Abstract
Cerebrovascular dysfunction is re-emerging as a major component of aging, and may contribute to the risk of developing Alzheimer’s disease (AD). Two important risk factors for cerebrovascular dysfunction are APOE and female sex, which are primarily researched in the context of high amyloid-β (Aβ) levels as found in AD. However, APOE4 and sex modulate Aβ-independent pathways that may induce cerebrovascular dysfunction as a downstream consequence. Therefore, testing the activity of factors that target cerebrovascular dysfunction in Aβ-independent models that incorporate APOE4 and female sex is crucial. We have previously demonstrated that peripheral administration of the epidermal growth factor (EGF) prevents cognitive dysfunction, cerebrovascular leakiness, and cerebrovascular coverage deficits in female mice that express APOE4 and overproduce Aβ, without affecting Aβ levels. These data raise the question of whether EGF protects the cerebrovasculature from general stress-induced damage. Therefore, the goal of this study was to determine whether EGF prevents Aβ-independent cerebrovascular dysfunction. In eight-month old mice that express human APOE, the interaction of APOE4 and female sex induced cognitive dysfunction, increased cerebrovascular leakiness and lowered vessel coverage. Importantly, in a prevention paradigm (from six to eight and a half months of age), EGF ameliorated cognitive decline and cerebrovascular deficits in female mice that express APOE4. Thus, developing treatment strategies based on EGF signaling could provide alternative therapeutic options for age-related cerebrovascular dysfunction and reduce AD risk.
Keyword: Neuroscience
1. Introduction
Cerebrovascular dysfunction is re-emerging as a major component of aging [1, 2, 3, 4] and Alzheimer’s disease (AD) [5, 6, 7, 8, 4, 9, 10, 11]. Outcomes of global cerebrovascular dysfunction are prevalent with aging in humans, including higher cerebrovascular leakiness [1, 2, 3, 4], which may also contribute to the risk of developing AD. Cerebrovascular dysfunction, including increased cerebrovascular leakiness, reduced cerebral blood flow, peripheral immune cell infiltration into the brain and indices of impaired nutrient/signaling supply are observed in AD patients [5, 6, 7, 8, 4, 9, 10, 11] and mouse models of AD-like pathology [12, 13, 14]. These cerebrovascular dysfunction outcomes are mediated by alterations in cells that comprise arteries/arterioles, capillary microvessels that define the blood-brain barrier (BBB), and venules/veins. However, changes at the BBB (comprised of brain endothelial cells, pericytes and astrocytic end-feet) may play a central role in many outcomes of global cerebrovascular dysfunction. Indeed, the BBB is the largest interface of blood-to-brain contact and is key for the homeostatic functions of the cerebrovasculature, including controlling general leakiness. In aging there is evidence of breaks in the BBB [2], and in AD, cerebral capillaries are fragmented, display atrophy, and there is lower total vessel coverage [15, 16, 17, 6, 18, 19, 20, 21, 22]. Breakdown of the BBB may lead to extravasation of plasma proteins (e.g. fibrinogen) into the brain, which in turn can induce neuroinflammation and direct neuronal dysfunction, contributing to cognitive decline [23, 24, 25]. Therefore, delineating the role of risk factors of aging and AD in cerebrovascular dysfunction is important.
Two important risk factors for cerebrovascular dysfunction are APOE and female sex (reviewed [26]), which are primarily researched in the context of high amyloid-β (Aβ) levels as found in AD. APOE4 is the greatest genetic risk factor for sporadic AD increasing risk up to 12-fold compared to APOE3 (reviewed in [27]), an effect that is greater in females [28, 29, 30]. The combination of APOE4, female sex, and Aβ induce cognitive decline and cerebrovascular dysfunction, including increased leakiness in vivo [31]. However, APOE4 and sex modulate Aβ-independent pathways, which may induce cerebrovascular dysfunction as a downstream consequence [32, 33, 26]. Indeed, there is higher cerebrovascular leakiness with APOE4 in humans and in vivo (reviewed in [26]), but the interaction among APOE4 and female sex is unclear. Therefore, identifying whether APOE4 and female sex induce Aβ-independent cerebrovascular dysfunction is critical.
Angiogenic growth factors are important for cerebrovascular homeostasis, particularly at the BBB. Therefore, one approach to determine the role of cerebrovascular dysfunction in aging is evaluating the in vivo activity of angiogenic growth factors. We have previously demonstrated in a prevention paradigm that one angiogenic growth factor, epidermal growth factor (EGF), ameliorates cognitive dysfunction, lowers cerebrovascular leakiness, and maintains vessel coverage in female mice that express APOE4 and overproduce Aβ [31]. However, EGF did not modulate Aβ levels. These data raise the question of whether EGF protects the cerebrovasculature from general stress-induced damage induced by aging and AD-relevant risk factors.
The goals of this study were to determine whether, in the absence of high Aβ levels, APOE4 and female sex interact to induce cognitive and cerebrovascular dysfunction, and if any detrimental changes can be prevented by EGF.
2. Materials and methods
2.1. Experimental design and animals
Ethical approval for all experiments were via the UIC Institutional Animal Care and Use Committee protocols. Breeding and colony maintenance were conducted as described in [31, 34]. EFAD mice were produced by crossing mice that express 5 Familial Alzheimer’s disease (FAD) mutations (APP K670N/M671L + I716 V + V717I and PS1 M146L + L286 V) with APOE-targeted replacement mice [34]. EFAD carriers are APOE+/+/5xFAD+/− (EFAD +) and non-carrier mice are APOE+/+ 5xFAD−/− (EFAD-). EFAD mice are maintained by inbreeding. For this study we used EFAD- mice. Detailed methods are described in [31].
2.2. Evaluation of cognitive and cerebrovascular changes in EFAD- mice at 8 months of age
Male (EFAD-M) and female (EFAD-F) mice were assessed using novel object recognition and spontaneous alternation (Y-maze) tests. Mice were then injected with 2% sodium fluorescein (NaFl, Sigma) and perfused with PBS. Right hemi-brains dissected and assessed for NaFl extravasation [31]. Left hemi-brains were frozen in cryomolds containing O.C.T compound (Tissue-Tek) for immunohistochemical (IHC) analysis. Investigators were blinded for APOE genotype. n = 7 (E3FAD-M), 6 (E3FAD-F), 6 (E4FAD-M), 8 (E4FAD-F).
