Abstract
Purpose
To assess the efficacy of oral azithromycin in the treatment of toxoplasmic retinochoroiditis.
Methods
A randomized interventional comparative study was conducted on 14 patients with ocular toxoplasmosis who were treated with oral azithromycin and 13 patients who were treated with oral trimethoprim/sulfamethoxazole for 6–12 weeks. The achievement of treatment criteria in the two groups and lesion size reduction were considered as primary outcome measures.
Results
The resolution of inflammatory activity, decrease in the size of retinochoroidal lesions, and final best corrected visual acuity (BCVA) did not differ between the two treatment groups. The lesion size declined significantly in all patients (P = 0.001). There was no significant difference in the reduction of the size of retinal lesions between the two treatment groups (P = 0.17).
Within each group, there was a significant improvement in BCVA after treatment; BCVA increased by 0.24 logMAR in the azithromycin group (P = 0.001) and by 0.3 logMAR in the trimethoprim/sulfamethoxazole group (P = 0.001).
Conclusions
Drug efficacy in terms of reducing the size of retinal lesions and visual improvement was similar in a regimen of trimethoprim/sulfamethoxazole or azithromycin treatment. Therefore, if confirmed with further studies, therapy with azithromycin seems to be an acceptable alternative for the treatment of ocular toxoplasmosis.
Keywords: Azithromycin, Trimethoprim/sulfamethoxazole, Toxoplasmic retinochoroiditis
Introduction
Ocular toxoplasmosis is the most common cause of retinochoroiditis worldwide and is responsible for about 25% and 54% of posterior uveitis in the United States and Iran, respectively.1, 2 The presentation of ocular toxoplasmosis varies depending on the retinal location of the lesion. Patients classically complain of a unilateral decrease in vision with associated floaters, and sometimes pain, redness, and photophobia. Recurrence is marked by the presence of active lesions in the setting of old pigmented retinal scars in either eye.3 A diagnosis of ocular toxoplasmosis is made by physical examination through a dilated funduscopic examination, and serologic findings can help for confirmation.4
For selecting a therapeutic regimen, there are some controversies and multiple choices. In considering whether to treat, the benefits of treatment must be weighed against the potential risks associated with antibiotic therapy. Available treatments include a combination of pyrimethamine and sulfadiazine plus corticosteroids as a classic and standard treatment, clindamycin (alone or in combination with the classic treatment), trimethoprim/sulfamethoxazole, azithromycin, ubiquinone analogues (atovaquone), and intravitreal injection of clindamycin.4, 5, 6, 7, 8, 9, 10, 11
Thrombocytopenia, leukopenia, and normochromic anemia have been associated with the use of pyrimethamine.12 Sulfadiazine, as a sulfonamide drug, can cause mild to severe skin rashes, Stevens–Johnson syndrome, and crystalluria.5 Due to these adverse reactions and the significant number of pills that patients should take in a day, the compliance for standard combination is poor and leads to discontinuation of treatment in approximately 25% of the patients.13
Recent studies have shown that trimethoprim/sulfamethoxazole is an alternative for the classic treatment,10, 14 but some adverse effects like fever, gastrointestinal upset, weight loss, Stevens–Johnson syndrome, toxic epidermal necrolysis, pancreatitis, serum sickness, hyperkalemia and thrombocytopenia have been reported.8
Azithromycin has a good tolerance in all age groups, and because of its long half-life, the once-daily regimen is suitable for most infections. Moreover, its side effects including stomach upset, diarrhea, nausea, vomiting, abdominal pain, abnormal liver function, arrhythmias like ventricular tachycardia, and hypotension are rare.15 In addition to the anti-replication effect of azithromycin on tachyzoites of Toxoplasma gondii, it even destroys the tissue cysts. Azithromycin also has a good intracellular penetration and can directly influence intracellular tachyzoites.5, 15
The main purpose of this prospective randomized study was to evaluate the efficacy, safety, and tolerability of azithromycin in the treatment of sight-threatening ocular toxoplasmosis and to compare this regimen with trimethoprim/sulfamethoxazole as another alternative for the treatment of ocular toxoplasmosis.
