Abstract
Background
Coronary bioresorbable scaffolds (BRS) were developed to overcome the limitations of standard metallic stents, especially to address late events after percutaneous coronary interventions. The aim of this meta-analysis was to evaluate the efficacy and safety of BRS, compared with Everolimus-eluting stents (EES), using the data available from randomized trials, with a focus on long-term outcomes.
Methods
Published randomized trials comparing BRS to EES for the treatment of coronary artery disease were searched for within PubMed, Cochrane Library and Scopus electronic databases up to April 4th 2017. The summary measure used was odds ratio (OR) with 95% confidence intervals.
Results
A total of 5 studies were eligible, including 5219 patients. At 2 years, BRS was associated with higher rates of target lesion failure (9.4% vs 7.2%; OR = 1.33; 95% CI 1.07 to 1.63; p = 0.008) and device thrombosis (2.3% vs 0.7%; OR = 3.22; 95% CI 1.86 to 5.57; p < 0.0001) compared with EES. The incidence of both early (within 30 days after implantation, 1.1% vs 0.5%, OR 1.97, 95% CI 1.02 to 3.81; p = 0.05) and very-late device thrombosis (>1 year, 0.6% vs 0.1%, OR 4.03, 95% CI 1.37 to 11.82; p = 0.01) was higher with BRS compared with EES.
Conclusions
BRS may be associated with worse two-years clinical outcomes compared with EES in patients with coronary artery disease.
Electronic supplementary material
The online version of this article (doi:10.1186/s12872-017-0586-2) contains supplementary material, which is available to authorized users.
Keywords: Stent thrombosis, Target lesion failure, Bioresorbable vascular scaffold
Background
The introduction of coronary stents has revolutionized interventional cardiology. However, despite significant improvement over the years, traditional metallic stents have some intrinsic limitations. In fact, their permanent structure hinders surgical myocardial revascularization, physiological vessel remodeling and exposes patients to the risk of stent thrombosis for an indefinite time. Coronary bioresorbable scaffolds (BRS) were developed to overcome some of these limitations of standard metallic stents, especially to address late events after percutaneous coronary interventions (PCI) [1]. BRS have been introduced in the last years as a novel promising approach to treat coronary stenosis by providing transient vessel support with drug delivery capability without the long-term limitations associated with vessel caging [2]. This technology has the potential to overcome many of the safety concerns associated with drug-eluting stents, with possible clinical benefits [3]. Although initial reports from single-arm studies in highly selected patients with simple coronary lesions were reassuring [4–6], recent data from “real-life” registries and randomized controlled trials reported that the rates of scaffold-related are not negligible, also at long-term [7–9].
The aim of this meta-analysis was to evaluate the efficacy and safety of BRS, compared with Everolimus-eluting stents (EES), using the data available from randomized trials, with a focus on long-term outcomes.
Methods
Search strategy and study selection
Published randomized trials comparing BRS to EES for the treatment of coronary artery disease were searched for within PubMed, Cochrane Library, Scopus electronic databases and scientific sessions abstracts, and relevant websites (www.clinicaltrialresults.org, www.escardio.org, www.tctmd.com) up to April 4th 2017. We checked the reference lists from all eligible studies to identify additional citations. The following keywords and the corresponding MeSH terms were used for search: “bioresorbable vascular scaffold”, “everolimus-eluting stent”, “coronary artery disease”, “randomized controlled trial”. Time of publication and language were not limiting criteria for our analysis. All reports including the search terms were independently screened by two investigators for relevance and eligibility (AP, RA). Additionally, references from relevant articles were also scanned for eligible studies. The authors discussed their evaluation and any disagreement was resolved through discussion and re-reading. All selected trials were thoroughly checked and classified by author’s institution in order to avoid any effect from duplicity of data.
Studies were considered eligible if the following statements were applying: a) they involved a study population with coronary artery disease; b) multicenter randomized controlled trials c) they compared BRS versus EES; d) follow-up length of 2 years; e) they reported outcome data: target lesion failure (TLF), device thrombosis (DvT), cardiac death, target-vessel myocardial infarction (TVMI), ischemia-driven target lesion revascularization (ID-TLR); f) minimum of 100 patients treated with BRS. Exclusion criteria were (just one was sufficient for study exclusion): duplicate publication, pre-specified endpoint, measure not specified. Studies reporting only lesion-based analyses were excluded from the present work.
