Summary
Background
Although overweight and obesity have been studied in relation to individual cardiometabolic diseases, their association with risk of cardiometabolic multimorbidity is poorly understood. Here we aimed to establish the risk of incident cardiometabolic multimorbidity (ie, at least two from: type 2 diabetes, coronary heart disease, and stroke) in adults who are overweight and obese compared with those who are a healthy weight.
Methods
We pooled individual-participant data for BMI and incident cardiometabolic multimorbidity from 16 prospective cohort studies from the USA and Europe. Participants included in the analyses were 35 years or older and had data available for BMI at baseline and for type 2 diabetes, coronary heart disease, and stroke at baseline and follow-up. We excluded participants with a diagnosis of diabetes, coronary heart disease, or stroke at or before study baseline. According to WHO recommendations, we classified BMI into categories of healthy (20·0–24·9 kg/m2), overweight (25·0–29·9 kg/m2), class I (mild) obesity (30·0–34·9 kg/m2), and class II and III (severe) obesity (≥35·0 kg/m2). We used an inclusive definition of underweight (<20 kg/m2) to achieve sufficient case numbers for analysis. The main outcome was cardiometabolic multimorbidity (ie, developing at least two from: type 2 diabetes, coronary heart disease, and stroke). Incident cardiometabolic multimorbidity was ascertained via resurvey or linkage to electronic medical records (including hospital admissions and death). We analysed data from each cohort separately using logistic regression and then pooled cohort-specific estimates using random-effects meta-analysis.
Findings
Participants were 120 813 adults (mean age 51·4 years, range 35–103; 71 445 women) who did not have diabetes, coronary heart disease, or stroke at study baseline (1973–2012). During a mean follow-up of 10·7 years (1995–2014), we identified 1627 cases of multimorbidity. After adjustment for sociodemographic and lifestyle factors, compared with individuals with a healthy weight, the risk of developing cardiometabolic multimorbidity in overweight individuals was twice as high (odds ratio [OR] 2·0, 95% CI 1·7–2·4; p<0·0001), almost five times higher for individuals with class I obesity (4·5, 3·5–5·8; p<0·0001), and almost 15 times higher for individuals with classes II and III obesity combined (14·5, 10·1–21·0; p<0·0001). This association was noted in men and women, young and old, and white and non-white participants, and was not dependent on the method of exposure assessment or outcome ascertainment. In analyses of different combinations of cardiometabolic conditions, odds ratios associated with classes II and III obesity were 2·2 (95% CI 1·9–2·6) for vascular disease only (coronary heart disease or stroke), 12·0 (8·1–17·9) for vascular disease followed by diabetes, 18·6 (16·6–20·9) for diabetes only, and 29·8 (21·7–40·8) for diabetes followed by vascular disease.
Interpretation
The risk of cardiometabolic multimorbidity increases as BMI increases; from double in overweight people to more than ten times in severely obese people compared with individuals with a healthy BMI. Our findings highlight the need for clinicians to actively screen for diabetes in overweight and obese patients with vascular disease, and pay increased attention to prevention of vascular disease in obese individuals with diabetes.
Funding
NordForsk, Medical Research Council, Cancer Research UK, Finnish Work Environment Fund, and Academy of Finland.
Introduction
With population ageing, it is becoming increasingly common for adults to have several co-occurring diseases.1, 2 Recently, the Emerging Risk Factor Collaboration of 91 cohort studies showed that a particular form of cardiometabolic multimorbidity, defined by the simultaneous coexistence of more than one of type 2 diabetes, coronary heart disease, and stroke, was associated with a risk of death substantially greater than that for each of these diseases on their own.3 For example, at age 60 years, people with one cardiometabolic disease had a life expectancy 6 to 10 years shorter than those with no such disease, whereas people with cardiometabolic multimorbidity had a life expectancy shorter by up to 15 years.3 Little is known about the risk factors for cardiometabolic multimorbidity because clinical trials often exclude patients with multimorbidity, and observational studies typically focus on single disease outcomes.
Research in context.
Systematic review
We searched PubMed up to November, 2016, with no date limits or language restrictions using the terms “body mass index”, “obesity”, “overweight” and “cardiometabolic multimorbidity”. We identified large-scale studies examining the consequences of cardiometabolic multimorbidity and treatment of obesity in patients with comorbid disorders. However, none of these studies focused on obesity or BMI as a risk factor for cardiometabolic multimorbidity.
Added value of the research
We collected a large set of individual-level data from 16 prospective cohort studies to assess the association between BMI and the risk of developing cardiometabolic multimorbidity (ie, at least two from: type 2 diabetes, coronary heart disease, and stroke). Data from our 10 year follow-up of more than 120 000 adults without cardiometabolic disease at baseline suggest that, compared with healthy weight people, being overweight is associated with double the risk of developing cardiometabolic multimorbidity; this risk increases to more than four in obese individuals, and more than ten in severely obese individuals. This association was noted in men and women, white and non-white, younger and older individuals, and was not attributable to lifestyle risk factors, such as physical activity, smoking, or alcohol consumption.
Interpretation
Our study quantifies the risk of developing cardiometabolic multimorbidity in overweight, obese, and severely obese individuals. The findings highlight the need for clinicians to actively screen for diabetes in overweight and obese patients with vascular disease, and pay increased attention to prevention of vascular disease in obese individuals with diabetes. The strong link between BMI and cardiometabolic multimorbidity, a disorder that increases mortality risk substantially beyond that associated with single cardiometabolic diseases, should be acknowledged in health policies and clinical guidelines.