Methods
This prospective randomized interventional study was conducted to compare the efficacy and tolerability of two different treatment regimens for active sight-threatening toxoplasmosis retinochoroiditis. This study was conducted between March 2010 and October 2013 in the Retinal Department of Farabi Eye Hospital, Tehran, Iran. The study was performed in accordance with the tenets of the Declaration of Helsinki. The study protocol was approved by the local Ethics Review Committee of Tehran University of Medical Sciences, and all individuals provided us with written informed consent prior to participation.
Patients were clinically diagnosed by the presence of an active white and bright focal retinal lesion with blurred margins with or without dark retinochoroidal scars. Confirmation was obtained by serum IgG and IgM antibody against T. gondii in all patients. The inclusion criteria were age between 16 and 75 years and lesions that matched the modified criteria formulated by Holland and associates; [1] a lesion within 3000 μm from the foveal center (zone 1) or [2] a lesion >2 disc diameters in size with 3–4 (+) vitreous inflammation within the region beyond the borders of zone 1 (zones 2 and 3).6
The exclusion criteria were the presence of other ocular diseases including other causes of uveitis, glaucoma, any retinal lesion, and systemic conditions such as uncontrolled diabetes, pregnancy, and any history of hypersensitivity to azithromycin and Sulfonamides, immunosuppression especially HIV or consumption of immunosuppressive drugs, history of any previous adverse drug reaction, corticosteroid treatment within 1 month prior to visit, and best corrected visual acuity (BCVA) less than 20/400 (1.3 logMAR) on the Snellen chart in either eye.
A total of 36 patients were clinically diagnosed with toxoplasmic retinochoroiditis, 5 patients used steroid drugs due to systemic diseases so they received the classic treatment, and 4 patients were lost to follow-up. Finally, using block randomization, 27 patients completed the study. Fourteen patients were treated with oral azithromycin, and 13 patients were treated with oral trimethoprim/sulfamethoxazole. The first group received 500 mg as a loading dose for one day followed by azithromycin 250 mg daily. In the second group, treatment included trimethoprim (160 mg)/sulfamethoxazole (800 mg) twice daily. Both groups were assigned to treatment for 6–12 weeks. Both groups received oral prednisone 1 mg/kg daily from the third day, and the dose was tapered over 2 weeks based on vitritis control.
A masked ophthalmologist examined patients on day 1 and then after 1, 2, 3, 5, 6, 8, and 12 weeks. The patients were observed for at least 9 months after treatment completion. On each visit, BCVA was measured with a Snellen chart (converted to the logMAR) for all patients, and anterior vitreous inflammation was estimated according to the system devised by Kimura et al16 which is the base of SUN group for uveitis classification. Fundus examination was done using a slit-lamp with a 90-diopter lens and indirect ophthalmoscopy. Goldmann applanation tonometry was also performed. Fundus photographs, fundus autofluorescence (FAF), and infrared reflectance (IR) imaging were taken on day one and then after 4 and 12 weeks of therapy (Retinal Angiography system, HRA2; Heidelberg Engineering, Dossenheim, Germany). Computer-assisted measurement of the initial and final lesion size was done in square millimeter on fundus photography, IR, and FAF using the Digimizer (version 4.2.2) image analysis software (MedCalc Software, Ostend, Belgium). This digital analyzer also measured the ratio of the lesion area to disc area in each patient's FAF. A change in the lesion size was calculated from these measurements. If the active lesion was adjacent to the old scar, the lesion size was generally considered as one lesion, while in 6 patients in which the active lesion was far from the old scar, the active lesion and old scar were calculated separately.
By definition, successful treatment was achieved when the lesion border became sharp with or without pigmentation or scar formation and when the absent or trace of inflammatory activity in the anterior chamber and the vitreous was also noted. A change in treatment to intravitreal clindamycin was considered for patients who still had active retinal lesions, dense vitreous inflammation, or both despite treatment for 4 complete weeks.7
The percentage of patients with successful treatment outcome in each group and lesion size reduction were considered as primary outcome measures. The BCVA, anterior chamber and vitreous inflammation status, number and size of satellite lesions before and after treatment, any correlation between the lesion size and satellite lesion, and adverse drug reactions were secondary outcome measures. Each patient was asked about compliance with the treatment regimen and any adverse event in every follow-up session. Bilirubin level in each follow-up was also measured. In this study, the accuracy of fundus photography, FAF, and IR in the detection of satellite lesions was also compared.