2.3. Treatment of E4FAD-F mice with EGF
Six month old E4FAD-F mice were treated with EGF (Shenandoah, 300 μg/kg per week) or vehicle control (water) by intraperitoneal injection (i.p.) until 8.5 months of age as described in [31]. Mice were sequentially assessed using the open field, novel object recognition, spontaneous alternation (Y-maze) and novel arm entry (Y-maze) tests. At the end-point the light-dark box and Morris water maze tests were also performed and food consumption was monitored (24 h). 30 min prior to sacrifice, mice were injected with EGF. Dissected right hemi-brains were stored at −80 °C until homogenization and left hemi-brains were stored as described above for IHC. NaFl extravasation was not conducted on the EGF or vehicle control treated E4FAD-F mice to enable additional biochemical analysis. Investigators were blinded for treatment. n = 7 per group. The exception is for western blot analysis, where n = 6 per group due to the loss of samples.
2.4. Behavioral analysis
Behavioral analysis was conducted in the mouse dark cycle and analyzed using the ANY-Maze software [31].
Open field. Mice were placed in the center of a white box (l38.5xw30xh30 cm) for 10 min, the distance traveled and average speed were measured.
Novel object recognition [31]. On day 1, mice were habituated for 20 min in a white box. On day 2, mice were introduced to two identical objects for 7 min and, 1 h later, with a familiar and novel object for 7 min. The preference index (ratio of time spent with the novel object divided by total investigation time for both objects) was calculated.
2.5. Spontaneous alternation (Y-maze)
Mice were placed in a Y-maze apparatus (l38.5xw8xh13 cm, spaced 120 degrees apart), allowed to explore for 7 min and the sequence of arm entries was recorded [31]. Spontaneous alternation was calculated as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (the number of arms entered minus 2) and multiplied by 100.
Novel arm entry (Y-maze). Mice were placed into the maze with one of the arms blocked for 10 min, returned to the home cage for 60 min, and placed back in the maze with access to all three arms for 5 min [31]. The time spent in the novel arm was calculated.
Light-dark box. Mice were placed into the light side of a light-dark box (l21xw42xh25 cm, 66.6% light side) and allowed to move freely for 5 min [31]. The time spent in each chamber was recorded.
Morris water maze (MWM) [31]. In the visual cue phase, mice were trained for 2 days to locate a flagged hidden platform (60 second trial time, 4 trials each day with a 20 min inter-trial interval (ITI)). 2 days later in the acquisition phase, mice were trained for 5 days (60 second trial time, 4 trials each day with a 20 min ITI) to locate the hidden platform. The entry quadrant varied but the platform location remained constant. 1 h after the final acquisition trial, a single 60 sec probe trial was conducted with the platform removed. The latency to the target area (the previous platform location) and the time spent in the target quadrant were recorded.
2.6. Sodium fluorescein extravasation
Mice were injected i.p. (200 μl) with 2% NaFl and sacrificed after 30 min. Dissected brain regions were weighed, homogenized in PBS, then mixed with an equal volume of 60% tricholoroacetic acid, vortexed, and centrifuged [31]. Fluorescence levels were measured using a microplate reader (SpectraMax i3x, Molecular Devices) and cleared volumes were calculated as: [1/plasma levels (fluorescence units/μl) x total brain fluorescence]/brain weight.
2.7. Biochemical analysis
Dissected brains were weighed and sequentially extracted in Tris-buffered saline (TBS) followed by TBS containing 1% Triton X100 (TBSX) [31]. Total protein was quantified in TBS (Ready to Use Bradford Reagent, Bio-Rad) and TBSX extracts (BCA Protein Assay Kit, Pierce).
For western blot analysis, 20 μg of protein (TBSX) was separated on 4–12% Bis-Tris gels (Invitrogen), transferred to PVDF membranes, blocked (5% non-fat milk), then incubated (4 °C, overnight) with primary antibodies for post synaptic density protein (PSD95, 1:1000, Cell Signaling) or actin (1 h room temperature, 1:20,000, Cell Signaling). Membranes were incubated in secondary antibodies (Jackson Immunoresearch), imaged and quantified using an Odyssey ® Fc Imaging System.
2.8. ELISA
EGF levels were measured in the plasma and TBS extracts by ELISA (Abcam).
2.9. Immunohistochemical analysis
General protocol. Frozen brains were sectioned at 12 μm and fixed in 10% neutral buffered formalin (Sigma). Slides were incubated in 52.8% formic acid (8 min), permeabilized with TBS containing 0.25% TBSX (3 × 5 min), blocked with 5% BSA (2 h), incubated with primary antibodies (4 °C, overnight), washed (3 × 5 min in TBSX), incubated with secondary antibodies (2 h), washed with TBSX (3 × 5 min) followed by TBS (1 × 5 min), and mounted. Nine nonadjacent sections (108 μm apart) were used for quantification per animal.
Fibrinogen. Fibrinogen (Rabbit anti-fibrinogen 1:200 from Dako, AlexaFlour 647 anti-rabbit 1:200 from Invitrogen) was co-stained with CD31 (Rat anti-CD31 from B&D Bioscience with AlexaFluor 405 anti-rat for characterization studies of 8 month old mice, and AlexaFluor 488 anti-rat for the EGF study, both at 1:200 from Invitrogen). 6 images from each cortex per section were captured on a Zeiss Axio Imager M1 under identical capture settings at 20x magnification. For representative images, Z-stack images were taken at 25x magnification on a Zeiss LSM 710Confocal Microscope and 3D reconstructions were produced using Imaris 7.7.2 software.
Laminin. Hemi-brain sections were imaged for laminin (rabbit anti-laminin 1:400 from Abcam, AlexaFlour 750 anti-rabbit 1:200 from Invitrogen) using the Zeiss Axio Mosaic setting. Quantification was performed on the deep layer isocortex and full isocortex.
Quantification. Images were thresholded equally to diminish background signal (NIH ImageJ software) and quantified using the Analyze Particles function.
2.10. Statistical analysis
All data are presented as mean +/− S.E.M and were analyzed using two-way ANOVA followed by either Tukey’s, Fisher’s LSD, or Sidak’s post hoc comparisons, or by using Student’s t-test with GraphPad Prism version 6.