Global rates of obesity (defined as a BMI of ≥30 kg/m2) range between 11% and 15%.4 Results of the Non-Communicable Disease Collaboration analyses of almost 20 million adults showed that the global prevalence of obesity doubled between 1975 and 2014. Despite an overarching aim of the WHO's 25 × 25 global prevention programme being to halt the rise in obesity, the population mean for BMI has continued to increase, with little sign of levelling off.4, 5, 6 High BMI is linked to a range of chronic disoders, disability, and reduced longevity.7, 8, 9 In particular, a large body of research shows that obesity increases the risk of individual cardiometabolic diseases.10 However, the extent to which obesity also contributes to cardiometabolic multimorbidity is currently unknown.
To address some of these limitations, we pooled individual-level data from prospective cohort studies to establish the risk of incident cardiometabolic multimorbidity in adults who are overweight and obese, compared with those who are a healthy weight. Importantly, the size of the dataset allowed us, for the first time, to differentiate between mild obesity (class I) and severe obesity (class II and III).
Methods
Study population
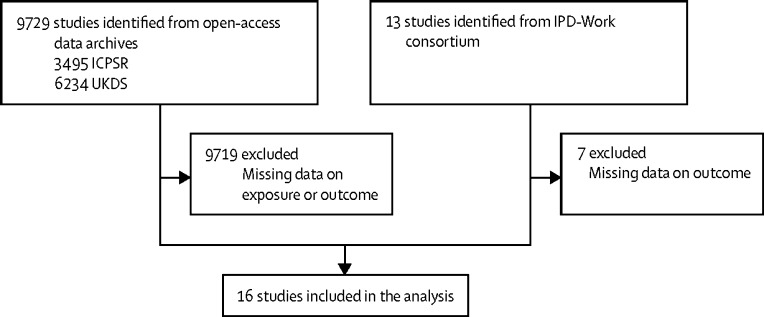
In this pooled analysis of individual-level data, we identified participants from prospective cohort studies from open-access data archives, Inter-University Consortium for Political and Social Research, and the UK Data Service, and from the Individual-Participant Meta-analysis in Working Populations (IPD-Work) consortium (figure 1).11 We included all cohort studies with individual-level data available for BMI at baseline and cardiometabolic multimorbidity at follow-up (ten studies from the open-access data archives and six from the IPD-Work consortium). Table 1 lists the studies included.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 The appendix provides a description of the studies (p 1).
Figure 1.
Study selection
ICPSR=Inter-University Consortium for Political and Social Research. UKDS=the UK Data Service. IPD-Work=The Individual-Participant Data Meta-analysis in Working Populations Consortium.
Table 1.
Baseline characteristics of the participants from 16 prospective cohort studies*
| Country | Baseline year | Number of participants | Number of women | Age (years) | BMI (kg/m2) | |
|---|---|---|---|---|---|---|
| ACL12 | USA | 1986 | 1080 | 706 (65%) | 52·1 (11·9), 35–96 | 26·3 (4·8) |
| ACS13 | USA | 1973–1974 | 1990 | 1095 (55%) | 48·2 (8·9), 35–103 | 24·3 (3·7) |
| ELSA14 | UK | 2004–2005 | 4165 | 2392 (57%) | 64·5 (8·8), 35–90 | 27·8 (4·8) |
| FPS21 | Finland | 2000 | 37 797 | 30 628 (81%) | 47·6 (7·0), 35–65 | 25·2 (4·0) |
| GAZEL22 | France | 1997 | 10 591 | 2992 (28%) | 50·2 (3·0), 43–58 | 25·3 (3·4) |
| HALS15 | UK | 1984–1985 | 2963 | 1668 (56%) | 51·3 (11·9), 35–97 | 25·1 (4·0) |
| HeSSup23 | Finland | 1998 | 11 246 | 6464 (57%) | 47·0 (5·1), 40–54 | 25·5 (4·0) |
| HRS16 | USA | 1993–1994 | 6337 | 4050 (64%) | 57·9 (7·4), 35–103 | 26·7 (4·6) |
| MIDUS17 | USA | 1995–1996 | 2610 | 1429 (55%) | 50·3 (10·2), 35–75 | 26·7 (5·0) |
| SHARE18 | Europe | 2004–2006 | 9808 | 5777 (59%) | 61·6 (8·7), 35–103 | 26·0 (4·1) |
| UKHLS19 | UK | 2012 | 9401 | 5339 (57%) | 56·7 (13·1), 35–102 | 28·2 (5·1) |
| Whitehall II24 | UK | 1991–1994 | 7477 | 2288 (31%) | 49·4 (6·0), 39–63 | 25·2 (3·7) |
| WLSG20 | USA | 1992–1994 | 5336 | 2906 (54%) | 53·7 (0·7), 52–57 | 26·5 (4·3) |
| WLSS20 | USA | 1992–1994 | 2626 | 1423 (54%) | 52·1 (6·8), 35–76 | 26·5 (4·5) |
| WOLF-N25 | Sweden | 1996–1998 | 3540 | 618 (17%) | 48·0 (7·5), 35–65 | 26·4 (3·6) |
| WOLF-S26 | Sweden | 1992–1995 | 3846 | 1670 (43%) | 47·3 (7·4), 35–69 | 25·1 (3·6) |
Data are n; n (%); or mean (SD), range.
In alphabetical order. ACL=the Americans' Changing Lives Study. ACS=the Alameda County Study. ELSA=the English Longitudinal Study of Ageing. FPS=the Finnish Public Sector Study. GAZEL=a cohort study of Électricité de France-Gaz de France employees. HALS=the British Health and Lifestyle Survey. HeSSup=the Health and Social Support Cohort Study. HRS=the Health and Retirement Study. MIDUS=the National Survey of Midlife Development in the United States. SHARE=the Survey of Health, Ageing and Retirement in Europe, including Austria, Belgium, Germany, Denmark, Spain, France, Italy, the Netherlands, Sweden, and Israel. UKHLS=the United Kingdom Household Panel Survey. Whitehall II=the Whitehall II Study. WLSG=the Wisconsin Longitudinal Study, graduate sample. WLSS=the Wisconsin Longitudinal Study, sibling sample. WOLF-N=the Work, Lipids and Fibrinogen Study, Norland. WOLF-S=the Work, Lipids and Fibrinogen Study, Stockholm.
