Abstract
OBJECTIVES:
Transcription Factor 21 represses steroidogenic factor 1, a nuclear receptor required for gonadal development, sex determination and the regulation of adrenogonadal steroidogenesis. The aim of this study was to investigate whether silencing or overexpression of the gene Transcription Factor 21 could modulate the gene and protein expression of steroidogenic factor 1 in adrenocortical tumors.
METHODS:
We analyzed the gene expression of steroidogenic factor 1 using qPCR after silencing endogenous Transcription Factor 21 in pediatric adrenal adenoma-T7 cells through small interfering RNA. In addition, using overexpression of Transcription Factor 21 in human adrenocortical carcinoma cells, we analyzed the protein expression of steroidogenic factor 1 using Western blotting.
RESULTS:
Transcription Factor 21 knockdown increased the mRNA expression of steroidogenic factor 1 by 5.97-fold in pediatric adrenal adenoma-T7 cells. Additionally, Transcription Factor 21 overexpression inhibited the protein expression of steroidogenic factor 1 by 0.41-fold and 0.64-fold in two different adult adrenocortical carcinoma cell cultures, H295R and T36, respectively.
CONCLUSIONS:
Transcription Factor 21 is downregulated in adrenocortical carcinoma cells. Taken together, these findings support the hypothesis that Transcription Factor 21 is a regulator of steroidogenic factor 1 and is a tumor suppressor gene in pediatric and adult adrenocortical tumors.
Keywords: TCF21, POD1, SF-1, siRNA-POD1, Adrenocortical Tumor Cells
INTRODUCTION
Transcription Factor 21 (Tcf21, POD1, capsulin, epicardin) is a basic helix-loop-helix (bHLH) transcriptional regulatory protein that is expressed in mesenchymal cells 1 at sites of mesenchymal-epithelial interaction in the developing urogenital 2, cardiovascular, respiratory, and gastrointestinal systems 3. In the adrenal gland, POD1 is expressed exclusively in the capsule region of the adrenal cortex, as shown in mice expressing a lacZ gene reporter under the control of the regulatory region of Pod1 4. In the testicles of fetal mice, POD1 represses steroidogenic factor 1 (SF-1/Nr5a1), an orphan member of the nuclear receptor family of transcription factors required for gonadal development, sex determination and the regulation of adrenal and gonadal steroidogenesis in adult mice 5. Alterations of SF-1 dosage regulate compensatory adrenal growth after unilateral adrenalectomy, proliferation and tumorigenesis in mice 6. In humans, SF-1 is associated with adrenocortical tumorigenesis both in children 7 and adults 8. POD1 represses Sf-1/SF-1/SF-1 expression in mouse 5, rat 9, and human adrenocortical cells 10. Moreover, POD1 is downregulated in adrenocortical carcinoma (ACC) 10,11, melanoma 12, lung, and head and neck squamous cell carcinomas 13. In human ACC cells, we showed that POD1 binds to the SF-1 E-box promoter sequence and inhibits SF-1 expression and steroidogenic acute regulatory (StAR) expression, which is controlled by SF-1 10. However, it is unknown whether silencing the POD1 gene promotes increased expression of the SF-1 gene. Accordingly, here, we analyzed the expression of the SF-1 gene after downregulation of endogenous POD1 expression in pediatric adrenocortical tumor cells. Moreover, we verified whether POD1 overexpression causes inhibition of SF-1 protein expression.
MATERIALS AND METHODS
Cell cultures and cell culture transfection
The NCI-H295R adult human ACC cell line 14 and ACC-T36 10, a 6th–10th passage secondary adult human ACC cell line, were cultured and transfected as described by França et al. 10. Cells from the ACA-T7 pediatric secondary cell line were obtained from a functioning adrenocortical adenoma (ACA) as described by Almeida et al. 15. Pediatric adenoma (weight: 10 g; stage I) was diagnosed in a 1.1-yr-old girl with mixed Cushing’s syndrome and virilization 15. The adrenocortical tumor cells were maintained at 37°C in a fully humidified 95% air-5% CO2 environment and cultured in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The experiments were performed at the 10th–15th passage of the pediatric ACA culture cells. Briefly, 1.5 x 105 ACA-T7 cells were plated into six-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA). After 24 h, the cells were transfected with small interfering RNA (siRNA-POD1) or positive or negative high CG RNAi Stealth (Invitrogen, Carlsbad, CA, USA) to a final concentration of 100 nM, combined with 9 µl of RNAiMax Lipofectamine® according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA).
Total RNA extraction and qPCR siRNA (qRT-PCR)
Total RNA was extracted using TRIzol® reagent (Invitrogen) 48 h after transfection. The synthesis of cDNA and RT-qPCR analysis were performed as described by França et al. 10. A cycle threshold (Ct) value in the log-linear phase of amplification was selected for each sample in triplicate and was normalized to the β-actin expression level. Reactions were conducted in triplicate. Data were analyzed using the 2-ΔΔCt method 16.
