Abstract
The immune system has evolved a variety of mechanisms to achieve and maintain tolerance both centrally and in the periphery. Central tolerance is achieved through negative selection of autoreactive T cells, while peripheral tolerance is achieved primarily via three mechanisms: activation-induced cell death, anergy, and the induction of regulatory T cells. Three forms of these regulatory T cells have been described: those that function via the production of the cytokine IL-10 (T regulatory 1 cells), transforming growth factor beta (Th3 cells), and a population of T cells that suppresses proliferation via a cell-contact-dependent mechanism (CD4+CD25+ TR cells). The present review focuses on the third form of peripheral tolerance – the induction of regulatory T cells. The review will address the induction of the three types of regulatory T cells, the mechanisms by which they suppress T-cell responses in the periphery, the role they play in immune homeostasis, and the potential these cells have as therapeutic agents in immune-mediated disease.
Keywords: interleukin-10, regulatory T cell, suppression, transforming growth factor beta, tolerance
Introduction
The ability of the immune system to distinguish between self-antigens and nonself-antigens, and between harmful and innocuous foreign antigens, is critical to the maintenance of immune homeostasis. Failure to maintain tolerance to self-antigens or innocuous antigen results in the development of autoimmune or allergic disease, respectively. To achieve this state of immune tolerance, the immune system has evolved a variety of mechanisms. These include deletion of self-reactive clones in the thymus through a process referred to as negative selection, or central tolerance [1]. Central tolerance is imperfect, however, and self-reactive T cells do appear in the periphery. Likewise, the immune system is continuously distinguishing between innocuous and pathogenic foreign antigens.
To deal with these situations the immune system has evolved a system of induced peripheral tolerance. Two well-characterized mechanisms of peripheral tolerance are the death of self-reactive T cells via negative selection and the induction of a state of nonresponsiveness, or anergy [2]. A third, less well-characterized, mechanism is the active suppression of T-cell responses. This latter mechanism involves a recently described T-cell subset, known as regulatory T cells, which are induced in the periphery in an antigen-specific fashion [3,4]. The present review will discuss the various types of regulatory T cells, as well as the mechanisms that have been described for their generation.
Natural versus acquired regulatory T cells
Several classes of regulatory T cells, capable of suppressing antigen-specific immune responses, have been identified and characterized. These subsets can be distinguished in a variety of ways; including whether suppression is cell-contact mediated or is mediated through soluble factors such as IL-10 and transforming growth factor beta (TGF-β). These cells can also be distinguished based on where they originate, in the thymus or in the periphery.
One prevailing model is that a class of regulatory T cells that originate in the thymus, are self-reactive and are involved in protection from autoimmune responses [5]. This class of cells referred to as 'natural' T regulatory cells (TR) are characterized by the expression of the IL-2 receptor α-chain (CD25), and more recently by the forkhead/winged-helix transcription factor FoxP3 [6-9]. These TR have the ability to suppress the activation of conventional T cells in a cell-contact-dependent, IL-10-independent and TGF-β-independent, manner [10,11]. On the other hand, 'acquired' regulatory T cells arise in the periphery, either during an immune response or after encountering a tolerogenic dendritic cell. These regulatory T cells are believed to differentiate from naïve precursors and are specific for antigens not presented in the thymus, such as food antigens, bacterial flora, pathogens, and self-antigens such as insulin [3]. They suppress the activation of conventional T cells in a cytokine-dependent manner: TGF-β for Th3 cells, and IL-10 for T regulatory 1 (Tr1) cells (Table 1) [4,12,13].
Table 1.
Cytokine expression profiles of the three classes of regulatory T cells
| Cytokine expressed | Th3 cells | T regulatory 1 cells | CD4+CD25+ TR cells |
| Interferon gamma | +/- | + | - |
| IL-4 | +/- | - | - |
| Transforming growth factor beta | +++ | ++ | +/- |
| IL-10 | +/- | +++ | +/- |
The production of cytokine is indicated as absent (-) or present (+) with relative quantities of cytokine indicated by +/- < + < ++ < +++.
