp46+CD27+KLRG1+ cells. We also determined the functional capacity of CD3-NKp46+CD27+KLRG1+ NK cells to restrict M. tb growth in the lungs of infected mice. This experiment was performed same as the above except CD3-NKp46+CD27+KLRG1+ and CD3-NKp46+CD27+KLRG1- cells were isolated three months after BCG vaccination or PBS treatment. Adoptive transfer of CD3-NKp46+CD27+KLRG1+ cells from three months BCG-vaccinated mice reduced lung CFU from 1.1 ± 0.14 × 106 to 0.12 ± 0.02 × 106 (a log reduction, p=0.0002, Figure 4B). However, adoptive transfer of CD3-NKp46+CD27+KLRG1- cells marginally reduced bacterial burden (1.1 ± 0.14 × 106 to 0.48 ± 0.05× 106 p=0.02, Figure. 4B).
Expansion and protection of memory-like NK cells depends on IL-21 production
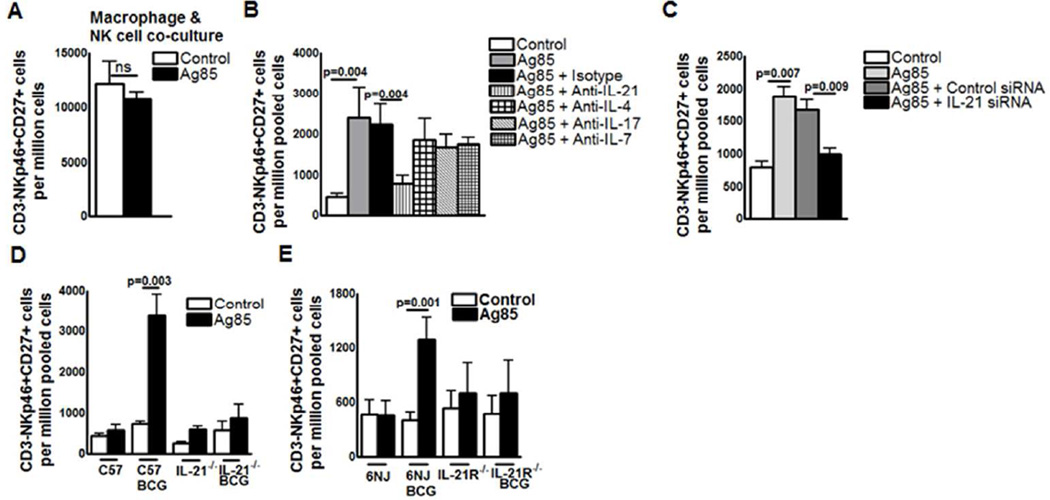
To determine the mechanisms involved in the expansion of memory-like NK cells in response to M.tb, we first asked whether antigen-presenting cells alone are sufficient for the expansion of CD3-NKp46+CD27+ cells. We cultured CD3-NKp46+ cells and autologous peritoneal macrophages from BCG-vaccinated mice in medium alone or with Ag85. After 5 days, Ag85 did not expand CD3-NKp46+CD27+ cells (12930 ± 2076 vs. 11300 ± 830.2 cells per 106 pooled NK cells, p=ns, Figure. 5A), suggesting that expansion of memory-like NK cells requires help from other cell populations. We next determined whether cytokines produced by activated T-cells contribute to expansion of memory-like NK cells. Spleen and peripheral lymph node cells from BCG-vaccinated mice (one month) were isolated, pooled, and cultured, with or without Ag85, and in the presence or absence of neutralizing antibodies to IL-4, IL-7, IL-17, and IL-21. These cytokines are known to play an important role in the expansion of memory T cells during infection with intracellular pathogens. Ag85-dependent expansion of CD3-NKp46+CD27+ cells was not affected by neutralization of IL-4, IL-7 and IL-17. In contrast, anti-IL-21 antibody significantly inhibited Ag85-dependent expansion of CD3-NKp46+CD27+ cells (Figure. 5B) and CD3-NKp46+CD27+ KLRG1+ cells (Supplementary Figure. 3A).
Figure 5. Expansion of memory-like NK cells in BCG-vaccinated mice depends on IL-21.
C57BL/6 mice were treated with PBS or immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS. One month after vaccination, spleen, and peripheral lymph node cells were pooled. (A) CD3- NK cells were isolated and cultured with peritoneal macrophages, with or without Ag85. (B) Pooled cells were cultured with or without Ag85, in the presence of isotype-matched control antibodies or antibodies to IL-4, IL-7, IL-17, or IL-21. (C) Pooled cells from one month BCG vaccinated C57BL/6 mice were transfected with either IL-21 or scrambled siRNA (control siRNA) and cultured with or without Ag85. (D) Pooled cells from one month BCG vaccinated IL-21 knockout and respective control mice were cultured with or without Ag85. (E) Pooled cells from one month BCG vaccinated IL-21R knockout and respective control mice were cultured with or without Ag85. In all panels, after five days, expansion of CD3-NKp46+CD27+ cells were measured by flow cytometry. Mean values and SEs are shown. Data are representative oftwo independent experiments.
To further confirm that the expansion of memory-like CD27+ NK cells is due to IL-21, we used IL-21 siRNA. Spleen and peripheral lymph node cells from BCG-vaccinated mice (three months after vaccination) were isolated, pooled, treated with scrambled siRNA or IL-21 -siRNA and cultured with Ag85. After 5 days, expansion of CD3-NKp46+CD27+ cells was quantified by flow cytometry. IL-21siRNA inhibited IL-21 mRNA expression by 70–80%, as quantified by real-time PCR (Supplementary Figure. 3B). In 5 BCG-vaccinated mice, IL-21 siRNA abrogated Ag85-dependent expansion of CD3-NKp46+CD27+ cells, reducing levels to those comparable to unstimulated cells (Figure. 5C). In contrast, scrambled siRNA had no effect (1675 ± 147.7 cells per 106 pooled cells for scrambled siRNA versus 1002 ± 90.91 cells per 106 pooled cells for IL-21 siRNA, p=0.009, Figure. 5C).