Statistical analysis
Data normality was confirmed using the Shapiro–Wilk test. Chi-square test was applied to compare gender distribution, vitreous inflammation before and after treatment, and to compare the lesion sharpness with reduction of satellite lesions. An independent-sample t-test was performed to compare age, lesion size reduction, and BCVA before and after treatment between the groups. Paired t-test was also used to compare BCVA and lesion size before and after treatment. This test also was used to compare the accuracy of fundus photography, FAF, and IR in detection of satellite lesions before and after treatment in each group, to evaluate the reduction in the number of satellite lesions in each group, and to investigate any correlation between the number of satellite lesions and the lesion size before treatment. P values less than 0.05 were considered significant.
Results
Twenty-seven patients including 14 patients in the azithromycin and 13 patients in the trimethoprim/sulfamethoxazole group were evaluated. The azithromycin group was comprised of 6 men (42.8%) and 8 women (57.2%), and the trimethoprim/sulfamethoxazole group consisted of 8 men (61.6%) and 5 women (38.4%) (P = 0.5). The mean age of the patients in the azithromycin therapy group and trimethoprim/sulfamethoxazole group was 24.9 ± 1.6 and 28 ± 1.5 years (P = 0.2). There was no significant difference in age and gender between the 2 groups. Also, old retinal scar was comparable in the two group (10 (71.4%) versus 9 (69.2%), P = 0.73).
Our serologic investigation showed that all of our patients were positive for anti toxoplasmosis IgG, and only 29.6% of our patients had positive IgM titers. Positive IgG and IgM titers had no significant difference between the two treatment groups (P = 0.21).
Overall, the mean lesion size was 6.8 mm2 (7.4–0.3) before treatment; it was 6.4 ± 1.4 mm2 in patients in the azithromycin group and 7.18 ± 1.3 mm2 in participants in the trimethoprim/sulfamethoxazole group (P = 0.24). After the treatment, the mean lesion size was 5.02 ± 0.9 mm2 in all patients; it was 5.1 ± 1.3 mm2 in the patients in the azithromycin group and 4.9 ± 1.2 mm2 in the patients in the trimethoprim/sulfamethoxazole group (P = 0.64). The lesion size decreased significantly in all patients (P = 0.001) and in both groups (azithromycin group: P = 0.002, trimethoprim/sulfamethoxazole group: P = 0.001). There was no significant difference in the retinal lesion size reduction between the 2 treatment groups; patients in the azithromycin group had a mean reduction of 24.2% ± 6.5%, and patients in the trimethoprim/sulfamethoxazole group demonstrated a mean reduction of 36.6% ± 4.6% in the lesion size (P = 0.17) (Table 1).
Table 1.
Visual acuity improvement, vitreous inflammatory cells clearance, and lesion size reduction after treatment in each group.
| Azithromycin | Trimethoprim/Sulfamethoxazole | P-value | |
|---|---|---|---|
| Vitreous inflammatory cells clearance | 7/14 (50%) | 10/13 (77%) | 0.2* |
| Mean lesion size reduction | 24.2 ± 6.5% | 36.6 ± 4.6% | 0.17** |
| Lesion sharpness at the end of 4 weeks after treatment (based on fundus photography) | 11 (78.6%) | 13 (100%) | 0.2* |
| Improvement in visual acuity (logMAR) | 0.24 ± 0.04 | 0.30 ± 0.01 | 0.17** |
| Mean of decrease in satellite lesion after treatment according to FAF (mm) | 1.85 ± 0.52 | 2.15 ± 0.62 | 0.3** |
*Chi square test.
**Independent-sample t-test.
FAF: Fundus autofluorescence.
In clinical fundus examination, 24 (88.9%) patients had sharp lesions 4 weeks after the start of treatment. Eleven (78.6%) patients in the azithromycin group and 13 (100%) patients in the trimethoprim/sulfamethoxazole group had sharp lesions 4 weeks after the start of treatment, so the treating ophthalmologist decided to considered intravitreal clindamycin injection for 3 patients in the azithromycin group who did not respond completely to azithromycin after 6 weeks of treatment. Both groups reached the treatment response criteria, so no patient needed to extend treatment beyond 6 weeks. There was also no significant difference in response time to treatment between the 2 treatment groups (P = 0.2, Table 1 and Fig. 1).