Recent work has shown that this distinction between natural and acquired Tregs may be simplistic. As discussed in the following, Tregs with the properties of CD4+CD25+ (TR) have been shown to be generated in vitro and in vivo in systems using both self-antigens and foreign antigens. The present review will summarize the recent findings on the development of acquired Tregs, and on their potential function in regulating immune responses to both self-antigens and foreign antigens.
Th3 cells
It has long been recognized that the oral administration of antigen can lead to immunological tolerance to that antigen. Recent work has begun to shed light on the mechanisms that underlie this process. Oral tolerance is established in the gut-associated lymphoid tissue, which consists of Peyer's patches, intraepithelial lymphoid cells, and scattered lymphoid cells in the lamina propria. Several lines of evidence have shown that oral tolerance is an active, ongoing process. For example, a high antigen dose leads to hyporesponsiveness mediated by anergy or deletion [14,15]. On the other hand, low doses of antigen lead to the generation of Th2 cells, as well as to active suppression through the generation of antigen-specific regulatory T cells known as Th3 cells [16].
Th3 cells produce TGF-β, but differ from classical Th2 cells in that TGF-β expression does not always correlate with IL-4 or IL-10 expression [12]. Importantly, these Th3 cells have been shown to transfer tolerance in vivo, and to suppress antigen-specific responses in vitro [16]. In both cases the suppression is mediated by TGF-β. Further support for the role of TGF-β in regulating immune responses comes from studies using mice that lack the ability to either produce or respond to TGF-β. In both instances the mice develop a fatal autoimmune lymphoproliferative disease [17]. As these mice have normal CD4+CD25+ TR cells (see later), these data suggest that a defect in either Th3 or Tr1 cells is responsible for the observed autoimmunity [17,18].
Tr1 cells
In addition to TGF-β, IL-10 has been shown to be a potent immunoregulatory cytokine [13,19]. The mechanism by which IL-10 regulates immune responses involves both T cells and antigen presenting cells (APCs). IL-10 treatment of dendritic cells results in the downmodulation of the co-stimulatory molecules CD80 and CD86, as well as MHC class II, and decreases the ability of these dendritic cells to activate T cells [20,21]. IL-10 can also have direct effects on CD4+ T cells. Constant antigen stimulation of T cells in the presence of IL-10, either in the presence or the absence of APCs, results in anergy [20,22-24]. Unlike other anergic CD4+ T cells, however, anergy in IL-10-treated T cells is not reversed by the addition of IL-2 or IL-15 [23]. When these IL-10-anergized T cells are driven to proliferate, they have a unique cytokine expression profile, producing high amounts of IL-10 and TGF-β, lesser amounts of interferon gamma, and no IL-2 or IL-4 [13,22]. CD4+ T cells with this phenotype are referred to as Tr1 cells.
In spite of the fact that Tr1 cells have poor proliferative capabilities, they express normal levels of T cell activation markers, including CD25, CD40L, and CD69, following TCR stimulation [20]. Tr1 cells have been shown to regulate immune responses both in vitro and in vivo. For example, co-culture of Tr1 cells with freshly isolated CD4+ T cells in the presence of allogeneic APCs results in the suppression of the proliferative allo-response [25,26]. Neutralization of IL-10 and/or TGF-β reverses this suppression [22]. Tr1 cells have also been shown to be capable of suppressing antibody production by B cells [27], and to decrease the ability of monocytes and dendritic cells to act as APCs. Kemper and colleagues [28] very recently showed that human Tr1 cells can be derived by stimulating CD4+ T cells through co-engagement of CD3 and the complement regulator CD46 in the presence of IL-2. These conditions resulted in IL-10-producing cells capable of inhibiting the activation of bystander T cells. Unlike the Tr1 cells described earlier, CD3/CD46-generated Tr1 cells exhibited strong and prolonged proliferation when stimulated. There thus appear to be multiple pathways capable of producing IL-10 secreting regulatory T cells.
Tr1 cells also have potent effects on in vivo immune responses. Studies with allograft systems have shown that long-term graft tolerance correlates with the presence of CD4+ T cells that suppress naïve T cells via IL-10 and TGF-β [29-31]. In mouse systems, CD4+ T cells with Tr1-like properties have been isolated following tolerance induction to allergens [32,33], as well as in models of autoimmunity [34,35] and in response to infectious pathogens [36,37].
