Abstract
Background
We explored the incidence of thromboembolic disease in relatives of women diagnosed with placental abruption, a condition that may be related to disordered coagulation.
Methods
Using data from a multicenter, case-control study of placental abruption, we assessed thromboembolic diseases in first-degree male and female relatives of women with and without abruption. The analysis was restricted to biologic parents and full siblings, below 65 years of age, and corrected for familial clustering.
Results
The prevalence of thromboembolic disease was 7.5% in 852 relatives of 212 placental abruption cases and 4.8% in 792 relatives of 206 controls. This increased risk was driven by an association among sisters of abruption probands (odds ratio = 6.8 [95% confidence interval = 1.8–26.0]), and to a lesser extent, among mothers (2.0 [1.0–4.2]). The risk of thromboembolic diseases was similar among the male relatives of placental abruption cases and controls.
Conclusions
These data suggest that thromboembolic diseases aggregate within female relatives of women with placental abruption.
Thromboembolic diseases are a leading cause of premature death. There are pregnancy-related conditions such as intrauterine growth restriction, preeclampsia, and placental abruption that resemble thromboembolic disease, with ischemic changes associated with abnormal clot formation at the placental-decidual interface. Such ischemic placental diseases account for over half of all medically-indicated preterm births.1 Although abruption is the rarest of these ischemic placental diseases (affecting approximately 1% of pregnancies2), it is the most severe.3–5
Placental abruption can be a condition of sudden onset, such as would occur after physical trauma to the abdomen, or use of vasoconstrictive agents such as cocaine. However, it is more often the result of a chronic process6,7 with origins in early pregnancy—even extending to the time of implantation.3 As with other thromboembolic diseases, the risk of abruption is associated with behavioral risk factors, such as smoking8–11 and cocaine use.2,12–15 The increased risk of abruption in women with a previous abruption suggests that there may also be a strong genetic component.16,17 Family history of thromboembolic disease is often a predisposing risk factor for thromboembolic disease in index patients.18 However, the potential for a familial role of abruption as a risk factor is less clear. One Swedish study reported a 3.4-fold higher risk of abruption in first-degree relatives with venous thromboembolism.19 However, it is unknown which specific subtypes of thromboembolic disease are associated with abruption or whether this potential association differs by sex of the parents.
METHODS
Subjects
Data for this study were derived from the New Jersey-Placental Abruption Study. This was a multicenter case-control study, carried out between 2002 and 2007. It was designed to evaluate epidemiologic, genetic, clinical, medical, and obstetric determinants, as well as reproductive and familial characteristics, of women diagnosed with placental abruption.20 Abruption was a clinical diagnosis that included painful vaginal bleeding or hemorrhage accompanied by fetal distress, uterine hypertonicity or tetanic uterine contractions. In addition, if the patient had a diagnosis of placental abruption on prenatal ultrasonography, or if the freshly delivered placenta showed signs of either retroplacental bleeding or clots, they were also recruited as cases. Following the recruitment of a case, a control patient was sought for recruitment from the same hospital. Potential controls were identified by reviewing daily delivery logs in both hospitals, and matched to cases on parity and maternal race/ethnicity. We excluded women with a diagnosis of placenta previa in the current pregnancy. In addition, women with history of abruption in any previous pregnancy were excluded from eligible controls. Only cases and controls with gestational age ≥20 weeks at delivery were eligible for recruitment. Further details about this study have been described in detail elsewhere.21
Thromboembolic Disease
We defined thromboembolic disease as a history of embolism, deep vein thrombosis, myocardial infarction, stroke, and other nonspecific thromboembolic events. Data on thromboembolic disease were ascertained in first-degree female (mother and sisters) and male (father and brothers) relatives of proband abruption cases and controls. Only data from biologic parents and full siblings were used. Interviews were performed by trained research nurses using a structured questionnaire. The interviews, lasting 25–30 minutes, were conducted after the delivery and before discharge. All recruited abruption cases and controls provided data on thromboembolic disease status in first-degree relatives as “present” or “absent,” if they did not know or were unsure, presumably they would have reported absent. Study participants also provided data on age, race, and smoking habits. Cases and controls were unaware that they might have a thromboembolic disease. The sampling fractions of abruption cases and controls were 35% and 0.5%, respectively.