As an additional means to determine the role of IL-21 and IL-21R signaling pathways in expansion of memory-like NK cells after BCG vaccination, we vaccinated IL-21 receptor (6NJ background) and IL-21 (C57BL/6 background) knockout mice and their control wild type mice. After one month, spleen and peripheral lymph node cells were isolated, pooled, and cultured with Ag85. After 5 days, in BCG-vaccinated IL-21 and IL-21R knockout mice, CD3-NKp46+CD27+ cells did not expand in response to Ag85 (Figure. 5D and E, respectively). In contrast, in BCG-vaccinated wild type mice, CD3-NKp46+CD27+ cells significantly expanded in response to Ag85 (744.0 ± 52.12 to 3377 ± 551.6 cells per 106 pooled cells, p = 0.003, Figure. 5D and E). These results provide additional evidence that IL-21 is required for expansion of CD3-NKp46+CD27+ cells in mice.
The data above suggest BCG vaccination primes Ag-specific T cell activation to release IL-21, which can non-specifically expand NK cells. We determine whether the above expansion of memory like NK cells requires prior BCG vaccination or IL-21 and antigen are sufficient. We cultured pooled spleen and peripheral lymph node cells from PBS-treated mice with recombinant IL-21 and Ag85. Under these conditions, Ag85 did not expand CD3-NKp46+CD27+ cells, with or without recombinant IL-21 (Supplementary Figure. 3C), suggesting that BCG vaccination induces development of memory-like CD27+ NK cells.
IL-21 is required at the time of BCG vaccination for the generation of memory-like NK cells
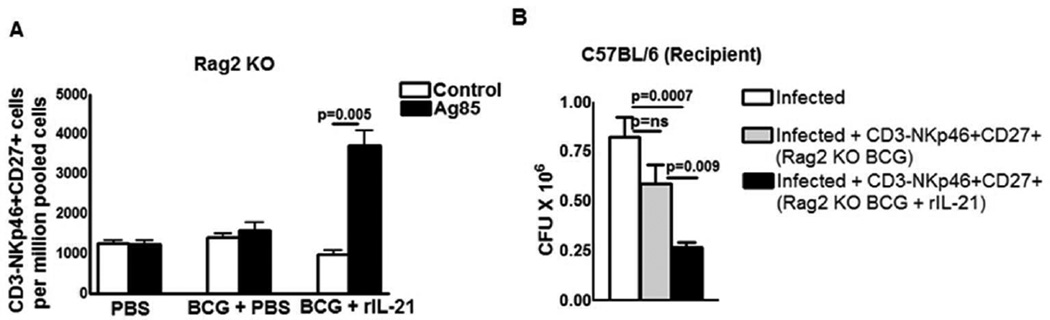
IL-21 is produced by T-cells and Rag2 knockout mice do not have functional T-cells. To determine whether IL-21 is required at the time of vaccination for the development of memory-like NK cells that respond to mycobacterial antigens, we vaccinated Rag2 knockout mice with BCG or treated with PBS as a control. Some BCG-vaccinated mice were given recombinant IL-21 at the time of vaccination. After one month, pooled spleen and peripheral lymph node cells from PBS-treated, BCG-vaccinated and BCG-vaccinated plus recombinant IL-21 treated Rag2 knockout mice were cultured with M. tb Ag85. After 5 days, in PBS and BCG-vaccinated Rag2 knockout mice, CD3-NKp46+CD27+ cells unable to expand in response to Ag85 alone (Figure. 6A). In contrast, in response to Ag85, CD3-NKp46+CD27+ cells significantly expanded in BCG vaccinated Rag2 knockout mice that received recombinant IL-21 (1122 ± 89.57 vs. 3759 ± 622.5 cells per 106 pooled cells, p = 0.005, Figure. 6A).
Figure 6. IL-21 is required at the time of BCG vaccination for the generation of memory-like NK cells.
C57BL/6 (Rag2 knockout) mice were treated with PBS or immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS. Some of the BCG vaccinated mice received 0.3 mg of recombinant IL-21 or PBS through tail vein at the time of BCG vaccination. One month after vaccination, spleen, and peripheral lymph node cells were pooled. (A) Spleen, and peripheral lymph node cells from the above groups of mice were cultured in the presence or absence of Ag85. After 5 days, expansion of CD3-NKp46+CD27+ cells was determined by flow cytometry. (B) Rag2 Knockout mice (5 mice per group) were immunized subcutaneously with 106 CFU of BCG and treated with or without recombinant IL-21. After one month, CD3-NKp46+CD27+ NK cells from pooled spleens and peripheral lymph node cells were isolated and adoptively transferred (1 × 106 cells once on day 0 of infection) to M. tb H37Rv-infected C57BL/6 mice. Infected mice were sacrificed thirty days post-infection, lung bacterial burden was measured. Mean values and SEs are shown. Data are representative of three independent experiments.
