Abstract
Background/Objectives
Many children do not consume the recommended daily allowance of calcium. Inadequate calcium intake in childhood may limit bone accrual. The objective of this study was to determine if a behavioral modification and nutritional education (BM-NE) intervention improved dietary calcium intake and bone accrual in children.
Subjects/Methods
139 (86 female) healthy children, 7–10 years of age, were enrolled in this randomized controlled trial conducted over 36 months. Participants randomized to the BM-NE intervention attended five sessions over a six-week period designed to increase calcium intake to 1500 mg/day. Participants randomized to the usual care (UC) group received a single nutritional counseling session. The Calcium Counts© Food Frequency Questionnaire was used to assess calcium intake; DXA was used to assess areal bone mineral density (aBMD) and bone mineral content (BMC). Longitudinal mixed effects models were used to assess for an effect of the intervention on calcium intake, BMC and aBMD.
Results
BM-NE participants had greater increases in calcium intake that persisted for 12 months following the intervention compared to UC. The intervention had no effect on BMC or aBMD accrual. Secondary analyses found a negative association between calcium intake and adiposity such that greater calcium intake was associated with lesser gains in BMI and fat mass index.
Conclusions
A family-centered BM-NE intervention program in healthy children was successful in increasing calcium intake for up to 12 months but had no effect on bone accrual. A beneficial relationship between calcium intake and adiposity was observed and warrants future study.
INTRODUCTION
Calcium is the primary mineral present in bone, and provides strength and stiffness to the skeleton.1 Calcium and bone mineral content (BMC) accrual are highest in the years surrounding the growth spurt,2 illustrating the necessity of adequate calcium intake during growth. Peak bone mass is attained in young adulthood3 and is an important predictor of osteoporosis and fracture in later years.4, 5 Greater calcium intake has also been shown to be associated with reductions in fat mass and obesity related complications in both children and adults.6 Given the potential benefits of calcium intake on life-long skeletal health and cardiometabolic disease, there is strong incentive to establish a pattern of adequate calcium intake during childhood.
Numerous studies have found that a large percentage of children do not meet the daily recommendation for calcium intake.7–12 A study from the National Health and Nutrition Examination Survey (NHANES) found that the majority of children in the US aged 9–18 years had inadequate calcium intake,13 a concerning finding given that these are the critical years for bone accrual. Calcium supplementation studies in pediatric populations have shown that increased calcium intake improves bone mineral density (BMD).14–18 The beneficial effects of supplementation may not persist beyond the study period; however, as long-term follow-up studies have shown that the relative gains in BMD were lost once supplementation was discontinued.19–21 An alternative approach to calcium supplementation is the institution of a behavioral modification and nutritional education (BM-NE) intervention to improve calcium intake from food selection. This strategy has shown promise in improving caloric intake in patients with cystic fibrosis,22 improving calcium intake in patients with chronic inflammatory diseases23, 24 and decreasing carbohydrate and fat consumption in obese children.25 The goal of this randomized controlled trial was to determine if a BM-NE program designed to increase dietary calcium intake in healthy children resulted in increased dietary calcium intake and bone accrual over a three year period.
METHODS
Study Participants
Healthy children 7 to 10 years of age were eligible if they were less than 130% of ideal body weight1 and did not have chronic diseases or medications that might impair growth, BMD or diet. Children were recruited from the clinics of The Children's Hospital of Philadelphia (CHOP), and through local advertisements. The study was approved by the CHOP Institutional Review Board, and written informed consent and assent were obtained from the parents/guardians and children, respectively. Study visits were conducted from 1999–2005.
During the baseline visit, children were classified as low-risk or high-risk for poor bone mineralization based on milk consumption, lactose intolerance, fracture history, and family history of osteoporosis. Children were stratified by age (7 and 8 vs 9 and 10 year olds), sex and risk status and then randomized 1:1 to participate in either the BM-NE intervention or usual care (UC) group using a computerized randomization program. Study statisticians performed the randomization. Technicians responsible for acquiring DXA, anthropometry and dietary data were blinded to group assignment and risk status for poor bone mineralization.
Usual Care
Children assigned to the UC group attended a 45-minute session with a dietitian where the child and primary caregiver received counseling and education materials regarding calcium requirements, reading food labels, dietary sources of calcium and suggestions on ways to improve calcium intake.
Nutrition Intervention
The BM-NE intervention program consisted of five 90-minute sessions over a 6-week period. Primary caregivers attended a class led by a psychologist and a dietitian. Children attended a simultaneous class led by trained study staff. A structured curriculum was created for both classes. The goal was to gradually increase total calcium intake to 1500 mg/day by the end of the program. This target was chosen because calcium absorption continues to increase up to 1500 mg/d and then plateaus,26 and it matched the NIH recommendation for calcium intake in children aged 11 to 24 at the time.27
The first session provided an overview of the program. Families completed a 7-day diet record following the first session in order to establish baseline calcium intake. This baseline calcium intake was used to develop an individualized plan for improving or maintaining calcium intake at each meal during the following weeks of the intervention. Subsequent sessions focused on a specific meal, starting with breakfast, and all activities were designed to reinforce methods of increasing dietary calcium for the specific meal. Examples of meal-specific calcium foods were provided, and nutrition labels were reviewed to illustrate calcium content. Weekly calcium goals were set at 400 mg/day for meals and 300 mg/day for snacks. The program is summarized in Supplemental Table 1.
Behavioral modification techniques were introduced at each session. Self-monitoring was achieved through the use of weighed food records. Children were reinforced for meeting daily calcium goal via sticker charts and parents were taught to use differential attention (praising/ignoring), contingency management, and behavioral contracting (identifying the rewards for children meeting calcium goals).
