Abstract
Preventing adverse health effects of environmental chemical exposure is fundamental to protecting individual and public health. When done efficiently and properly, chemical risk assessment enables risk management actions that minimize the incidence and effects of environmentally induced diseases related to chemical exposure. However, traditional chemical risk assessment is faced with multiple challenges with respect to predicting and preventing disease in human populations, and epidemiological studies increasingly report observations of adverse health effects at exposure levels predicted from animal studies to be safe for humans. This discordance reinforces concerns about the adequacy of contemporary risk assessment practices for protecting public health.
It is becoming clear that to protect public health more effectively, future risk assessments will need to use the full range of available data, draw on innovative methods to integrate diverse data streams, and consider health endpoints that also reflect the range of subtle effects and morbidities observed in human populations.
Considering these factors, there is a need to reframe chemical risk assessment to be more clearly aligned with the public health goal of minimizing environmental exposures associated with disease.
For the past several decades, human health risk assessment has been a pillar of environmental health protection. In general, the products of risk assessment have been numerical risk values derived from animal toxicology studies of observable effects at high doses of individual chemicals. Although this approach has contributed to our understanding of overt health outcomes from chemical exposures, it does not always match our understanding from epidemiology studies of the consequences of real-world exposures in human populations, which are characterized by exposure to multiple pollutants, often chronically, at concentrations that can fluctuate over wide ranges; susceptible populations and life stages; potential interactions between chemicals and nonchemical stressors and background disease states; and lifestyle factors that modify exposures (e.g., airtight houses).1 These and other issues are particularly important when determining risk of complex diseases, such as cardiovascular disease.
Ten years ago, the National Research Council offered a new paradigm for evaluating the safety of chemicals on the basis of chemical characterization, testing using a toxicity pathway approach, and modeling and extrapolating the dose–response relationship from in vitro testing, all embedded in a risk context and considering population-based data and exposure.2 Efforts such as the Tox21 Consortium3,4 and ToxCast program5 have helped us better understand the biological interactions of large numbers of chemicals using high-throughput assay systems, and we are witnessing early adoption of new technologies and approaches for screening chemicals for integrated testing.6
Several other factors are also changing the way environmental health professionals think about chemical risks and how to most effectively protect public health, especially for complex diseases like cardiovascular disease. It is estimated that intrinsic factors (e.g., those that result in mutations stemming from random errors in DNA replication) account for only 10% to 30% of many common cancers.7 Similarly, only 30% to 40% of birth defects can be attributed to known causes such as genetics, fetal alcohol syndrome, maternal smoking, and folate insufficiency.8 Other studies have concluded that nongenetic environmental factors and gene by environment interactions are the primary causes of chronic diseases.9 The ability to evaluate and quantify the role of environmental factors on public health is a clear opportunity, but it is limited by the lack of readily available models for prominent clinical outcomes.
CURRENT CHALLENGES
Understanding public health risk from environmental chemical exposures is complicated by many factors, such as population variability and susceptibility, long latencies between critical exposures and disease manifestations, and background environmental exposures. Issues of population variability and susceptibility are poorly understood and difficult to characterize and incorporate into risk assessments. For example, a person’s unique microbiome may modulate his or her response to environmental exposures.10,11 Although studies are limited in this emerging area, knowledge about the microbiome may inform interindividual variability and unexplained susceptibility observed in populations. Scientists have begun to appreciate the role of the microbiome in the lack of reproducibility and interpretability of animal studies.12
Another example is the effects of early life environmental exposures on health outcomes later in life. Advances in the field of epigenetics have revealed that developmental exposure to endocrine disrupting chemicals can alter epigenetic programming of gene regulation and thus may play a role in the risk of obesity later in life.13 Similar to microbiome research, studies in this area are limited, and a better understanding of the link between chemical exposure, epigenetic gene regulation, and health outcomes through epidemiological research can help us better address factors that are currently difficult to account for in traditional risk assessment. Finally, there are also methodological challenges in determining attributable risks in populations with background environmental exposures, as these background exposures may change the population health baselines or affect the response of the target chemical. Other examples of important factors to incorporate in risk assessments can be found in Table 1.