We also determined whether CD3-NKp46+CD27+ cells from BCG vaccinated Rag2 knockout mice that received recombinant IL-21 inhibits M. tb growth in naive C57BL/6 mice. After one month, CD3-NKp46+CD27+ cells from pooled spleen and peripheral lymph node cells from BCG-vaccinated and BCG-vaccinated Rag2 knockout mice that received recombinant IL-21 were isolated. The above isolated cells were adoptively transferred to wild type C57BL/6 mice at the time of M. tb infection. Adoptive transfer of CD3-NKp46+CD27+ cells from BCG-vaccinated, IL-21-treated mice markedly reduced CFU (0.8 ± 0.1 × 106 to 0.26 ± 0.02 × 106 p=0.0007, Figure. 6B). In contrast, adoptive transfer of CD3-NKp46+CD27+ cells from BCG-vaccinated Rag2 mice that did not receive IL-21 had no effect on CFU (0.8 ± 0.1 × 106 to 0.58 ± 0.09 × 106, p=ns, Figure. 6B). Our results also confirms that CD3-NKp46+CD27+ cell mediated inhibition of M. tb growth in Figure.4 is not due to contaminating T cell population.
Memory-like NK cells enhance cytokine and anti-microbial peptide expression in M. tb-infected mice lungs
To determine the mechanism(s) involved in the control of M. tb infection by CD3-NKp46+CD27+ NK cells in the lungs of infected mice, we measured cytokine and anti-microbial peptide expression in M. tb-infected mice lungs after adoptive transfer of CD3-NKp46+CD27+ or CD3-NKp46+CD27- NK cells. C57BL/6 mice were vaccinated with BCG. After thirty days, CD3-NKp46+CD27+ and CD3-NKp46+CD27- cells from spleen and lymph nodes were isolated and adoptively transferred to C57BL/6 mice at the time of infection with H37Rv. Adoptive transfer of CD3-NKp46+CD27+ cells significantly enhanced IFN-γ, TNF-α, IL-1β, IL-12, and β-defensin mRNA in lungs of recipient mice, whereas adoptive transfer of CD3-NKp46+CD27-cells had no effect (Supplementary Figure. 4). Adoptive transfer of CD3-NKp46+CD27+ cells and CD3-NKp46+CD27-cells had no effect on the expression of IL-15 (Supplementary Figure. 5) and IL-18 (Supplementary Figure. 4) expression in M. tb-infected mice lungs.
Expansion of memory-like NK cells in individuals with latent tuberculosis infection (LTBI)
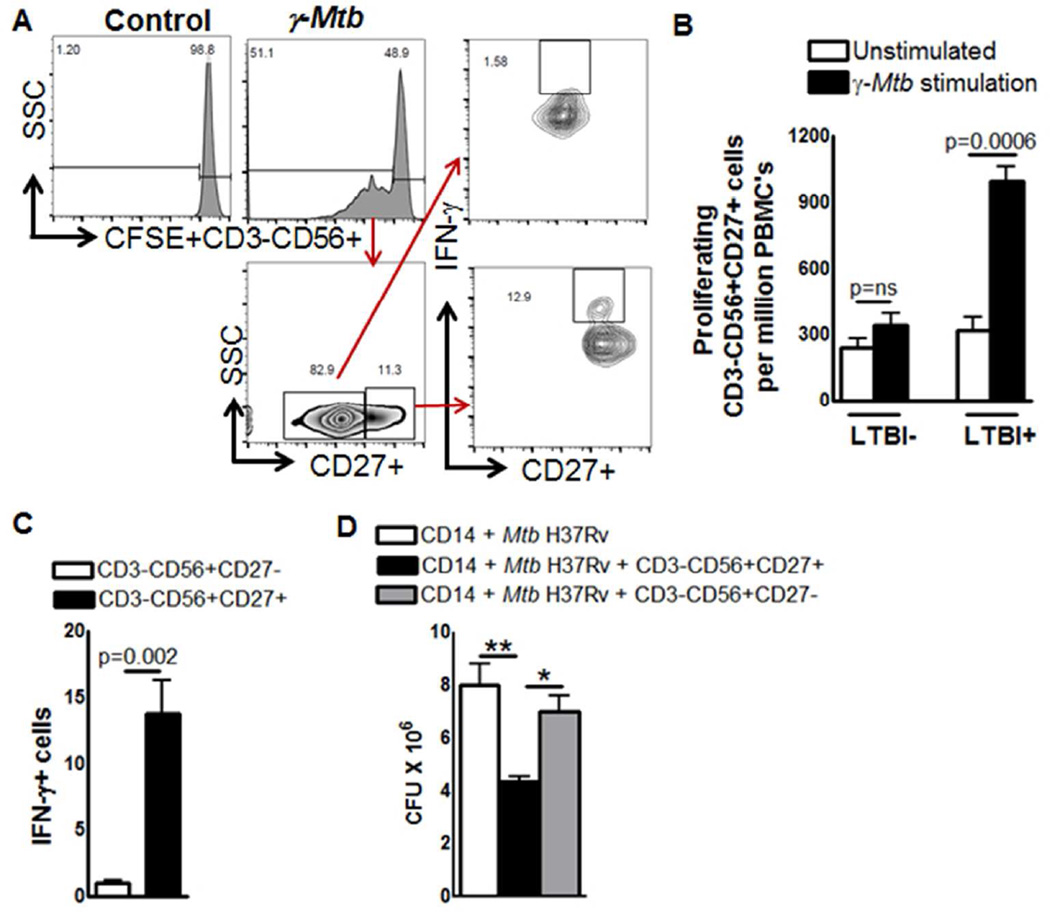
Persons with LTBI have a substantial degree of protective immunity against TB. To determine if protective immunity is associated with expansion of memory-like NK cells, we evaluated persons with or without LTBI. PBMC from 5 individuals with LTBI and 5 individuals without LTBI were labeled with CFSE and cultured with or without γ-M. tb. After 5 days, the percentages of proliferating CD3-CD56+CD27+ cells were measured by flow cytometry. γ-M. tb significantly expanded CD3-CD56+CD27+ cells in LTBI+ individuals but not in LTBI-individuals (947.4 ± 156.28 vs. 381.2 ± 57.91, p=0.0006, Figure. 7A and B). In five LTBI+ individuals, we found that γ-M. tb expanded CD3-CD56+CD27+ NK cells are the major source of IFN-γ but not the CD3-CD56+CD27- NK cells (13.8 ± 2.4 vs. 1.0 ± 0.19, p=0.002, Figure. 7A and C).