Immunoblotting
For the protein assay, H295R and ACC-T36 cells were plated and transfected as described by França et al. 17. The cells were lysed 72 h post-transfection in RIPA buffer containing protease and phosphatase inhibitors (Sigma Aldrich Gmbh, Steinheim, Germany). The total protein concentration was determined using the Bradford assay. Total protein lysates (30 µg) were resolved by 12% SDS-PAGE and gels were blotted onto nitrocellulose membranes after electrophoresis. Non-specific binding sites were blocked for 2 h with 0.1% bovine serum albumin (BSA) or 5% non-fat dried milk in TBST (TRIS-buffered saline solution containing 1% Tween 20). All washes and antibody incubations were performed using TBST. The following primary antibodies were used: anti-SF-1 (RD Systems Inc., Minneapolis, MN, USA; 1:1000) in blocking buffer (5% non-fat dried milk in TBST) and anti-actin (1:1000) in TRIS-buffered saline containing 1% Tween 20. Proteins were visualized using enhanced chemiluminescence (ECL) detection with secondary HRP-conjugated anti-rabbit (Amersham Hybond ECL, Freiburg, Germany) or anti-mouse (Jackson ImmunoResearch Inc., West Grove, PA, USA) antibodies. Immunoblot results were quantified on a densitometer using GeneSnap and GeneTools software (SynGene-Synoptic Ltd., Cambridge, United Kingdom). Protein transfer and loading were monitored using Ponceau S staining of the membranes. The experiments were repeated in full at least three times, and SF-1 protein expression was normalized to the levels of β-actin.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of three independent replicate experiments. Data were analyzed using the Kruskal-Wallis test (non-parametric one-way ANOVA) or paired t-tests, when indicated. The results were considered significant when p<0.05.
RESULTS AND DISCUSSION
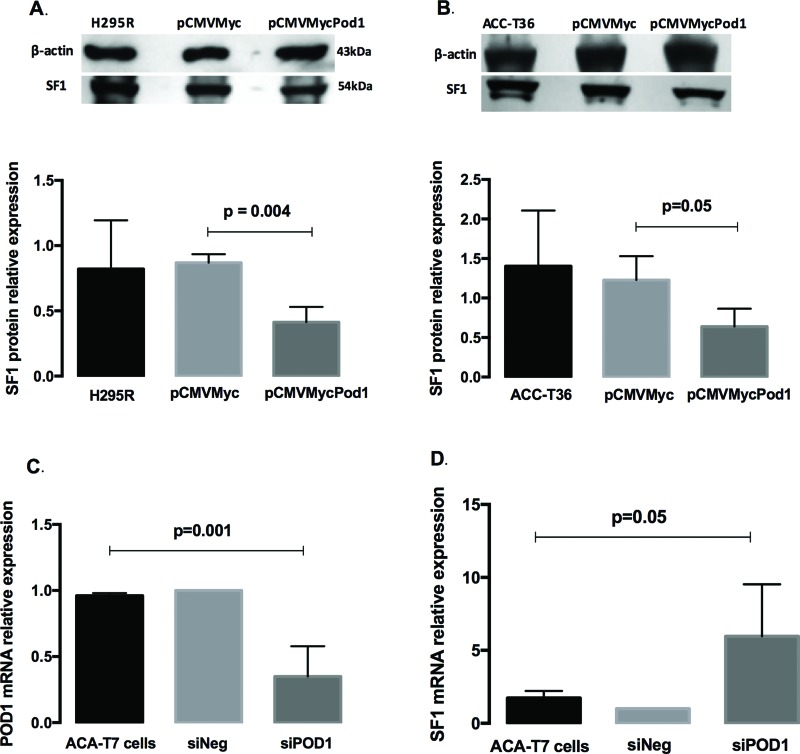
SF-1 protein levels in the H295R cell line (Figure 1A) decreased 0.41±0.11-fold (p=0.004) compared to those in the empty plasmid controls, whereas in ACC-T36 cells (Figure 1B), SF-1 protein expression decreased 0.64±0.22-fold (p=0.05). These results are in agreement with a previous study showing that POD1 inhibited SF-1 mRNA expression in ACC cells 10. Next, we investigated whether knockdown of POD1 could increase SF-1 expression. To knockdown POD1, we used siRNA in ACA-T7 cells that expressed POD1 constitutively (data not shown) and determined the mRNA levels of POD1 and SF-1 in ACA-T7-siRNA-transfected and ACA-T7-siPOD1-transfected cells. POD1 mRNA expression was significantly lower in T7-siPOD1 transfected cells (0.35±0.22-fold), with a 65% decrease compared to that in transfected controls (ANOVA, p=0.001; Figure 1C), whereas the mRNA levels of SF1 increased 5.97±0.22-fold in T7-siPOD-1 cells compared to those in transfected controls (ANOVA, p=0.05; Figure 1D). These results support a role for POD1/TCF21 as a regulator of SF-1 expression in adrenocortical tumor cells. Considering that POD1 is a repressor of SF-1 expression, that increased SF-1 dosage can trigger human adrenocortical cell proliferation 18, and that SF1 amplification 19 and SF1 overexpression 7 are characteristic of childhood adrenocortical tumors, the results of our study improve the knowledge of how the tumorigenic process is controlled in adrenocortical tumor cells. Taken together, the findings of this study and the POD1 inactivation observed in several types of tumors suggest that POD1 may act as a tumor suppressor in pediatric and adult adrenocortical tumors.