Data abstraction, validity assessment and analysis
Baseline characteristics as well as numbers of events were extracted from the single studies, through carefully scanning of the full article by two independent reviewers (AP, SDR). Divergences were resolved by consensus. In particular, the following data were abstracted: year of publication, location, number of study patients, study design, clinical outcome data, baseline patients’ characteristics. Selection and data abstraction was performed according to the PRISMA statement [10]. The primary analysis was based on the composite endpoint of TLF (Cardiac death, TVMI, ID-TLR). Furthermore, meta-analysis results of single endpoints are also provided (Probable/definite DvT; early/late/very late DvT; ischemia-driven target vessel revascularization; target vessel myocardial infarction; cardiac death). Since some of the RCTs pool definite and probable DvT, we evaluated only the incidence of the composite endpoint “definite/probable DvT”.
Statistical analysis
The summary measure used was the Odds Ratio (OR) with 95% confidence interval. The random-effects model was used, as previously described, to combine the collected values [11]. This model calculates a weighted average of the relative risks by incorporating within-study and between-study variations. Heterogeneity was assessed by means of the Cochrane Q test using a chi-squared function, with p values <0·10 considered significant for heterogeneity, as previously described [12]. Additionally, I2 values were calculated for estimation of variation in weighted mean differences among studies attributable to heterogeneity. Any I2 value >20% was considered significant. Small study effects were evaluated through graphical inspection of funnel plots, as already previously described [13]. Forest plots were used to graphically display the results of the meta-analysis, as already previously described [14]. Briefly, the measure of effect (OR) for each single study included (represented by a square) is plotted, together with confidence intervals, represented by horizontal lines. The area of each square is proportional to the study’s weight in the meta-analysis. The overall measure of effect is reported on the bottom line of the plot as a diamond, whose lateral ends indicate the confidence interval for the summary effect. Analyses were performed by means of RevMan 5.3.
Results
Search results
Our search retrieved a total of 590 entries, which were reduced to 30 studies after an initial pre-screening. Fifteen studies were then excluded for one of the following reasons: a) they were not related to our research question b) they weren’t original articles. In the assessment of eligibility further 10 studies were excluded. Finally, a total of 5 studies were available for the analysis including 5219 patients [15–19]. The study selection procedure is reported in detail in Fig. 1.
Fig. 1.
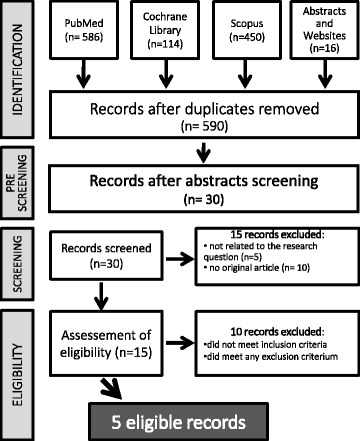
Study selection flow chart
Study characteristics
Only multicenter, randomized, trials were included in the present meta-analysis. Table 1 summarizes the most relevant characteristics of the selected studies. Not surprisingly, quality assessment revealed a high study quality (Additional file 1: Figure S1). Moreover, endpoint assessment and data analysis was blinded in all included studies.
Table 1.
Characteristics and Endpoint definitions of included randomized trials
| Study | Year | Location | Number | Study design | Primary endpoint | Definition of TLF | Definition of ST | Follow up (months) | Lost to FU (%) |
|---|---|---|---|---|---|---|---|---|---|
| AIDA | 2017 | Multicenter | 1845 | RCT | TVF | Cardiac death, TVMI, ID-TLR |
ARC | 24 | 2.8 |
| ABSORB III | 2017 | Multicenter | 2008 | RCT | TLF | Cardiac death, TVMI, ID-TLR |
ARC | 24 | 2.1 |
| ABSORB China | 2016 | Multicenter | 480 | RCT | IS-LL | Cardiac death, TVMI, ID-TLR |
ARC | 24 | 3.7 |
| ABSORB II | 2016 | Multicenter | 501 | RCT | Vasomotion, MLD | Cardiac death, TVMI, ID-TLR |
ARC | 24 | 4.2 |
| ABSORB Japan | 2016 | Multicenter | 400 | RCT | TLF | Cardiac death, TVMI, ID-TLR |
ARC | 24 | 3 |
Abbreviations: TLF target lesion failure, TVF target vessel failure, IS-LL in-segment late loss, MLD minimal lumen diameter, TVMI target vessel myocardial infarction, ID-TLR ischemia driven target lesion revascularization, RCT randomized clinical trials
Across the studies, patients were predominantly male and approximately one fourth of patients had diabetes mellitus. Although prevalence of single cardiovascular risk factors was not equal among the studies, treatment arms were generally well balanced. Dual antiplatelet therapy was used at least 1 year in all the studies. More details on patients’ characteristics are provided in Table 2.
Table 2.