Measurements
The primary outcome was calcium intake as measured using the Calcium Counts!© food frequency questionnaire, which had been validated against 7-day weighed food records for estimating calcium intake in children.28 Because the questionnaire systematically overestimated calcium intake, an adjusted calcium intake was calculated based on the validation study and used for analyses. Calcium intake was assessed at all study visits (baseline, 6-,12-,18-,24-,30-, and 36-months).
Secondary outcomes included BMC (g), areal BMD (aBMD; g/cm2), and body composition assessed by DXA at baseline, 6-,12-,24-, and 36-month visits. DXA scans were obtained using a Hologic QDR 4500a densitometer (Hologic, Inc, Bedford, MA, USA) and analyzed in software version 12.3. Whole body (WB) and L1–L4 lumbar spine (LS) BMC and aBMD were assessed and converted into sex- and race- specific Z-scores respective to age and adjusted for height Z-scores using reference data from the Bone Mineral Density in Childhood Study.29 WB fat and lean body mass were expressed as fat mass index [FMI; FM (kg)/m2] and lean body mass index [LBMI; LBM (kg)/m2].30 LBM did not include BMC.
Height was assessed by stadiometer (Holtrain, Crymych), and weight by digital scale (Scaletronix, White Plains, NY, USA). Body mass index (BMI) was calculated as kg/m2. Height and BMI were converted into age- and sex-specific Z-scores using the 2000 CDC reference data.31 Pubertal maturation was assessed according to Tanner32 using a validated self-assessment questionnaire.33, 34 Non-fasting, serum 25-hydroxy vitamin D (25OHD) was assessed at baseline by 125I-labeled radioimmunoassay using a commercially available kit (DiaSorin, Stillwater, MN).35 Physical activity was assessed by self-report using a questionnaire and reported as hours/day.
Statistical Analysis
Data for all participants were analyzed according to assigned treatment group, regardless of adherence to study protocol (intention to treat analysis). Participants were allowed to continue in the protocol if BM-NE sessions or follow-up study visits were missed. Sample size calculation assumed 10% attrition, accounted for repeated measures and was determined in order to have 80% power at α=0.05 to detect a difference of at least 300 mg/day in calcium intake at study conclusion between the BM-NE and UC groups. Continuous variables were assessed for normality and reported as mean ± SD or median [interquartile range (IQR)], as appropriate. Longitudinal mixed effects models adjusted for covariates of interest were used to assess for an effect of the intervention on calcium intake, bone, and body composition outcomes over the study period. Initial models were developed to look at the main effects of time, intervention, and the time*treatment interaction (the main contrast of interest). Males and females were analyzed together as there were no sex*treatment or sex*time*treatment interactions. Secondary analyses were performed to look at the main effects of time and calcium intake and the time*calcium intake interaction on bone and body composition outcomes. Goodness of fit was assessed using the Akaike information criteria (AIC). Final models included a random intercept for subjects and random slope for visit. Analyses were performed using Stata 13.1 (Stata Corporation, College Station, TX), and a two-sided p-value of <0.05 was used to determine statistical significance.
RESULTS
Participants
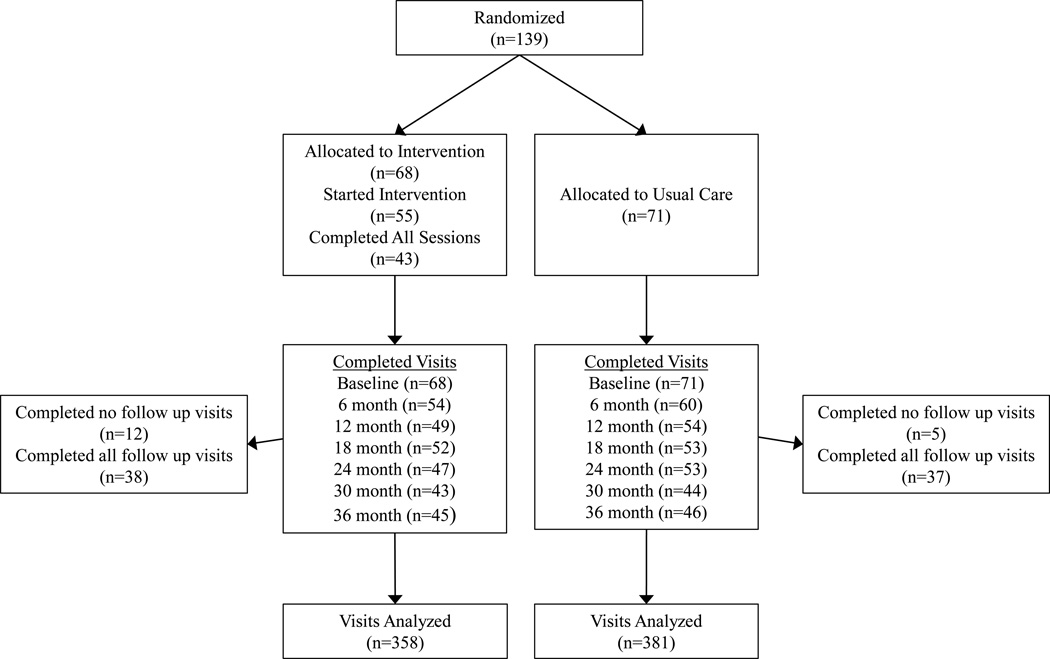
A total of 139 participants met inclusion criteria and were randomized into the BM-NE (n=68) or UC (n=71) treatment groups (Figure 1). The characteristics and baseline measures of calcium intake, bone and body composition outcomes, physical activity, and vitamin D levels did not differ between the two groups (Table 1). Vitamin D deficiency [defined as 25OHD <50 nmol/L(20 ng/mL)] was present in 16.7% of BM-NE and 14.1% of UC participants. The percentage of children in early puberty (Tanner Stage 1 or 2) did not differ by treatment group or sex at baseline (90.3% males versus 87.2% females, p=0.57) or at last follow-up (48.7% males versus 34.6% females, p=0.18).