3. Results
3.1. Female E4FAD- mice are cognitively impaired at 8 months of age
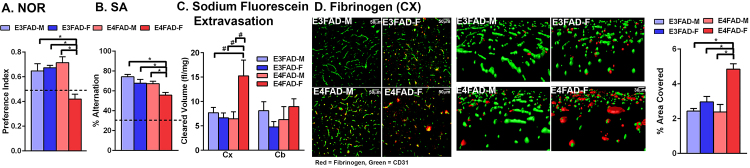
EFAD+ mice overproduce Aβ42 (via 5xFAD mutations) and express human APOE3 or APOE4, whereas EFAD- mice are non-carries for 5xFAD. 8-month-old E4FAD+ female (E4FAD + F) mice are cognitively impaired, have higher cerebrovascular leakiness and lower cerebrovascular coverage [31]. However, evidence suggests that APOE4 induces cognitive [35, 36, 37] and cerebrovascular deficits, including higher leakiness and lower vessel coverage [32, 33], in the absence of high Aβ levels. Therefore, we initially assessed the cognition of E3FAD-M, E3FAD-F, E4FAD-M and E4FAD-F mice at 8 months of age to identify a group for EGF treatment. EFAD mice are an inbred strain. We focused on the EFAD- mice, rather than other models that express APOE3 or APOE4 in the absence of Aβ, to facilitate data comparisons within the EFAD mouse model (e.g. does female sex and APOE4 within the same model induce deficits in the absence of the FAD mutations). In the novel object recognition test (Fig. 1A) there was an interaction between sex and APOE genotype (F(1,23) = 12.53, p < 0.05). Post-hoc analysis revealed that the only significant change was impaired cognition in E4FAD-F mice compared to all other groups (Tukey’s post hoc analysis comparing all groups, *p < 0.05). For spontaneous alternation (Fig. 1B), there was a sex and genotype effect (F(1,23) = 9.855 for sex and 10.65 for genotype, p < 0.05), and similar to novel object recognition the E4FAD-F mice were impaired compared to all other groups (Tukey’s post-hoc). In both behavioral tests the cognitive performance of E4FAD-F mice was ∼30% lower. Therefore, E4FAD-F mice were cognitively impaired compared when assessed by both novel object recognition and spontaneous alternation in agreement with previous reports that APOE4 and female sex induce cognitive impairment at younger ages [35] and at older ages [38].
Fig. 1.
Cognitive and cerebrovascular dysfunction in E4FAD-F mice. E4FAD-F mice are cognitively impaired compared to E3FAD-M mice, E3FAD-F and E4FAD-M mice, when assessed by novel object recognition (NOR; A.) and spontaneous alternation (SA; B.). The dashed line in A. represents no preference and in B. chance alternation. C. After intraperitoneal injection, levels of sodium fluorescein are higher in the cortex (CX) of E4FAD-F mice compared to E3FAD-M mice, E3FAD-F or E4FAD-M. Cleared volume represents the levels of sodium fluorescein in the brain after normalization to plasma levels and brain weight. D. Levels of the plasma protein fibrinogen are higher in the cortex when assessed by quantitative immunohistochemical analysis. Representative confocal images from the cortex highlight the higher levels of fibrinbogen (red) in the cortex, and also indicate lower vessel coverage (CD31 green) in E4FAD-F mice compared to all groups. n = 7 (E3FAD-M), 6 (E3FAD-F), 6 (E4FAD-M), 8 (E4FAD-F). Data expressed as mean +/− S.E.M. *p < 0.05 by two-way ANOVA and Tukey’s post hoc comparisons. #p < 0.05 by two-way AVOVA followed Fisher’s LSD test.
3.2. Higher cerebrovascular leakiness and lower vessel coverage in E4FAD-F mice
It was critical to determine whether APOE4 and female sex induce cerebrovascular dysfunction. We focused on leakiness, or extravasation, of molecules from the blood into the brain. Previous data support that APOE4 is associated with higher cerebrovascular leakiness in vivo [32, 33], however the effect of sex is unknown. Further, we have demonstrated that there is higher cerebrovascular leakiness in the cortex, but not the cerebellum of EFAD + F mice at eight-months of age [31]. The higher cerebrovascular leakiness in the cortex may be related to Aβ deposition in EFAD+ mice, or increased susceptibility to age-related vascular deficits. Therefore, brain levels of NaFl were assessed after i.p. injection (Fig. 1C). There was a sex and APOE genotype interaction (F(1,23) = 4.1, p < 0.05), with higher NaFl levels in the cortex of E4FAD-F mice compared to all other groups (significance only with Fisher’s LSD post-hoc analysis but not by Tukey’s post-hoc). However, there were no APOE −genotype or sex- induced increased leakiness in the cerebellum (F(1,23), p > 0.05). As a complementary measure, brain levels of the endogenous blood clotting factor fibrinogen were assessed by IHC analysis in the cortex. Previous research has demonstrated higher fibrinogen levels in the brain in vivo and in AD patients [33, 39]. Visually, fibrinogen levels appeared higher in the cortex of E4FAD-F mice surrounding the vessels (Fig. 1D). Quantitatively, cortical fibrinogen levels were ∼ 65% higher in E4FAD- female mice compared to all other groups (F(1,23) = 9.7 (interaction), 22.46 (sex), 8.36 (APOE genotype), p < 0.05, Tukey’s post-hoc comparison, *p < 0.05). Therefore, there is higher cerebrovascular leakiness in female E4FAD- mice at 8 months of age.
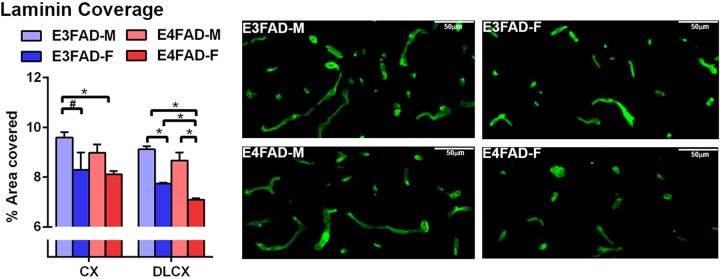
An important measure of cerebrovascular dysfunction is total vessel coverage, which we also propose is a target of EGF. Indeed, our previous data have demonstrated lower vessel coverage in female E4FAD+ mice, particularly in the deep layer cortex, which is prevented by EGF. However, whether there is similar lowering of vessel coverage in E4FADF- mice is unknown. Therefore, the cerebral coverage of laminin (basement membrane protein) was assessed as an indication of total vessel coverage (Fig. 2). In the whole cortex, E4FAD-F mice had lower laminin coverage compared to E3FAD-M mice (F(1,23) = 9.0 for sex p < 0.05, Tukey’s post-hoc analysis *p < 0.05), and E3FAD-F mice had a lower coverage compared to E3FAD-M mice (only significant by Fisher’s post-hoc analysis). We previously demonstrated that the deep layer cortex exhibits pronounced changes in vessel coverage in EFAD+ mice (lowest with EFAD + F), which may be driven by the accumulation of Aβ in the deep layer cortex, and/or increased susceptibility of vessels in the deep layer cortex to damage. In this study, laminin coverage in the deep layer cortex from highest to lowest was: E3FAD-M = E4FAD-M > E3FAD‐F > E4FAD-F (F(1,23) = 86.82 for sex, 11.78 for APOE genotype, p < 0.05, Tukey’s post-hoc analysis *p < 0.05). These data indicate that the even in the absence of high human Aβ levels, vessels in the deep layer cortex are particularly susceptible to changes induced by APOE4 and female sex.