TABLE 1—
Examples of Current Risk Assessment Challenges and Opportunities
| Risk Assessment Challenge | Description | Impact on Risk Assessment | Public Health Opportunity |
| Molecular initiating events and subsequent key events in adverse outcome pathways | Early biological changes or precursor effects in response to chemical exposures may be identified by in vitro, animal, or epidemiological studies | Useful for qualitative and quantitative understanding of ultimate health effect of early biological changes | Improved public health protection without need for long-term toxicology or epidemiology studies |
| Background exposures | Population exposures to a myriad of environmental chemicals at low concentrations | Exposures to background chemicals may affect response to target chemical exposures and may change population health baselines | Increased public health protection if baseline exposures are taken into account when determining prevention strategies |
| Nonchemical stressors | Physical and psychosocial stressors, including noise, temperature, socioeconomic status, social stress, and limited resources | Impact on baseline susceptibility and potential effect modification | Potential role in cumulative assessment, improved identification of vulnerable populations, potential target for public health interventions (e.g., stress management) |
| Early life determinants of health | Biological characteristics and exposures that can determine chronic and lifelong health outcomes | Effect of exposures during early life may play a role in later disease states (e.g., endocrine disruptors, epigenetic changes) | Potential for early life interventions for prevention and management of later disease |
| Baseline health status | Individual health status, with a focus on potential health susceptibilities | Baseline health status may affect response to additional environmental chemical exposures | Increased public health protection if baseline health status is taken into account |
| Microbiome | Microorganisms that reside within and on our bodies and interact with the environment | Exposure modification, susceptibility and resilience to environmental pollutants, important as an early life determinant of health | Potential targets for prevention and intervention, management of allergic responses, and precision risk management |
OPPORTUNITIES FOR USING MULTIPLE DATA TYPES
Concurrent with these challenges, science and technology are advancing rapidly and in ways that create opportunities for risk assessment. Public health disciplines help us understand how baseline health status can influence the effect of population-level chemical exposures. We also need to consider how environmental pollutants may contribute to overall disease burden for endpoints not traditionally considered in chemical risk assessment (e.g., metabolic disorders, autism). New methods in epidemiological research help us evaluate complex interactions among multifactorial causes of disease ranging from macro (societal, neighborhood) to micro (molecular) factors, relevance of exposures during sensitive life stages, and a better understanding of interrelatedness of disease across the life span.14
Advances in high-throughput technologies and computational modeling (e.g., ToxCast, Tox21, and ExpoCast efforts) are providing data on hazard and exposure potential for a large number of data-poor chemicals. The increased generation of data for both hazard and exposure from these advances can be used to better understand the biological pathways that lead to adverse health effects in ways that were not possible in the past. But linking these observations to specific disease endpoints is challenging because the translation of effects across levels of biological organization is not well understood. One approach with the potential to advance our understanding of how chemical exposures can affect health is the use of adverse outcome pathways, which integrate various types of biological information to link molecular initiating events to downstream key events and ultimately unwanted health outcomes.15,16
To fully realize the potential of adverse outcome pathway–based approaches and to integrate biological findings across disciplines, we must strengthen our ability to detect precursor events in human populations and to identify biologically relevant exposure metrics, ideally measurable in individuals. Another advancement that has a great potential to advance our understanding of data-poor chemicals is the use of nontesting approaches (e.g., quantitative structure–activity relationship) that allow us to predict toxicity when adequate testing data are absent—especially when we combine knowledge of chemical structural features and in vitro bioactivity determinations. Advances in the development of chemical libraries, cheminformatics, and read-across predictions and integration with molecular data and adverse outcome pathways have significantly improved their application and predictive capacity, which will allow more comprehensive assessment of the health effects of exposures.17,18
Effectively predicting population risk by integrating a variety of data streams (e.g., epidemiology, toxicology, high-throughput testing) and considering multiple sources and pathways of exposure can better inform environmental public health decisions. Advances in technology and computational capabilities have fostered new opportunities for generating and analyzing molecular, animal, and human data on effects and exposures, which can be integrated into chemical risk assessments. At the same time, probabilistic and high-throughput approaches for risk assessment have been advancing. Table 2 highlights various data types available and challenges in applying these data types to inform risk assessment.