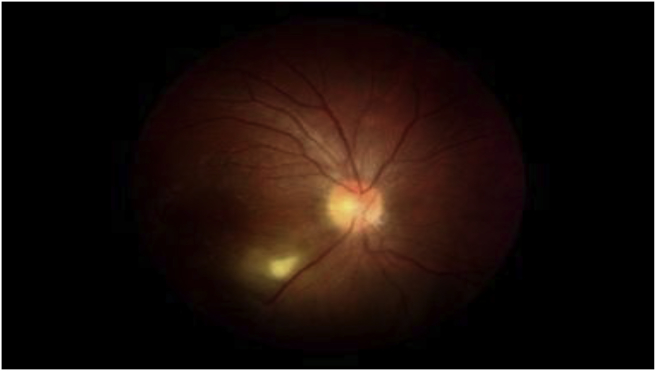
Fig. 1.
A 32 years old man with toxoplasmic retinochoroiditis. fundus-photo of the patient before treatment, reveals obvious white active retinochoroiditis patch located in inferior arcade.
Analysis of the fundus photographs disclosed that sharpening of the retinal lesions at the end of the 4th week was rather similar in both groups. 8/14 (57.2%) of the patients in azithromycin group and 8/13 (61.6%) of the patients in the trimethoprim/sulfamethoxazole group had sharp lesions on fundus photography at that time (P = 0.9).
The mean BCVA was 0.47 ± 0.06 and 0.20 ± 0.02 logMAR in all patients before and after the treatment, respectively (P = 0.001). There was a significant improvement in VA after treatment; it increased by 0.24 ± 0.04 logMAR in the azithromycin group (P = 0.001) and by 0.30 ± 0.01 logMAR in the trimethoprim/sulfamethoxazole group (P = 0.001). Visual acuity improved in all cases after treatment except for 7 patients (25.9%); 4 patients (14.8%) in the azithromycin group and 3 patients (11.1%) in the trimethoprim/sulfamethoxazole group due to an old retinal scar in the macular area (logMAR >0.4). In these patients, there was no significant relationship between the primary size of the lesion in FAF and loss of vision (P = 0.17).
Of 27 patients, 17 (63%) showed no vitreous inflammatory cells while 10 (37%) showed 0.5 to 1 plus vitreous inflammatory cells after completion of treatment. There was no significant difference in vitreous inflammation reduction between the two groups; 7 patients (50%) in the azithromycin group and 3 patients (23%) in the trimethoprim/sulfamethoxazole group showed (0.5–1) plus inflammatory cells after the treatment (P = 0.2) (Table 1).
In this study, we calculated the lesion/optic disc size ratio based on FAF. The mean ratio decreased from 3.6 ± 0.5 at baseline to 2.6 ± 0.4 after treatment; the decrease was 2.35 ± 0.4 in the azithromycin group and 2.84 ± 0.6 in the trimethoprim/sulfamethoxazole group. There was no significant difference in the reduction of the retinal lesion size/optic disc ratio between the two treatment groups (P = 0.6).
The evaluation of retinal satellite lesions in our patients revealed that on the initial examination; 25/27 (92.5%) of the patients on FAF, 23/27 (85.1%) of the patients on IR imaging,12 and 19/27 (70.3%) of the patients on fundus photography had active satellite lesions. The mean number of satellite lesions detected by FAF, IR, and fundus photography was 3.59, 2.29, and 1.96, respectively. Therefore, FAF showed significantly more satellite lesions in comparison with IR and fundus photography (P = 0.001). There was no significant difference in detecting satellite lesions between IR and fundus photography (P = 0.4).
There was no significant difference in the decrease in active satellite lesions after the treatment between the two treatment groups (P = 0.3, Table 1 and Fig. 2).