Participants included in the analyses were 35 years or older, had data available for BMI at baseline and for type 2 diabetes, coronary heart disease, and stroke at baseline and follow-up. We excluded participants with a diagnosis of diabetes, coronary heart disease, or stroke at or before study baseline. All studies providing individual participant data for the meta-analysis had obtained ethical approval.
Baseline assessment
BMI was calculated using the formula: weight in kg divided by height in metres squared. Weight and height at baseline were measured in the English Longitudinal Study of Ageing (ELSA);14 the British Health and Lifestyle Survey (HALS);15 the Whitehall II Study (Whitehall II);24 the Work, Lipids and Fibrinogen Study, Norland (WOLF-N);25 the Work, Lipids and Fibrinogen Study, Stockholm (WOLF-S);26 and were self-reported in the Americans' Changing Lives Study (ACL);12 the Alameda County Study (ACS);13 the Finnish Public Sector Study (FPS);21 a cohort study of Électricité de France-Gaz de France employees (GAZEL);22 the Health and Social Support Cohort Study (HeSSup);23 the Health and Retirement Study (HRS);16 the National Survey of Midlife Development in the United States (MIDUS);17 the Survey of Health, Ageing and Retirement in Europe, including Austria, Belgium, Germany, Denmark, Spain, France, Italy, the Netherlands, Sweden, and Israel (SHARE);18 the United Kingdom Household Panel Survey (UKHLS);19 the Wisconsin Longitudinal Study, graduate sample (WLSG);20 and the Wisconsin Longitudinal Study, sibling sample (WLSS).20 We classified BMI into categories of healthy (20·0–24·9 kg/m2), overweight (25·0–29·9 kg/m2), class I (mild) obesity (30·0–34·9 kg/m2) and class II and III (severe) obesity (≥35·0 kg/m2) according to WHO recommendations.27 As in previous studies, we used an inclusive definition of underweight (<20 kg/m2) to achieve sufficient case numbers for analysis.9
Demographic and lifestyle factors used as covariates, included age, sex, ethnic origin (white or non-white), leisure time physical activity (low, intermediate, or high),28 smoking (current, ex-smoker, or never),11 and alcohol consumption (none, moderate, or heavy),29 ascertained at baseline using self-administered questionnaires. Ethnic origin was self-defined according to pre-set categories by participants in each study. These categories were designated as white or non-white by the relevant research team.
Follow-up of cardiometabolic diseases
In the ten studies with repeated resurveys (ACL, ACS, ELSA, HALS, HRS, MIDUS, SHARE, UKHLS, WLSG, and WLSS), non-fatal cardiometabolic diseases were ascertained via repeated self-reports of physician or health professional diagnosis of coronary heart disease, heart attack, angina, heart failure, heart trouble, or other heart problems; stroke; and diabetes or high blood glucose.
In the six studies linked to electronic medical records (FPS, GAZEL, HeSSup, Whitehall II, WOLF-N, and WOLF-S), information about fatal or non-fatal incident coronary heart disease during the follow-up period was ascertained from national hospitalisation and death registries. The one exception was the GAZEL study in which hospitalisation registry data were not available and non-fatal events were based on annual self-report questionnaires. Date of diagnosis, hospital admission due to myocardial infarction, or date of coronary death was used to define coronary heart disease incidence, coded using MONICA definitions or International Classification of Disease (ICD) codes.30, 31 For mortality and hospital records, only the main diagnosis was used. We included all non-fatal myocardial infarctions that were recorded as International Classification of Diseases, version 10 (ICD-10) I21-I22 and coronary deaths recorded as ICD-10 I20-I25.
In the six record-linkage studies, we defined fatal or non-fatal incident stroke using national hospital admission and death registers (ICD-10 codes I60, I61, I63, I64). In Whitehall II, additional information came from self-reports in follow-up questionnaires and stroke event tracing.32 In GAZEL, only self-reports from annual follow-up surveys and mortality records were available. Cases of fatal or non-fatal type 2 diabetes, diagnosed using ICD-10 code E11, were identified from hospital admission and discharge registers, and from mortality records with mention of type 2 diabetes in any of the diagnostic codes.33 Additionally, in FPS and HeSSup, participants were defined as diabetes cases the first time they appeared in the nationwide drug reimbursement register having been prescribed medication for the treatment of diabetes. In Whitehall II, type 2 diabetes was ascertained by 2 h oral glucose tolerance test administered every 5 years using WHO criteria (fasting glucose ≥7 mmol/L or 2 h post-load glucose ≥11·1 mmol/L),34 and complemented by self-reports of diabetes diagnosis and medication.35 In the GAZEL study, diabetes cases were based on self-report from annual questionnaires or mortality records.
For coronary heart disease, stroke, and type 2 diabetes, the onset date for incident disease was defined as the date of the first record during the follow-up period ascertained via any of the sources described above. For cardiometabolic multimorbidity the date of onset was defined as the date the second incident cardiometabolic disease was ascertained. In ACL, HALS, MIDUS, and UKHLS, dates of disease onset were not available and cardiometabolic multimorbidity was therefore recorded if the participant reported at least two of the three cardiometabolic conditions.
Outcomes
The main outcome was cardiometabolic multimorbidity (ie, developing at least two out of type 2 diabetes, coronary heart disease, and stroke). Additionally, we decomposed cardiometabolic multimorbidity into two distinct endpoints on the basis of the temporal order of disease occurrence: diabetes followed by vascular disease (ie, coronary heart disease or stroke) and vascular disease followed by diabetes.