Baseline patient’s and procedural characteristics
| AIDA 2017 |
ABSORB III 2017 |
ABSORB China 2016 |
ABSORB II 2016 |
ABSORB Japan 2016 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRS | EES | BRS | EES | BRS | EES | BRS | EES | BRS | EES | |
| N of patients, n | 924 | 921 | 1322 | 686 | 238 | 237 | 335 | 166 | 266 | 134 |
| Age, yrs. | 64 | 64 | 63 | 64 | 57 | 58 | 62 | 61 | 67 | 67 |
| Male, % | 72 | 76 | 71 | 70 | 72 | 73 | 76 | 80 | 79 | 74 |
| Hypertension, % | 51 | 50 | 85 | 85 | 59 | 60 | 69 | 72 | 78 | 80 |
| Diabetes, % | 18 | 17 | 31 | 33 | 25 | 23 | 24 | 24 | 36 | 36 |
| Dyslipidaemia, % | 38 | 38 | 86 | 86 | 42 | 38 | 75 | 80 | 82 | 82 |
| Prior MI, % | 18 | 19 | 21 | 22 | 17 | 16 | 28 | 28 | 16 | 24 |
| STEMI, % | 25 | 0 | 0 | 0 | 0 | |||||
| NSTEMI, % | 20 | 0 | 0 | 0 | 0 | |||||
| UA, % | 8 | 26 | 64 | 21 | 12 | |||||
| SA, % | 40 | 58 | 19 | 64 | 64 | |||||
| Silent Ischaemia, % | NR | 10 | 5 | 12 | 23 | |||||
| Intracoronary imaging, % | NR | 100 | 100 | 0.4 | 0.4 | 100 | 100 | 100 | 100 | |
| Pre-dilation, % | 97 | 91 | 100 | 100 | 99.6 | 98 | 100 | 99 | 100 | 100 |
| Post dilation, % | 74 | 49.1 | 65.5 | 51.2 | 63 | 54.4 | 61 | 59 | 82.2 | 77.4 |
yrs years, MI myocardial infarction BRS = bioresorbable vascular scaffold, EESeverolimus-eluting stent, STEMI ST-elevation myocardial infarction, NSTEMI No ST-elevation myocardial infarction;UA = unstable angina, SA stable angina, NR not reported
Meta-analysis results
At 2 years, BRS was associated with higher rates of TLF compared with EES (9.4% vs 7.2%; OR = 1.33; 95% CI 1.07 to 1.63; p = 0.008, I2 = 0%) (Fig. 2a). This result was driven by a major incidence of TLR (5.1% vs 4.0%; OR = 1.32; 95% CI 1.01 to 1.74; p = 0.05, I2 = 0%) (Fig. 2b) and TV-MI (5.7% vs 3.3%; OR = 1.66; 95% CI 1.25 to 2.21; p = 0.0005, I2 = 0%)(Fig. 3a) in BRS group compared with EES. No difference in Cardiac death (1.2% vs 1.4%; OR = 0.94; 95% CI 0.56 to 1.57; p = 0.80, I2 = 0%) was observed between both groups (Fig. 3b).
Fig. 2.
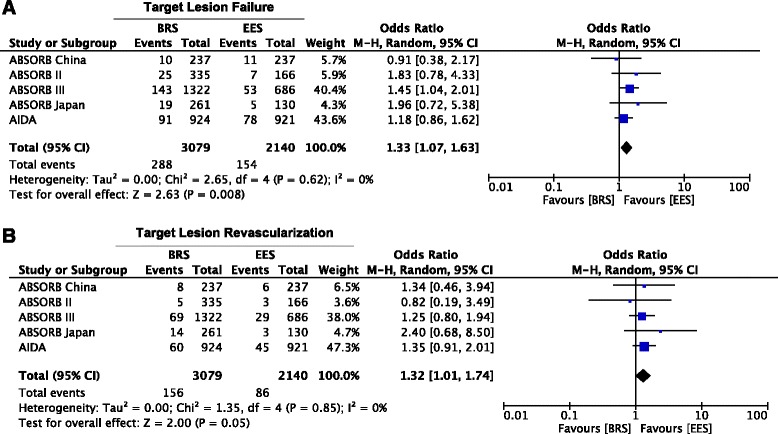
Meta-analysis of Target lesion failure and Target lesion revascularization. Panel a. Forest plot and summary effect of the difference in the incidence of TLF, showing a significantly lower incidence in the EES arm (p = 0.008). Panel b. Forest plot and summary effect of the difference in the incidence of TLR, showing a significantly lower incidence in the EES arm (p = 0.05)
Fig. 3.