Figure 1. Randomization and follow up of study participants.
Table 1.
Baseline characteristics of 139 participants in a behavioral modification and nutritional educational (BM-NE) trial to improve calcium intake
| BM-NE Intervention | Usual Care1 | |
|---|---|---|
| N | 68 | 71 |
| Female, n (%) | 43 (63.2) | 43 (60.6) |
| Age (yrs), median (range) | 9 (7.2 to 11) | 9.1 (7.2 to 11) |
| African American, n (%) | 19 (27.9) | 23 (32.4) |
| Recent fracture, n (%) | 0 (0) | 1 (1.4) |
| High risk2, n (%) | 54 (79.4) | 58 (81.7) |
| Lactose intolerant, n (%) | 6 (8.8) | 5 (7.1) |
| Milk refusal, n (%) | 35 (51.5) | 38 (53.5) |
| Family history osteoporosis, n (%) | 36 (52.9) | 47 (66.2) |
| Height Z-Score, mean ± SD | 0.17 ± 1.0 | 0.16 ± 0.94 |
| BMI Z-Score, median (IQR) | 0.12 (−0.49–0.79) | 0.19 (−0.39–0.86) |
| Tanner Stage 1–2, n (%) | 61 (89.7) | 61 (87.1) |
| 25-Hydroxy Vitamin D (ng/mL), median (IQR)3 | 30.1 (24.5–36.1) | 30.4 (25.6–39.2) |
| 25-Hydroxy Vitamin D (nmol/L), median (IQR)3 | 75.1 (61.2–90.1) | 75.9 (63.9–97.8) |
| Physical Activity (hrs/day), median (IQR) | 2.5 (1.5–3.3) | 2.3 (1.7–3.3) |
| Calcium Intake | ||
| Calcium intake (mg)4, median (IQR) | 876 (735–1039) | 910 (748–1078) |
| Meeting RDA for calcium, n (%) | 9 (13.2) | 14 (19.7) |
| DXA Bone and Body Composition | ||
| WB BMC (gm), median (IQR) | 932 (838–1079) | 953 (859–1068) |
| WB BMC Z-score, median (IQR) | −0.58 (−1.16–−0.09) | −0.54 (−0.93–−0.2) |
| WB aBMD (gm/cm2), mean ± SD | 0.744 ± 0.07 | 0.751 ± 0.07 |
| WB aBMD Z-score, mean ± SD | −0.97 ± 0.82 | −0.91 ± 0.75 |
| LS BMC (gm), median (IQR) | 22 (19 to 25) | 22 (19 to 25) |
| LS BMC Z-Score, mean ± SD | −0.16 ± 0.9 | −0.08 ± 0.8 |
| LS aBMD (gm/cm2), mean ± SD | 0.555 ± 0.07 | 0.568 ± 0.07 |
| LS aBMD Z-score, mean ± SD | −0.52 ± 0.88 | −0.39 ± 0.80 |
| FMI (kg/m2), median (IQR) | 3.8 (3.0–5.1) | 3.7 (3.0–4.8) |
| LBMI (kg/m2), median (IQR) | 12.4 (11.7–13.2) | 12.4 (11.8–13.4) |
No significant differences between BM-NE and usual care participants in any characteristic
Based on milk consumption, fracture history, family history of osteoporosis, lactose intolerance
n=54 (BM-NE), n= 57 (Usual Care)
Mg/day, adjusted by study dietician as described previously36
Abbreviations: BMC, Bone Mineral Content; BMD, Bone Mineral Density; BMI, Body Mass Index; BM-NE, Behavioral Modification and Nutritional Education; DXA, Dual Energy X-ray Absorptiometry; FMI, Fat Mass Index; LBMI, Lean Body Mass Index; LS, Lumbar Spine; RDA, Recommended Daily Allowance; WB, Whole Body
Of the 68 participants allocated to the BM-NE group, 55 (80.9%) started the intervention and 43 (63.2%) completed all educational sessions. A total of 17 (12.2%) participants (12 BM-NE and 5 UC) dropped out after the baseline visit and were lost to follow-up. Compared to participants completing one or more follow-up visit, those who were lost to follow-up were more likely to be African American, and had higher WB BMC. All other characteristics including calcium intake were similar. The percentage of participants who completed all study visits did not differ between the BM-NE and UC group, at 44.2% and 47.9%, respectively. Participants who missed at least one visit were more likely to be African American compared to those who completed all visits; there were no differences in other characteristics. The study ended with conclusion of planned study visits.