Statistical analysis
We analysed BMI as a categorical variable (<20·0, 20·0–24·9, 25·0–29·9, 30·0–34·9, ≥35 kg/m2) and as a continuous variable. In the main analysis, the associations between BMI and cardiometabolic multimorbidity were examined in two steps. First, we studied the association separately in each cohort study using logistic regression. We adjusted odds ratios and their 95% confidence intervals for age, sex, ethnic origin, and lifestyle factors (smoking, physical activity, and alcohol consumption). Cases were individuals who developed at least two of the three cardiometabolic disorders. To form a comparable group of disease-free non-cases as in analyses of individual cardiometabolic diseases and a uniform group in terms of cardiometabolic disease, we considered participants with none of the three disorders as non-cases and excluded those with one only from these analyses. Second, we pooled the study-specific odds ratios using random-effects meta-analysis.
To assess whether the association between BMI and cardiometabolic multimorbidity was robust, we did several sensitivity and subgroup analyses; stratifying by method of exposure assessment (measured vs self-reported weight and height) and outcome ascertainment (electronic health records vs self-report), sex (men vs women), age group (<50 vs ≥50 years), and ethnic origin (white vs non-white). To achieve sufficient case numbers in these analyses, we pooled data from all cohort studies and did the logistic analysis in one step. The statistical significance of subgroup differences was evaluated by BMI category using meta-regression.
For the six cohort studies linked to electronic medical records, we additionally fitted Cox proportional hazards models and computed hazard ratios for the associations between BMI and cardiometabolic multimorbidity to examine whether hazard ratios and odds ratios were comparable.
To assess the association of BMI with different combinations of cardiometabolic disease, we formed a multicategory outcome variable with four disease endpoints: vascular disease only including incident coronary heart disease, incident stroke or both of these conditions; vascular disease followed by diabetes; diabetes only; and diabetes followed by vascular disease. The associations between BMI and this multicategory outcome variable were examined using multinomial regression, an extension of logistic regression that allows for a dependent variable with more than two categories. This set of analyses included participants with one disorder only.
We used Stata (version 13.1) for data analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MK, EK, and MJ had full access to the data. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
Overall, we included 120 813 adults from Europe or the USA in the analyses. We excluded 9841 participants with a diagnosis of either diabetes, coronary heart disease, or stroke at or before study baseline. Table 1 shows baseline characteristics of the study participants: 71 445 (59%) of 120 813 participants were women, the mean age was 51·4 years (range 35–103), and the mean BMI was 25·9 kg/m2 (SD 4·1). Across the BMI categories, 5039 (4%) participants were underweight, 51 488 (43%) were a healthy weight, 46 132 (38%) were overweight, 13 943 (12%) were mildly obese, and 4211 (4%) were severely obese.
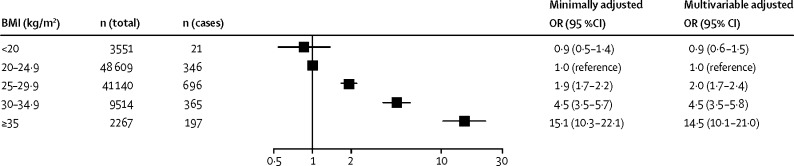
During a mean follow-up of 10·7 years, 1627 participants developed cardiometabolic multimorbidity (incidence 12·6 per 10 000 person-years). Figure 2 shows odds ratios for developing cardiometabolic multimorbidity adjusted for age, sex, and ethnic origin in overweight, mildly obese, and severely obese participants compared with healthy weight participants. After adjustment for sociodemographic and lifestyle factors, compared with individuals with a healthy weight, the risk of developing cardiometabolic multimorbidity in overweight individuals was twice as high (odds ratio [OR] 2·0, 95% CI 1·7–2·4; p<0·0001), almost five times higher for individuals with class I obesity (4·5, 3·5–5·8; p<0·0001), and almost 15 times higher for individuals with classes II and III obesity combined (14·5, 10·1–21·0; p<0·0001). For continuous BMI, the odds ratio for cardiometabolic multimorbidity was 1·9 (95% CI 1·8–2·3) per 5 kg/m2 increase in BMI, and there was further evidence of a curvilinear association (linear term 2·3, 95% CI 2·0–2·5, quadratic term of 0·9, 0·9–1·0 when BMI was centered at 25 kg/m2; data not shown). The appendix provides study-specific findings (appendix p 2).
Figure 2.
Associations of BMI categories with incident cardiometabolic multimorbidity
OR=odds ratio. ORs were adjusted for age, sex, ethnic origin (minimally adjusted), and lifestyle factors (multivariable adjusted).