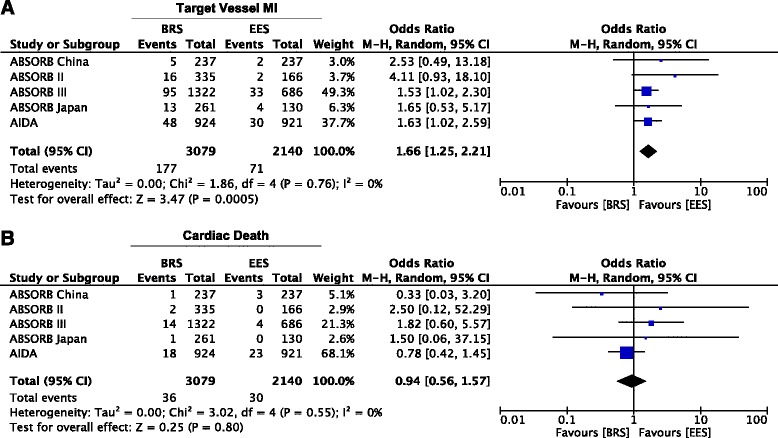
Meta-analysis of Target vessel myocardial infarction and Cardiac Death. Panel a. Forest plot and summary effect of the difference in the incidence of TV-MI, showing a significantly lower incidence in the EES arm (p = 0.0005). Panel b. Forest plot and summary effect of the difference in the incidence of Cardiac death, showing no difference between BRS and EES (p = 0.80)
BRS was associated with higher rates of DvT compared with EES (2.3% vs 0.7%; OR = 3.22; 95% CI 1.86 to 5.57; p < 0.0001, I2 = 0) (Fig. 4). Interestingly, the incidence of both early (within 30 days after implantation, 1.1% vs 0.5%, OR 1.97, 95% CI 1.02 to 3.81; p = 0.05) and very late DvT (>1 year, 0.6% vs 0.1%, OR 4.03, 95% CI 1.37 to 11.82; p = 0.01)) was higher with BRS compared with EES (Fig. 5a, c), with the majority of events occurring within 30 days (n = 44). Conversely, although numerically higher with BRS compared to EES, the incidence of late DvT (30 days to 1 year) was not statistically different between devices (0.5% vs 0.1%, OR 3.44%, 95% CI 0.62 to 19.12; p = 0.16) (Fig. 5b).
Fig. 4.
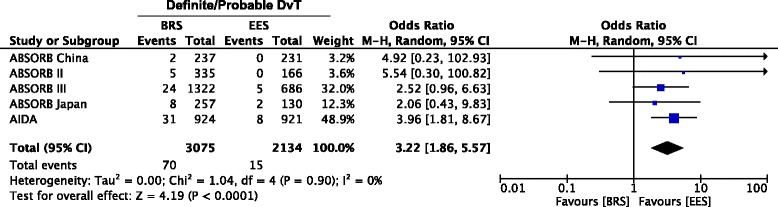
Meta-analysis of Definite/Probable Scaffold Thrombosis. Forest plot and summary effect of the difference in the incidence of Definite/Probable DvT, showing a significantly lower incidence in the EES arm (p < 0.0001)
Fig. 5.
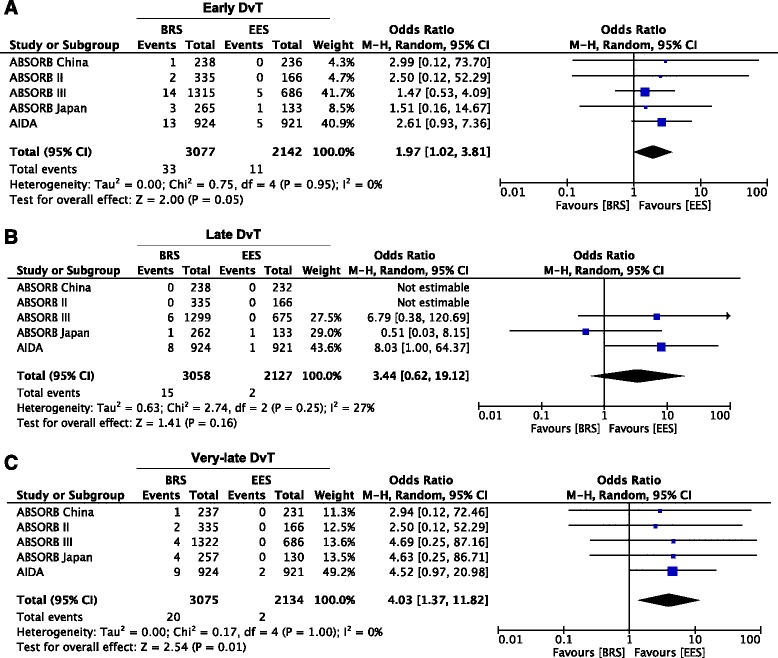
Meta-analysis of Early, Late and Very-late Scaffold Thrombosis. Panel a. Forest plot and summary effect of the difference in the incidence of Early DvT, showing a significantly lower incidence in the EES arm (p = 0.05). Panel b. Forest plot and summary effect of the difference in the incidence of Late DvT, showing no difference between BRS and EES. Panel c. Forest plot and summary effect of the difference in the incidence of Very-late DvT, showing a significantly lower incidence in the EES arm (p = 0.01)
These results were unchanged when fixed effects model was used. There was no evidence of publication bias by visual inspection of Funnel plots and by Egger’s test (Additional file 2: Figure S2).