Figure 7. Expansion of memory-like NK cells in individuals with LTBI.
PBMC from 5 individuals with LTBI and 5 individuals without LTBI were labeled with CFSE and cultured, with or without γ-M. tb. After 5 days, proliferating CD3-CD56+CD27+ cells were measured by flow cytometry. (A) A representative flow cytometry plot is shown. NK cells were identified by sequentially gating on lymphocytic singlet population and then on CD3−CD56+ NK cells. The events within the gated CD3-CD56+ NK cells were analyzed for CFSE+ cells and plotted in the histograms. Total PBMC CFSE+CD3-CD56+CD27+ NK cell numbers are shown. (B) Absolute number of proliferating CD3-CD56+CD27+ cells. (C) Absolute number of CD3-CD56+CD27+ IFN-γ cells. Five independent experiments each time with 1 LTBI+ and one LTBI- donor was performed in panel A, B and C. (D) PBMC from 5 individuals with LTBI were cultured, with or without γ-M. tb. After 3 days, CD3-CD56+CD27+ and CD3-CD56+CD27- cells were isolated by magnetic selection. CD14+ monocytes (106/well) were isolated from fresh PBMC and differentiate them to macrophages (MDMs) for 3 days. MDMs were infected with M. tb H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). To some wells, the above isolated CD3-CD56+CD27+ or CD3-CD56+CD27- cells were added, at a ratio of 1 NK cell:9 MDMs. Infected macrophages were cultured for 5 days, and bacterial burden was determined. Mean values and SEs are shown.The data shown in panel D was performed six times, each time with PBMC obtained from one LTBI+ donor.
Memory-like NK cells in individuals with LTBI inhibits M. tb growth in autologous macrophages
To determine the effect of memory like NK cells (CD3-CD56+CD27+) on intracellular mycobacterial growth, freshly isolated PBMC from 6 LTBI were cultured with γ-irradiated M. tb H37Rv. After three days, CD3-CD56+CD27+ and CD3-CD56+CD27- cells were isolated, as outlined in the methods. Autologous monocyte derived macrophages (MDMs) were generated, infected with M. tb H37Rv at a MOI of 1:2.5 and cultured with M. tb-expanded CD3-CD56+CD27+ or CD3-CD56+CD27- cells. After 5 days, 8.0 ± 0.8 X 106 CFU per well were present in MDMs cultured alone. Addition of CD3-NKp46+CD27+ cells reduced CFU to 4.3 ± 0.2 X 106 (>45% inhibition, p = 0.01, Figure. 7D). CD3-CD56+CD27+ NK cells significantly reduced the bacterial burden in MDMs compared to addition of CD3-NKp46+CD27- cells (7.0 ± 0.6 X 106 CFU vs.4.3 ± 0.2 X 106 CFU, p< 0.05, >35% inhibition Figure. 7D). This result indicates that CD3-CD56+CD27+ cells inhibit M. tb H37Rv growth in macrophages more efficiently compared to CD3-CD56+CD27- cells.
Expansion of memory-like NK cells in individuals with LTBI depends on IL-21
In supplementary Fig. 1, we found expansion of NKp46+CD27+KLRG1+ cells in BCG vaccinated mice. M. tb antigen ESAT-6 is not expressed by BCG and used to distinguish BCG vaccination vs. latent M. tb infection. We determined the expansion of NKp46+CD27+ KLRG1+ cells in 5 individuals with LTBI and 5 individuals without LTBI. PBMC were cultured, with or without ESAT-6 and after 5 days, the absolute number of NKp46+CD27+KLRG1+ cells were measured by flow cytometry. ESAT-6 significantly expanded NKp46+CD27+KLRG1+ cells in LTBI+ individuals but not in LTBI- individuals (229.44 ± 64.105 vs. 561.10 ± 141.7, p=0.004, Supplementary Figure. 5A).
We asked whether IL-21 is responsible for the expansion of NKp46+CD27+KLRG1+ cells in persons with LTBI. PBMC from 5 individuals with LTBI were cultured with or without ESAT-6. Some cells were treated with scrambled siRNA or IL-21siRNA before culture with ESAT6. After 5 days, expansion of NKp46+CD27+KLRG1+ cells was determined by flow cytometry. IL-21 siRNA inhibited IL-21 mRNA expression by 70–80%, as quantified by real-time PCR (Supplementary Figure. 5C). IL-21 siRNA reduced ESAT6-dependent expansion of NKp46+CD27+KLRG1+ cells compared to scrambled siRNA (557.3 ± 96.9 vs. 193.1 ± 48.1 p=0.004, Supplementary Figure. 5B).