Statistical Analysis
Associations between familial thromboembolic disease and placental abruption were expressed as odds ratios (ORs) and 95% confidence intervals (CIs) derived from logistic regression models, based on generalized estimating equations.22 All models were adjusted for age, race, and smoking habits of first-degree relatives.
RESULTS
Demographic characteristics of first-degree relatives of abruption cases and controls are shown in Table 1. The association between thromboembolism in first-degree relatives and placental abruption is shown in Table 2. Relatives of abruption cases were at increased risk of thromboembolic diseases. This association was larger among female relatives (P = 0.052 for interaction by sex), with the difference seen mostly in sisters and to a lesser extent among mothers. The distribution of specific thromboembolic diseases, by sex in relatives of abruption cases and controls are shown in Table 3. The Figure shows adjusted ORs by sex for myocardial infarction and stroke in relatives in relation to placental abruption.
TABLE 1.
Characteristics of First-degree Relatives of Women With Placental Abruption and Controls
| Probands and First-degree Relatives | Abruption Cases | Controls |
|---|---|---|
| Index probands; no. | 212 | 206 |
| Age (years); mean (SD) | 30.2 (6.0) | 30.5 (6.3) |
| Smoking; % | 9 | 4 |
| African-American race; % | 18 | 17 |
| Probands biologic mother; no. | 188 | 170 |
| Age (years); mean (SD) | 53.1 (7.3) | 52.5 (7.8) |
| Smoking; % | 25 | 18 |
| African-American race; % | 19 | 17 |
| Probands biologic father; no. | 170 | 159 |
| Age (years); mean (SD) | 53.9 (7.6) | 54.4 (8.4) |
| Smoking; % | 40 | 37 |
| African-American race; % | 19 | 20 |
| Probands sisters; no. (range) | 258 (1–6) | 268 (1–6) |
| Age (years); mean (SD) | 31.5 (9.9) | 32.3 (10.2) |
| Smoking; % | 13 | 13 |
| African-American race; % | 11 | 15 |
| Probands brothers; no. (range) | 236 (1–6) | 195 (1–9) |
| Age (years); mean (SD) | 31.7 (10.1) | 30.6 (11.0) |
| Smoking; % | 25 | 25 |
| African-American race; % | 17 | 15 |
TABLE 2.
Association of Thromboembolic Disease in First-degree Relatives of Index Placental Abruption Cases and Controls
| First-degree Relatives | Abruption Cases (n = 212) |
Controls (n = 206) |
Odds Ratio (95% Confidence Interval) |
|||
|---|---|---|---|---|---|---|
| Total Members | Thromboembolic Diseasea No. (%) | Total Members | Thromboembolic Diseasea No. (%) | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) | |
| All relativesc | 852 | 64 (7.5) | 792 | 38 (4.8) | 1.6 (1.1, 2.4) | 1.6 (1.0, 2.5) |
| Parents | 358 | 56 (15.6) | 329 | 35 (10.6) | 1.6 (1.0, 2.5) | 1.5 (0.9, 2.5) |
| Siblings | 494 | 8 (1.6) | 463 | 3 (0.7) | 2.5 (0.7, 9.6) | 2.6 (0.7, 9.7) |
| Female relatives | 446 | 32 (7.2) | 438 | 13 (3.0) | 2.5 (1.3, 4.9) | 2.6 (1.3, 5.0) |
| Biologic mother | 188 | 26 (13.8) | 170 | 12 (7.1) | 2.1 (1.0, 4.3) | 2.0 (1.0, 4.2) |
| Full sisters | 258 | 6 (2.3) | 268 | 1 (0.4) | 6.4 (0.8, 53.2) | 6.8 (1.8, 25.7) |
| Male relatives | 406 | 32 (7.9) | 354 | 25 (7.1) | 1.1 (0.7, 1.9) | 1.1 (0.6, 1.9) |
| Biologic father | 170 | 30 (17.1) | 159 | 23 (14.5) | 1.3 (0.7, 2.3) | 1.3 (0.7, 2.3) |
| Full brothers | 236 | 2 (0.9) | 195 | 2 (1.0) | 0.8 (0.1, 5.9) | 0.7 (0.2, 3.9) |
Thromboembolic disease among male relatives includes one or more of myocardial infarction, embolism, stroke, deep-vein thrombosis, and blood clots and restricted to relatives ≤65 years old.