Discussion
This is the most comprehensive and updated meta-analysis of randomized studies comparing the long-term outcome after treatment of coronary artery disease with everolimus-eluting BRS or the equivalent metallic stent (EES). Summing up the best clinical evidence available to date, including 5 randomized studies and 5219 patients, we found that: a) BRS was associated with higher rates of TLF compared with EES; b) BRS was associated with higher rates of TV-MI compared with EES; c) BRS was associated with higher rates of DvT compared with EES; d) very-late DvT was higher with BRS compared with EES.
These results are not surprising, as similar trends were reported both in randomized and observational studies [20, 21]. In particular, a previously published patient-level meta-analysis reported a higher incidence of device thrombosis with the BRS, compared with the equivalent metallic EES [22]. However, their observation period was limited to 1 year and the studies were heterogeneous with regards to the enrollment of acute coronary syndrome and stable CAD patients [23]. Similarly, a recent published meta-analysis of RCTs and observational studies had already reported a higher risk for TLF and DvT in BRS-treated patients [24–26]. In this context, our results represent a robust confirmation of the association between use of BRS and a higher rate of DvT, and are the first report of differences in DvT early, late, and very-late after implantation.
The pathophysiology of scaffold thrombosis, and the possible explanations for the increased risk as compared to drug-eluting stents (DES), and particularly the role of the implantation technique [27, 28] have been previously investigated. The implantation technique was not homogeneous across studies and instructions for use were not systematically followed in all studies. In fact, appropriate sizing of the balloon to be used for pre-dilation was not warranted in all patients enrolled in the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial (AIDA). More generally, the BRS-specific implantation protocol, prescribing long inflation times and systematic high-pressure post-dilation with a non-compliant balloon, was not homogeneously adopted in all included studies, despite it was strongly recommended to achieve optimal implantation results [29–31]. In addition, intravascular imaging guiding during BRS implantation has been reported to have a major positive impact on patients’ outcome but was not frequently used across the selected studies [32–34]. Thus, a potential explanation for the observed increased risk of DvT in the randomized trials may relate to suboptimal implantation techniques, rather than to the intrinsic properties of BRS. Findings of this meta-analysis have relevant potential implications. The concern raised about BRS thrombosis, together with the lack of a clear advantage in terms of clinical efficacy, potentially undermines the future development of this promising class of coronary devices. In fact, the added value of the so-called vascular restoration therapy is still waiting for a proof of evidence, while interventional cardiologists have largely experienced the technical challenges in implanting the device, including worse trackability than best-in-class equivalent EES, longer procedural times, larger amounts of contrast medium necessary for successful implantation.
Collectively, the data emphasize the importance of appropriate lesion selection and accurate application of proper implantation technique. As well, a new generation of BRS should warrant a better radial strength, a sleeker endoluminal profile, a smaller footprint, and resorption processes that do not interact with the vessel wall.
Study limitations
Although no large heterogeneity was found between the randomized studies included in the present analysis, it entails possible limitations of the original studies included. There were differences in the study design, patients’ and procedural characteristics. To account for these potential sources of heterogeneity, we used a random effects model for all analyses. Even though the present analysis only included high quality, randomized studies, some potential source for bias may still persist. Unfortunately, a patient level meta-analysis couldn’t be possible because of lack of data. Additionally, in two of the trials, 2-year results had been presented but not have not been published yet [15, 19]. In addition, since angina recurrence was not systematically reported in the original studies, we could not analyze the impact of BRS on this specific outcome. Moreover, we only focused on the Absorb, as this was the only type of BRS with several randomized studies and reporting long-term outcomes. Hence, our findings may not entirely apply to other BRS platforms. As well, the assessment of publication bias is limited by the small number of trials included, preventing a definitive exclusion of potential small study effect. Finally, further data on the impact of a BRS-specific implantation technique and the role of sizing on DvT are still needed, to confirm that BRS can deliver the same results as DES with the appropriate implantation techniques [35].