TABLE 2—
Data Streams and Opportunities and Challenges for Informing Risk Assessment
| Data Type | Description | Opportunity | Challenge |
| Nontesting data | Nontesting approaches, such as quantitative structure–activity relationship models and read-across allow us to predict toxicity when adequate testing data are absent | Advances in the field have significantly improved their application and predictive capacity | Developing principles for acceptance, for characterizing and incorporating uncertainties into predictions, and for developing objective metrics of performance |
| Molecular | Biochemical and cell-based bioactivity data and “omics-based” data on thousands of chemicals | Can help inform our understanding of the health outcomes of environmental exposures, using data that are potentially more human relevant | Lack of scientific consensus on inferring hazard from bioactivity in vitro assay and omics-based data and providing quantitative dose–response information on exposure metrics |
| Animal | Traditional animal testing provides a hazard based point of departure for risk assessments | Targeted animal testing can be performed on the basis of the results of bioactivity data to focus on key health outcomes | Potential uncertainties with using traditional animal testing to estimate human risk (e.g., extrapolating from animal to human or high to low doses and accounting for human population variability and life stage susceptibility) |
| Human | Epidemiological and other human data support holistic assessment of the effects of chemical exposures on public health | Newer exposure science and statistical techniques advance the understanding of human variability that can be obtained from epidemiology and individual sequencing; understanding effect modification by nonchemical stressors and baseline health status | Often limited mechanistic and dose–response data, and exposure misclassification can bias results to the null; possibility of unmeasured confounders often undermines confidence in observed associations, and it may require multiple studies and many years to rule out chance, bias, and confounding as possible explanations for observed associations |
| Exposure | Exposure characterization that captured the variability in time, space, and within and across populations; better toxicokinetic data link external to internal dosimetry and relevant environmental exposure concentrations with biological significance | Targeted and nontargeted biomonitoring, application of sensors, and other new technologies are greatly advancing population exposure characterization; high-throughput exposure models allow exposure predictions on thousands of chemicals with associated uncertainty | Estimating and incorporating the inter- and intraindividual variability in exposures into current designs of toxicity testing and risk assessments; extrapolating relevant target tissue and organ dose information from external exposures and in vitro assays; accounting for multiple exposures; sample collection, data management, and analysis; and covering or extrapolating to a broader chemical space |
| Digital data | The ongoing revolution in social media use and communication has provided a new source of data used in exposure science and environmental epidemiology for local and timely information about disease and health dynamics | A significant source of untapped data | The collection and application of these data have significant ethical implications that need to be understood and managed, particularly taking into account personal identifiable information; methods to evaluate the quality of the data and build confidence in the applications are needed |
A PUBLIC HEALTH PERSPECTIVE
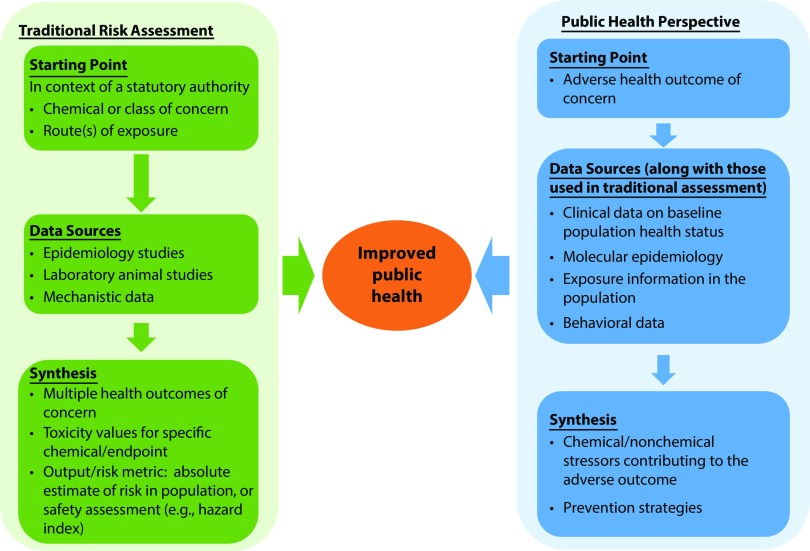