Associations were adjusted for subjects’ age, smoking status, and race.
Analysis for “all relatives” were further adjusted for sex.
TABLE 3.
Distribution of Thromboembolic Disease Types in Families of Placental Abruption Cases (n = 212) and Controls (n = 206)
| Abruption Cases (n = 852) No. (%) |
Controls (n = 792) No. (%) |
|
|---|---|---|
| Myocardial infarction | 34 (4.0) | 16 (2.0) |
| Female relativesa | 10 (2.2) | 2 (0.5) |
| Male relativesa | 24 (5.9) | 14 (4.0) |
| Embolism | 2 (0.2) | 0 (—) |
| Female relatives | 1 (0.2) | 0 (—) |
| Male relatives | 1 (0.3) | 0 (—) |
| Stroke | 14 (1.6) | 10 (1.3) |
| Female relatives | 9 (2.0) | 4 (0.9) |
| Male relatives | 5 (1.2) | 6 (1.7) |
| Deep-vein thrombosis | 8 (0.9) | 2 (0.3) |
| Female relatives | 4 (0.9) | 1 (0.2) |
| Male relatives | 4 (1.0) | 1 (0.3) |
| Other thrombosis/embolism | 9 (1.1) | 12 (1.5) |
| Female relatives | 7 (1.6) | 5 (1.1) |
| Male relatives | 2 (0.5) | 7 (2.0) |
Female relatives include mother and sisters; male relatives include father and brothers.
Family members may have experienced more than 1 thromboembolic condition.
FIGURE.
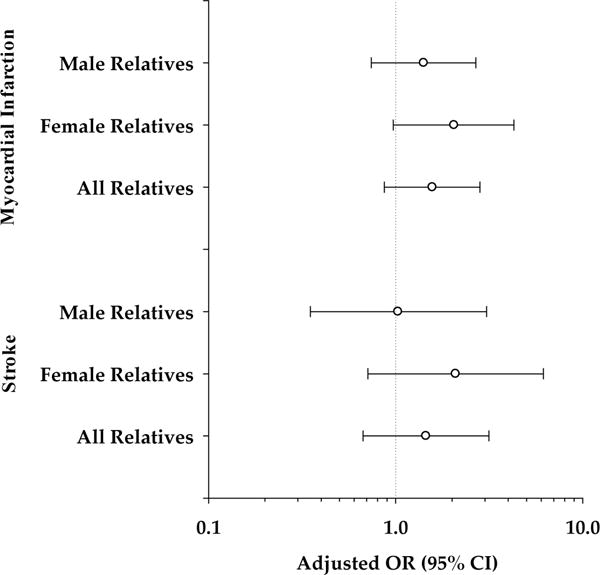
Adjusted OR (95% CI) of myocardial infarction and stroke in first-degree male and female relatives of women with placental abruption.
DISCUSSION
Thromboembolic disease aggregates within families. However, little is known about the impact of abruption as a component of the aggregation of thromboembolic diseases in families. We found that female relatives of women who experience abruption are at increased risk for thromboembolic diseases in general, and myocardial infarction in particular. There was little evidence of associations of thromboembolic disease in male relatives.
Diseases of pregnancy with a likely ischemic or thromboembolic etiology1 (such as preeclampsia, abruption, and intrauterine growth restriction) may share genetic cause with other common thromboembolic diseases. In a large retrospective cohort study, abruption or infarction was associated with increased risk of subsequent premature cardiovascular disease in women.23 Preeclampsia, another form of ischemic placental disease, is associated with increased risk for hypertension later in life24 as well as adult cardiovascular disease.23,24 A meta-analysis evaluating preeclampsia as a risk factor for development of cardiovascular disease concluded that women with preeclampsia had a 3.7-fold (95% CI = 2.7–5.0) increased risk of developing hypertension, a 2.2-fold (1.9–2.5) increased risk of developing ischemic heart disease, a 1.8-fold (1.4–2.3) risk of stroke, and a 1.8-fold (1.4–2.3) increased risk of venous thromboembolism.25 Women giving birth to infants in the lowest birthweight quintile were also found to be at increased risk (OR = 2.4 [95% CI = 1.3–4.4]) of dying of ischemic heart disease later in life.26
Family history of thromboembolic disease as a risk factor for developing pregnancy-related complications has also been explored. A history of venous thromboembolism in first-degree relatives (mother, father or siblings) was more frequent for women with abruption than controls (OR = 3.4 [95% CI = 2.1–5.6]).19 Ness et al27 found that women who reported 2 or more family members with a history of heart disease or stroke had a 3.2-fold (95% CI = 1.4–7.7) increased risk for preeclampsia. Neither of these studies examined sex-specific effects of thromboembolic disease.