DISCUSSION
Using a mouse model of TB infection, we found that BCG vaccination induces the development of memory-like CD3-NKp46+CD27+ NK cells. These memory-like NK cells after adoptive transfer in M. tb-infected mice, were able to proliferate, produce IFN-γ and reduce bacterial burden, providing the first evidence that these cells contribute to immunity against a bacterial pathogen. Using antibodies, siRNA and gene-deleted mice, we found that expansion of memory-like NK cells depends on IL-21. In healthy individuals with LTBI and protective immunity against M. tb, memory-like NK cells expanded from PBMC in response to M. tb antigen in an IL-21-dependent fashion. In contrast, memory-like NK cells did not expand from PBMC of healthy persons who were not infected with M. tb. The sum of these data demonstrate that: 1) memory-like NK cells contribute to vaccine-induced protective immunity against M. tb; 2) expansion of memory-like NK cells in humans correlates with protective immunity against M. tb; and 3) IL-21 is required for expansion of memory-like NK cells in both humans and mice.
NK cells play a central role in innate immunity to viruses, bacteria, and parasites through destruction of infected cells and by secretion of cytokines that shape the adaptive immune response 5,10,16. NK cells are traditionally considered as innate cells because they possess germ-line encoded receptors and lack structurally unique receptors, somatic hypermutation, and clonal expansion. However, recent studies suggest that NK cells can be long-lived and can behave like memory T-cells. In a mouse model, dendritic cell-activated NK cells provided protection against B16 melanoma for up to one year 17. NK cells undergo homeostatic proliferation in a lymphopenic environment 18,19 and generate long-lived NK cells that respond to viral infection 20, suggesting that memory-like NK cells may contribute to immune defenses in viral infections and cancer. This concept is also supported by findings that CXCR6+ liver NK cells can generate antigen-specific memory responses 12,21, mouse NK cells expressing KLRG1 can recognize viral proteins and protect mice against secondary infection 13,20 and cytokine-dependent memory NK cells protect the host against tumors 22. chronic viral infections in mice and human increased KLRG1 expression has been reported in virus-specific expanded NK and CD8+ T-cells. However, limited information is available about the factors that induce memory NK cell expansion and long-term survival, and the role of memory NK cells in vaccine-induced immunity. Our current study found that memory-like NK cells expand during BCG vaccination in mice, and contribute to vaccine-induced protective immunity against M. tb. Furthermore, our human studies suggest that, like T-cells, memory-like NK cells can survive in the host for long periods in persons with LTBI.
In pleural fluid of patients with tuberculous pleuritis, a subpopulation of NK cells that express the memory-associated marker, CD45RO, exert robust immune responses when stimulated by IL-12 23. These cells produce IFN-γ in response to BCG 24, but it is uncertain if they are memory-like NK cells and the factors that induce their development and expansion are unknown. In infections due to Leishmania major, Pneumocystis pneumoniae, and simian immunodeficiency virus, CD4+ T-cell help is essential to activate NK cells to control infection 25,26. Similarly, CD4+ T-cell help is required for expansion of memory-like NK cells and for their prolonged anti-tumor effects 27,28. The above studies found that IL-2 produced by activated T-cells is essential for NK cell activation. However, in P. falciparum infection, exogenous IL-2 (100 IU/ml) in the absence of CD4+ T-cells is insufficient to elicit memory-like IFN-γ responses by NK cells 29. This suggests the need for additional factors produced by CD4+ T-cells for induction and activation of memory-like NK cells. Using neutralizing antibodies, siRNA, and mice with deleted IL-21 and IL-21R genes, the current study provides evidence that IL-21 is essential for the expansion of memory-like NK cells that contribute to vaccine-induced immunity against M. tb and are associated with protective immunity against TB in humans. Our unpublished studies indicate that antigen-stimulated CD4+ T-cells are the major source for IL-21 in the setting of M. tb infection.
IL-21 is a member of the class 1 family of cytokines, which fold into a four-helix-bundle structure 30. The biological effects of IL-21 are mediated through IL-21R, which uses the common gamma chain (γc), as do other members of this family, including IL-2, IL-4, IL-7, IL-9, and IL-15 30,31. Activated CD4+ T-cells are the major sources for IL-2130, which also affects the proliferation of T and NK cells 32. IL-21 has anti-tumor effects and is in phase 2 clinical trials in patients with metastatic melanoma 33. In viral infections, IL-21 contributes to the control of persistent lymphocytic choriomeningitis virus 34 and improves T and NK cell function in HIV-infected persons 35,36. However, limited information is available on the role of IL-21 in M. tb infection. Intranasal BCG vaccination provides greater protection against M. tb than subcutaneous BCG vaccination, and is associated with increased expression of IL-21 37. IL-21 increases the immunogenicity of a DNA vaccine encoding Ag85A 38 and this is enhanced by a cationic nanoparticle formulation 39. A recombinant mouse cytomegalovirus expressing Ag85A provides protection against M. tb, and this protective effect is abrogated by depletion of NK cells and blockade of IL-21 40, supporting our current findings that IL-21-mediated expansion of memory-like NK cells plays a significant role in vaccine-induced protective immunity to mycobacterial infection.
The molecular mechanisms that control expansion of memory-like NK cells in response to mycobacteria remain uncertain. BCG and M. tb can directly interact with NK cells to induce effector functions like IFN-γ production and cytotoxicity 41. We speculate that memory-like NK cells may develop through engagement of specific Toll-like receptors (TLRs). NK cells express TLR2, TLR3, TLR7, TLR8, and TLR9 41. TLR2 can bind to bacterial products, and TLR2 expressed by NK cells can directly recognize M. tb 41. TLR3 engagement on NK cells can directly upregulate natural killer cell receptor expression and TLR7 or TLR8 ligands expressed by HIV can directly activate NK cells 42. Activation of NK cells through TLR9 can also enhance NK cell function 43. The sum of these studies suggests that early interaction between TLRs on NK cells and BCG may induce development of memory-like NK cells. During M. tb infection after BCG vaccination, some memory-like NK cells may interact with M. tb, through receptors like CD27, and expand to effector NK cells. CD27 is a member of the TNF receptor super-family and is essential for the generation and maintenance of long-term T-cell immunity 44. CD27 is also associated with priming of NK cells in response to microbial ligands 45, and CD27high splenic NK cells display greater effector function than CD27- cells 46,47. CD27-deficient mice show normal NK cell differentiation but impaired function upon stimulation 48.