A public health perspective for chemical risk assessment would approach risk assessment from a new lens. It would address population health with a focus on the health and societal burden of disease; use and integrate all available types of data—including traditional toxicology, human epidemiological findings, and newer and emerging data streams and information, such as digital epidemiology,19 high-throughput and high-content data, and adverse outcome pathways; and draw on public health approaches, such as attributable risk or relative risk. This new perspective may be especially important for some historically challenging aspects of risk assessment, such as understanding cumulative risks of exposures to multiple chemical and nonchemical stressors. Internationally, scientists have raised concerns about the large number of ubiquitous chemicals people are exposed to and called for rethinking approaches to evaluating the health effects of chemicals.16 Figure 1 presents a conceptual model for a public health perspective for risk assessment.
FIGURE 1—
Conceptual Model for a Public Health Perspective for Chemical Risk Assessment
Note. This conceptual model illustrates how the starting point in a public health–focused risk assessment would differ from that of traditional risk assessment. In traditional risk assessment, the starting point is focused on specific chemicals or classes of chemicals of concern, with multiple data streams saying what the critical effects from that chemical are. A public health perspective would focus on the adverse health outcome of concern with multiple data streams, informing our understanding of hazard and exposure in the context of public health decisions related to that outcome and not necessarily focused on just 1 chemical or class of chemicals.
Although approaching assessments from the perspective of health outcomes may be challenging, it provides the opportunity to evaluate exposures and effects across the life span that are relevant to population health. Advances in science and technology, such as adverse outcome pathway development, the broader availability of chemical and biological data, and the applications of statistical and bioinformatics tools, bring this previously aspirational approach well within reach.20
EXAMPLE: CARDIOVASCULAR DISEASE
A public health approach may inform the challenge of cardiovascular disease. Cardiovascular disease is the number 1 cause of mortality worldwide and is a major US public health burden.21,22 Annual costs of cardiovascular disease in the United States were estimated to be $317 billion in 2011 and 2012, considering direct medical costs and lost productivity because of premature mortality.22 This estimate is likely to substantially underestimate the social cost of cardiovascular disease because of limitations in the estimation of indirect costs associated with morbidity and premature mortality.23
Although much is known about the biochemical and behavioral risk factors associated with cardiovascular disease, particularly compared with other diseases and health conditions, the traditional risk factors fail to account for 10% to 25% of its prevalence.24 Environmental factors, including air pollution25 and chemical exposures26 are thought to contribute to the unexplained fraction. Although mortality stemming from cardiovascular disease has decreased over the past few decades in the developed world as a result of reductions in behavioral risk factors, the rising prevalence of obesity and diabetes might account for the deceleration in the rate of improvement in annual cardiovascular mortality in the United States over the past few years.27
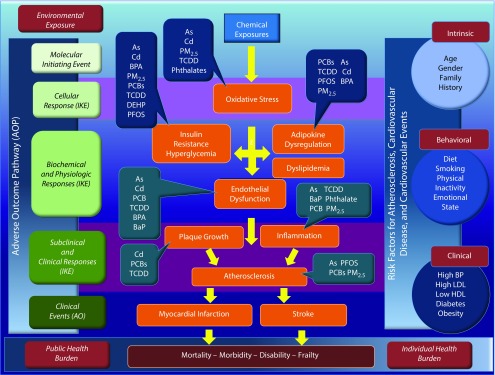
There is an urgent need to better understand the biological pathways through which environmental exposures to chemical and nonchemical stressors act to stimulate and accelerate atherosclerosis and promote adverse cardiovascular health effects. Applying the adverse outcome pathway framework,28 the initial molecular response to a chemical exposure will often be receptor activation and changes in metabolism and, ultimately, changes in tissue and organ function. Such changes can be modified by both intrinsic (e.g., gender, age, genetic, and epigenetic background) and extrinsic factors (e.g., coexposures to other chemical and nonchemical stressors; Figure 2). Over time, these changes produce subclinical effects, such as changes in electrical and mechanical cardiac function, vascular function, and nonobstructive atherosclerotic vascular changes. With the persistence of metabolic changes that stimulate the progression of vascular disease, clinical cardiovascular events such as heart attacks, strokes, heart failure, and abnormal heart rhythms follow.