As already described, IL-10-treated dendritic cells are capable of driving the generation of Tr1 cells in vitro. However, the nature of the in vivo dendritic cell subset responsible for Tr1 cell differentiation remains unclear. Several groups have isolated specific dendritic cell subsets from nonlymphoid peripheral tissues that are capable of inducing tolerance. These subsets have been isolated from a variety of tissues, including the liver, the lung, and the intestine, and they appear to function via IL-10 secretion [32,38-40]. Wakkach and colleagues [41] recently identified a subset of dendritic cells in the spleen and lymph node that appear to be a natural tolerizing dendritic cell subset. The cells have a plasmacytoid morphology and remain immature even after in vitro activation with lipopolysaccharide or CpG, they have an unusual cell-surface phenotype (CD11clo/CD45RBhi), and they produce large amounts of IL-10 when stimulated. These cells are capable of directly generating Tr1 cells in vitro and in vivo, and may represent a naturally occurring dendritic cell subset involved in eliciting tolerance in vivo [41]. The identification of this dendritic cell subset, as well as the demonstration that Tr1 cells can regulate immune responses in vivo, thus enhances the possible therapeutic uses of Tr1 cells as a means to regulate immune responses in a variety of diseases.
CD4+CD25+, cell-contact-dependent TR cells
A third regulatory T cell population has been identified, which is characterized by the expression of the cell surface markers CD4 and CD25 (referred to as TR cells). These CD25+CD4+ (TR) cells are anergic, but upon activation suppress the proliferation and IL-2 production of naive and memory CD4+ T cells through a contact-dependent, cytokine-independent mechanism [10].
In mice, TR cells are thought to represent a population of T cells that are thymically derived and suppress autoreactive CD4+ T cells. This is supported by the finding that thymectomy of mice at day 3 of life leads to a lack of TR cells and produces a spectrum of spontaneous organ-specific autoimmune manifestations including autoimmune gastritis, oophoritis, orchitis, and thyroiditis [42]. Mice that have undergone thymectomy are rescued by the infusion of CD4+CD25+ T cells [43,44], and the removal of CD4+CD25+ using depleting antibody leads to a similar autoimmune phenotype seen in mice after thymectomy [45]. Studies of experimental autoimmune encephalomyelitis have demonstrated the protective effect of these regulatory cells in the response to inflammation directed against self-antigens [46], and additional studies of thyroiditis [47], diabetes [48], and nerve injury [49] have suggested that the TR responses are specific for self-antigens. It is thought that TR cells in mice represent those thymocytes with the highest affinity for self-peptide but that are below the threshold of negative selection [50]. Only a small number of TR cells are thus selected, all of which are more sensitive to self-antigens than other circulating autoreactive T cells.
The molecular basis for the development and function of TR cells remains unclear. Work in mice with targeted mutations suggests a role for several molecules in the development and function of TR cells. One gene clearly associated with the development and function of TR cells is FoxP3. Mice carrying the X-linked scurfy mutation develop a lymphoproliferative disease, display a multi-organ autoimmune disease, and lack conventional CD4+CD25+ TR cells [6,7,51-53]. In mice, FoxP3 has been shown to be expressed exclusively in CD4+CD25+ TR cells and is not induced upon activation of CD25- T cells. When FoxP3 is introduced via retrovirus or enforced transgene expression, however, naive CD4+CD25- T cells are converted to TR cells [8]. Thus, in mice, FoxP3 is both necessary and sufficient for the development and function of CD4+CD25+ TR cells.
TR cells with properties similar to those described in the mouse are present in humans. These cells represent 1–3% of all CD4+ T cells and require activation to induce suppressor function, which is mediated via cell–cell contact, and is abrogated by the addition of IL-2 [54,55]. In humans, TR cells have been shown to regulate T-cell responses to both foreign antigen and self-antigen [56], including TR cells specific for alloantigens [57]. TR cells in humans, as in mice, express FoxP3 [58], and individuals with a mutation in the FoxP3 gene develop immunodysregulatory, polyendocrinopathy, enteritis X linked syndrome, a disease similar to that seen in scurfy mice [59].