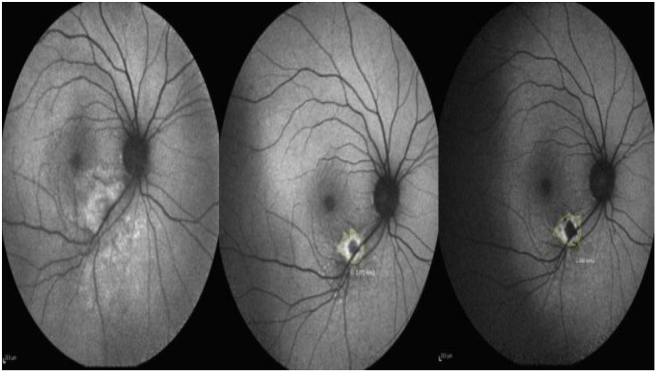
Fig. 2.
Fundus autofluorescence (FAF) of the same patient with toxoplasmic retinochoroiditis. Inferior arcade hyper autofluorescence area with blurred margins within inferior arcade before treatment (left figure). The lesion size is decreased and the satellite lesions are appeared after 2 weeks of treatment (middle figure). Satellite lesions were decreased in number and lesion margins became well-defined after 6 weeks of treatment as documented with a hypo-autofluorescence area with hyper-autofluorescence margins (right figure).
Adverse drug reactions were seen in 4 patients (28.5%) in the azithromycin group; one patient (7.1%) had skin irritation, one (7.1%) had an increase in the serum bilirubin level to borderline values, and 2 patients (14.2%) developed mild diarrhea (see Fig. 3, Fig. 4). However, none of them discontinued the treatment. In 3 patients (23%) receiving trimethoprim/sulfamethoxazole, the drug reaction was limited to skin rashes in 1 patient, and fixed drug eruption in 3 patients (23%). Also, at the time of stopping full dose treatment, 1 patient had a serum bilirubin level of 14 mg/dl; however, no major adverse events were observed in patients in the two groups.
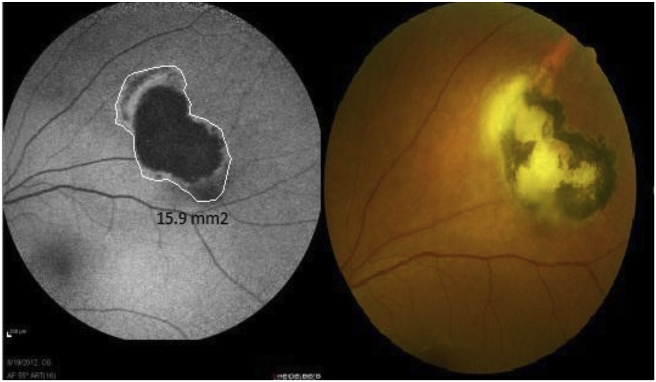
Fig. 3.
A 24 years old woman with toxoplasmic retinochoroiditis. Fundus-photo of the patient before treatment, shows old toxoplasmic scar with adjacent white active lesion (right). fundus autofluorescence (FAF) before treatment (left).
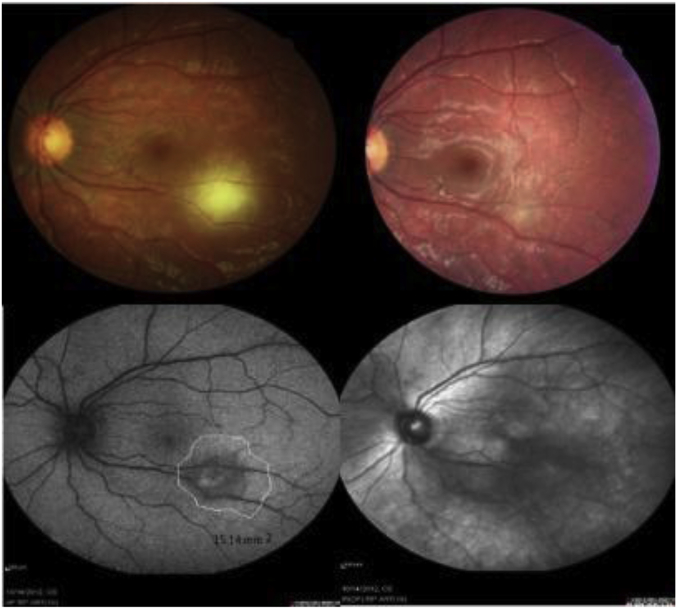
Fig. 4.