Effect of BM-NE intervention on calcium intake
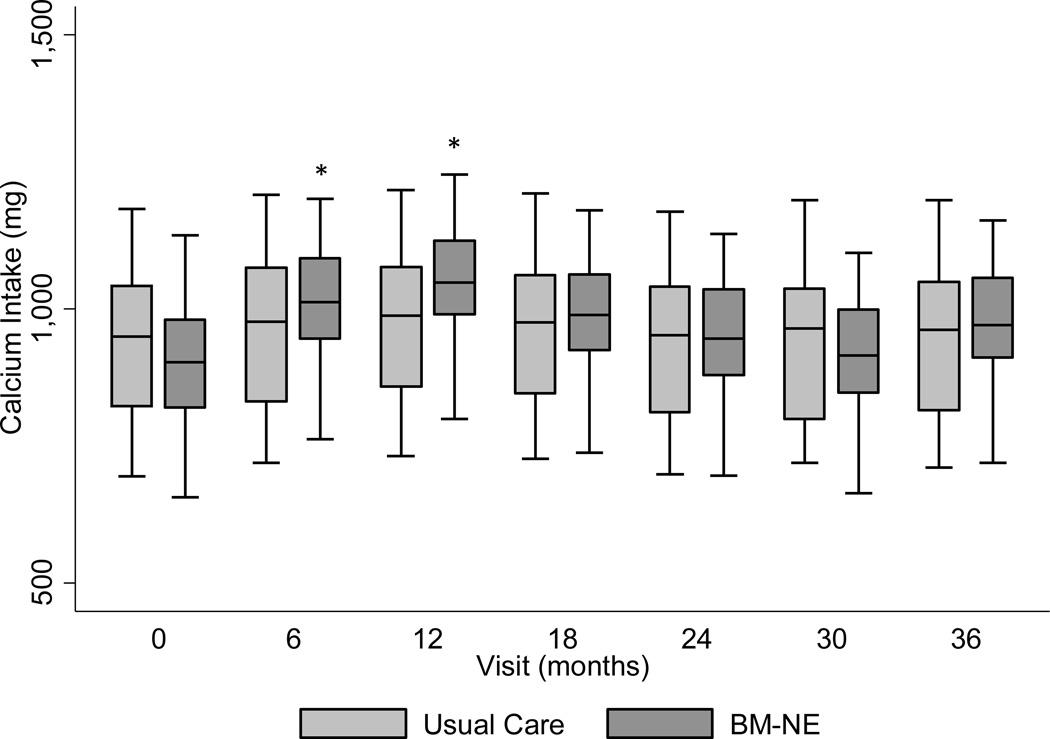
Calcium intake ranged from 876 (IQR: 735–1039) mg/day at baseline to 951 (IQR: 814–1126) mg/day at end of study in the BM-NE group and from 910 (IQR: 748–1078) mg/day at baseline to 897 (IQR: 750–1079) mg/day at end of study in the UC group (Table 2). There was no significant difference in calcium intake at end of study between the BM-NE and UC groups; and calcium intake at end of study was not significantly different from baseline in either group. Results of the longitudinal mixed model assessment of the effect of the BM-NE intervention on calcium intake over the study period are shown in Figure 2. Adjusted for age, sex, history of milk refusal, and African American ancestry, participants randomized to the BM-NE intervention had a significantly greater increase in calcium intake from baseline at 6 months (p=0.04) and 12 months (p=0.01), compared to those in the UC group.
Table 2.
Dietary calcium intake over time in behavioral modification-nutritional intervention (BM-NE) compared to usual care (UC) groups2
| BM-NE Intervention1 | Usual Care1 | p-value2 | adjusted p-value3 | |
|---|---|---|---|---|
| Baseline | 876 (735–1039)4 | 910 (748–1078) | 0.39 | -- |
| 6 months | 1008 (804–1164) | 967 (815–1093) | 0.34 | 0.04 |
| 12 months | 1008 (841–1217) | 956 (779–1100) | 0.09 | 0.01 |
| 18 months | 969 (806–1146) | 917 (750–1074) | 0.31 | 0.13 |
| 24 months | 937 (807–1088) | 903 (726–1059) | 0.35 | 0.27 |
| 30 months | 880 (772–1087) | 876 (758–1019) | 0.75 | 0.88 |
| 36 months | 951 (814–1126) | 897 (750–1079) | 0.24 | 0.17 |
Mg/day, adjusted by study dietician as described previously36
p-value represents pairwise comparison of raw dietary calcium intake at each visit
p-value represents group difference in calcium intake over time adjusted for age, sex, African American ancestry, and milk refusal
Median (inter-quartile range), all such values
Figure 2. Median adjusted calcium intake in the usual care compared to behavioral modification-nutritional education (BM-NE) intervention group.
Predicted calcium intake adjusted for age, sex, history of milk refusal, black racial group, BM-NE intervention*visit interaction. Calcium intake from food frequency questionnaire was adjusted by nutritionists to account for overestimation of calcium intake by participants.
* indicates significantly different from usual care, p<0.05
Effect of BM-NE intervention on bone outcomes
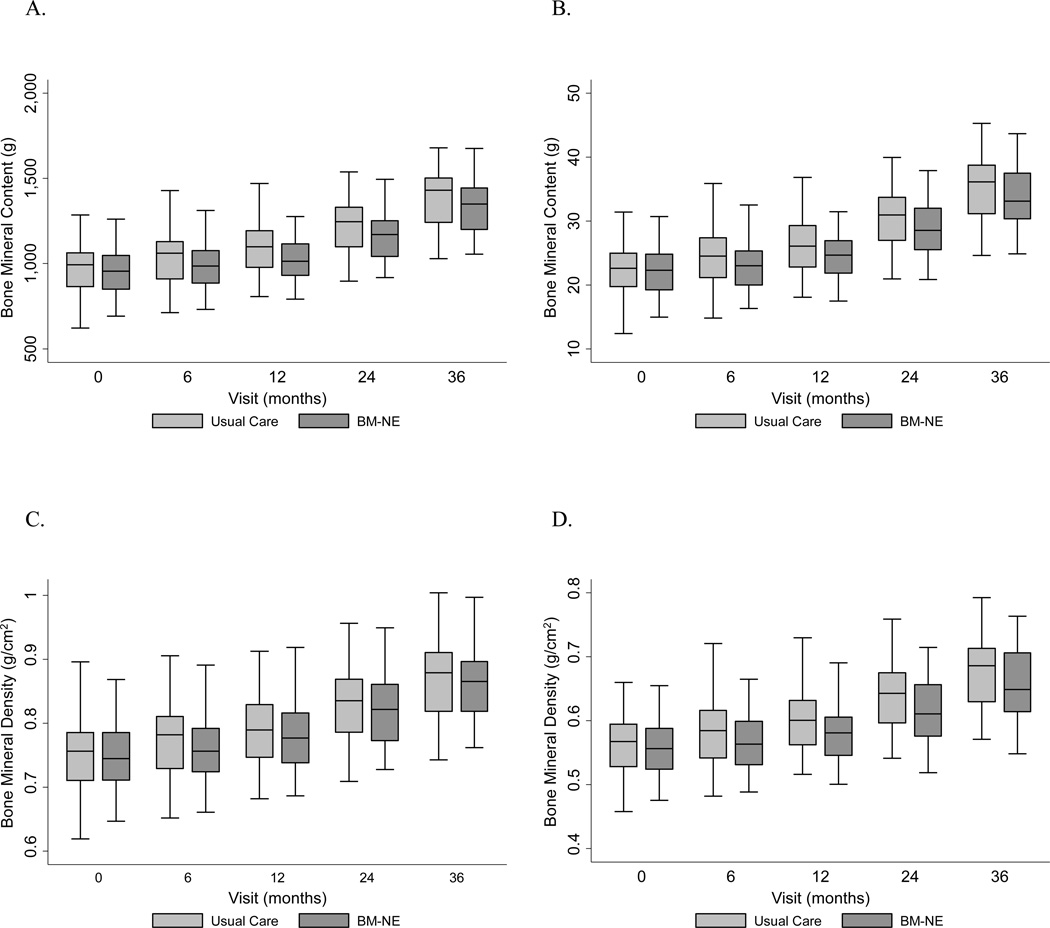
There were no significant differences between WB or LS aBMD or BMC between groups at end of study. Longitudinal mixed effects analysis adjusted for sex, age, height, African American ancestry and LBMI did not reveal a significant effect of the BM-NE intervention on BMC or aBMD accrual at any point over the study period (Figure 3). The results were similar when bone outcomes were converted into Z-scores, and again showed no effect of the intervention (Supplemental Figures 1 and 2). Additional analysis revealed no effect of calcium intake, irrespective of treatment group, on bone accrual (Table 3). Sensitivity analyses that (1) were restricted to participants that completed all study visits, (2) incorporated baseline 25OHD level and (3) incorporated self-reported physical activity all confirmed the lack of an effect of the intervention on bone accrual over 36 months.