However, the source of the CD4+CD25+ TR cells found in the peripheral blood of humans, whether thymic or peripheral, is not known. CD4+CD25+ TR cells have been identified in the human thymus [60], and TR cells with a naïve phenotype have been identified in cord blood [61]. Those TR cells isolated from adult peripheral blood are CD4+CD25+ CD45RO+ CD45Rblow [62] and have a short telomere length, however, both of which suggest that this population of TR cells is thought to be derived from highly differentiated memory cells [56]. Yet, in humans, it is impossible to prove whether the TR cells isolated from peripheral blood originate in the thymus, and are expanded in the periphery, or whether they have been generated in the periphery. The possibility exists that, due to differences between mouse and man in life expectancy, thymic involution, and antigen exposures, the development of CD4+CD25+ TR cells may occur in the periphery in man.
Induction of CD4+CD25+ TR cells in the periphery
The induction of TR cells that resemble the 'natural' or thymically derived TR cells described in mice has been described in man. The induction is based upon the ability to create CD4+CD25+ T cells from nonregulatory cells that suppress proliferation of T cells in a contact-dependent, cytokine-independent manner. In all cases, although the conditions under which these cells are induced differ, activation of CD4+ T cells is required to generate a TR cell. Both in vivo and in vitro studies in mice support the idea that these cells can arise outside of the thymus. TR cells have been identified in the periphery of mice under conditions that do not favor TR cell development in the thymus [63]. The administration of oral, subcutaneous, intravenous antigen [64-66] or a repeated [67-70] exposure to superantigen [67] have been reported to induce CD4+CD25+ TR cells in the periphery of mice.
Induction of TR cells from peripheral CD4+CD25- T cells in vitro has been reported by several groups. Duthoit and colleagues have demonstrated that recently activated T cells (4 days post stimulation) are anergic, express FoxP3, and suppress the proliferation of naïve T cells via a cell-contact-dependent mechanism in co-culture experiments [68]. Additionally, in vitro induction of TR cells by activation of CD4+CD25- T cells in the presence of TGF-β has been reported by two groups [69,70]. In the most recent of these reports, Chen and colleagues have shown that the induction of both FoxP3 expression and TR cell function in previously nonregulatory CD4+CD25- T cells required both TCR activation and TGF-β exposure [70]. However, Piccirillo and colleagues [66] have found that TR cell function is normal in the absence of either TGF-β production or responsiveness. We have also shown that T cells from mice expressing a T-cell-specific transgene encoding a dominant-negative TGF-βRII have normal levels of FoxP3 (K Newton, SF Ziegler, unpublished data).
In humans, CD4+CD25+ T cells with regulatory activity requiring only cell-cell contact have been induced via activation under several different conditions. Taams and colleagues [56] used T-cell clones to demonstrate that activation of these clones with peptide only, in the absence of co-stimulation, leads to T cells that are anergic and suppress proliferation of other T-cell clones via cell contact [56]. TR cells specific to allogeneic antigens have been generated in vitro by activation with IL-10-treated allogeneic dendritic cells [20]. Induction of TR cells from CD4+CD25- T cells has also been successful by activation of CD4+CD25- T cells with mature, allogeneic dendritic cells, and these T cells also expressed FoxP3. The specificity of the TR cells was determined by the type of mature dendritic cells used: autologous dendritic cells generate TR specific for self-antigens [71], and allogeneic dendritic cells produce alloreactive TR cells (MR Walker, JH Buckner, SF Ziegler, unpublished data).
The induction of CD4+CD25+ TR cells in the absence of APCs has also been achieved in vitro. Our group has recently demonstrated that activation of CD4+CD25- T cells with plate-bound anti-CD3 and soluble anti-CD28 can induce a group of CD4+CD25+ T cells with regulatory function that express FoxP3. These TR cells are derived from highly purified CD4+CD25- cells; they become CD25+FoxP3+ within 4 days of activation and regulate in a contact-dependent, cytokine-independent manner. The function and cell surface markers of these cells are indistinguishable from the CD4+CD25+ T cells directly isolated from the peripheral blood that have been defined as 'natural' TR cells [58]. Unlike that reported in the mouse, induction of FoxP3 in this system does not require the presence of TGF-β. However, the induction does require engagement of the TCR and co-stimulation through CD28. Similarly, induction of TR cells with mature dendritic cells also required MHC II and CD80/86 co-stimulation to induce TR cells [71]. The induction of TR cells in vitro has also been shown using αCD3 and a novel antibody 4C8 [72] or exposure to staphylococcal enterotoxin B for 7 days in culture [67].