In summary, using a mouse model, we found that vaccination with BCG induces expansion of memory-like NK cells that contribute to protection against subsequent M. tb infection. Memory-like NK cells also expand in healthy persons with LTBI, suggesting that their expansion correlates with protective immunity. Expansion of memory-like NK cells in mice and humans depends on IL-21. Our work provides the first evidence that memory-like NK cells contribute to the efficacy of vaccination against microbial challenge. These findings are important for development of improved vaccines against TB, can facilitate development of interventions to prevent progression of LTBI to TB, and may be relevant to protection against other intracellular pathogens.
METHODS
Animals
All animal studies were performed on specific-pathogen-free 6–8-week-old female mice according to institutional guidelines. C57BL/6 (CD45.1 and CD45.2 congenic strains), Rag2 knockout, C57BL/6 NJ (6NJ) and IL-21R were from Jackson Laboratory. IL-21 knockout mice was provided by Dr. Roza Nurieva, University of Texas M. D. Anderson Cancer Center, Houston, Texas, USA.
Patient population
After obtaining written informed consent, blood was obtained from 12 healthy persons with positive QuantiFERON-TB Gold tests, indicative of LTBI, 12 healthy persons with negative QuantiFERON-TB Gold tests. All subjects were 18 to 65 years old. Individuals with or without LTBI did not have a history of TB or HIV infection, and were not receiving therapy with immunosuppressive drugs.
Ethics statement
The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler approved all the protocols. All animal procedures involving the care and use of mice were in accordance with the guidelines of NIH / OLAW (Office of Laboratory Animal Welfare). All human studies were approved by the Institutional Review Board of the University of Texas Health Science Center at Tyler (protocol#889).
Abs and other reagents
For flow cytometry, we used FITC anti-CD3, PE anti-CD27, PE/Cy7 anti-NKp46, FITC anti-CD8, PE anti-CD11b, PE anti-CD56, allophycocyanin KLRG1, (all from BioLegend). For neutralization, we used mAb to IL-4, IL-7, IL-17, IL-21, or isotype-matched control antibody (eBioscience). Recombinant mouse IL-21 was obtained from eBioscience. We used γ-irradiated M. tb H37Rv (γ-M. tb), ESAT6, and Ag85a (all from BEI Resources), and the BCG Tice strain (Organon USA Inc.).
Flow Cytometry
Surface and intracellular staining was performed using our published methods 9.
BCG vaccination and aerosol infection with M. tb H37Rv
C57BL/6 mice were immunized subcutaneously with 106 CFU of BCG in 100 µl of PBS, or with PBS alone. In some experiments Rag2 knockout mice were given 0.3 mg of recombinant IL-21 or PBS intravenously at the time of BCG vaccination. One, three and six months after vaccination, mice were infected with 50–100 CFU of M. tb H37Rv in an aerosol exposure chamber, using our published methods 9,49.
Cell isolation and adoptive transfer of NK cells
NK cells from pooled spleens and peripheral lymph nodes of one or three or six months BCG-vaccinated or PBS-treated mice were isolated by negative immunomagnetic selection (Miltenyi Biotec). From the negatively selected NK cells (>97% CD3-NKp46+), CD27+ cells were isolated by positive selection and CD3-NKp46+CD27+ and CD3-NKp46+CD27- cells were used for adoptive transfer. To isolate CD3-NKp46+CD27+KLRG1+ cells, the above isolated CD3-NKp46+CD27+ cells were stained using PE-conjugated anti-KLRG1 antibody and then sorted using anti-PE MultiSort Kit according to manufacturer’s instruction. In some experiments, CD3-CD56+ cells were isolated from human peripheral blood mononuclear cell (PBMC) by negative immunomagnetic selection (Miltenyi Biotec). From the negatively selected NK cells (>97% CD3-NKp46+), CD27+ cells were isolated by positive selection. Purity of the isolated cells was more than 96% as determined by flow cytometry. In the purified NK cell population less than 0.1% of the cells were T and B cells. One million CD3-NKp46+CD27+ or CD3-NKp46+CD27- or CD3-NKp46+CD27+KLRG1+ or CD3-NKp46+CD27+KLRG1- cells were adoptively transferred to mice through the tail vein injection, 30 minutes before M. tb infection.
Culture of lung, spleen, and lymph node cells
BCG-vaccinated mice, uninfected or infected with M. tb H37Rv, were sacrificed, and cells from the lungs, spleens, or peripheral lymph nodes were cultured in 24-well plates at 2 ×106 cells/well in RPMI-1640 containing penicillin (Life Technologies) and 10% heat-inactivated FCS, with or without γ-irradiated H37Rv (10 µg/ml) or Ag85a (3 µg/ml) at 37°C and 5% CO2. For cell proliferation experiments, cells were cultured in the presence of carboxyfluorescein succinimidyl ester (CFSE) (5µM). After 5 days, culture supernatants were collected to determine cytokine levels, and the expansion of CD3-NKp46+CD27+ or CD3-NKp46+CD27+IFN-γ+ cells was measured by immunolabeling and flow cytometry. For cytokine neutralization experiments, 10 µg/ml neutralizing antibody to IL-17, IL-2, IL-21, and IL-4 were added to the cultures. For intracellular staining, freshly isolated lung cells were immunostained to determine IFN-γ-positive CD3-NKp46+CD27+ or CD3-NKp46+CD27- or CD3-NKp46+CD27+KLRG1+ or CD3-NKp46+CD27+KLRG1- cells.