FIGURE 2—
Adverse Outcome Pathway for Cardiovascular Outcomes
Source. Action of specific chemicals and metals adapted from Kirkley and Sargis.26
Note. As = Arsenic; AO = adverse outcome; BaP = benzo[a]pyrene; BPA = bisphenol A; Cd = Cadmium; DEHP = di(2-ethylhexyl) phthlate; DES = diethylstilbestrol; HDL = high-density lipoprotein; IKE = intermediate key event; LDL = low-density lipoprotein; PCB = polychlorinated biphenyl; PFOS = perfluorooctane sulfonic acid; PM2.5 = particulate matter ≤ 2.5 μm; TCDD = tetrachlorodibenzo-p-dioxin. This figure illustrates the biological pathway leading from exposure to adverse cardiovascular outcomes for a variety of chemicals. On the left-hand side of the figure these pathways are linked to the adverse outcome pathway, and on the right-hand side of the figure we see the traditional risk factors for adverse cardiovascular outcomes.
To date, the most comprehensive application of this approach has been in the study of population-level health effects of air pollution exposure.28 Epidemiological data at the population level has provided support that air pollutant exposure (e.g., ambient particular matter and NO2) accelerates the development and progression of coronary atherosclerosis.25 Xenobiotic metals such as arsenic, cadmium, lead, and mercury are also associated with atherosclerosis.29 Gene–environment interaction alters the risk of vascular disease30; for example, the residential proximity to highways (representing exposure to a mixture of traffic-related air pollutants) is associated with peripheral vascular disease, which is modified by the gene encoding bone morphogenic protein.7,31
Because of the complexity of the drivers of atherosclerosis, a medical model treating blood pressure and high cholesterol and advising dietary modification and exercise will be inadequate to fully address this disease. Likewise, identifying the chemicals that increase risk on an individual basis will be inadequate to prevent vascular disease. Instead an integrated systems approach is needed to fully account for all known risk factors and formulate the problem to define the most effective strategy to decrease individual risk and societal burden. Accomplishing this will require clinical data that fully reflect a population under consideration as well as exposures to traditional risk factors, biomonitoring data documenting exposures to multiple chemicals, and molecular responses from in vitro and in vivo studies indicative of the activation of biochemical pathways that accelerate atherosclerosis.