A European study, however, found that paternal, but not maternal, early-onset stroke or cardiovascular disease was associated with early-onset severe preeclampsia.28 Although our goal was slightly different, in that we evaluated placental abruption as a risk factor for thromboembolic disease in first-degree relatives, we found associations mostly for female relatives. It is possible that genetic factors predisposing women to preeclampsia are different from those predisposing them to abruption, even though both diseases include a thromboembolic component.
Our finding of an association of thromboembolic disease in female relatives of abruption cases suggests that family history may be a sex-specific risk factor for thromboembolic disease. Some of these observations may be driven by a cohort influence of hormone use between mothers and sisters of probands. Pregnant women are at increased risk for stroke (although uncommon), possibly due to hormone-induced vascular changes that accompany pregnancy, such as hypervolemia, increased cardiac output, and hypercoagulapathies.29 Acquired thrombophillias are more frequent in women with abruption and could potentially also increase their risk for thromboembolic diseases.
Strengths, Limitations, and Biases
This study has a number of strengths. First, it is a large study on placental abruption that included over 400 case and control probands. Each case of abruption was carefully evaluated using well-defined clinical criteria and controls matched to cases on maternal race/ethnicity and parity. The study was based on robust statistical models for family data to adjust for within-family clustering.
There are, however, some limitations to our study. Our results were from a case-control study designed to test a different hypothesis. The possibility of recall bias, although nondifferential between case and control families with thromboembolic diseases, may be present. Second, we were unable to confirm the diagnosis of thromboembolic events with those reported by the probands. The possibility of residual confounding due to unmeasured factors is likely. Finally, we did not specifically distinguish early-onset from late-onset thromboembolic diseases. A myocardial infarction or stroke is a common cause of death in old age, but unusual at a younger age.
A family history of thromboembolic disease may be higher in the setting of placental abruption. This increased risk may be greatest in female relatives, suggesting that placental abruption may share common genetic mechanisms with other vascular diseases that may have expression only in women. Further research may identify how this risk may be modified by hormonal factors and specific genes that may be responsible for this increased risk.
Acknowledgments
Supported by the National Institutes of Health (HD038902) awarded (to C.V.A.) and also supported by the Robert Wood Johnson Research Foundation and the National Institutes of Health Loan Repayment Program (to M.R.P.).
APPENDIX
Investigators who have participated in the New Jersey-Placental Abruption Study include Cande V. Ananth (Principal investigator), Darios Getahun, Neela Srinivas, Celeste DeMarco, Denise Elsasser, Yu-Ling Lai, and Shelby Pitts (Division of Epidemiology and Biostatistics) and John C. Smulian, Wendy L. Kinzler, Morgan R. Peltier, and Marian Lake (Division of Maternal-Fetal Medicine), all in the Department of Obstetrics, Gynecology, and Reproductive Sciences, UMDNJ-Robert Wood Johnson Medical School; Claire Philipp (Department of Medicine), UMDNJ-Robert Wood Johnson Medical School; and George G. Rhoads (Department of Epidemiology), and Dirk F. Moore (Department of Biostatistics) at UMDNJ-School of Public Health.
Other investigators who were involved with the study included Rima Rozen and Jacques Genest (McGill University, Montreal, Canada); Susan Shen-Schwarz, MD (Department of Pathology, Saint Peter’s University Hospital, New Brunswick, NJ), and Vinay Prasad (Department of Pathology, Nationwide Children’s Hospital, Ohio State University, Columbus, OH).