Conclusions
BRS may be associated with worse two-years clinical outcomes compared with EES in patients with CAD. In particular, the current data expand previous observations of an increased risk of early DvT to long-term follow-up periods.
Additional files
Risk of bias. Summary of the study quality analysis. (PPTX 80 kb)
Funnel plots. Funnel plots for TLF, TLR, TV-MI, Cardiac Death, Definite/Probable DvT, Early, Late and Very-late DvT, demonstrating no evidence of publication bias. Each circle represents a study. Study precision (reported on the y-axis as the Standard Error of the Log OR) is plotted against the summary effect. (PPTX 158 kb)
Acknowledgements
None.
Funding
None.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors’ contributions
AP, SDR, and TG designed the study and acquired, analysed, and interpreted data. AP and RA did the literature search and study selection procedures. TM and CI drafted the manuscript, with critical revisions for important intellectual content from all authors. All authors read and approved the final manuscript.
Competing interests
TG, TM and CI have received speaker fees from Abbott Vascular. AP is a fellow of the European Association of Percutaneous Coronary Interventions. There is no other conflict with this research. Abbott Vascular had no role in any phase of this research. All other authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AIDA
Amsterdam investigator-initiated absorb strategy all-comers trial
- BRS
Bioresorbable scaffolds
- CI
Confidence interval
- DES
Drug eluting stent
- DvT
Device thrombosis
- EES
Everolimus eluting stent
- ID
Ischemia-driven
- MI
Myocardial infarction
- OR
Odds ratio
- PCI
Percutaneous coronary intervention
- RCT
Randomized controlled trial
- TLF
Target lesion failure
- TLR
Target lesion revascularization
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12872-017-0586-2) contains supplementary material, which is available to authorized users.
Contributor Information
Alberto Polimeni, Email: polimeni@unicz.it.
Remzi Anadol, Email: remzi.anadol@gmail.com.
Thomas Münzel, Email: tmuenzel@uni-mainz.de.
Ciro Indolfi, Email: indolfi@unicz.it.
Salvatore De Rosa, Email: saderosa@unicz.it.
Tommaso Gori, Phone: +49 6131 17 2829, Email: tommaso.gori@unimedizin-mainz.de.
References
- 1.Indolfi C, De Rosa S, Colombo A. Bioresorbable vascular scaffolds - basic concepts and clinical outcome. Nat Rev Cardiol. 2016;13:719–729. doi: 10.1038/nrcardio.2016.151. [DOI] [PubMed] [Google Scholar]
- 2.Gori T, Münzel T. First Evidence of Complete Resorption 4 Years After Bioresorbable Scaffold Implantation in the Setting of ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2017;10:200–202. doi: 10.1016/j.jcin.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Ormiston JA, Serruys PW. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009;2:255–260. doi: 10.1161/CIRCINTERVENTIONS.109.859173. [DOI] [PubMed] [Google Scholar]
- 4.Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, Onuma Y, Dudek D, Smits PC, Koolen J, Chevalier B, et al. J Am Coll Cardiol. 2011;58:1578–1588. doi: 10.1016/j.jacc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Gori T, Schulz E, Hink U, Wenzel P, Post F, Jabs A, et al. Early outcome after implantation of Absorb bioresorbable drug-eluting scaffolds in patients with acute coronary syndromes. EuroIntervention. 2014;9:1036–1041. doi: 10.4244/EIJV9I9A176. [DOI] [PubMed] [Google Scholar]
- 7.Indolfi C, Mongiardo A, Spaccarotella C, Caiazzo G, Torella D, De Rosa S. Neointimal proliferation is associated with clinical restenosis 2 years after fully bioresorbable vascular scaffold implantation. Circ Cardiovasc Imaging. 2014;7:755–757. doi: 10.1161/CIRCIMAGING.114.001727. [DOI] [PubMed] [Google Scholar]
- 8.Gori T, Jansen T, Weissner M, Foin N, Wenzel P, Schulz E, et al. Coronary evaginations and peri-scaffold aneurysms following implantation of bioresorbable scaffolds: incidence, outcome, and optical coherence tomography analysis of possible mechanisms. Eur Heart J. 2016;37:2040–2049. doi: 10.1093/eurheartj/ehv581. [DOI] [PubMed] [Google Scholar]