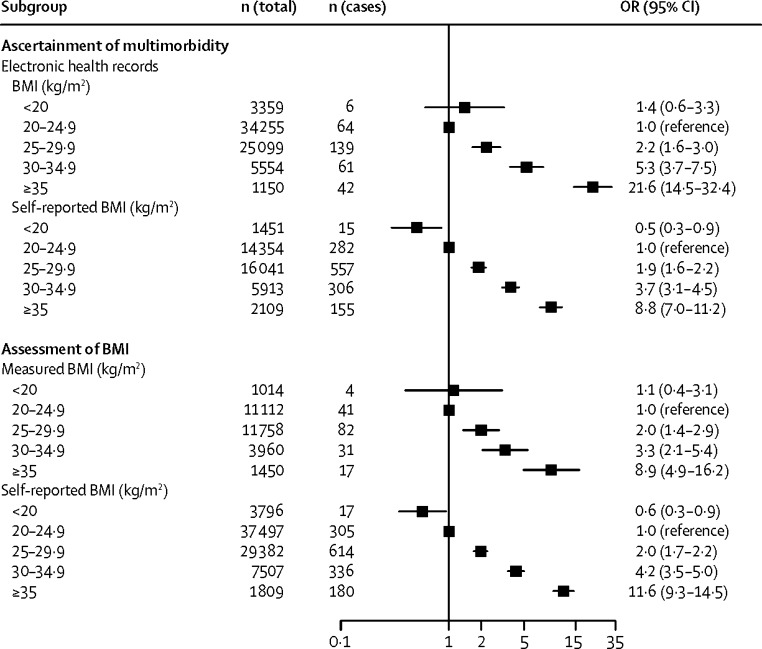
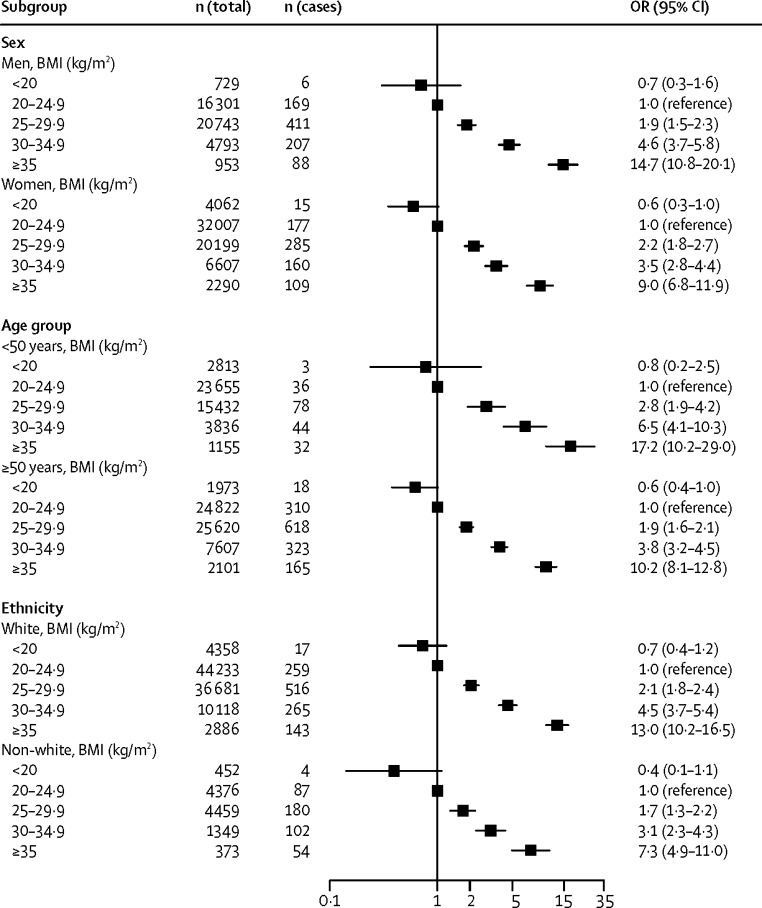
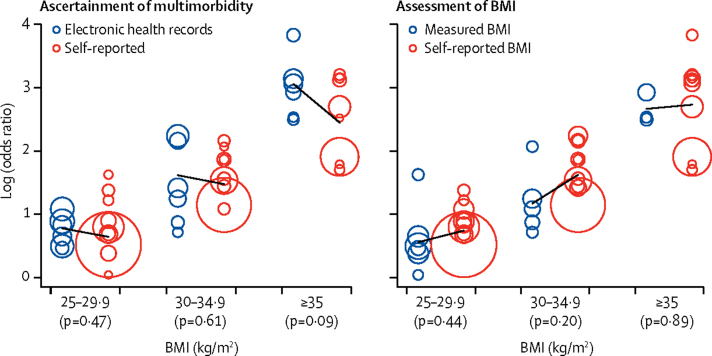
Figure 3, Figure 4 present the odds ratios for developing cardiometabolic multimorbidity in stratified analyses. The association between BMI and cardiometabolic multimorbidity was recorded irrespective of whether weight and height were measured or self-reported, and whether multimorbidity was ascertained using electronic health records or using repeated self-report surveys. Analyses stratified by sex suggested associations between BMI and cardiometabolic multimorbidity among men and women, as well as among younger and older, and among white and non-white participants. Although the odds ratio for BMI higher than 35 kg/m2 seems higher in studies using electronic health records to ascertain multimorbidity (21·6, 95% CI 14·5–32·4) than in studies using repeated self-report surveys (8·8, 7·0–11·2), this difference is not significant when taking into account heterogeneity in study-specific effect estimates (figure 5). There was no evidence for any real differences in outcome associations related to other BMI categories or for subgroup analyses by sex, age, ethnic origin, and method of BMI assessment (appendix p 3). The findings were also independent of the method of statistical analysis; results from logistic regression models were similar to those from Cox proportional regression models (appendix p 4).
Figure 3.
Associations of BMI categories with incident cardiometabolic multimorbidity by method of exposure assessment and outcome ascertainment
OR=odds ratio.
Figure 4.
Associations of BMI categories with incident cardiometabolic multimorbidity by subgroup
OR=odds ratio.
Figure 5.
Comparison of study-specific effect estimates for BMI as a predictor of multimorbidity between studies with electronic health records (blue circles) and those with self-reported data (red circles) and between studies with measured BMI (blue circles) and those with self-reported BMI (red circles)
The size of each circle is proportional to the study's weight in the analysis. p values are for the difference between the two groups of studies.