Culturing of mouse peritoneal exudate macrophages and NK cells
Peritoneal exudate macrophages (PEM) were isolated as previously described 50. In some experiments PEM and purified NK cells were cultured at the ratio of 1:5 in 24-well plates containing RPMI-1640 supplemented with penicillin (Life Technologies) and 10% heat-inactivated FCS, with or without Ag85a (3 µg/ml) at 37°C and 5% CO2. After 5 days, IFN-γ-positive CD3-NKp46+CD27+ or CD3-NKp46+CD27- or CD3-NKp46+CD27+KLRG1+ or CD3-NKp46+CD27+KLRG1- cells were determined by flowcytometry.
Isolation and culture of PBMC
PBMC were isolated by differential centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech). PBMC were cultured in 12-well plates at 2 × 106 cells/well in RPMI-1640 containing 10% heat-inactivated human serum, and ESAT-6 (5 µg/ml) at 37°C. In some experiments cells were cultured in the presence of CFSE (5µM). After 5 days, expansion of CD3-CD56+CD27+, CD3-CD56+CD27-, CD3-CD56+CD27+IFN-γ+, CD3-CD56+CD27+IFN-γ-, CD3-NKp46+CD27+KLRG1+ and CD3-NKp46+CD27+KLRG1- cells were determined by immunolabeling and flow cytometry.
Real-time PCR for quantification of IL-21, IL-1β, IL-12, IL-15, IL-18, IFN-γ, TNF-α, cathelicidin, and beta-defensin mRNA
The above gene expression in M. tb infected mice lungs and spleens was performed as previously described9. Mouse primer sequences were given in Supplementary Table 1.
siRNA
Freshly isolated human PBMC or mouse spleen cells were transfected with siRNA for IL-21 or control siRNA, using transfection reagents (all from Santa Cruz Biotechnology). The efficiency of siRNA knockdown was measured by real-time PCR of human or mouse IL-21 mRNA expression. Briefly, 106 cells were resuspended in 500 µl of transfection medium and transfected with siRNA (6 pmoles). After 6 h, an additional 500 µl of 2X RPMI-1640 complete medium was added, and cells were cultured overnight in a 24-well plate. The next day, cells were washed and stimulated with either Ag85a or ESAT6, or kept in medium alone as a control. Expansion of memory like NK cell subpopulations was determined after 5 days.
Infection of human macrophages with M. tb, and coculture with autologous NK cells
CD14+ monocytes (106/well) were plated in 12-well plates in 1 ml of antibiotic-free RPMI 1640 containing 10% heat-inactivated human serum, and incubated at 37°C in a humidified 5% CO2 atmosphere for 3 days to differentiate into macrophages. At the same time, PBMC were cultured with γ-M. tb for 3 days, and CD3-CD56+CD27+ or CD3-CD56+CD27- cells were isolated, as outlined above. MDMs were infected with M. tb H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM), incubated for 2 hr at 37°C, washed to remove extracellular bacilli, and cultured in RPMI 1640 containing10% heat-inactivated human serum. To some wells, CD3-CD56+CD27+ or CD3-CD56+CD27- cells were added, at a ratio of 1 NK cell:9 MDMs. Infected macrophages were co-cultured for 5 days, at which point macrophage viability was >90%. The supernatant was aspirated, and macrophages were lysed. The supernatant was centrifuged to pellet bacteria, and the pellets were added to the cell lysates. Bacterial suspensions were ultrasonically dispersed, serially diluted, and plated in triplicate on 7H10 agar. The number of colonies was counted after 3 weeks.
Statistical analysis
Results are shown as the mean ± SE. For data that were normally distributed, comparisons between groups were performed by a paired or unpaired t test, as appropriate. For data that were not normally distributed, the Wilcoxon rank-sum test was used.