Figure 3. Median whole body (A) and lumbar spine (B) bone mineral content and whole body (C) and lumbar spine (D) bone mineral density in the usual care compared to behavioral modification-nutritional education (BM-NE) intervention group.
Predicted whole body and lumbar spine bone mineral content and bone mineral density adjusted for age, sex, height, LBMI, and black racial group. No significant effect of the BM-NE was observed at any of the study time points for any bone outcome.
Table 3.
Effect of time*calcium intake interaction on bone and body composition outcomes
| Beta Coefficient | 95% CI | P-value | |
|---|---|---|---|
| Whole Body BMC1 | |||
| Calcium intake | −0.0004 | −0.04,0.04 | 0.98 |
| Calcium*6 month visit | 0.0001 | −0.04,0.04 | 0.99 |
| Calcium*12 month visit | 0.0124 | −0.03,0.56 | 0.57 |
| Calcium*24 month visit | 0.0035 | −0.05,0.06 | 0.91 |
| Calcium*36 month visit | 0.0652 | −0.01,0.14 | 0.08 |
| _cons | −986.1 | −1192.5,−779.5 | <0.0001 |
| Lumbar Spine BMC1 | |||
| Calcium intake | 0.0013 | −0.001,0.003 | 0.05 |
| Calcium*6 month visit | −0.0002 | −0.001,0.001 | 0.72 |
| Calcium*12 month visit | −0.0008 | −0.002,0.001 | 0.26 |
| Calcium*24 month visit | −0.0009 | −0.003,0.001 | 0.29 |
| Calcium*36 month visit | 0.0002 | −0.002,0.003 | 0.841 |
| _cons | −41.7 | −48.7,−34.6 | <0.0001 |
| Body Mass Index2 | |||
| Calcium intake | 0.0006 | −0.001,0.001 | 0.09 |
| Calcium*6 month visit | −0.0003 | −0.001,0.001 | 0.41 |
| Calcium*12 month visit | −0.0005 | −0.001,0.001 | 0.17 |
| Calcium*18 month visit | −0.0012 | −0.002,−0.001 | 0.002 |
| Calcium*24 month visit | −0.0012 | −0.002,−0.001 | 0.007 |
| Calcium*30 month visit | 0.0003 | −0.001,0.001 | 0.58 |
| Calcium*36 month visit | 0.0001 | −0.001,0.001 | 0.77 |
| _cons | 10.6 | 7.3,13.9 | <0.0001 |
| Fat Mass Index2 | |||
| Calcium intake | 0.0003 | −0.001,0.001 | 0.21 |
| Calcium*6 month visit | −0.0001 | −0.001,0.001 | 0.57 |
| Calcium*12 month visit | −0.0002 | −0.001,0.001 | 0.27 |
| Calcium*24 month visit | −0.0008 | −0.002,−0.001 | 0.02 |
| Calcium*36 month visit | 0.0002 | −0.001,0.001 | 0.57 |
| _cons | −0.2 | −2.5,2.1 | 0.86 |
additional covariates included sex, African American ancestry group, age, height, lean body mass
additional covariates included sex, African American ancestry group, age
Abbreviations: BMC, bone mineral content
Effect of calcium intake on adiposity
Median BMI increased from 16.7 (IQR: 15.3 to 18.0) to 17.9 (IQR:17.1 to 20.2) kg/m2 and 16.6 (IQR:15.3 to 18.4) to 19.1 (IQR:16.4 to 20.4) kg/m2 over the study in the BM-NE and UC groups, respectively. Changes in other anthropometric outcomes over the study are provided in Supplemental Table 2. Longitudinal mixed effects analysis adjusted for African American ancestry, age and sex did not reveal a significant effect of the BM-NE intervention on BMI over the study period. However, secondary longitudinal analyses did reveal a significant negative effect of calcium intake on BMI, irrespective of treatment group, such that higher calcium intake was associated with less increase in BMI at 18- and 24-month visits (Table 3). A similar negative association between calcium intake and FMI was found at the 24-month visit. Inclusion of pubertal status did not alter the effect of calcium intake on BMI and did not improve goodness of fit; therefore it was not included in final models.