A 29 years old woman with toxoplasmic retinochoroiditis. Fundus-photo of the patient before treatment, reveals white active lesion with poor defined margin in inferior arcade (Top left). fundus-photo 6 weeks after treatment with azithromycin shows resolved lesion with minimal scar (Top right). fundus autofluorescence (FAF) before treatment shows central hyper-autofluoresence with hypo and hyper autofluorescence margin (bottom left). infrared reflectance (IR) imaging after 6 weeks treatment (bottom right).
Discussion
The self-limiting nature of ocular toxoplasmosis makes it difficult to compare its therapeutic options. A combination of host, parasitic, and environmental factors affect the severity of ocular toxoplasmosis, making it difficult to clearly identify the characteristics of the ideal drug for ocular toxoplasmosis.6 The effectiveness of different treatment regimens for ocular toxoplasmosis is difficult to evaluate because ocular toxoplasmosis varies widely in clinical presentation, location of the lesions, and severity of inflammation. The combination of pyrimethamine and sulfadiazine is probably most effective against toxoplasmosis and therefore recommended as the standard treatment for sight-threatening ocular toxoplasmosis. However, a combination of trimethoprim/sulfamethoxazole is reported to be a good alternative to standard therapy.4, 17 Our study revealed no significant difference between treatment with azithromycin and trimethoprim/sulfamethoxazole for ocular toxoplasmosis retinochoroiditis in terms of vision improvement, vitreous inflammation, duration of treatment, sharpness of lesions, reduction of the lesion size, and satellite lesions.
In accordance with Bosch-Driessen et al,5 we determined the dose of azithromycin based on two in vitro studies showing that the concentration of azithromycin in the brain tissue of patients with brain tumors reached a level of 3.64 ± 3.81 μg/g 48 hrs after the administration of 500 mg azithromycin.18, 19 This value is between the IC 50 and IC 90 (50% and 90% inhibitory concentrations, respectively) for parasite growth in the presence of pyrimethamine. However, this concentration of azithromycin clearly reached the IC 50 for parasite growth when used alone in the same study18 and also can pass blood retinal barrier.20
In our study, the response to treatment measured by a change in BCVA revealed a non-significant difference between the treatment groups with a 0.24 logMAR improvement in the azithromycin group and a 0.30 logMAR improvement in patients receiving trimethoprim/sulfamethoxazole. In our study, 25.9% of the patients had BCVA higher than 0.4 logMAR after treatment. In a study by Soheilian et al, the patients in the classic therapy group improved by 0.56 logMAR and patients receiving trimethoprim/sulfamethoxazole improved by 0.52 logMAR.10 In a study by Bosch-Driessen et al, an improvement of at least 0.5 logMAR in visual acuity was noted in 21% of the patients receiving azithromycin and 28% of the patients receiving sulfadiazine.5
Neither the duration of the inflammatory activity of the retinochoroidal lesions or vitritis nor the change in the size of retinal lesions on FAF during treatment showed a significant difference between the azithromycin and the trimethoprim/sulfamethoxazole groups that could be explained by retinal pigment epithelium (RPE) death due to inflammation, which is not reversible with treatment. The mean reduction in the size of retinal lesions on FAF was 24% in the azithromycin group and 35.5% in the trimethoprim/sulfamethoxazole group. In a similar prospective study by Soheilian et al, the mean reduction in the size of retinal lesions was 61% in the classic therapy group and 59% in the trimethoprim/sulfamethoxazole group.10 In a study by Bosch-Driessen et al, the mean reduction in the size of retinal lesions was 41% in both groups of treatment with classic therapy group and azithromycin plus pyrimethamine group.5 Less reduction in the size of the lesions on FAF in our study was probably due to summation of the size of the previous scar with the new lesion in 19 (70.45%) patients in whom the new lesions were on or just close to the old scar, so it was not possible to measure the lesion size separately.
We also found an analogous effect in terms of vitreous inflammatory response in both treatment groups. Twelve weeks after the treatment, the signs of vitreous inflammation resolved in 50% of the patients receiving azithromycin therapy and 76.9% of the patients on trimethoprim/sulfamethoxazole. In a prospective study by Soheilian et al, at the end of 6 weeks therapy, the resolution of vitreous inflammation was observed in 56.7% of the patients on trimethoprim/sulfamethoxazole.10 Bosch-Driessen et al reported the resolution of vitreous inflammation in 71% of their patients who were treated with classic therapy and 70% of their patients in the azithromycin plus pyrimethamine therapy group.5 The higher clearance of vitreous inflammation in this study may be due to the effect of combination therapy with azithromycin plus pyrimethamine.