References
- 1.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Oyelese Y, Ananth CV. Placental abruption. Obstet Gynecol. 2006;108:1005–1016. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV, Wilcox AJ. Placental abruption and perinatal mortality in the United States. Am J Epidemiol. 2001;153:332–337. doi: 10.1093/aje/153.4.332. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen S, Irgens LM, Bergsjo P, Dalaker K. Perinatal mortality and case fatality after placental abruption in Norway 1967–1991. Acta Obstet Gynecol Scand. 1996;75:229–234. doi: 10.3109/00016349609047092. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen S, Irgens LM, Bergsjo P, Dalaker K. The occurrence of placental abruption in Norway 1967–1991. Acta Obstet Gynecol Scand. 1996;75:222–228. doi: 10.3109/00016349609047091. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol. 2006;107:785–792. doi: 10.1097/01.AOG.0000207560.41604.19. [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC. Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol. 2006;128:15–21. doi: 10.1016/j.ejogrb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Voigt LF, Hollenbach KA, Krohn MA, Daling JR, Hickok DE. The relationship of abruptio placentae with maternal smoking and small for gestational age infants. Obstet Gynecol. 1990;75:771–774. [PubMed] [Google Scholar]
- 9.Williams MA, Lieberman E, Mittendorf R, Monson RR, Schoenbaum SC. Risk factors for abruptio placentae. Am J Epidemiol. 1991;134:965–972. doi: 10.1093/oxfordjournals.aje.a116181. [DOI] [PubMed] [Google Scholar]
- 10.Raymond EG, Mills JL. Placental abruption. Maternal risk factors and associated fetal conditions. Acta Obstet Gynecol Scand. 1993;72:633–639. doi: 10.3109/00016349309021156. [DOI] [PubMed] [Google Scholar]
- 11.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881–889. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 12.Flowers D, Clark JF, Westney LS. Cocaine intoxication associated with abruptio placentae. J Natl Med Assoc. 1991;83:230–232. [PMC free article] [PubMed] [Google Scholar]
- 13.Slutsker L. Risks associated with cocaine use during pregnancy. Obstet Gynecol. 1992;79:778–789. [PubMed] [Google Scholar]
- 14.Kain ZN, Rimar S, Barash PG. Cocaine abuse in the parturient and effects on the fetus and neonate. Anesth Analg. 1993;77:835–845. doi: 10.1213/00000539-199310000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Addis A, Moretti ME, Ahmed Syed F, Einarson TR, Koren G. Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol. 2001;15:341–369. doi: 10.1016/s0890-6238(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 16.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol. 2007;110:128–133. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 17.Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O. Prepregnancy risk factors for placental abruption. Acta Obstet Gynecol Scand. 2006;85:40–44. doi: 10.1080/00016340500324241. [DOI] [PubMed] [Google Scholar]
- 18.Fischer M, Mayer B, Baessler A, et al. Familial aggregation of left main coronary artery disease and future risk of coronary events in asymptomatic siblings of affected patients. Eur Heart J. 2007;28:2432–2437. doi: 10.1093/eurheartj/ehm377. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist PG, Happach C. Risk and risk estimation of placental abruption. Eur J Obstet Gynecol Reprod Biol. 2006;126:160–164. doi: 10.1016/j.ejogrb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Ananth CV, Elsasser DA, Kinzler WL, et al. Polymorphisms in methionine synthase reductase and betaine-homocysteine S-methyltransferase genes: risk of placental abruption. Mol Genet Metab. 2007;91:104–110. doi: 10.1016/j.ymgme.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananth CV, Peltier MR, De Marco C, et al. Associations between 2 polymorphisms in the methylenetetrahydrofolate reductase gene and placental abruption. Am J Obstet Gynecol. 2007;197:385 e1–e7. doi: 10.1016/j.ajog.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 23.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 24.Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334:291–295. doi: 10.1097/MAJ.0b013e3180a6f094. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 27.Ness RB, Markovic N, Bass D, Harger G, Roberts JM. Family history of hypertension, heart disease, and stroke among women who develop hypertension in pregnancy. Obstet Gynecol. 2003;102:1366–1371. doi: 10.1016/j.obstetgynecol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Rigo J, Jr, Boze T, Derzsy Z, et al. Family history of early-onset cardiovascular disorders is associated with a higher risk of severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2006;128:148–151. doi: 10.1016/j.ejogrb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Feske SK. Stroke in pregnancy. Semin Neurol. 2007;27:442–452. doi: 10.1055/s-2007-991126. [DOI] [PubMed] [Google Scholar]