Each of these systems used to induce TR cells have differences; however, several common factors are present. TR cells can be generated from peripheral CD4+CD25- T cells, but only in response to activation. The activation conditions required for that induction might differ between mouse and man. However, differences in culture conditions and assays used to measure suppression make these comparisons difficult, and more work is needed to clarify these apparent differences. For example, differences between the species in the expression of surface molecules on T cells may contribute. Human T cells, unlike those from rodents, express HLA class II and co-stimulatory molecules upon activation, which may allow induction of TR cells to occur in the absence of an APC. In addition, if the differentiation and function of TR cells are regulated by the expression of FoxP3 then, as the regulation of FoxP3 expression becomes better understood, the requirements for TR cell induction will become more apparent and the differences between mouse and man will be better understood.
Our ability to generate TR cells in the periphery suggests a larger question: Do TR cells represent a lineage of T cells or a state of activation that may be achieved by any T cell under the appropriate conditions of activation? The induction of TR cells in the periphery allows for a dynamic immune response when the body is threatened by infection or injury. In this setting T cells will become activated, and will recruit other T cells and other inflammatory cells and mediators to the site. As the response becomes mature, a group of regulatory T cells will develop locally as a result of the local milieu allowing for a resolution of inflammation and regulating the responses directed to self-antigens exposed during the inflammatory response.
Role of peripherally generated TR cells in the immune response to foreign antigens
Regulation of immune responses is required to protect individuals from autoreactive T cells that have escaped into the periphery. Regulation of autoreactivity is present at the level of thymic selection, but also in the periphery. Those autoreactive cells that escape negative selection must be restrained in the periphery, and it is thought that CD4+CD25+ TR cells generated in the thymus perform that role. In addition to regulation of autoimmune responses, the T-cell response to foreign antigens must be regulated as well. This regulation occurs in several forms: activation induced cell death once antigen or co-stimulation becomes limiting at the site of inflammation, the production of cytokines that lead to inhibition of T-cell responses, or the development of Tr1 or Th3 cells.
These regulatory phenomena are very important in the resolution of inflammation, to rein in the T cell response, to control bystander responses to self-antigens and to limit the resulting T cells for future responses, so as to avoid overwhelming immunologic reactions upon a repeated exposure to an antigen. In addition, regulation of the immune response may allow the fittest T cells to survive in a nutrient-limiting environment in order to proceed to become memory T cells. Evidence that this balancing act involves both cytokines and CD4+CD25+ TR cells has been found with cutaneous infection of mice with Leishmania major, where the presence of TR cells leads to a low-level persistence of the pathogen but allows for the development of long-term immunity, whereas animals that lack TR cells or IL-10 are able to completely clear the infection but do not have any resistance to a second infection [73].
As we have already described, TR cells may be generated at the site of inflammation through the activation of CD4+CD25- T cells, inducing the expression of FoxP3 and CD25. In this way, activation itself would lead to a limitation of the extent to which the T-cell response could proceed. It is not yet known whether the CD4+CD25- T cells capable of differentiating into TR cells following activation are derived from a separate lineage of CD4+ T cells, and whether their induction is a result of the initial activation early in the inflammatory process or occurs late upon depletion of growth signals (e.g. cytokines) in the local environment. We propose that these cells act by inhibiting further proliferation of T cells as the inflammatory process has peaked and nutrients are limiting. This allows a limited number of the most fit T cells to survive and become memory cells, while the remaining T cell response resolves through activation induced cell death (Fig. 1). In addition, CD4+CD25+ TR cells leading to 'infectious tolerance', thus inducing the local induction of Th3 or Tr1 cells [74], extends this paradigm. The fate of the TR cells induced at the site is not known – whether these cells exist only transiently then die, whether they persist in the body as TR cells, or whether they return to their previous role as nonregulatory resting T cells remains unknown.