In our study, a previous retinal scar was noted in 19 (70.4%) out of 27 patients. Rothova et al and Bosch-Driessen reported that 69% and 60% of their patients had previous retinal scars, respectively.5, 11 This finding is different from the results of the study by Soheilian et al in which 50.8% of the patients did not have any old scars.10 Our serologic investigation showed that only 29.6% of our patients had positive IgM titers.
The analysis of the photographs disclosed that sharpening of retinal lesions occurred within 4 weeks.
Our study revealed that FAF showed significantly more retinal toxoplasmosis satellite lesions in comparison with IR and fundus photography. By using FAF some very small satellite lesions were discovered which could not be determined in routine fundus photography. This finding shows that RPE dysfunction in FAF may occur before morphologic changes on fundus photography. Moreover, it revealed that there was no significant difference between either treatment groups in decreasing satellite lesions within treatment.
We noticed no previously described severe side effects. We found no significant difference in severe adverse drug reactions between treatment groups; the side effects were limited to bilirubin elevation to 14 mg/dl in 1 case (7.6%) in the trimethoprim/sulfamethoxazole therapy group and fixed drug eruption in 3 patients (23%). In a study by Rothova et al, the rate of adverse drug reactions in patients receiving trimethoprim/sulfamethoxazole was only 4%.11 Another study by Bosch-Driessen et al showed adverse drug reactions in 33% of the patients receiving pyrimethamine and azithromycin.5 The different rate of adverse drug reactions in this study may be due to using pyrimethamine. Konstantinos Balaskas et al reported that no patients receiving azithromycin developed adverse drug reactions while one patient developed a skin rash 45 days after starting treatment with sulfadiazine/pyrimethamine. All patients who received sulfadiazine/pyrimethamine reported weak treatment tolerance, with symptoms being malaise, dizziness, headaches, and gastrointestinal disorders.21 Soheilian et al found that adverse drug reactions were limited to 1 case (3.4%) in the classic therapy group and 1 patient (3.3%) receiving trimethoprim/sulfamethoxazole.10
Our study had several limitations. The small sample size of our study was the most important limitation. Small differences between the two groups may not be detected by this sample size. Therefore, it should be kept in mind that this study was rather a pilot investigation, and larger clinical trials are warranted to confirm the results. In addition, the single-blind design of our study was potentially a source of bias, although the routine examiner was masked to treatment regimes. Also, we did not have a control group of patients treated with standard regimen of pyrimethamine and sulfadiazine.
In summary, the positive response of toxoplasmic retinochoroiditis to azithromycin was shown by visual improvement, lesion size, sharpening of margins of the lesions, and reduction of satellite lesions. Although there was no significant difference in time to respond to treatment between the 2 treatment groups, the difference may be clinically important. On the basis of our findings, treatment with azithromycin could be an appropriate alternative to treatment with trimethoprim/sulfamethoxazole, and therefore, it might be an acceptable choice instead of standard treatment with pyrimethamine and sulfadiazine for ocular toxoplasmosis if confirmed with further large scale studies. Treatment with azithromycin is, however, better tolerated and has fewer side effects.