DISCUSSION
This randomized controlled trial found that children who completed a six-week BM-NE program increased their dietary calcium intake to a greater extent compared to children who underwent a single educational session with a dietician. The positive effect on dietary calcium intake in the BM-NE group persisted for 12 months following completion of the intervention program. These results suggest that the BM-NE program effectively promoted changes in dietary behavior in regards to calcium intake. However, sustained dietary behavior changes beyond 12 months were not seen and may require more prolonged reinforcement than a six-week intervention.
Achieving and sustaining adequate calcium intake in childhood may be beneficial to lifelong skeletal health.36 Reduced bone mass is a known contributor to fracture risk in children and adults;37 and cross-sectional studies support a link between low milk intake in childhood and diminished BMD and greater fracture risk across the lifespan.38 Bone mineral accretion peaks in early to mid-puberty in conjunction with the adolescent growth spurt.39 Kinetic studies have shown that both calcium absorption from the gastrointestinal track and bone calcium deposition increase during early puberty;40 however increased absorption in the absence of appropriate intake may not be sufficient to achieve adequate calcium deposition into bone.41
Despite the greater calcium intake observed in BM-NE participants in the year following the intervention, we did not find an effect of the intervention on bone accrual over the study period. These results are in contrast to a calcium related behavioral intervention trial in children with rheumatoid arthritis which reported increased BMC accrual over 12 months in the intervention group.23 Another study of calcium intake and bone accrual following a nutritional and physical activity behavioral intervention in healthy children found an effect of the intervention to increase aBMD accrual at the hip in boys but not girls.42 Our study was conducted in healthy children and did not specifically target individuals with low calcium intake or impaired calcium metabolism. We speculate that our failure to detect an effect of the intervention on BMC or aBMD accrual was because the children in our study had baseline calcium intakes that were only modestly low, and they were able to achieve adequate calcium deposition into bone through increased calcium absorption. It is also notable that 60% of participants had a family history of osteoporosis. This may be a reason for the below average BMC and aBMD Z-scores at baseline. There is a strong heritable component to peak bone mass43 and it is possible that families with a history of osteoporosis may be more inclined to participate in a clinical trial.
In addition to the known relationship between calcium intake and skeletal health, there is growing evidence to suggest a beneficial association between calcium intake and obesity related cardiometabolic outcomes. In our study, greater calcium intake was associated with lesser increases in BMI and FMI irrespective of intervention group in study participants. These results are in keeping with the findings of previous prospective and randomized controlled trials which have identified positive effects of calcium supplementation on body composition;44, 45 although another study did not find an effect of calcium supplementation in addition to caloric restriction to promote weight loss.46 The mechanism linking calcium intake to obesity is unknown. It may be the result of dietary choice of nutrient rich milk versus calorically dense but nutrient poor sugar-sweetened beverages,47 a regulatory role for calcium or calciotropic hormones in the control of thermogenesis, fat oxidation, and lipolysis,48, 49 or an effect of gastrointestinal calcium content to decrease fat absorption.50 We used a food frequency questionnaire that did not capture total daily caloric intake, so we were unable to evaluate for potential relationships among calcium intake, caloric intake, and BMI in the current study.
Although the BM-NE intervention program was successful in promoting dietary change, there were limitations. The program consisted of five sessions over a six-week period. This represented a significant time commitment for families and study staff. The cost of offering an equivalent clinical program is unknown. Additionally, the families who agreed to participate in a study involving a nutrition intervention may have been motivated to institute dietary change, and may not be a representative sample of the population. However, we recruited families from a broad range of socioeconomic status and across the greater metropolitan area to minimize possible sources of sample bias. Future studies are necessary to determine whether the program can be simplified and if interval follow-up activities can be implemented to sustain increased calcium intake beyond 12 months. An additional limitation is the number of participants who did not complete all study visits. Longitudinal mixed methods is an accepted approach for dealing with missing data, and it appears that the characteristics of the participants who missed visits were similar between both treatment groups. Finally, blood samples were only collected at baseline, therefore we were unable to assess for an effect of the intervention on vitamin D status or to completely assess for an effect of vitamin D status to moderate the association between calcium intake and bone accrual.
In summary, this study found that a BM-NE intervention program designed to change dietary habits was successful in increasing calcium intake for up to 12 months in healthy children. These findings support the implementation and future study of BM-NE programs to help children improve dietary calcium intake.
Supplementary Material
Predicted whole body and lumbar spine bone mineral content Z-scores adjusted for LBMI. Z-scores are sex- and race-specific respective to age and adjusted for height Z-score. No significant effect of the BM-NE was observed at any of the study time points.
Predicted whole body and lumbar spine bone mineral density Z-scores adjusted for LBMI. Z-scores are sex- and race-specific respective to age and adjusted for height Z-score. No significant effect of the BM-NE was observed at any of the study time points.
Acknowledgments
This study was supported by R01HD037748. DRW was supported by NIH grants K12DK094723 and K12HD068373. The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. ClinicalTrials.gov identifier: NCT00063037
Footnotes
Conflict of interest statement: None of the other authors have a relevant conflict of interest to disclose.
The study was initiated prior to the publication of the CDC 2000 growth charts, so percent of ideal body weight was used instead of BMI percentiles.