Figure 1.
A and B: Immunoblotting analysis of the SF-1 protein levels relative to α-Actin in H295R (A) and ACC-T36 cells (B) that were transiently transfected with pCMVMycPod1 or empty pCMVMyc vectors. C and D: Quantitative reverse transcription PCR (qRT-PCR) analysis of the mRNA expression levels of POD1 (C) and SF1 (D) relative to β-Actin in ACA-T7 pediatric adrenocortical adenoma culture cells. The expression levels were compared using paired t-tests (A and B) or one-way ANOVA Kruskal-Wallis tests (C and D). The values represent the mean ± standard deviation of three experiments.
AUTHOR CONTRIBUTIONS
Lotfi CF conceived the project. Lotfi CF and França MM designed the experiments. Lotfi CF, França MM, Lerario AM and Fragoso MC analyzed the data. França MM performed the experiments. França MM and Lotfi CF wrote the manuscript.
ACKNOWLEDGMENTS
M.M.F. received a scholarship (2010/00771-9) from the São Paulo Research Foundation (FAPESP) and C.F.P.L. received funding from FAPESP (2012/21839-6), the National Council for Scientific and Technological Development (CNPq), and the Office of the Dean for Research at the University of São Paulo.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lu J, Richardson JA, Olson EN. Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev. 1998;73((1)):23–32. doi: 10.1016/S0925-4773(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 2.Quaggin SE, Vanden Heuvel GB, Igarashi P. Pod-1, a mesoderm-spcific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev. 1998;71((1-2)):37–48. doi: 10.1016/S0925-4773(97)00201-3. [DOI] [PubMed] [Google Scholar]
- 3.Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, et al. epicardin: A novel basic helix-loop-helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Dev Dyn. 1998;213((1)):105–13. doi: 10.1002/(SICI)1097-0177(199809)213:1<105::AID-AJA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30((3)):241–63. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura M, Kanno Y, Chuma S, Saito T, Nakatsuji N. Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev. 2001;102((1-2)):135–44. doi: 10.1016/S0925-4773(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 6.Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143((8)):3122–35. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- 7.Pianovski MA, Cavalli LR, Figueiredo BC, Santos SC, Doghman M, Ribeiro RC, et al. SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer. 2006;42((8)):1040–3. doi: 10.1016/j.ejca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010;95((10)):E161–71. doi: 10.1210/jc.2010-0653. [DOI] [PubMed] [Google Scholar]
- 9.França MM, Abreu NP, Vrechi TAM, Lotfi CF. POD-1/Tcf21 overexpression reduces endogenous SF-1 and StAR expression in rat adrenal cells. Braz J Med Biol Res. 2015;48((12)):1087–94. doi: 10.1590/1414-431X20154748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.França MM, Ferraz-de-Souza B, Santos MG, Lerario AM, Fragoso MC, Latronico AC, et al. POD-1 binding to the E-box sequence inhibits SF-1 and StAR expression in human adrenocortical tumor cells. Mol Cell Endocrinol. 2013;371((1-2)):140–7. doi: 10.1016/j.mce.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009;15((2)):668–76. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arab K, Smith LT, Gast A, Weichenhan D, Huang JP, Claus R, et al. Epigenetic deregulation of TCF21 inhibits metastasis suppressor KISS1 in metastatic melanoma. Carcinogenesis. 2011;32((10)):1467–73. doi: 10.1093/carcin/bgr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LT, Lin M, Brena RM, Lang JC, Schuller DE, Otterson GA, et al. Epigenetic regulation of the tumor suppressor gene <italic>TCF21</italic> on 6q23-q24 in lung and head and neck cancer. Proc Natl Acad Sci USA. 2006;103((4)):982–7. doi: 10.1073/pnas.0510171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazdar AF, Oie HK, Shackleton CH, Chen TR, Triche TJ, Myers CE, et al. Establishment and Characterization of a Human Adrenocortical Carcinoma Cell Line That Expresses Multiple Pathways of Steroid Biosynthesis. Cancer Res. 1990;50((17)):5488–96. [PubMed] [Google Scholar]
- 15.Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93((9)):3524–31. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25((4)):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.França MM, Ferraz-de-Souza B, Lerario AM, Fragoso MCBV, Lotfi CFP. POD-1/TCF21 Reduces SHP Expression, Affecting LRH-1 Regulation and Cell Cycle Balance in Adrenocortical and Hepatocarcinoma Tumor Cells. Biomed Res Int. 2015;2015:841784. doi: 10.1155/2015/841784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21((12)):2968–87. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 19.Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, et al. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90((2)):615–9. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]