Although this approach might not be practical currently, it is not unrealistic to think about future states where it could become standard practice. Our proposed innovative approach to chemical risk assessment is occurring contemporaneously during the formative stages of the National Institutes of Health–sponsored Precision Medicine Initiative, which will drive integration of genomics, data sciences, and bioinformatics as the basis for improved individual health care, disease prevention, and public health. The Affordable Care Act has accelerated electronic medical record adoption in health care practices and hospital systems, potentially offering a valuable source of information for population-level health monitoring. Recent research has used big data to study the early stages of disease and better classify and predict disease progression and could be used to inform personalized medicine to optimize wellness in healthy populations.32–34
Moreover, the anticipated integration and development of technologies and analytical tools have the potential to improve public health and increase the spatial and temporal resolution of environmental health surveillance. The establishment of a long-term representative precision medicine cohort, if integrated with the proposed National Biomonitoring Network,35 could have enormous benefit in helping us understand the relationship between chemical exposures and disease and in managing some of the most challenging clinical problems more effectively.
Applying this framework would potentially expand our understanding of the origins of vascular disease and its progression, helping define strategies for primary prevention to thwart the initiation of the process we ultimately call atherosclerosis. Thus, such a framework would provide new and ongoing insights into the associations between environmental exposures that contribute the greatest burden to public health. This approach would facilitate accounting for sensitive populations and could inform suggested individual health or behavioral measures in which there have been past exposures or in which current exposure cannot be reduced enough to protect those most at risk.
CONCLUSIONS
The proposed conceptual model is grounded in public health principles and focused on identifying the greatest opportunity to reduce environmental exposures to improve health outcomes. Along with traditional risk assessment, this perspective can better inform public health decision-making. Although there are clear benefits to operating within a public health–focused framework and moving away from individual chemicals and apical endpoints, there are also challenges.
Informing Decision-Making
Since the 1980s, the Environmental Protection Agency’s decision-making has been grounded on traditional risk assessments that are conducted within the constraints of the Environmental Protection Agency’s statutes and programs. Although program-targeted risk assessments will remain an important component, the disease-based approach draws on information in a holistic fashion that cuts across organizational and legal boundaries, integrating traditional inputs and newer data streams. These assessments will provide decision-makers with critical information to inform exposure-reduction efforts to affect the selected health outcomes and, ultimately, improve public health. Because those exposure-reduction efforts would take place within the existing statutory construct, an important implementation step would be to move from findings of disease-based risk assessments to assessments of specific risk management actions under the relevant statutory authorities.
Priorities for Screening and Testing
A health outcome–focused framework can inform priorities for screening and testing the toxicity of chemicals. Efforts to develop and synthesize approaches for screening large numbers of chemicals using high-throughput toxicity testing and exposure prediction should continue to provide data for data-poor chemicals. For example, in the recently announced Cancer Moonshot,36 high-throughput approaches could screen a large set of chemicals for potential carcinogenicity and identify a suite of chemicals for additional animal toxicity testing.
Examining noncancer endpoints will also be challenging, which is why developing adverse outcome pathways and networks to contextualize and interpret nonapical hazard data in relation to population health is of increasing value. Epidemiology studies can be designed to inform and validate high-throughput testing approaches by identifying both chemical stressors and nonchemical stressors that modify responses to chemical exposures; they can also be designed to test relationships between disease and early markers of exposure and biological response (e.g., epigenetic changes).
The Impact of Cumulative Exposures
Although cumulative risk assessment has been of high interest for the past few decades, putting cumulative assessment approaches into practice has been challenging. This framework provides a new construct for considering cumulative risk. By focusing on a health endpoint of concern, one could consider the multiple exposures that may contribute to a health outcome. Past National Research Council recommendations have encouraged assessors to evaluate the combined effects of exposures to all chemicals that affect a common adverse outcome, for example, male reproductive development.37 Challenges include gaining adequate understanding of individual chemical effects to group chemicals by health outcome. Increased research into the biological pathways by which chemicals affect health status can help inform approaches for estimating the joint effect of chemicals without testing all permutations or combinations.
One example of an alternative approach is health impact assessment, which uses a systems approach to array data sources and analytic methods and considers input from stakeholders to determine potential effects of a proposed action or decision on the health of a population and the distribution of those effects in the population.38 Using health impact assessment approaches for chemical risk assessments made through this framework can offer a method to organize various data streams that can influence our understanding of a health effect, inform potential multiple contributors to adverse health outcomes, and provide recommendations to decision-makers for monitoring and managing these outcomes.
