Abstract
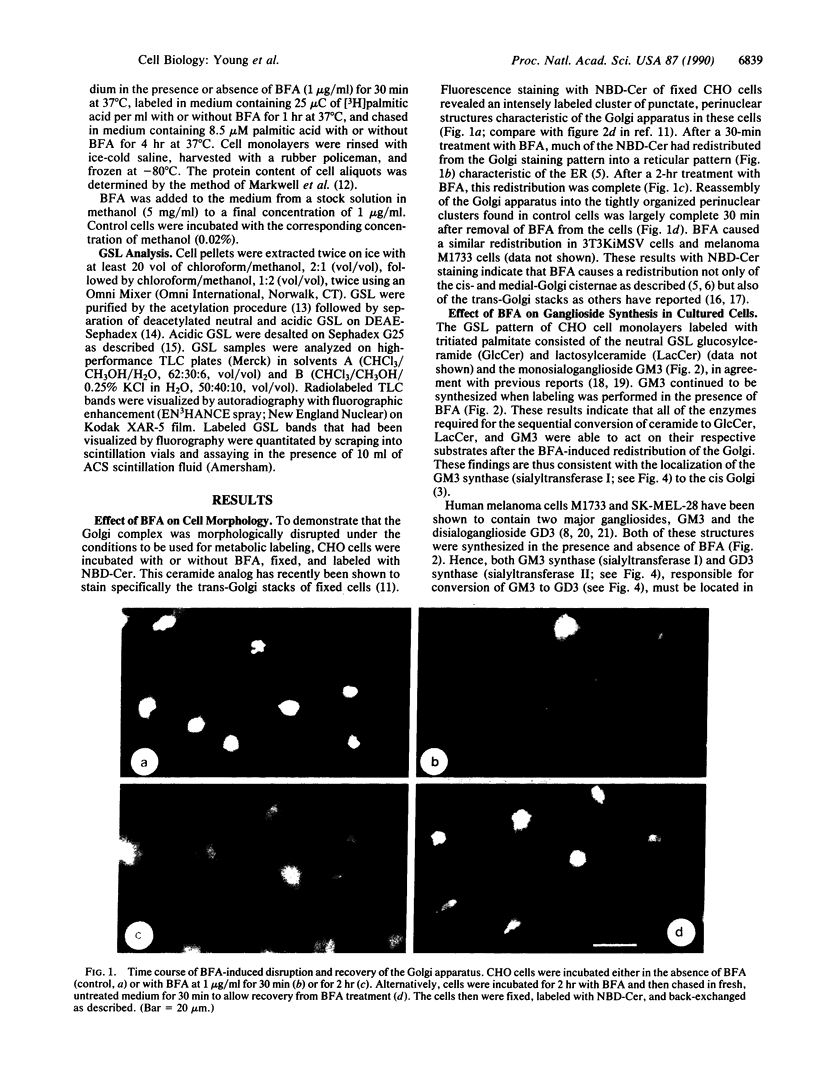
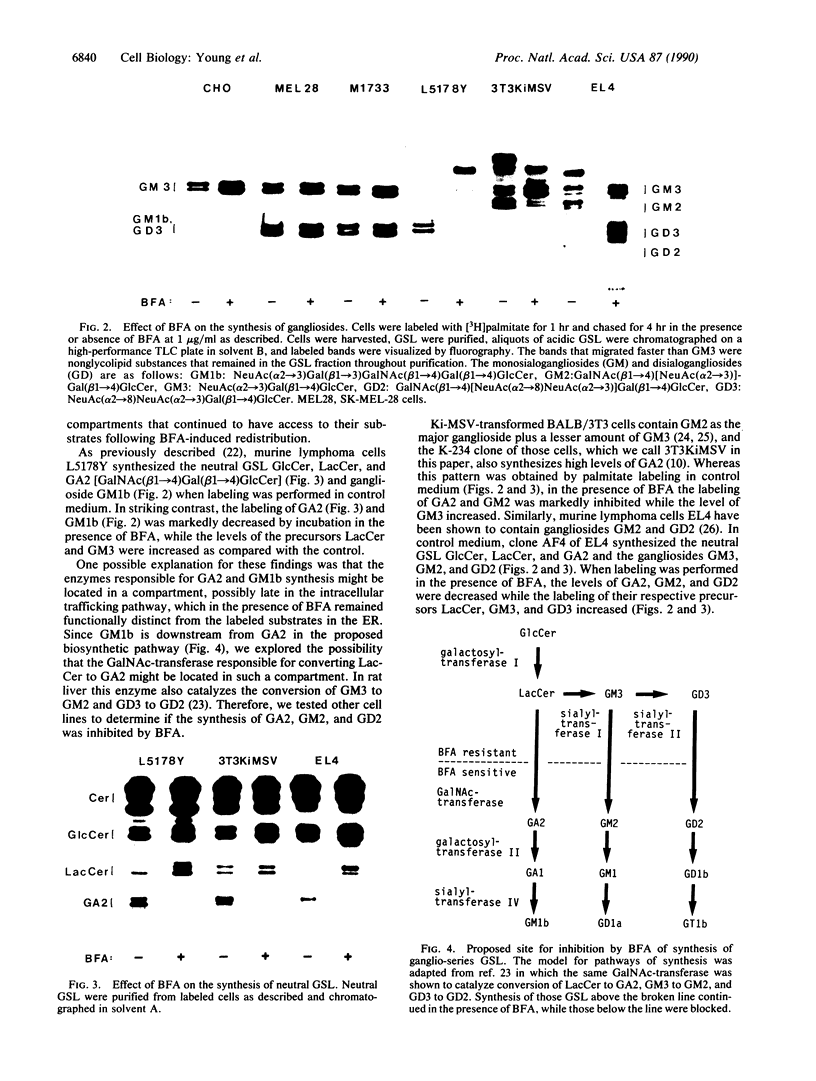
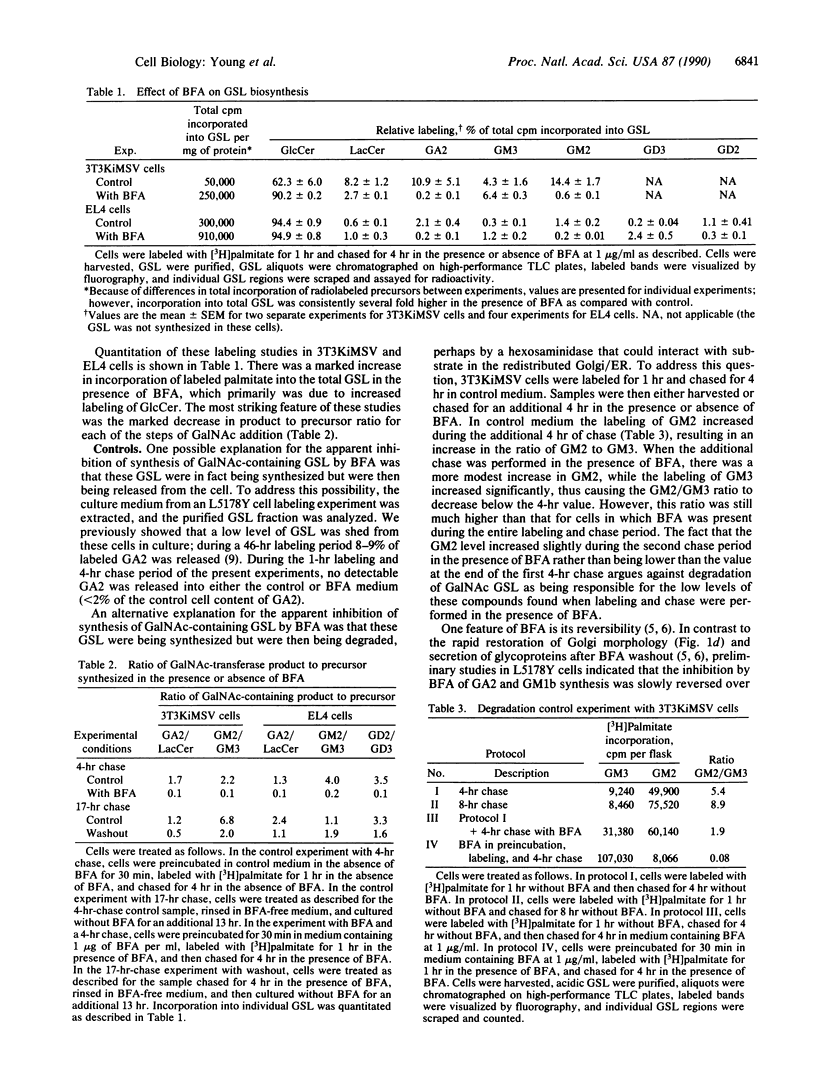
Brefeldin A (BFA) induces the rapid redistribution of the Golgi complex into the endoplasmic reticulum (ER), causing the glycoproteins that are retained in the ER to be processed by Golgi enzymes. We have examined the effects of BFA on the synthesis of glycosphingolipids (GSL) to map the intracellular sites of GSL synthesis. In several cultured cell types, BFA inhibited the synthesis of the neutral GSL gangliotriaosylceramide (GA2) and monosialoganglioside GM2 and disialoganglioside GD2, where GD2 is GalNAc(beta 1----4)- [NeuAc(alpha 2----8)NeuAc(alpha 2----3)]Gal(beta 1----4)GlcCer, GM2 lacks the NeuAc(alpha 2----8) unit, and GA2 lacks both NeuAc(alpha 2----8) and NeuAc(alpha 2----3) units. The observed decrease in labeling of GA2, GM2, and GD2 in the presence of BFA was not due either to enhanced degradation of these glycolipids or to shedding of these glycolipids from the cells. In rat liver all three of these glycolipids have been shown by others to be synthesized by the same enzyme, GA2/GM2/GD2 synthase, which catalyzes the addition of N-acetylgalactosamine to lactosylceramide (Lac-Cer), GM3 [NeuAc(alpha 2----3)Gal(beta 1----4)GlcCer], and GD3 [NeuAc(alpha 2----8)NeuAc-(alpha 2----3)Gal(beta 1----4)GlcCer], respectively. Studies with a fluorescent glycolipid analog indicated that BFA redistributed the trans-Golgi stacks into a reticular pattern characteristic of the ER. These studies localize GA2/GM2/GD2 synthase, a key enzyme involved in the synthesis of complex gangliosides, to a compartment late in the intracellular trafficking pathway, which remains functionally distinct from the ER in the presence of BFA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles E. B., Li E., Kornfeld S. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J Biol Chem. 1977 Feb 10;252(3):1107–1116. [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O., Aaronson S. T. A comparison of membrane glycoconjugates from mouse cells transformed by murine and primate RNA sarcoma viruses. Biochemistry. 1976 Jan 13;15(1):201–208. doi: 10.1021/bi00646a031. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O., Bradley R. M., Aaronson S. A., Todaro G. J. Absence of a specific ganglioside galactosyltransferase in mouse cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1974 Feb;71(2):298–301. doi: 10.1073/pnas.71.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Ikehara Y. Dynamic distribution of the Golgi marker thiamine pyrophosphatase is modulated by brefeldin A in rat hepatoma cells. Cell Struct Funct. 1989 Oct;14(5):605–616. doi: 10.1247/csf.14.605. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988 Dec 5;263(34):18545–18552. [PubMed] [Google Scholar]
- Griffiths G., Fuller S. D., Back R., Hollinshead M., Pfeiffer S., Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989 Feb;108(2):277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Stroup R., Cochran N. A., Urdal D. L., Young W. W., Jr, Hakomori S. Factors affecting expression of glycolipid tumor antigens: influence of ceramide composition and coexisting glycolipid on the antigenicity of gangliotriaosylceramide in murine lymphoma cells. Cancer Res. 1983 Oct;43(10):4997–5005. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nudelman E., Hakomori S., Kannagi R., Levery S., Yeh M. Y., Hellström K. E., Hellström I. Characterization of a human melanoma-associated ganglioside antigen defined by a monoclonal antibody, 4.2. J Biol Chem. 1982 Nov 10;257(21):12752–12756. [PubMed] [Google Scholar]
- Pagano R. E., Sepanski M. A., Martin O. C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989 Nov;109(5):2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pohlentz G., Klein D., Schwarzmann G., Schmitz D., Sandhoff K. Both GA2, GM2, and GD2 synthases and GM1b, GD1a, and GT1b synthases are single enzymes in Golgi vesicles from rat liver. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7044–7048. doi: 10.1073/pnas.85.19.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder G., Young W. W., Jr, Hakomori S. I. Association of the glycolipid pattern with antigenic alterations in mouse fibroblasts transformed by murine sarcoma virus. Cancer Res. 1977 May;37(5):1333–1339. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Trinchera M., Ghidoni R. Two glycosphingolipid sialyltransferases are localized in different sub-Golgi compartments in rat liver. J Biol Chem. 1989 Sep 25;264(27):15766–15769. [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Targeting and processing of glycophorins in murine erythroleukemia cells: use of brefeldin A as a perturbant of intracellular traffic. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6992–6996. doi: 10.1073/pnas.86.18.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Yeh M. Y., Hellström I., Abe K., Hakomori S., Hellström K. E. A cell-surface antigen which is present in the ganglioside fraction and shared by human melanomas. Int J Cancer. 1982 Mar 15;29(3):269–275. doi: 10.1002/ijc.2910290308. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Murray R. K., Wright J. A. Glycosphingolipids of wild-type and mutant lectin-resistant Chinese hamster ovarian cells. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1010–1016. doi: 10.1016/s0006-291x(74)80289-5. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Borgman C. A., Wolock D. M. Modes of shedding of glycosphingolipids from mouse lymphoma cells. J Biol Chem. 1986 Feb 15;261(5):2279–2283. [PubMed] [Google Scholar]
- van Echten G., Iber H., Stotz H., Takatsuki A., Sandhoff K. Uncoupling of ganglioside biosynthesis by Brefeldin A. Eur J Cell Biol. 1990 Feb;51(1):135–139. [PubMed] [Google Scholar]
- van Echten G., Sandhoff K. Modulation of ganglioside biosynthesis in primary cultured neurons. J Neurochem. 1989 Jan;52(1):207–214. doi: 10.1111/j.1471-4159.1989.tb10918.x. [DOI] [PubMed] [Google Scholar]