- 9.Gori T, Schulz E, Hink U, Kress M, Weiers N, Weissner M, et al. Clinical, Angiographic, Functional, and Imaging Outcomes 12 Months After Implantation of Drug-Eluting Bioresorbable Vascular Scaffolds in Acute Coronary Syndromes. JACC Cardiovasc Interv. 2015;8:770–777. doi: 10.1016/j.jcin.2014.12.244. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polimeni A, De Rosa S, Sabatino J, Sorrentino S, Indolfi C. Impact of intracoronary adenosine administration during primary PCI: A meta-analysis. Int J Cardiol. 2016;203:1032–1041. doi: 10.1016/j.ijcard.2015.11.086. [DOI] [PubMed] [Google Scholar]
- 12.Polimeni A, Passafaro F, De Rosa S, Sorrentino S, Torella D, Spaccarotella C, et al. Clinical and Procedural Outcomes of 5-French versus 6-French Sheaths in Transradial Coronary Interventions. Medicine (Baltimore) 2015;94:e2170. doi: 10.1097/MD.0000000000002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santarpia G, De Rosa S, Polimeni A, Giampà S, Micieli M, Curcio A, et al. Efficacy and Safety of Non-Vitamin K Antagonist Oral Anticoagulants versus Vitamin K Antagonist Oral Anticoagulants in Patients Undergoing Radiofrequency Catheter Ablation of Atrial Fibrillation: A Meta-Analysis. PLoS One. 2015;10:e0126512. doi: 10.1371/journal.pone.0126512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rosa S, Torella D, Caiazzo G, Giampà S, Indolfi C. Left radial access for percutaneous coronary procedures: from neglected to performer? A meta-analysis of 14 studies including 7,603 procedures. Int J Cardiol. 2014;171:66–72. doi: 10.1016/j.ijcard.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Gao R. ABSORB China: two-year clinical results in patients with coronary artery disease randomized to the ABSORB bioresorbable vascular scaffold versus metallic drug-eluting stents. Transcatheter Cardiovascular Therapeutics. 2016; https://www.tctmd.com/slide/absorb-china-two-year-clinical-outcomes-prospective-randomized-trial-everolimus-eluting. Accessed 30 Oct 2016
- 16.Onuma Y, Sotomi Y, Shiomi H, Ozaki Y, Namiki A, Yasuda S, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention. 2016;12:1090–1101. doi: 10.4244/EIJY16M09_01. [DOI] [PubMed] [Google Scholar]
- 17.Chevalier B, Onuma Y, van Boven AJ, Piek JJ, Sabaté M, Helqvist S, et al. Randomised comparison of a bioresorbable everolimus-eluting scaffold with a metallic everolimus-eluting stent for ischaemic heart disease caused by de novo native coronary artery lesions: the 2-year clinical outcomes of the ABSORB II trial. EuroIntervention. 2016;12:1102–1107. doi: 10.4244/EIJY16M08_01. [DOI] [PubMed] [Google Scholar]
- 18.Wykrzykowska JJ, Kraak RP, Hofma SH, Van der Schaaf RJ, Arkenbout EK, Ijsselmuiden AJ, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. doi:10.1056/NEJMoa1614954. [DOI] [PubMed]
- 19.Ellis GS. Everolimus-eluting Bioresorbable Vascular Scaffolds in Patients with Coronary Artery Disease: ABSORB III Trial 2-Year Results. Am Coll Cardiol. 2017; https://www.tctmd.com/slide/everolimus-eluting-bioresorbable-vascular-scaffolds-patients-coronary-artery-disease-absorb. Accessed 19 Mar 2017
- 20.Kraak RP, Grundeken MJ, Hassell ME, Elias J, Koch KT, Henriques JP, et al. Two-year clinical outcomes of Absorb bioresorbable vascular scaffold implantation in complex coronary artery disease patients stratified by SYNTAX score and ABSORB II study enrolment criteria. EuroIntervention. 2016;12:e557–e565. doi: 10.4244/EIJV12I5A95. [DOI] [PubMed] [Google Scholar]
- 21.Capodanno D, Gori T, Nef H, Latib A, Mehilli J, Lesiak M, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144–1153. doi: 10.4244/EIJY14M07_11. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Gao R, Kimura T, Kereiakes DJ, Ellis SG, Onuma Y, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. 2016;387:1277–1289. doi: 10.1016/S0140-6736(15)01039-9. [DOI] [PubMed] [Google Scholar]
- 23.Cassese S, Byrne RA, Ndrepepa G, Kufner S, Wiebe J, Repp J, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet. 2016;387:537–544. doi: 10.1016/S0140-6736(15)00979-4. [DOI] [PubMed] [Google Scholar]