Contributor Information
David R. Weber, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester NY, 14642, Phone: 585-275-7744; david_weber@urmc.rochester.edu; Fax: 585-244-6097.
Lori J. Stark, Cincinnati Children’s Hospital, 3333 Burnet Avenue, Cincinnati, Ohio, 45229, Phone: 513-636-4336 lori.stark@cchmc.org; Fax: 513-636-3677.
Richard F. Ittenbach, Cincinnati Children’s Hospital, 3333 Burnet Avenue, Cincinnati, Ohio, 45229, Phone: 513-803-3310 richard.ittenbach@cchmc.org; Fax: 513-636-1254.
Virginia A. Stallings, The Children’s Hospital of Philadelphia and Perelman School of Medicine at the University of Pennsylvania, 3535 Market Street, Room 1558, Philadelphia PA, 19104, Phone: 215-590-3630 stallingsv@email.chop.edu; Fax : 215.590.0604.
Babette S. Zemel, The Children’s Hospital of Philadelphia and Perelman School of Medicine at the University of Pennsylvania, 3535 Market Street, Room 1560, Philadelphia PA, 19104, Phone: 215-590-1669; zemel@email.chop.edu; Fax : 215.590.0604.
References
- 1.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 2.Martin AD, Bailey DA, McKay HA, Whiting S. Bone mineral and calcium accretion during puberty. Am J Clin Nutr. 1997;66(3):611–615. doi: 10.1093/ajcn/66.3.611. [DOI] [PubMed] [Google Scholar]
- 3.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaney RP. Effect of calcium on skeletal development, bone loss, and risk of fractures. Am J Med. 1991;91(5b):23s–28s. doi: 10.1016/0002-9343(91)90243-q. [DOI] [PubMed] [Google Scholar]
- 5.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. Bmj. 1991;303(6808):961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85(1):54–59. doi: 10.1093/ajcn/85.1.54. [DOI] [PubMed] [Google Scholar]
- 7.Alaimo K, McDowell MA, Briefel RR, Bischof AM, Caughman CR, Loria CM, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Adv Data. 1994;(258):1–28. [PubMed] [Google Scholar]
- 8.Salamoun MM, Kizirian AS, Tannous RI, Nabulsi MM, Choucair MK, Deeb ME, et al. Low calcium and vitamin D intake in healthy children and adolescents and their correlates. Eur J Clin Nutr. 2005;59(2):177–184. doi: 10.1038/sj.ejcn.1602056. [DOI] [PubMed] [Google Scholar]
- 9.Looker AC, Loria CM, Carroll MD, McDowell MA, Johnson CL. Calcium intakes of Mexican Americans, Cubans, Puerto Ricans, non-Hispanic whites, and non-Hispanic blacks in the United States. J Am Diet Assoc. 1993;93(11):1274–1279. doi: 10.1016/0002-8223(93)91954-o. [DOI] [PubMed] [Google Scholar]
- 10.Joo NS, Dawson-Hughes B, Yeum KJ. 25-Hydroxyvitamin D, calcium intake, and bone mineral content in adolescents and young adults: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3, 2008–2009 and V-1, 2010) J Clin Endocrinol Metab. 2013;98(9):3627–3636. doi: 10.1210/jc.2013-1480. [DOI] [PubMed] [Google Scholar]
- 11.Delahanty L, Kriska A, Edelstein S, Amodei N, Chadwick J, Copeland K, et al. Self-reported dietary intake of youth with recent onset of type 2 diabetes: results from the TODAY study. J Acad Nutr Diet. 2013;113(3):431–439. doi: 10.1016/j.jand.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucholz EM, Desai MM, Rosenthal MS. Dietary intake in Head Start vs non-Head Start preschool-aged children: results from the 1999–2004 National Health and Nutrition Examination Survey. J Am Diet Assoc. 2011;111(7):1021–1030. doi: 10.1016/j.jada.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston CC, Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327(2):82–87. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 15.Stear SJ, Prentice A, Jones SC, Cole TJ. Effect of a calcium and exercise intervention on the bone mineral status of 16–18-y-old adolescent girls. Am J Clin Nutr. 2003;77(4):985–992. doi: 10.1093/ajcn/77.4.985. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd T, Andon MB, Rollings N, Martel JK, Landis JR, Demers LM, et al. Calcium supplementation and bone mineral density in adolescent girls. Jama. 1993;270(7):841–844. [PubMed] [Google Scholar]
- 17.Lee WT, Leung SS, Wang SH, Xu YC, Zeng WP, Lau J, et al. Double-blind, controlled calcium supplementation and bone mineral accretion in children accustomed to a low-calcium diet. Am J Clin Nutr. 1994;60(5):744–750. doi: 10.1093/ajcn/60.5.744. [DOI] [PubMed] [Google Scholar]
- 18.Nowson CA, Green RM, Hopper JL, Sherwin AJ, Young D, Kaymakci B, et al. A co-twin study of the effect of calcium supplementation on bone density during adolescence. Osteoporos Int. 1997;7(3):219–225. doi: 10.1007/BF01622292. [DOI] [PubMed] [Google Scholar]
- 19.Lee WT, Leung SS, Leung DM, Cheng JC. A follow-up study on the effects of calcium-supplement withdrawal and puberty on bone acquisition of children. Am J Clin Nutr. 1996;64(1):71–77. doi: 10.1093/ajcn/64.1.71. [DOI] [PubMed] [Google Scholar]
- 20.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density during the development of peak skeletal mass. J Bone Miner Res. 1997;12(4):676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 21.Ward KA, Cole TJ, Laskey MA, Ceesay M, Mendy MB, Sawo Y, et al. The effect of prepubertal calcium carbonate supplementation on skeletal development in Gambian boys-a 12-year follow-up study. J Clin Endocrinol Metab. 2014;99(9):3169–3176. doi: 10.1210/jc.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers SW, Stark LJ, Chamberlin LA, Filigno SS, Sullivan SM, Lemanek KL, et al. Behavioral and nutritional treatment for preschool-aged children with cystic fibrosis: a randomized clinical trial. JAMA Pediatr. 2015;169(5):e150636. doi: 10.1001/jamapediatrics.2015.0636. [DOI] [PubMed] [Google Scholar]