Fig. 2.
Lower laminin coverage in female E4FAD- mice. When assessed by quantitative IHC analysis, laminin staining is lower in the deep layer cortex of EFAD-F mice. n = 7 (E3FAD-M), 6 (E3FAD-F), 6 (E4FAD-M), 8 (E4FAD-F). Data expressed as mean +/− S.E.M. *p < 0.05 by two-way ANOVA and Tukey’s post hoc comparisons. #p < 0.05 by two-way AVOVA followed Fisher’s LSD test.
Overall, E4FAD-F mice are cognitively impaired, have higher cerebrovascular leakiness and lower vessel coverage in the cortex compared to all other groups.
3.3. EGF prevents cognitive decline in E4FAD-F mice
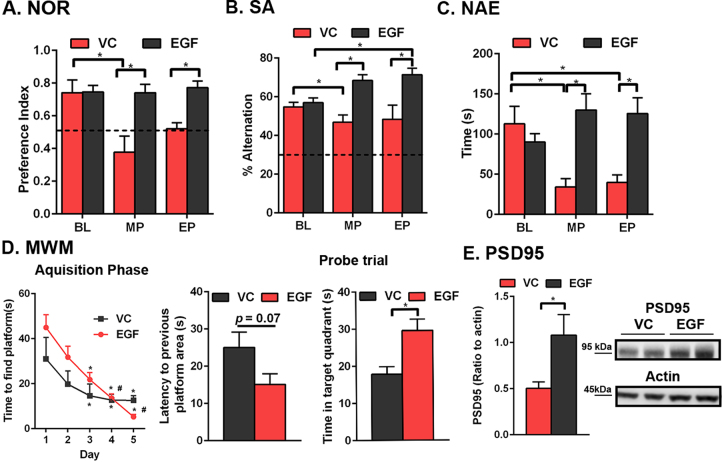
The primary goal of this study was to assess the protective effect of peripheral EGF in a model of Aβ-independent cerebrovascular dysfunction. Therefore, E4FAD-F mice were treated with EGF (300 μg/kg per week) or vehicle control (VC) by i.p. from 6 to 8.5 months. The dose and treatment duration were selected to match our previous study in E4FAD+ mice [31]. Here, EGF prevented age-dependent cognitive decline in E4FAD-F mice. In general, cognitive ability of VC treated mice declined over the course of the study. Indeed, for VC treated mice there was cognitive decline in: novel object recognition from baseline to mid-point (Fig. 3A, F(1,24) = 4.7 (interaction), 4.8 (time), F(1,12) = 19.6 (treatment), p < 0.05, Sidak’s post-hoc comparison for same animal retesting, *p < 0.05); spontaneous alternation from baseline to mid-point (Fig. 3B, F(1,24) = 11.9 (interaction), F(1,12) = 36.78 (treatment), p < 0.05, Sidak’s post-hoc, *p < 0.05); and novel arm entry for baseline to mid-point and end-point (Fig. 3C, F(1,24) = 10.13 (interaction), F(1,12) = 11.3 (treatment), p < 0.05, Sidak’s post-hoc comparison, *p < 0.05). In contrast, EGF-treated E4FAD-F mice did not decline in cognition and, at the end-point, performed ∼30% higher in novel object recognition and spontaneous alternation and, spent ∼300% more time in the novel arm (Y maze) compared to the VC.
Fig. 3.
Peripheral EGF administration prevents cognitive deficits in E4FAD-F mice. Key: EP, end-point; MP, mid-point; BL, baseline. EGF prevents cognitive decline when assessed by novel object recognition (NOR; A.), spontaneous alternation (SA; B.), novel arm entry (NAE; C) and by the Morris water maze (MWM, D.). The dashed line in A. represents no preference and in B. chance alternation. E. PSD95 levels are higher in the cortex of EGF-treated E4FAD-F mice compared to the VC. The full, non-adjusted blot is presented in supplementary Fig. 1. n = 7 per group, except for E, where n = 6. Data expressed as mean +/− S.E.M. *p < 0.05 by 2-way ANOVA and Sidak’s post-hoc analysis (A-C, and acquisition phase in D). *p < 0.05 by Students t-test (probe trial in D, and E).
At the end-point, in the Morris water maze test, both EGF- (day 1 versus 3, 4 and 5, day 2 versus 4 and 5) and VC- (day 1 versus 3, 4 and 5) treated E4FAD-F mice learned the location of the platform (Fig. 3D, (4,48) = 17.5 (time), p < 0.05, Sidak’s post-hoc comparison, *p < 0.05). In the probe trial EGF-treated mice spent more time in the target quadrant (VC = 17.9 seconds and EGF = 29.9 seconds, Student’s t-test, *p < 0.05). Furthermore, levels of the postsynaptic protein PSD95 were higher in the cortex of EGF-treated mice (Fig. 3E and Supplementary Fig. 1 for full blot, Student’s t-test, *p < 0.05). These data support that EGF prevents cognitive and post-synaptic deficits in E4FAD-F mice.
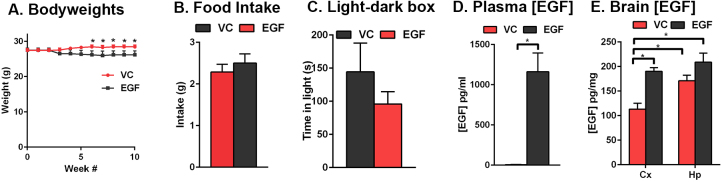
A potential confounding factor for behavioral read-outs is anxiety-like behavior. One marker of anxiety is changes in bodyweight. EGF treated mice lost ∼6% in bodyweight at week 5 and then remained steady for the remainder of the study (Fig. 4A, F(10,120) = 3.6 (interaction), p < 0.05, Sidak’s post-hoc comparison, *p < 0.05). The changes in bodyweight were not related to general locomotion (open field test, data not shown), food intake over 24 hours (Fig. 4B, Student’s t-test, p > 0.05) or anxiety-like behavior as assessed in the light-dark box test (Fig. 4C, Student’s t-test, p > 0.05). These data support that the improved cognition associated with peripheral EGF administration compared to the VC was not related to differences in general locomotion or anxiety-like behavior.