Table 2 shows multinomial regression with the different cardiometabolic conditions decomposed and analysed separately. Cohort studies with no data for the order of disease onset were excluded (ACL, HALS, MIDUS, UKHLS). Of the remaining 104 730 participants (mean follow-up 10·2 years), 6227 developed a vascular disease (50·4 per 10 000 person-years) and 6609 participants were diagnosed with diabetes (53·6 per 10 000 person-years). Overweight and obesity were associated with increased odds ratios for all the cardiometabolic endpoints (table 2). For overweight, the odds ratios varied between 1·2 and 3·3 depending on the endpoint. The corresponding range of odds ratios was from 1·5 to 9·4 for mild obesity and from 2·2 to 29·8 for severe obesity. Odds ratios were highest for the outcomes of diabetes only and diabetes followed by vascular disease.
Table 2.
Multinomial logistic regression analysis of the association of BMI with different combinations of cardiometabolic disease, adjusted for age, sex and ethnic origin (total n=104 730)
| Vascular disease only (n=5214) | Vascular disease followed by diabetes (n=415) | Diabetes only (n=5596) | Diabetes followed by vascular disease (n=598) | |
|---|---|---|---|---|
| <20·0 kg/m2 (underweight) | 0·9 (0·7–1·0) | 1·1 (0·5–2·2) | 0·7 (0·5–0·9) | 0·5 (0·2–1·4) |
| 20·0–24·9 kg/m2 (healthy weight) | 1·0 (reference) | 1·0 (reference) | 1·0 (reference) | 1·0 (reference) |
| 25·0–29·9 kg/m2 (overweight) | 1·2 (1·1–1·3) | 2·4 (1·8–3·1) | 3·3 (3·0–3·6) | 3·1 (2·4–4·1) |
| 30·0–34·9 kg/m2 (obese, class I) | 1·5 (1·3–1·6) | 4·9 (3·6–6·7) | 9·4 (8·6–10·3) | 8·5 (6·4–11·2) |
| ≥35·0 kg/m2 (obese, classes II and III) | 2·2 (1·9–2·6) | 12·0 (8·1–17·9) | 18·6 (16·6–20·9) | 29·8 (21·7–40·8) |
| Continuous BMI, per 5 units | 1·3 (1·2–1·3) | 2·3 (2·1–2·6) | 2·7 (2·6–2·7) | 2·9 (2·7–3·2) |
Data are odds ratio (95% CI). In the Americans' Changing Lives Study (ACL), the British Health and Lifestyle Survey (HALS), the National Survey of Midlife Development in the United States (MIDUS), and the United Kingdom Household Panel Survey (UKHLS), the order of disease onset could not be determined. 6227 participants developed vascular disease (3924 had coronary heart disease, 1816 stroke, and 487 had both) and 6609 were diagnosed with diabetes. Vascular disease followed by diabetes refers to participants with coronary heart disease or stroke who subsequently developed comorbid diabetes. Diabetes followed by vascular disease refers to participants with diagnosed diabetes who subsequently developed comorbid coronary heart disease or stroke.
Discussion
Our analyses of more than 120 000 adults free of cardiometabolic diseases at study entry suggest that, compared with being a healthy weight, being overweight is associated with double the risk of developing cardiometabolic multimorbidity; in participants with mild and severe obesity, the risk is four and ten times bigger, respectively. Obesity is associated with unhealthy lifestyles such as physical inactivity, smoking, and alcohol consumption.36 However, after accounting for such behaviours, the associations of overweight and obesity with cardiometabolic multimorbidity remained strong.
Data from previous studies have suggested that obese men and women, compared with those of a healthy weight, have a 1·6 times increased risk of coronary heart disease37 and a 1·6–1·8 times increased risk of stroke and admittance to hospital for any cardiovascular disease.38, 39 In our study, the odds ratios for vascular disease without subsequent diabetes associated with mild obesity and with severe obesity, each compared with a healthy weight, were 1·5 and 2·2; for vascular disease followed by comorbid diabetes these were as high as 4·9 and 12·0. This finding reflects a substantially increased risk of diabetes among obese participants with vascular disease. In the Emerging Risk Factor Collaboration analysis of 91 cohort studies, vascular disease without diabetes only doubled risk of death, but vascular disease with diabetes almost quadrupled it.3 Together, evidence from our study and the Emerging Risk Factor Collaboration indicates that high BMI is associated with serious forms of vascular disease. Data from Mendelian randomisation studies of BMI and cardiovascular disease suggest this association is probably causal.40, 41, 42
The link between high BMI and diabetes is especially strong, with randomised controlled trials, Mendelian randomisation analyses, and large-scale observational studies all supporting causality.43, 44, 45, 46 A recent meta-analysis of cohort studies found that obesity is associated with more than a seven times increased risk of diabetes.46 Our data for diabetes-related multimorbidity add to this evidence by suggesting that high BMI is more strongly linked to diabetes followed by vascular disease (that is, diabetes with cardiovascular complications) than diabetes only. Odds ratios for the association between obesity and diabetes varied between 8·5 and 9·5, which is in agreement with previous findings.46 Severe obesity was linked to substantially greater disease risk, the odds ratios being almost 19-fold for diabetes alone and 30-fold for diabetes followed by vascular disease. The contribution of high BMI to diabetes development is marked and clearly exceeds its effect on vascular disease.47, 48
Obesity increases the risk of dyslipidaemia and systemic inflammation, which could be common pathways to the development of both diabetes and vascular disease.48, 49 However, there are additional pathways that might explain the stronger association of BMI with diabetes followed by vascular disease than vascular disease followed by diabetes. These include strong causal associations of overweight and obesity with metabolic abnormalities that are directly linked to diabetes and that develop early in the course of adiposity-related pathophysiology, such as reduced peripheral glucose uptake, insulin resistance and glucotoxicity, finally leading to elevated blood glucose.48, 50 By contrast, longer durations of obesity might be required for the development of atherosclerosis, which typically precedes overt vascular disease.51 This order of disease onset is supported by pooled analyses that show diabetes as an important mediator between obesity and coronary heart disease and stroke.52
An alternative temporal sequence, from obesity to vascular disease and diabetes, is also biologically plausible because obesity could induce changes that are more strongly related to vascular events than diabetes, such as increased thrombogenic factors and raised blood pressure resulting from increased peripheral vascular resistance and renal salt retention.49, 52 Additionally, people who develop vascular disease before diabetes might have early-onset susceptibility factors, which are independent of obesity, such as genetic predisposition to high blood pressure and dyslipidaemia, thus weakening the association between obesity and multimorbidity. It would be interesting to probe and quantify in subsequent studies the role of these and other factors, such as increased sympathetic nervous system activity, in the pathways from obesity to cardiometabolic multimorbidity.