Consider Public Health Concepts
This new approach takes a systematic view of collective factors that contribute to a health outcome or disease state, including those that are not regulated by a single federal entity. Any single health outcome may be influenced by multiple factors beyond chemical exposures, such as nutrition, genetics, and social stressors. Because those factors are not regulated, it is important for environmental regulatory agencies to understand what fraction of the disease burden is influenced by the regulated environmental exposure.
Public health approaches, such as attributable risk, can help inform this understanding. Challenges may include incorporating these approaches, which are typically used in epidemiology, to animal and advanced toxicity testing data; ensuring adequate training with the approaches; and communicating risk in a way that acknowledges the influence of nonregulated factors.
Public Health Implications
Understanding the health effects of chemicals has real implications for public health. This proposed approach for chemical risk assessment starts at the health endpoint and incorporates multiple data streams, including data developed using newer technologies such as high-throughput screening. In parallel with more traditional risk assessment approaches, this will lead to a better understanding of mechanisms of single chemicals as well as cumulative exposures that lead to specific disease endpoints.
This new lens will need to be applied to the complete risk assessment process—problem formulation, data considerations, and data synthesis through multipathway methods, including cumulative assessment and health impact assessment—with an eye to the prevention of adverse effects. This approach draws on the best available science to improve our understanding of the health effects of environmental chemicals and informs decision-making to prevent, reduce, or mitigate exposure and ultimately improve public health.
ACKNOWLEDGMENTS
The authors would like to acknowledge the assistance of Laura Romano in preparing the article.
Note. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the US Environmental Protection Agency.
Footnotes
See also Greenberg, p. 1020.
REFERENCES
- 1.Birnbaum LS, Burke TA, Jones JJ. Informing 21st-century risk assessments with 21st-century science. Environ Health Perspect. 2016;124(4):A60–A63. doi: 10.1289/ehp.1511135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 3.Kavlock RJ, Austin CP, Tice RR. Toxicity testing in the 21st century: implications for human health risk assessment. Risk Anal. 2009;29(4):485–487. doi: 10.1111/j.1539-6924.2008.01168.x. discussion 492–497. [Comment on Toxicity testing in the 21st century: implications for human health risk assessment. Risk Anal. 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tice RR, Austin CP, Kavlock RJ, Bucher JR. Improving the human hazard characterization of chemicals: a Tox21 update. Environ Health Perspect. 2013;121(7):756–765. doi: 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavlock R, Chandler K, Houck K et al. Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chem Res Toxicol. 2012;25(7):1287–1302. doi: 10.1021/tx3000939. [DOI] [PubMed] [Google Scholar]
- 6.Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol. 2015;49(14):8804–8814. doi: 10.1021/acs.est.5b02641. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinhold B. Environmental factors in birth defects: what we need to know. Environ Health Perspect. 2009;117(10):A440–A447. [Google Scholar]
- 9.Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A. The blood exposome and its role in discovering causes of disease. Environ Health Perspect. 2014;122(8):769–774. doi: 10.1289/ehp.1308015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietert RR, Silbergeld EK. Biomarkers for the 21st century: listening to the microbiome. Toxicol Sci. 2015;144(2):208–216. doi: 10.1093/toxsci/kfv013. [DOI] [PubMed] [Google Scholar]
- 11.Patterson AD, Turnbaugh PJ. Microbial determinants of biochemical individuality and their impact on toxicology and pharmacology. Cell Metab. 2014;20(5):761–768. doi: 10.1016/j.cmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servick K. Of mice and microbes. Science. 2016;353(6301):741–743. doi: 10.1126/science.353.6301.741. [DOI] [PubMed] [Google Scholar]