- 24.Toyota T, Morimoto T, Shiomi H, Yoshikawa Y, Yaku H, Yamashita Y, et al. Very Late Scaffold Thrombosis of Bioresorbable Vascular Scaffold: Systematic Review and a Meta-Analysis. JACC Cardiovasc Interv. 2017;10:27–37. doi: 10.1016/j.jcin.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Nairooz R, Saad M, Sardar P, Aronow WS. Two-year outcomes of bioresorbable vascular scaffold versus drug-eluting stents in coronary artery disease: a meta-analysis. Heart. 2017; doi:10.1136/heartjnl-2016-310886. [DOI] [PubMed]
- 26.Ha FJ, Nerlekar N, Cameron JD, Bennett MR, Meredith IT, West NE, et al. Midterm Safety and Efficacy of ABSORB Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent: An Updated Meta-Analysis. JACC Cardiovasc Interv. 2017;10:308–310. doi: 10.1016/j.jcin.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 27.Puricel S, Cuculi F, Weissner M, Schmermund A, Jamshidi P, Nyffenegger T, et al. Bioresorbable Coronary Scaffold Thrombosis: Multicenter Comprehensive Analysis of Clinical Presentation, Mechanisms, and Predictors. J Am Coll Cardiol. 2016;67:921–931. doi: 10.1016/j.jacc.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka A, Latib A, Jabbour RJ, Mitomo S, Regazzoli D, Leone PP, et al. Bioresorbable Vascular Scaffolds and Very Late Scaffold Thrombosis: Searching an Explanation and a Solution. JACC Cardiovasc Interv. 2017;10:745–746. doi: 10.1016/j.jcin.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Sorrentino S, De Rosa S, Ambrosio G, Mongiardo A, Spaccarotella C, Polimeni A, et al. The duration of balloon inflation affects the luminal diameter of coronary segments after bioresorbable vascular scaffolds deployment. BMC Cardiovasc Disord. 2015;15:169. doi: 10.1186/s12872-015-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imori Y, D'Ascenzo F, Gori T, Münzel T, Fabrizio U, Campo G, et al. Impact of postdilatation on performance of bioresorbable vascular scaffolds in patients with acute coronary syndrome compared with everolimus-eluting stents: A propensity score-matched analysis from a multicenter "real-world" registry. Cardiol J. 2016;23:374–383. doi: 10.5603/CJ.a2016.0052. [DOI] [PubMed] [Google Scholar]
- 31.Biscaglia S, Ugo F, Ielasi A, Secco GG, Durante A, D'Ascenzo F, et al. Bioresorbable Scaffold vs. Second Generation Drug Eluting Stent in Long Coronary Lesions requiring Overlap: A Propensity-Matched Comparison (the UNDERDOGS study) Int J Cardiol. 2016;208:40–45. doi: 10.1016/j.ijcard.2016.01.202. [DOI] [PubMed] [Google Scholar]
- 32.Biscaglia S, Secco GG, Tumscitz C, Di Mario C, Campo G. Optical coherence tomography evaluation of overlapping everolimus-eluting bioresorbable vascular scaffold implantation guided by enhanced stent visualization system. Int J Cardiol. 2015;182:1–3. doi: 10.1016/j.ijcard.2014.12.103. [DOI] [PubMed] [Google Scholar]
- 33.Biscaglia S, Campo G, Tebaldi M, Tumscitz C, Pavasini R, Fileti L, et al. Bioresorbable vascular scaffold overlap evaluation with optical coherence tomography after implantation with or without enhanced stent visualization system (WOLFIE study): a two-centre prospective comparison. Int J Cardiovasc Imaging. 2016;32:211–223. doi: 10.1007/s10554-015-0756-1. [DOI] [PubMed] [Google Scholar]
- 34.Caiazzo G, Longo G, Giavarini A, Kilic ID, Fabris E, Serdoz R, et al. Optical coherence tomography guidance for percutaneous coronary intervention with bioresorbable scaffolds. Int J Cardiol. 2016;221:352–358. doi: 10.1016/j.ijcard.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Ielasi A, Varricchio A, Campo G, Leoncini M, Cortese B, Vicinelli P, et al. A prospective evaluation of a standardized strategy for the use of a polymeric everolimus-eluting bioresorbable scaffold in ST-segment elevation myocardial infarction: Rationale and design of the BVS STEMI STRATEGY-IT study. Catheter Cardiovasc Interv. 2016; doi:10.1002/ccd.26801. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias. Summary of the study quality analysis. (PPTX 80 kb)
Funnel plots. Funnel plots for TLF, TLR, TV-MI, Cardiac Death, Definite/Probable DvT, Early, Late and Very-late DvT, demonstrating no evidence of publication bias. Each circle represents a study. Study precision (reported on the y-axis as the Standard Error of the Log OR) is plotted against the summary effect. (PPTX 158 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article.