Figure 1.
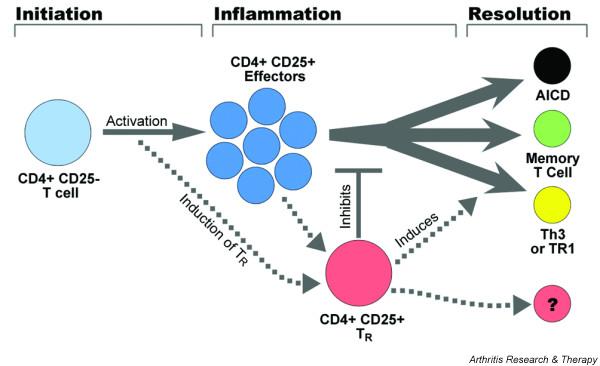
Schematic representation of the fate of CD4 T cells at a localized site of inflammation. Naïve or memory CD4+CD25- T cells are recruited to the site, then become activated upon exposure to antigen and co-stimulation. These cells proliferate and become CD4 T effectors. Activation also induces a subset of CD4+CD25- T cells to upregulate CD25 and FoxP3 and acquire CD25+CD4+ (TR) cell function. These cells may result from activation at the initiation of the response or, more probably, as the response matures. As antigen and IL-2 is depleted, effector T cells undergo activation induced cell death, TR cells lead to the induction of T regulator 1 (Tr1) cells and Th3 cells, which feed back to inhibit inflammation, and the TR cells inhibit proliferation of antigen-specific and bystander T cells. This results in a small number of CD4+ T cells surviving, which persist as memory T cells.
The balancing act required by the immune system to attack foreign invaders, to retain memory for future exposures to an antigen while reining in an inflammatory response once the danger has passed, and to retain tolerance to self probably combines many mechanisms both centrally and in the periphery. Our understanding of regulation is now expanding with the identification of peripherally generated Tr1, Th3 and TR cells. The implication is that these cells may play a role in human disease. TR cells have been isolated from tumors and could contribute to inadequacy of the immune response against these tumors. While inflammatory disease such as allergy and autoimmunity may occur when the T regulatory response is inadequate, a lack of TR cell function has been demonstrated in autoimmune polyglondular syndrome II [75]. It is likely that more subtle defects in the generation of regulatory response in the periphery could lead to manifestations of autoimmunity. Our ability to generate such regulatory cells holds promise for the development of new therapies to enhance regulation to treat autoimmune disease. A better understanding of how these forces work together will allow us to understand immunologic settings where either the immune response is inadequate, such as the response to tumors, or it is misdirected, as in the case of autoimmune disease and allergy.
Competing interests
None declared.
Abbreviations
APC = antigen presenting cell; IL = interleukin; MHC = major histocompatibility complex; TCR = T-cell receptor; TGF-β = transforming growth factor beta; Th = T helper cell; Tr1 = T regulatory 1; TR = CD4+CD25+ T regulatory cell.
Acknowledgments
Acknowledgements
The authors thank Matt Warren for assistance with the preparation of this manuscript. This work was supported by NIH NIAID grants AI48779 and AI54610, and by grants from the American Diabetes Association and Juvenile Diabetes Research Foundation International to SFZ.