The strength of this study lies in its novelty, the use of individual-participant data from prospective cohort studies from Europe and the USA, and the statistical power resulting from 16 studies which allowed us to determine associations between multiple BMI categories and cardiometabolic multimorbidity. This study also has limitations. Ascertainment of multimorbidity was based on repeat surveys in ten cohort studies. Electronic health records were available for six studies. Neither of our outcome ascertainment methods captured undiagnosed disease and in self-reported data fatal cardiometabolic disease was additionally missing. However, the pattern of results was similar in both sets of studies suggesting that self-reports did not substantially bias our findings. We did not have data for BMI during the follow-up or time-dependent covariates, such as dietary factors or medication use.53 This prevented us from assessing potential biological and behavioural mediators for the observed associations.36 Reverse causation caused by weight loss after disease onset may lead the status of risk factor to be underestimated. This is an unlikely source of major bias in the present study because BMI was assessed in participants with no history of coronary heart disease, stroke, or diabetes. Not all of our cohorts were population based. However, the generalisability of our findings was strengthened by individual data from 16 independent studies in 18 different countries with recruitment over three decades.
In conclusion, our findings show that even being overweight represents a major risk factor for cardiometabolic multimorbidity and that the excess risk associated with mild obesity and severe obesity reaches levels that are seldom seen in modern epidemiology.54 This study has practical implications. In view of the excess risk of death attributable to cardiometabolic multimorbidity, it is important that clinicians and their patients are informed that high BMI not only increases the risk of diabetes and vascular disease separately, but also of their more severe, comorbid forms. Our data support recommendations that clinicians treating overweight or obese patients with vascular disease screen for the development of diabetes. Screening for vascular disease in overweight or obese patients with diabetes is also clearly important. Given that overweight and obesity are increasing, acknowledging the high risk of cardiometabolic multimorbidity associated with these conditions in clinical guidelines and public health policies is vital.
Acknowledgments
Acknowledgments
The IPD-Work Consortium has received funding from NordForsk (the Nordic Research Programme on Health and Welfare). MK receives funding from the UK Medical Research Council (K013351), the Academy of Finland (311492), and the Finnish Work Environment Fund. EK, STN, and RL were supported by NordForsk. AT has received funding from the UK Medical Research Council (K013351) and JAB has received funding from Cancer Research UK (C18281/A19169). All other authors declare no competing interests.
Contributors
All authors participated in designing the study, generating hypotheses, interpreting the data and critically reviewing the paper. MK wrote the first draft of the report. Other members of the writing group were AGT, JAB, JEF, and MJ. MJ and EK, with support from STN and RL, analysed the data. MJ, EK, and MK had full access to anonymised individual-participant data from all constituent studies.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Glynn LG. Multimorbidity: another key issue for cardiovascular medicine. Lancet. 2009;374:1421–1422. doi: 10.1016/S0140-6736(09)61863-8. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition-multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Angelantonio E, Kaptoge S, Wormser D, for the Emerging Risk Factors Collaboration Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Global action plan for the prevention and control of noncommunicable diseases 2013–2020. World Health Organization; Geneva, Switzerland: 2013. [Google Scholar]
- 6.Pearce N, Ebrahim S, McKee M. The road to 25x25: how can the five-target strategy reach its goal? Lancet Global Health. 2014;2:e126–e128. doi: 10.1016/S2214-109X(14)70015-4. [DOI] [PubMed] [Google Scholar]
- 7.Fruhbeck G, Toplak H, Woodward E. Obesity: the gateway to ill health—an EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes Facts. 2013;6:117–120. doi: 10.1159/000350627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Hartge P, Cerhan JR. Body-mass index and mortality among 1·46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global BMI Mortality Collaboration. Di Angelantonio E, Bhupathiraju ShN. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 11.Kivimaki M, Jokela M, Nyberg ST. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603 838 individuals. Lancet. 2015;386:1739–1746. doi: 10.1016/S0140-6736(15)60295-1. [DOI] [PubMed] [Google Scholar]
- 12.House JS, Lantz PM, Herd P. Continuity and change in the social stratification of aging and health over the life course: evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans' Changing Lives Study) J Gerontol B Psychol Sci Soc Sci. 2005;60:15–26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- 13.Berkman L, Breslow L. Health and ways of living: the Alameda County Study. Oxford University Press; New York: 1983. [Google Scholar]
- 14.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42:1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox BD. Health and Lifestyle Survey, 1984–1985 (HALS1) User Manual. Economic and Social Data Service; Essex/Manchester: 1988. [Google Scholar]
- 16.Juster FT, Suzman R. An overview of the Health Retirement Study. J Hum Res. 1995;30:S7–S56. [Google Scholar]
- 17.Brim OG, Baltes PB, Bumpass LL, Cleary PD, Featherman DL, Hazzard WR. National Survey of Midlife Development in the United States (MIDUS), 1995–1996. [computer file]. ICPSR02760-v4. Harvard Medical School, Department of Health Care Policy; Ann Arbor, MI: 2007. [Google Scholar]
- 18.Börsch-Supan A. Survey of Health, Ageing and Retirement in Europe (SHARE) Wave 1. Release version: 5.0.0. SHARE-ERIC. Data set. 2016. DOI:10.6103/SHARE.w1.500