- 23.Stark LJ, Davis AM, Janicke DM, Mackner LM, Hommel KA, Bean JA, et al. A randomized clinical trial of dietary calcium to improve bone accretion in children with juvenile rheumatoid arthritis. J Pediatr. 2006;148(4):501–507. doi: 10.1016/j.jpeds.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Stark LJ, Hommel KA, Mackner LM, Janicke DM, Davis AM, Pfefferkorn M, et al. Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2005;40(4):501–507. doi: 10.1097/01.mpg.0000157913.32465.45. [DOI] [PubMed] [Google Scholar]
- 25.Reinehr T, Schaefer A, Winkel K, Finne E, Toschke AM, Kolip P. An effective lifestyle intervention in overweight children: findings from a randomized controlled trial on "Obeldicks light". Clin Nutr. 2010;29(3):331–336. doi: 10.1016/j.clnu.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Matkovic V, Heaney RP. Calcium balance during human growth: evidence for threshold behavior. Am J Clin Nutr. 1992;55(5):992–996. doi: 10.1093/ajcn/55.5.992. [DOI] [PubMed] [Google Scholar]
- 27.Opitmal Calcium Intake. NIH Consensus Statement. 1994;12(4):1–31. [PubMed] [Google Scholar]
- 28.Zemel BS, Carey LB, Paulhamus DR, Stallings VA, Ittenbach RF. Quantifying calcium intake in school age children: development and validation of the Calcium Counts! food frequency questionnaire. Am J Hum Biol. 2010;22(2):180–186. doi: 10.1002/ajhb.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 32.Tanner J. Growth at Adolescence. 2nd. Oxford: Blackwell Scientific Publication; 1962. [Google Scholar]
- 33.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 34.Schall JI, Semeao EJ, Stallings VA, Zemel BS. Self-assessment of sexual maturity status in children with Crohn's disease. J Pediatr. 2002;141(2):223–229. doi: 10.1067/mpd.2002.125907. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–533. [PubMed] [Google Scholar]
- 36.Institute of Medicine. Dietary references intakes for calcium and vitamin D. 2010 [Google Scholar]
- 37.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21(9):1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77(1):257–265. doi: 10.1093/ajcn/77.1.257. [DOI] [PubMed] [Google Scholar]
- 39.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(Suppl 3(3)):S191–S194. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 40.Abrams SA, Copeland KC, Gunn SK, Gundberg CM, Klein KO, Ellis KJ. Calcium absorption, bone mass accumulation, and kinetics increase during early pubertal development in girls. J Clin Endocrinol Metab. 2000;85(5):1805–1809. doi: 10.1210/jcem.85.5.6508. [DOI] [PubMed] [Google Scholar]
- 41.Abrams SA, Stuff JE. Calcium metabolism in girls: current dietary intakes lead to low rates of calcium absorption and retention during puberty. Am J Clin Nutr. 1994;60(5):739–743. doi: 10.1093/ajcn/60.5.739. [DOI] [PubMed] [Google Scholar]
- 42.Hovell MF, Nichols JF, Irvin VL, Schmitz KE, Rock CL, Hofstetter CR, et al. Parent/Child training to increase preteens' calcium, physical activity, and bone density: a controlled trial. Am J Health Promot. 2009;24(2):118–128. doi: 10.4278/ajhp.08021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krall EA, Dawson-Hughes B. Heritable and life-style determinants of bone mineral density. J Bone Miner Res. 1993;8(1):1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- 44.DeJongh ED, Binkley TL, Specker BL. Fat mass gain is lower in calcium-supplemented than in unsupplemented preschool children with low dietary calcium intakes. Am J Clin Nutr. 2006;84(5):1123–1127. doi: 10.1093/ajcn/84.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. 2004;12(4):582–590. doi: 10.1038/oby.2004.67. [DOI] [PubMed] [Google Scholar]
- 46.Shapses SA, Heshka S, Heymsfield SB. Effect of calcium supplementation on weight and fat loss in women. J Clin Endocrinol Metab. 2004;89(2):632–637. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller KL, Kirzner J, Pietrobelli A, St-Onge MP, Faith MS. Increased sweetened beverage intake is associated with reduced milk and calcium intake in 3- to 7-year-old children at multi-item laboratory lunches. J Am Diet Assoc. 2009;109(3):497–501. doi: 10.1016/j.jada.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. Faseb j. 2000;14(9):1132–1138. [PubMed] [Google Scholar]
- 49.Ping-Delfos WC, Soares M. Diet induced thermogenesis, fat oxidation and food intake following sequential meals: influence of calcium and vitamin D. Clin Nutr. 2011;30(3):376–383. doi: 10.1016/j.clnu.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen R, Lorenzen JK, Toubro S, Krog-Mikkelsen I, Astrup A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int J Obes (Lond) 2005;29(3):292–301. doi: 10.1038/sj.ijo.0802785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted whole body and lumbar spine bone mineral content Z-scores adjusted for LBMI. Z-scores are sex- and race-specific respective to age and adjusted for height Z-score. No significant effect of the BM-NE was observed at any of the study time points.
Predicted whole body and lumbar spine bone mineral density Z-scores adjusted for LBMI. Z-scores are sex- and race-specific respective to age and adjusted for height Z-score. No significant effect of the BM-NE was observed at any of the study time points.