Fig. 4.
EGF did not modulate food intake or anxiety like behavior. A. Body weight decreased at 5 weeks, but remained constant until 10 weeks with EGF treatment. B. EGF treatment had no effect on food intake (over 24 h at the end point or C. performance in the light-dark box test. D. EGF-treated mice had higher plasma and E. brain levels of EGF, measured by ELISA. n = 7 per group. Data expressed as mean +/− S.E.M. *p < 0.05 by 2-way ANOVA and Sidak’s post-hoc analysis (A). *p < 0.05 by Students t-test (B-D).
3.4. Plasma and brain EGF levels were increased after EGF treatment
For data interpretation, it was important to determine whether brain EGF levels were increased after treatment. 30 minutes after the final treatment, EGF levels were measured in the plasma (Fig. 4D) and cortex (Fig. 4E). Plasma EGF levels were ∼6 pg/ml in VC treated mice and ∼1163 pg/ml in EGF treated mice (Student’s t-test, *p < 0.05). Interestingly, brain EGF levels were higher in the cortex after EGF treatment (∼110 pg/ml VC, ∼190 pg/ml EGF, Student’s t-test, *p < 0.05), but not to the same extent as the plasma. Therefore, in this study, we cannot draw a conclusion on whether higher peripheral or brain levels of EGF underlie the cognitive benefits.
3.5. Fibrinogen leakiness is lower and vessel coverage higher in EGF-treated E4FAD-F mice
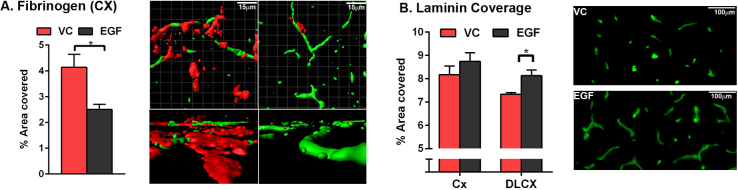
In order to determine the effect of EGF on cerebrovascular dysfunction, fibrinogen extravasation was assessed by IHC. Quantitatively, levels of fibrinogen were ∼40% lower in the cortex of EGF-treated, compared to VC-treated, E4FAD-F mice (Fig. 5A, Student’s t-test, *p < 0.05). To assess whether EGF also modulated total vessel coverage, laminin staining was quantified (Fig. 5B, Student’s t-test, *p < 0.05). In the deep layer cortex, laminin coverage was ∼20% higher in EGF-treated mice compared to VC-treated. Collectively these data demonstrate that in E4FAD-F mice, EGF treatment is associated with lower cerebrovascular leakiness and higher vessel coverage.
Fig. 5.
Peripheral EGF administration prevents cerebrovascular dysfunction in E4FAD-F mice. Fibrinogen levels are lower in the cortex (CX) of EGF-treated E4FAD-F mice (A.) and laminin coverage is higher in the CX and deep layer CX of EGF-treated E4FAD-F mice (B.) when compared to vehicle control treated mice. n = 7 per group. Data expressed as mean +/− S.E.M.*p < 0.05 by Students t test.
4. Discussion
4.1. Sex and APOE4-induced cognitive and CV dysfunction in EFAD-mice
The first important finding from this study is that independent of high Aβ levels, APOE4 and female sex interact to induce cognitive and cerebrovascular dysfunction in eight-month-old EFAD- mice. These data raise important questions surrounding the significance of APOE- and sex-modulated cerebrovascular damage outcomes for cognitive function, the underlying mechanisms, and how our data compare to EFAD+ mice.
Our behavioral data are consistent with previous reports that APOE4 and female sex induce cognitive function in vivo [38, 35, 36, 37]. In addition, human data demonstrate that APOE4 is associated with declining cognitive ability during aging (reviewed in [26]), and APOE4 and female sex interact to increase AD risk and/or progression (reviewed in [40]). Our data also support that cerebrovascular dysfunction, as evident from higher leakiness and lower vessel coverage, is co-incident with cognitive deficits in female E4FAD- mice. Although Aβ-independent APOE4-induced cerebrovascular dysfunction has been reported previously [32, 33], including lower vessel coverage and higher leakiness, our novel data support that the effect is more pronounced in female mice. APOE can modulate multiple processes that impact neuronal function (see below), however a tempting speculation is that cerebrovascular dysfunction plays a significant role in the development of cognitive deficits in E4FAD- female mice. For example, the higher leakiness of plasma proteins into the brain can drive neuroinflammation and potentially directly impair neuronal function [23, 24, 25]. Alternatively, more subtle cerebrovascular deficits prior to overt leakiness may contribute to impaired cognition with APOE4 and female sex. For example, lower cerebral blood flow (controlled by smooth muscle cells in arterioles or pericytes at capillaries), disrupted levels of specialized homeostatic brain endothelial cell proteins at the BBB (e.g. glucose transporter 1) and/or changes at the post capillary venule (e.g. inflammatory cell activation). Therefore, fully dissecting the temporal sequence of cerebrovascular dysfunction outcomes independent of Aβ in female E4FAD-F mice may provide critical insights on the contribution of each outcome to cognitive dysfunction.
In E4FAD-F mice the cortex (not cerebellum) was more permeable to sodium fluorescein and lower vessel coverage was particularly pronounced in the deep layer cortex. Potential explanations for the brain region-specific increased susceptibility of the cerebrovasculature to damage include, but are not limited to; 1. Deeper layer cortical vessels, most likely capillaries, are more sensitive to damage and/or less able to recover due to the greater distance from the larger blood vessels; 2. Capillaries are the main vessels that are modulated by APOE4 and female sex in aging, and are more prevalent in the deep layer cortex; 3. There is a differential gene expression profile in brain endothelial cells by brain region and; 4. All cells types in the deep layer cortex are modulated by APOE4 and sex during aging, which is reflected by cerebrovascular damage. Closer investigation of vessel coverage in the deep layer cortex indicates that female sex may be an overall driver of cerebrovascular impairments. In the deep layer cortex, female E3FAD-F have lower vessel coverage than E3FAD-M and E4FAD-M male mice. Thus, longitudinally, E3FAD-F mice may exhibit cognitive impairments prior to male E4FAD-M mice. Alternatively, cerebrovascular dysfunction may not be critical for APOE3 carriers.