Footnotes
Conflicts of interest: Authors declare no financial support or relationships that may pose conflict of interest.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Jones J.L., Holland G.N. Annual burden of ocular toxoplasmosis in the US. Am J Trop Med Hyg. 2010;82:464–465. doi: 10.4269/ajtmh.2010.09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soheilian M., Heidari K., Yazdani S., Shahsavari M., Ahmadieh H., Dehghan M. Patterns of uveitis in a tertiary eye care center in Iran. Ocul Immunol Inflamm. 2004;12:297–310. doi: 10.1080/092739490500174. [DOI] [PubMed] [Google Scholar]
- 3.Saffra N.A., Seidman C.J., Weiss L.M. Ocular toxoplasmosis: controversies in primary and secondary prevention. J Neuroinfectious Dis. 2013;4:4–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Lima G.S., Saraiva P.G., Saraiva F.P. Current therapy of acquired ocular toxoplasmosis: a review. J Ocul Pharmacol Ther. 2015;31:511–517. doi: 10.1089/jop.2015.0059. [DOI] [PubMed] [Google Scholar]
- 5.Bosch-Driessen L.H., Verbraak F.D., Suttorp-Schulten M.S. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:34–40. doi: 10.1016/s0002-9394(02)01537-4. [DOI] [PubMed] [Google Scholar]
- 6.Holland G.N., Lewis K.G. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:102–114. doi: 10.1016/s0002-9394(02)01526-x. [DOI] [PubMed] [Google Scholar]
- 7.Kishore K., Conway M.D., Peyman G.A. Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg lasers. 2001;32:183–192. [PubMed] [Google Scholar]
- 8.Opremcak E.M., Scales D.K., Sharpe M.R. Trimethoprim-sulfamethoxazole therapy for ocular toxoplasmosis. Ophthalmology. 1992;99:920–925. doi: 10.1016/s0161-6420(92)31873-1. [DOI] [PubMed] [Google Scholar]
- 9.Reich M., Becker M.D., Mackensen F. Influence of drug therapy on the risk of recurrence of ocular toxoplasmosis. Br J Ophthalmol. 2016;2:195–202. doi: 10.1136/bjophthalmol-2015-306650. [DOI] [PubMed] [Google Scholar]
- 10.Soheilian M., Sadoughi M.M., Ghajarnia M. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005;112:1876–1882. doi: 10.1016/j.ophtha.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Rothova A., Meenken C., Buitenhuis H.J. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115:517–523. doi: 10.1016/s0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- 12.Pissinate K., dos Santos Martins-Duarte E., Schaffazick S.R., de Pyrimethamine-loaded lipid-core nanocapsules to improve drug efficacy for the treatment of toxoplasmosis. Parasitol Res. 2014;113:555–564. doi: 10.1007/s00436-013-3715-6. [DOI] [PubMed] [Google Scholar]
- 13.Iaccheri B., Fiore T., Papadaki T. Adverse drug reactions to treatments for ocular toxoplasmosis: a retrospective chart review. Clin Ther. 2008;30:2069–2074. doi: 10.1016/j.clinthera.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 14.de-la-Torre A., Stanford M., Curi A., Jaffe G.J., Gomez-Marin J.E. Therapy for ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:314–320. doi: 10.3109/09273948.2011.608915. [DOI] [PubMed] [Google Scholar]
- 15.Araujo F.G., Shepard R.M., Remington J.S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur J Clin Microbiol Infect Dis Eur J Clin Microbiol Infect Dis. 1991;10:519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- 16.Kimura S.J., Thygeson P., Hogan M.J. Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol. 1959;47:171–176. doi: 10.1016/s0002-9394(14)78240-6. [DOI] [PubMed] [Google Scholar]
- 17.Fajardo R.V., Furgiuele F.P., Leopold I.H. Treatment of toxoplasmosis uveitis. Arch Ophthalmol. 1962;67:712–720. doi: 10.1001/archopht.1962.00960020712004. [DOI] [PubMed] [Google Scholar]
- 18.Cantin L., Chamberland S. In vitro evaluation of the activities of azithromycin alone and combined with pyrimethamine against Toxoplasma gondii. Antimicrob Agents Chemother. 1993;37:1993–1996. doi: 10.1128/aac.37.9.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaruratanasirikul S., Hortiwakul R., Tantisarasart T., Phuenpathom N., Tussanasunthornwong S. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother. 1996;40:825–826. doi: 10.1128/aac.40.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothova A., Bosch-Driessen L.E., van Loon N.H., Treffers W.F. Azithromycin for ocular toxoplasmosis. Br J Ophthalmol. 1998;82:1306–1308. doi: 10.1136/bjo.82.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balaskas K., Vaudaux J., Boillat-Blanco N., Guex-Crosier Y. Azithromycin versus Sulfadiazine and Pyrimethamine for non-vision-threatening toxoplasmic retinochoroiditis: a pilot study. Medical science monitor. Int J Clin Exp Med. 2012;18:296–302. doi: 10.12659/MSM.882735. [DOI] [PMC free article] [PubMed] [Google Scholar]