- 19.University of Essex . Understanding Society: Waves 1-5, 2009–2014 [computer file] 7th edn. Institute for Social and Economic Research and NatCen Social Research; Colchester, Essex: 2015. [Google Scholar]
- 20.Wollmering E. Wisconsin Longitudinal Study Handbook. University of Wisconsin; Madison, WI: 2007. [Google Scholar]
- 21.Kivimäki M, Lawlor DA, Davey Smith G. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector study. Am J Public Health. 2007;97:874–879. doi: 10.2105/AJPH.2005.078691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg M, Leclerc A, Bonenfant S. Cohort profile: the GAZEL Cohort Study. Int J Epidemiol. 2007;36:32–39. doi: 10.1093/ije/dyl247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkeila K, Suominen S, Ahvenainen J. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol. 2001;17:991–999. doi: 10.1023/a:1020016922473. [DOI] [PubMed] [Google Scholar]
- 24.Marmot MG, Davey Smith G, Stansfeld S. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 25.Alfredsson L, Hammar N, Fransson E. Job strain and major risk factors for coronary heart disease among employed males and females in a Swedish study on work, lipids and fibrinogen. Scand J Work Environment Health. 2002;28:238–248. doi: 10.5271/sjweh.671. [DOI] [PubMed] [Google Scholar]
- 26.Peter R, Alfredsson L, Hammar N, Siegrist J, Theorell T, Westerholm P. High effort, low reward, and cardiovascular risk factors in employed Swedish men and women: baseline results from the WOLF Study. J Epidemiol Community Health. 1998;52:540–547. doi: 10.1136/jech.52.9.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organization; Geneva: 2000. [PubMed] [Google Scholar]
- 28.Fransson EI, Heikkilä K, Nyberg ST. Job strain as a risk factor for leisure-time physical inactivity: an individual-participant meta-analysis of up to 170 000 men and women—The IPD-Work Consortium. Am J Epidemiology. 2012;176:1078–1089. doi: 10.1093/aje/kws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heikkila K, Nyberg ST, Fransson EI. Job strain and alcohol intake: a collaborative meta-analysis of individual-participant data from 140,000 men and women. PLoS One. 2012;7:e40101. doi: 10.1371/journal.pone.0040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . International Statistical Classification of Diseases and Related Health Problems 10th Revision. World Health Organization; Geneva: 2010. [Google Scholar]
- 31.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 32.Britton A, Milne B, Butler T. Validating self-reported strokes in a longitudinal UK cohort study (Whitehall II): Extracting information from hospital medical records versus the Hospital Episode Statistics database. BMC Med Res Methodol. 2012;12:83. doi: 10.1186/1471-2288-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyberg ST, Fransson EI, Heikkilä K. Job strain as a risk factor for type 2 diabetes: A pooled analysis of 124 808 men and women. Diabetes Care. 2014;7:2268–2275. doi: 10.2337/dc13-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO . Definition, diagnosis and classification of diabetes mellitus and its complications. World Health Organization; Geneva: 1997. [Google Scholar]
- 35.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimaki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 37.Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015;3:437–449. doi: 10.1016/S2213-8587(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 39.Joshy G, Korda RJ, Attia J, Liu B, Bauman AE, Banks E. Body mass index and incident hospitalisation for cardiovascular disease in 158 546 participants from the 45 and Up Study. Int J Obesity. 2014;38:848–856. doi: 10.1038/ijo.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Palmer TM, Benn M. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med. 2012;9:e1001212. doi: 10.1371/journal.pmed.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes MV, Lange LA, Palmer T. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet. 2014;94:198–208. doi: 10.1016/j.ajhg.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagg S, Fall T, Ploner A. Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int J Epidemiol. 2015;44:578–586. doi: 10.1093/ije/dyv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbin LJ, Richmond RC, Wade KH. BMI as a modifiable risk factor for type 2 diabetes: Refining and understanding causal estimates using Mendelian randomization. Diabetes. 2016;65:3002–3007. doi: 10.2337/db16-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y, Sha Y, Yao G. Roux-en-Y Gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3462. doi: 10.1097/MD.0000000000003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab. 2014;16:719–727. doi: 10.1111/dom.12270. [DOI] [PubMed] [Google Scholar]
- 46.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Murthy VL, Abbasi SA, Siddique J. Transitions in metabolic risk and long-term cardiovascular health: Coronary artery risk development in young adults (CARDIA) study. J Am Heart Assoc. 2016;5:e003934. doi: 10.1161/JAHA.116.003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017 doi: 10.1016/S0140-6736(17)30058-2. http://dx.doi.org/10.1016/S0140-6736(17)30058-2 published online Feb 9. [DOI] [PubMed] [Google Scholar]
- 49.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 50.Bell J, Hamer M, Batty GD, Singh-Manoux A, Sabia S, Kivimäki M. Incidence of metabolic risk factors among healthy obese adults: 20-year follow-up. J Am Coll Cardiol. 2015;66:871–873. doi: 10.1016/j.jacc.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reis JP, Loria CM, Lewis CE. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration Metabolic mediators of the effect of body mass index, overweight and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383:370–383. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blundell JE, Dulloo AG, Salvador J, Fruhbeck G, BMI ESWGo Beyond BMI-phenotyping the obesities. Obes Facts. 2014;7:322–328. doi: 10.1159/000368783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yusuf S, Hawken S, Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.