The question of how the apoE isoforms differentially modulate a diverse range of biological processes to affect cognition is an ongoing research focus of a number of groups. In general, proximally, apoE4 may induce detrimental changes in apoE-containing lipoprotein structure, lipidation, stability, toxic apoE fragment production, apoE levels, apoE receptor recycling, and/or receptor activity in a number of cell types (reviewed in [27, 41, 26]). As a consequence, interlinked processes in the periphery (e.g. cholesterol metabolism and inflammation) and brain (e.g. neuronal function, neuroinflammation) are modified. Thus, APOE-modulated processes in the periphery and brain could signal to brain endothelial cells of the BBB to modulate function. For example, higher levels of detrimental cytokines (e.g. cytokines considered pro-inflammatory), or lower levels of protective cytokines (e.g. cytokines considered anti-inflammatory) produced by peripheral inflammation and neuroinflammation can signal in brain endothelial cells. More specific APOE4 modulated pathways have also been identified that are linked to BBB dysfunction and leakiness. For example, one proposal is that apoE4 produced by astrocyte is impaired in its ability to activate the low density lipoprotein receptor-related protein 1 (LRP1) in pericytes, resulting in higher matrix metalloproteinase 9 production, basement membrane degradation and impaired brain endothelial function [33, 42]. In addition, unlike apoE4, apoE3 suppresses pericyte mobility in a pathway involving LRP1 in vitro [43]. Pericyte migration can lead to vessel wall instability, and therefore BBB leakiness. ApoE3 signaling via LRP1 is also higher than apoE4 in brain endothelial cells in vitro, resulting in higher occludin phosphorylation [44]. Therefore, the multifunctional detrimental effects of apoE4, either as a loss of protective function or gain of toxic function, can converge to induce brain endothelial cell disruption. These mechanistic pathways might be compounded by female sex, or as discussed above, vice versa. Frequently, the higher risk of AD in females is attributed to the loss of sex hormones after menopause [45, 40]. However, here in the absence of ovariectomy, we still observed cognitive and cerebrovascular deficits in E4FAD-F mice. As for APOE4, there are a number of proximal mechanisms and processes that are modulated by sex that are relevant for cerebrovascular dysfunction. These include, but are not limited to, peripheral inflammation, neuroinflammation, cellular senescence and brain vulnerability to stress-induced damage. In addition, although 17-β estradiol is beneficial to brain endothelial cells in vitro [46], it may be less protective than male sex hormones. Thus, the collective changes modified by sex over time may converge to induce dysfunction in all cell types at the BBB. Interesting areas for future study include, whether sex hormones in development increase susceptibility of the BBB to age-related damage, if sex hormone levels or receptor signaling become blunted with age in brain endothelial cells, the differences between central and peripheral sex hormone levels and signaling [47] and how apoE4 interacts with the sex related changes.
A limitation of the current study is that EFAD- mice that lack the FAD mutations, were not compared side by side with EFAD+ mice, which overproduce Aβ. However, and although not ideal, we can compare our previous data in EFAD+ mice [31] to data obtained in EFAD- mice in this study. This comparison supports that Aβ exacerbates the cognitive decline in female mice that express APOE4. Indeed, in the Y-maze test, the number of spontaneous alternations were ∼40 E4FAD+ mice, whereas in this study are ∼60 for E4FAD-F mice. Further, a comparison of the vehicle control groups of the EGF E4FAD + F [31] and E4FAD-F mice, demonstrate a similar exacerbation of cognitive dysfunction in the acquisition and probe trial phase of the Morris water maze test. Therefore, in EFAD mice APOE4 and female sex induce cognitive deficits, an effect exacerbated by Aβ. For fibrinogen extravasation (cortex) and vessel coverage (deep layer cortex), a similar comparison demonstrates that Aβ exacerbates dysfunction when comparing E4FAD-F and E4FAD + F mice [48, 31]. Further comparisons among EFAD mice highlight interesting differences by APOE genotype, sex and Aβ. Although we are careful not to over interpret across studies, markers of cerebrovascular dysfunction in the cortex are more pronounced in EFAD+ mice compared to EFAD- mice, and are similar for E4FAD-F, E3FAD + F, and E4FAD + M, or are slightly more pronounced in E4FAD-F mice. Therefore, cerebrovascular dysfunction is likely a prominent feature for female sex + APOE3 + Aβ, male sex + APOE4 + Aβ and female sex + APOE4. Future studies on temporal changes in EFAD+ and EFAD- mice will provide critical insight on the role of APOE genotype, sex, and Aβ in cognitive and cerebrovascular dysfunction
4.2. EGF prevents cognitive and cerebrovascular dysfunction in E4FAD-F mice
The second key finding of this study is that EGF prevents cognitive and cerebrovascular dysfunction in E4FAD-F mice. These novel data support that the protective effects of EGF are not limited to signaling cascades induced by Aβ.
It is important to discuss the potential mechanism(s) through which EGF induced beneficial effects on cognition and cerebrovascular dysfunction in E4FAD-F mice. Strongly linked to this issue is whether cerebrovascular dysfunction or other mechanistic pathways are the driving force behind cognitive deficits. One potential mechanism is that EGF directly targeted brain endothelial cells at the BBB. Brain endothelial cells in the deep layer cortex and other cortical areas may have become dysfunctional in EFAD-F mice, leading to lower levels of tight junction proteins, higher leakiness, vessel degeneration, and neuronal dysfunction. EGF, through signaling via the EGF receptor in brain endothelial cells, could have prevented the signaling deficits and prevented cerebrovascular dysfunction. This hypothesis is consistent with our in vitro data [49] and the general protective functions of EGF in wound healing, stroke, and epithelial cell disorders (reviewed in [50]). Further, during the course of treatment, EGF may have prevented a loss of key proteins involved in the homeostatic functions of brain endothelial cells prior to vessel degeneration. An alternative explanation is that the beneficial effects of EGF were mediated by non-brain endothelial targets in the brain, such as neurons, astrocytes, pericytes, and/or in the periphery. Indeed, EGF levels in the brain were increased by EGF treatment, however to a lesser extent than in the plasma. Theoretically, EGF can enter the brain via the brain endothelial cells though diffusion or internalization by the EGF receptor and eventual non-selective transcytosis [51], and/or via areas of the brain with a leakier cerebrovasculature (e.g. circumventricular organs). In the brain, neurons, astrocytes, pericytes and microglia all express the EGF receptor. Functionally, EGF directly enhances long-term potentiation in slice cultures [52], EGF receptor signaling in cortical astrocytes promotes neuronal survival [53], EGF receptor ligands protect pericytes from anoxic damage [54] and in microglia EGF modulates inflammation [55]. In the periphery, EGF can signal in cells of virtually every organ in the body and is associated with changes in glucose metabolism and insulin signaling, both of which may protect the cerebrovasculature [56, 57]. An indicator of the peripheral effects is the lower body weight in EGF treated mice. However, the weight loss induced by EGF may not be indicative of a beneficial process initiated by EGF. Dementia in AD is associated with frailty and weight loss [58]. Although the underlying cause is unclear, potential pathophysiology of frailty include oxidative stress and inflammation. Therefore, EGF may have modulated these processes, which over the longer term could prove detrimental. Alternatively, as E4FADF- mice treated mice lost weight at weak 4 of treatment with no further loss over time, EGF could have modified peripheral metabolism in a neutral or even beneficial manner. Our data provide the basis for further studies dissecting if brain endothelial cells are the primary target of EGF.