- 13.Stel J, Legler J. The role of epigenetics in the latent effects of early life exposure to obesogenic endocrine disrupting chemicals. Endocrinology. 2015;156(10):3466–3472. doi: 10.1210/en.2015-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck Louis GM, Bloom MS, Gatto NM, Hogue CR, Westreich DJ, Zhang C. Epidemiology’s continuing contribution to public health: the power of “then and now.”. Am J Epidemiol. 2015;181(8):e1–e8. doi: 10.1093/aje/kwv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ankley GT, Bennett RS, Erickson RJ et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 16.Goodson WH, III, Lowe L, Carpenter DO et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis. 2015;36(suppl 1):S254–S296. doi: 10.1093/carcin/bgv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansouri K, Abdelaziz A, Rybacka A et al. CERAPP: Collaborative Estrogen Receptor Activity Prediction Project. Environ Health Perspect. 2016;124(7):1023–1033. doi: 10.1289/ehp.1510267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judson R, Houck K, Martin M et al. Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol Sci. 2016;153(2):409. doi: 10.1093/toxsci/kfw148. [Erratum in Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol Sci. 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker KM, Martinez-Bakker ME, Helm B, Stevenson TJ et al. Digital epidemiology reveals global childhood disease seasonality and the effects of immunization. Proc Natl Acad Sci USA. 2016;113(24):6689–6694. doi: 10.1073/pnas.1523941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academies of Sciences, Engineering, and Medicine. Using 21st Century Science to Improve Risk-Based Evaluations. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 21.Moran AE, Forouzanfar MH, Roth GA et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 23.US Environmental Protection Agency. Guidelines for Preparing Economic Analyses. Washington, DC: 2010. [Google Scholar]
- 24.Kannel WB, Vasan RS. Adverse consequences of the 50% misconception. Am J Cardiol. 2009;103(3):426–427. doi: 10.1016/j.amjcard.2008.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman JD, Adar SD, Barr RG et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388(10045):696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkley AG, Sargis RM. Environmental endocrine disruption of energy metabolism and cardiovascular risk. Curr Diab Rep. 2014;14(6):494. doi: 10.1007/s11892-014-0494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidney S, Quesenberry CP, Jr, Jaffe MG et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594–599. doi: 10.1001/jamacardio.2016.1326. [DOI] [PubMed] [Google Scholar]
- 28.Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12(11):627–642. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 29.Solenkova NV, Newman JD, Berger JS et al. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168(6):812–822. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanobetti A, Baccarelli A, Schwartz J. Gene-air pollution interaction and cardiovascular disease: a review. Prog Cardiovasc Dis. 2011;53(5):344–352. doi: 10.1016/j.pcad.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward-Caviness CK, Neas LM, Blach C et al. Genetic variants in the bone morphogenic protein gene family modify the association between residential exposure to traffic and peripheral arterial disease. PLoS One. 2016;11(4):e0152670. doi: 10.1371/journal.pone.0152670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinov ID, Heavner B, Tang M et al. Predictive big data analytics: a study of Parkinson’s disease using large, complex, heterogeneous, incongruent, multi-source and incomplete observations. PLoS One. 2016;11(8):e0157077. doi: 10.1371/journal.pone.0157077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood L, Lovejoy JC, Price ND. Integrating big data and actionable health coaching to optimize wellness. BMC Med. 2015;13:4. doi: 10.1186/s12916-014-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury MJ, Evans JP. A public health perspective on a national precision medicine cohort: balancing long-term knowledge generation with early health benefit. JAMA. 2015;313(21):2117–2118. doi: 10.1001/jama.2015.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Association of Public Health Laboratories National Biomonitoring Plan. Silver Spring, MD: 2015 [Google Scholar]
- 36.Mitchell E. Moonshot toward a cure for cancer. J Natl Med Assoc. 2016;108(2):104–105. doi: 10.1016/j.jnma.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 37.National Research Council. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 38.National Research Council. Improving Health in the United States: The Role of Health Impact Assessment. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]