Supplementary Material
Acknowledgments
We thank Dr. Lewis Lanier for his helpful discussion. This work was supported by grants from the National Institutes of Health (AI054629, AI073612 and A1085135 to R.V), the Cain Foundation for Infectious Disease Research, CRDF Global and the Department of Pulmonary Immunology.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
REFERENCE
- 1.Zumla A, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob. Health. 2015;3:e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat. Med. 2000;6:1327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JL, Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay CH, Szomolanyi-Tsuda E, Welsh RM. Control of infections by NK cells. Curr. Top. Microbiol. Immunol. 1998;230:193–220. doi: 10.1007/978-3-642-46859-9_12. [DOI] [PubMed] [Google Scholar]
- 6.Vankayalapati R, et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J. Immunol. Baltim. Md 1950. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 7.Vankayalapati R, et al. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J. Immunol. Baltim. Md 1950. 2004;172:130–137. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, et al. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. Baltim. Md 1950. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 9.Dhiman R, et al. NK1.1+ cells and IL-22 regulate vaccine-induced protective immunity against challenge with Mycobacterium tuberculosis. J. Immunol. Baltim. Md 1950. 2012;189:897–905. doi: 10.4049/jimmunol.1102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int. Immunol. 2008;20:1155–1167. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 11.Schierloh P, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J. Immunol. Baltim. Md 1950. 2005;175:6852–6860. doi: 10.4049/jimmunol.175.10.6852. [DOI] [PubMed] [Google Scholar]
- 12.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigenspecific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune ‘memory’. J. Immunol. Baltim. Md 1950. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Verges S, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun JC, et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu. Rev. Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Fujii S. DC therapy induces long-term NK reactivity to tumors via host DC. Eur. J. Immunol. 2009;39:457–468. doi: 10.1002/eji.200838794. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. Baltim. Md 1950. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 19.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Leary JG, Goodarzi M, Drayton DL, Andrian UH. von T cell- and B cellindependent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X, et al. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO- natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41–49. doi: 10.1111/j.1365-2567.2011.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Yang B, Lao S, Fan Y, Wu C. Human memory-like NK cells migrating to tuberculous pleural fluid via IP-10/CXCR3 and SDF-1/CXCR4 axis produce IFN-γ in response to Bacille Calmette Guerin. Clin. Immunol. Orlando Fla. 2013;148:113–123. doi: 10.1016/j.clim.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MN, et al. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. Baltim. Md 1950. 2013;190:285–295. doi: 10.4049/jimmunol.1200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bihl F, et al. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J. Immunol. Baltim. Md 1950. 2010;185:2174–2181. doi: 10.4049/jimmunol.1001486. [DOI] [PubMed] [Google Scholar]
- 27.Leong JW, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu K, Asakura M, Fujii S. Prolonged antitumor NK cell reactivity elicited by CXCL10-expressing dendritic cells licensed by CD40L+ CD4+ memory T cells. J. Immunol. Baltim. Md 1950. 2011;186:5927–5937. doi: 10.4049/jimmunol.1003351. [DOI] [PubMed] [Google Scholar]
- 29.McCall MBB, et al. Memory-like IFN-γ response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur. J. Immunol. 2010;40:3472–3477. doi: 10.1002/eji.201040587. [DOI] [PubMed] [Google Scholar]
- 30.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 31.Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J. Allergy Clin. Immunol. 2003;112:1033–1045. doi: 10.1016/j.jaci.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J. Leukoc. Biol. 2002;72:856–863. [PubMed] [Google Scholar]
- 33.Davis ID, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 34.Johnson LDS, Jameson SC. Immunology. A chronic need for IL-21. Science. 2009;324:1525–1526. doi: 10.1126/science.1176487. [DOI] [PubMed] [Google Scholar]
- 35.Iannello A, et al. IL-21 enhances NK cell functions and survival in healthy and HIVinfected patients with minimal stimulation of viral replication. J. Leukoc. Biol. 2010;87:857–867. doi: 10.1189/jlb.1009701. [DOI] [PubMed] [Google Scholar]
- 36.Strbo N, et al. IL-21 augments natural killer effector functions in chronically HIV-infected individuals. AIDS Lond. Engl. 2008;22:1551–1560. doi: 10.1097/QAD.0b013e3283089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derrick SC, Kolibab K, Yang A, Morris SL. Intranasal administration of Mycobacterium bovis BCG induces superior protection against aerosol infection with Mycobacterium tuberculosis in mice. Clin. Vaccine Immunol. CVI. 2014;21:1443–1451. doi: 10.1128/CVI.00394-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dou J, et al. Protection against Mycobacterium tuberculosis challenge in mice by DNA vaccine Ag85A-ESAT-6-IL-21 priming and BCG boosting. Int. J. Immunogenet. 2012;39:183–190. doi: 10.1111/j.1744-313X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 39.Yu F, et al. Nanoparticle-based adjuvant for enhanced protective efficacy of DNA vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis infection. Nanomedicine Nanotechnol. Biol. Med. 2012;8:1337–1344. doi: 10.1016/j.nano.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Beverley PCL, et al. A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J. Immunol. Baltim. Md 1950. 2014;193:2306–2316. doi: 10.4049/jimmunol.1302523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiesa M Della, et al. Human NK cell response to pathogens. Semin. Immunol. 2014;26:152–160. doi: 10.1016/j.smim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J. Immunol. Baltim. Md 1950. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 43.Sivori S, et al. A novel KIR-associated function: evidence that CpG DNA uptake and shuttling to early endosomes is mediated by KIR3DL2. Blood. 2010;116:1637–1647. doi: 10.1182/blood-2009-12-256586. [DOI] [PubMed] [Google Scholar]
- 44.Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 45.Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN-gammadependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J. Immunol. Baltim. Md 1950. 2008;181:5323–5330. doi: 10.4049/jimmunol.181.8.5323. [DOI] [PubMed] [Google Scholar]
- 46.Brady J, et al. The interactions of multiple cytokines control NK cell maturation. J. Immunol. Baltim. Md 1950. 2010;185:6679–6688. doi: 10.4049/jimmunol.0903354. [DOI] [PubMed] [Google Scholar]
- 47.Marquardt N, Wilk E, Pokoyski C, Schmidt RE, Jacobs R. Murine CXCR3+CD27bright NK cells resemble the human CD56bright NK-cell population. Eur. J. Immunol. 2010;40:1428–1439. doi: 10.1002/eji.200940056. [DOI] [PubMed] [Google Scholar]
- 48.Colvenaer V De, et al. CD27-deficient mice show normal NK-cell differentiation but impaired function upon stimulation. Immunol. Cell Biol. 2011;89:803–811. doi: 10.1038/icb.2010.171. [DOI] [PubMed] [Google Scholar]
- 49.Venkatasubramanian S, et al. A rho GDP dissociation inhibitor produced by apoptotic Tcells inhibits growth of Mycobacterium tuberculosis. PLoS Pathog. 2015;11:e1004617. doi: 10.1371/journal.ppat.1004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Ed. John E Coligan Al. 2008 doi: 10.1002/0471142735.im1401s83. Chapter 14, Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.