There are a number of important questions that remain regarding the role of EGF signaling in cognitive and cerebrovascular function, which are also important for any future therapeutic programs based on EGF. The first is dissecting whether EGF reverses cognitive and cerebrovascular deficits in female E4FAD+ and E4FAD- mice. A beneficial response is dependent on a number of factors, including the ability of brain endothelial cells to recover from damage and also the function of EGF within the brain. Regarding the latter, treatment of mice with a leaky cerebrovasculature will likely lead to much higher brain EGF levels than observed in the prevention paradigm, and induce EGF receptor activation in astrocytes, pericytes and neurons. Currently, the consequence of raising brain EGF levels in an aging or an FAD-model is unclear and is a focus of our ongoing research. The second question is whether EGF will induce beneficial effects in mice that express APOE3, or in male mice expressing APOE4, either in the presence or absence of high Aβ levels. As yet, we have not fully characterized cognitive and cerebrovascular dysfunction over the lifespan of EFAD+ and EFAD- mice, information that is critical prior to initiating treatments. Additional considerations include, identifying the exact cell type(s) and signaling cascades through which EGF impact cognition, whether levels of the EGF family or other angiogenic growth factors in the plasma or brain are modulated by APOE genotype, female sex and aging in mice and humans, and if longer term EGF treatment increases the risk of side effects (e.g. cancer).
In summary, our data demonstrate that EGF prevents cognitive decline in female mice that express APOE4. Therefore, developing treatment strategies based on EGF signaling could provide alternative therapeutic options for age-related cerebrovascular dysfunction and reducing AD risk.
Declarations
Author contribution statement
Riya Thomas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Alan W.J. Morris: Performed the experiments.
Leon M. Tai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Leon M. Tai is supported by University of Illinois at Chicago start-up funds.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The full, non-adjusted blot of Fig. 3E.
References
- 1.Farrall A.J., Wardlaw J.M. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol. Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Goodall E.F., Wang C., Simpson J.E., Baker D.J., Drew D.R., Heath P.R., Saffrey M.J., Romero I.A., Wharton S.B. Age-associated changes in the blood brain barrier: Comparative studies in human and mouse. Neuropathol. Appl. Neurobiol. 2017 doi: 10.1111/nan.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Love S., Miners J.S. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131:645–658. doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., Harrington M.G., Chui H.C., Law M., Zlokovic B.V. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Torre J.C. Vascular risk factor detection and control may prevent Alzheimer's disease. Ageing Res. Rev. 2010;9:218–225. doi: 10.1016/j.arr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Farkas E., Luiten P.G. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 7.Henry-Feugeas M.C. Alzheimer's disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunction. Med. Hypotheses. 2008;70:866–875. doi: 10.1016/j.mehy.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 9.Stanimirovic D.B., Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J. Cereb. Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Haar H.J., Burgmans S., Hofman P.A., Verhey F.R., Jansen J.F., Backes W.H. Blood-brain barrier impairment in dementia: Current and future in vivo assessments. Neurosci. Biobehav. Rev. 2015;49C:71–81. doi: 10.1016/j.neubiorev.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickstein D.L., Biron K.E., Ujiie M., Pfeifer C.G., Jeffries A.R., Jefferies W.A. Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer disease. FASEB J. 2006;20:426–433. doi: 10.1096/fj.05-3956com. [DOI] [PubMed] [Google Scholar]
- 13.Paul J., Strickland S., Melchor J.P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J. Exp. Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ujiie M., Dickstein D.L., Carlow D.A., Jefferies W.A. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 15.Ambrose C.T. Neuroangiogenesis: a vascular basis for Alzheimer's disease and cognitive decline during aging. J. Alzheimers Dis. 2012;32:773–788. doi: 10.3233/JAD-2012-120067. [DOI] [PubMed] [Google Scholar]
- 16.Brown W.R., Thore C.R. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron D.J., Galvin C., Alkam T., Sidhu H., Ellison J., Luna S., Ethell D.W. Alzheimer's-related peptide amyloid-beta plays a conserved role in angiogenesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grammas P., Martinez J., Sanchez A., Yin X., Riley J., Gay D., Desobry K., Tripathy D., Luo J., Evola M., Young A. A new paradigm for the treatment of Alzheimer's disease: targeting vascular activation. J. Alzheimers Dis. 2014;40:619–630. doi: 10.3233/JAD-2014-132057. [DOI] [PubMed] [Google Scholar]
- 19.Parham C., Auckland L., Rachwal J., Clarke D., Bix G. Perlecan domain V inhibits amyloid-beta induced brain endothelial cell toxicity and restores angiogenic function. J. Alzheimers Dis. 2014;38:415–423. doi: 10.3233/JAD-130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel N.S., Mathura V.S., Bachmeier C., Beaulieu-Abdelahad D., Laporte V., Weeks O., Mullan M., Paris D. Alzheimer's beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J. Neurochem. 2010;112:66–76. doi: 10.1111/j.1471-4159.2009.06426.x. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel-Coelho P.M., Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer's disease. Eur. J. Neurosci. 2012;35:1917–1937. doi: 10.1111/j.1460-9568.2012.08126.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang P., Xie Z.H., Guo Y.J., Zhao C.P., Jiang H., Song Y., Zhu Z.Y., Lai C., Xu S.L., Bi J.Z. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer's disease. Biochem. Biophys. Res. Commun. 2011;411:620–626. doi: 10.1016/j.bbrc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Cortes-Canteli M., Mattei L., Richards A.T., Norris E.H., Strickland S. Fibrin deposited in the Alzheimer's disease brain promotes neuronal degeneration. Neurobiol. Aging. 2015;36:608–617. doi: 10.1016/j.neurobiolaging.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu J.K., McLarnon J.G. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J. Cell Mol. Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamolodchikov D., Strickland S. A possible new role for Abeta in vascular and inflammatory dysfunction in Alzheimer's disease. Thromb. Res. 2016;141(Suppl. 2):S59–S61. doi: 10.1016/S0049-3848(16)30367-X. [DOI] [PubMed] [Google Scholar]