References
- Sprent J, Kishimoto H. The thymus and negative selection. Immunol Rev. 2002;185:126–135. doi: 10.1034/j.1600-065X.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Groux H. An overview of regulatory T cells. Microbes Infect. 2001;3:883–889. doi: 10.1016/S1286-4579(01)01448-4. [DOI] [PubMed] [Google Scholar]
- Cottrez F, Groux H. Specialization in tolerance: innate CD(4+)CD(25+) versus acquired TR1 and TH3 regulatory T cells. Transplantation. 2004;77:S12–S15. doi: 10.1097/01.TP.0000106471.23410.32. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/S1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/S1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263–276. doi: 10.1159/000067596. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993;23:935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191:1187–1196. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.V99.7.2468. [DOI] [PubMed] [Google Scholar]
- Koppelman B, Neefjes JJ, de Vries JE, de Waal MR. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–871. doi: 10.1016/S1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecart S, Boulay V, Raison-Peyron N, Bousquet J, Meunier L, Yssel H, Pene J. Phenotypic characterization of human CD4+regulatory T cells obtained from cutaneous dinitrochlorobenzene-induced delayed type hypersensitivity reactions. J Invest Dermatol. 2001;117:318–325. doi: 10.1046/j.1523-1747.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- Cavani A, Nasorri F, Prezzi C, Sebastiani S, Albanesi C, Girolomoni G. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Invest Dermatol. 2000;114:295–302. doi: 10.1046/j.1523-1747.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- Kitani A, Chua K, Nakamura K, Strober W. Activated self-MHC-reactive T cells have the cytokine phenotype of Th3/T regulatory cell 1 T cells. J Immunol. 2000;165:691–702. doi: 10.4049/jimmunol.165.2.691. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Holler E, Roncarolo MG, Hintermeier-Knabe R, Eissner G, Ertl B, Schulz U, Knabe H, Kolb HJ, Andreesen R, Wilmanns W. Prognostic significance of increased IL-10 production in patients prior to allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:237–241. doi: 10.1038/sj.bmt.1702126. [DOI] [PubMed] [Google Scholar]
- VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal MR, de Vries JE, Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- Chen C, Lee WH, Yun P, Snow P, Liu CP. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. J Immunol. 2003;171:733–744. doi: 10.4049/jimmunol.171.2.733. [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Netzer N, Karin N. Tr1 cell-dependent active tolerance blunts the pathogenic effects of determinant spreading. J Clin Invest. 2002;110:701–710. doi: 10.1172/JCI200215176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23:450–455. doi: 10.1016/S1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Dendritic cells: regulators of allo-immunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065X.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Bonham CA, Liang X, Chen Z, Li W, Wang L, Watkins SC, Nalesnik MA, Schlissel MS, Demestris AJ, Fung JJ, Qian S. Liver-derived DEC205+B220+ J Immunol. 2001;166:7042–7052. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/S1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Thymus and autoimmunity: capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of auto-immune disease. J Exp Med. 1990;172:537–545. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4(+)CD25(+) regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–882. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–191. doi: 10.1016/S1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci USA. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey VL, Rouse BT, Wilkinson JE. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am J Pathol. 1994;145:281–286. [PMC free article] [PubMed] [Google Scholar]
- Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem. 2001;276:37672–37679. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::AID-IMMU1122>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, Ebeling SB, Lombardi G, Rustin MH, Bijlsma JW, Lafeber FP, Salmon M, Akbar AN. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–2744. doi: 10.1182/blood.V98.9.2736. [DOI] [PubMed] [Google Scholar]
- Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI200319441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::AID-IMMU1247>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–199. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto A, Nakajima H, Ikeda K, Kubo S, Nakayama T, Taniguchi M, Saito Y, Iwamoto I. CD4(+)CD25(+) T-cell development is regulated by at least 2 distinct mechanisms. Blood. 2002;99:555–560. doi: 10.1182/blood.V99.2.555. [DOI] [PubMed] [Google Scholar]
- Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundstrom S, Cederbom L, Sundstedt A, Scheipers P, Ivars F. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4(+)CD25(+) regulatory T cells in vivo. J Immunol. 2003;170:5008–5017. doi: 10.4049/jimmunol.170.10.5008. [DOI] [PubMed] [Google Scholar]
- Duthoit CT, Nguyen P, Geiger TL. Antigen nonspecific suppression of T cell responses by activated stimulation-refractory CD4(+) T cells. J Immunol. 2004;172:2238–2246. doi: 10.4049/jimmunol.172.4.2238. [DOI] [PubMed] [Google Scholar]
- Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–772. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- Masuyama J, Kaga S, Kano S, Minota S. A novel costimulation pathway via the 4C8 antigen for the induction of CD4+regulatory T cells. J Immunol. 2002;169:3710–3716. doi: 10.4049/jimmunol.169.7.3710. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–1291. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]