- 26.Tai L.M., Thomas R., Marottoli F.M., Koster K.P., Kanekiyo T., Morris A.W., Bu G. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 2016;131:709–723. doi: 10.1007/s00401-016-1547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altmann A., Tian L., Henderson V.W., Greicius M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretsky P.M., Buckwalter J.G., Seeman T.E., Miller C.A., Poirier J., Schellenberg G.D., Finch C.E., Henderson V.W. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Payami H., Zareparsi S., Montee K.R., Sexton G.J., Kaye J.A., Bird T.D., Yu C.E., Wijsman E.M., Heston L.L., Litt M., Schellenberg G.D. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am. J. Hum. Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas R., Zuchowska P., Morris A.W., Marottoli F.M., Sunny S., Deaton R., Gann P.H., Tai L.M. Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol. Commun. 2016;4:111. doi: 10.1186/s40478-016-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alata W., Ye Y., St-Amour I., Vandal M., Calon F. Human apolipoprotein E varepsilon4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J. Cereb. Blood Flow Metab. 2015;35:86–94. doi: 10.1038/jcbfm.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., Zlokovic B.V. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youmans K.L., Tai L.M., Nwabuisi-Heath E., Jungbauer L., Kanekiyo T., Gan M., Kim J., Eimer W.A., Estus S., Rebeck G.W., Weeber E.J., Bu G., Yu C., Ladu M.J. APOE4-specific Changes in Abeta Accumulation in a New Transgenic Mouse Model of Alzheimer Disease. J. Biol. Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grootendorst J., Bour A., Vogel E., Kelche C., Sullivan P.M., Dodart J.C., Bales K., Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav. Brain Res. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez G.A., Burns M.P., Weeber E.J., Rebeck G.W. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn Mem. 2013;20:256–266. doi: 10.1101/lm.030031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villasana L., Acevedo S., Poage C., Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat. Res. 2006;166:883–891. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 38.Bour A., Grootendorst J., Vogel E., Kelche C., Dodart J.C., Bales K., Moreau P.H., Sullivan P.M., Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Hultman K., Strickland S., Norris E.H. The APOE varepsilon4/varepsilon4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J. Cereb. Blood Flow. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riedel B.C., Thompson P.M., Brinton R.D. Age APOE and sex: Triad of risk of Alzheimer's disease. J. Steroid Biochem. Mol. Biol. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai L.M., Mehra S., Shete V., Estus S., Rebeck G.W., Bu G., Ladu M.J. Soluble apoE/Abeta complex: mechanism and therapeutic target for APOE4-induced AD risk. Mol. Neurodegener. 2014;9:2. doi: 10.1186/1750-1326-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halliday M.R., Rege S.V., Ma Q., Zhao Z., Miller C.A., Winkler E.A., Zlokovic B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J. Cereb. Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casey C.S., Atagi Y., Yamazaki Y., Shinohara M., Tachibana M., Fu Y., Bu G., Kanekiyo T. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA-mediated pathway. J. Biol. Chem. 2015 doi: 10.1074/jbc.M114.625251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishitsuji K., Hosono T., Nakamura T., Bu G., Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlamangla A.S., Lachman M.E., Han W., Huang M., Greendale G.A. Evidence for Cognitive Aging in Midlife Women: Study of Women's Health Across the Nation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Na W., Lee J.Y., Kim W.S., Yune T.Y., Ju B.G. 17beta-Estradiol Ameliorates Tight Junction Disruption via Repression of MMP Transcription. Mol. Endocrinol. 2015;29:1347–1361. doi: 10.1210/ME.2015-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pike C.J. Sex and the development of Alzheimer's disease. J. Neurosci. Res. 2017;95:671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai L.M., Balu D., Avila-Munoz E., Abdullah L., Thomas R., Collins N., Valencia-Olvera A.C., LaDu M.J. EFAD Transgenic Mice as a Human APOE Relevant Preclinical Model of Alzheimer's Disease. J. Lipid Res. 2017 doi: 10.1194/jlr.R076315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koster K.P., Thomas R., Morris A.W., Tai L.M. Epidermal growth factor prevents oligomeric amyloid-beta induced angiogenesis deficits in vitro. J. Cereb. Blood Flow Metab. 2016;36:1865–1871. doi: 10.1177/0271678X16669956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J., Zeng F., Forrester S.J., Eguchi S., Zhang M.-Z., Harris R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016;96 doi: 10.1152/physrev.00030.2015. [DOI] [PubMed] [Google Scholar]
- 51.Brandli A.W., Adamson E.D., Simons K. Transcytosis of epidermal growth factor. The epidermal growth factor receptor mediates uptake but not transcytosis. J. Biol. Chem. 1991;266:8560–8566. [PubMed] [Google Scholar]
- 52.Tang Y., Ye M., Du Y., Qiu X., Lv X., Yang W., Luo J. EGFR signaling upregulates surface expression of the GluN2B-containing NMDA receptor and contributes to long-term potentiation in the hippocampus. Neuroscience. 2015;304:109–121. doi: 10.1016/j.neuroscience.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Wagner B., Natarajan A., Grunaug S., Kroismayr R., Wagner E.F., Sibilia M. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. EMBO J. 2006;25:752–762. doi: 10.1038/sj.emboj.7600988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu X., Radulescu A., Chen C.L., James I.O., Besner G.E. Heparin-binding EGF-like growth factor protects pericytes from injury. J. Surg. Res. 2012;172:165–176. doi: 10.1016/j.jss.2010.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu W.S., Liu J.L., Li C.Y., Li X., Xie M.J., Wang W., Tian D.S. Rapidly activated epidermal growth factor receptor mediates lipopolysaccharide-triggered migration of microglia. Neurochem. Int. 2015;90:85–92. doi: 10.1016/j.neuint.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Siddiqui S., Fang M., Ni B., Lu D., Martin B., Maudsley S. Central role of the EGF receptor in neurometabolic aging. Int. J. Endocrinol. 2012;2012:739428. doi: 10.1155/2012/739428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zielinski R., Przytycki P.F., Zheng J., Zhang D., Przytycka T.M., Capala J. The crosstalk between EGF, IGF, and Insulin cell signaling pathways–computational and experimental analysis. BMC Syst. Biol. 2009;3:88. doi: 10.1186/1752-0509-3-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura B.K., Masaki K.H., Blanchette P. Weight loss in patients with Alzheimer's disease. J. Nutr. Elder. 2007;26:21–38. doi: 10.1300/j052v26n03_02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full, non-adjusted blot of Fig. 3E.