Abstract
Objective: Curcumin is known for its anti-oxidative, anti-inflammatory and anti-tumorigenic qualities at concentrations ranging from 3.7µg/ml to 55µg/ml. Therefore it is pre-destined for tumour therapy. Due to high oral doses that have to be administered and the low bioavailability of curcumin new therapy concepts have to be developed. One of these therapy concepts is the combination of low curcumin concentrations and UVA or visible light. Aim of our study was to investigate the influence of this treatment regime on oral squamous cell carcinoma cells.
Materials and Methods: A human oral squamous cell carcinoma cell line (HN) was pre-incubated with low curcumin concentrations (0.01µg/ml to 1µg/ml). Thereafter cell cultures were either left un-irradiated or were irradiated either with 1J/cm2 UVA or for 5min with visible light. Quantitative analysis of proliferation, membrane integrity, oxidative potential and DNA fragmentation were done.
Results: It could be shown that low curcumin concentrations neither influenced proliferation, nor cell morphology, nor cell integrity nor apoptosis. When combining these curcumin concentrations with UVA or visible light irradiation cell proliferation as well as development of reactive oxygen species was reduced whereas DNA fragmentation was increased. Concentration as well as light entity specific effects could be observed.
Conclusions: The present findings substantiate the potential of the combination of low curcumin concentrations and light as a new therapeutic concept to increase the efficacy of curcumin in the treatment of cancer of the oral mucosa.
Keywords: head and neck cancer, curcumin, apoptosis, proliferation, UVA, VIS.
Introduction
Oral squamous cell carcinomas are among the widest spread malign tumours 1. Late diagnosis due to the asymptomatic, fast and aggressive progression 2, 3 and early occurring metastasis of the tumour are responsible for a 5 year survival rate of 39-43% 4, 5. Local excision, chemotherapy, radiotherapy and combinations thereof are common treatment regimens 6-9. To increase the survival rate new treatment regimens and drugs are required.
Natural compounds play a significant role in drug discovery and development 10, 11. One of these natural compounds with broad spectrum of physiological effects is curcumin. The phytochemical can be isolated from the rhizome of the ginger plant Curcuma longa. For thousands of years it has been a part of the traditional Asian medicine. Among others it is known for its anti-inflammatory and anti-oxidative potential 12. Taking into account the hardly existing toxicity, curcumin is a promising substance to develop anti-tumorigenic therapeutic strategies. Obstacles are the low bioavailability of curcumin due to low resorption from the gastrointestinal tract, low solubility 13 and fast metabolisation 14. Therefore even after administering high curcumin concentrations pharmacological relevant effects are not achievable 15, 16. Inhibiting metabolic degradation 17, 18, encapsulating curcumin in micelles 19 or binding to nanoparticles 20-22 are strategies to increase the curcumin concentration in the serum. Another possible therapy concept is the combination of low concentrations of curcumin (0.1-2 µg/ml) with UVA or visible light (VIS) 23-25. Aim of this study was to investigate the influence of curcumin in combination with light on oral squamous cell carcinoma cells.
Materials and Methods
Cell culture and treatment
The human oral squamous cell carcinoma cell line (HN ACC 417, DSMZ, Leipzig, Germany), the spontaneous immortalized human keratinocyte cell line HaCaT 26 and the human epidermoid carcinoma cell line A431 (ATCC® CRL1555™) were cultured in Dulbecco's Modified Eagle's Medium (D-MEM, Gibco, Karlsruhe, Germany) with GlutaMax supplemented with 10% (v/v) fetal calf serum (FCS, PAA, Cölbe, Germany) and 1% (v/v) penicillin/streptomycin solution (Gibco) in 7.5% CO2 atmosphere at 37°C. Curcumin (Sigma-Aldrich Taufkirchen, Germany) was dissolved as previously described 23, 27. Briefly, cells were incubated for 1h with medium containing 0.01µg/ml to 1µg/ml curcumin. For irradiation either with 1J/cm2 ultraviolet A (UVA, Waldmann, Villingen-Schwenningen, Germany) or visible light (VIS, 5500 lx, Philips GmbH, Hamburg, Germany) the medium was replaced with PBS (Gibco) or PBS curcumin. After irradiation PBS was replaced with culture medium containing no curcumin.
Morphological properties
HN cells were cultivated in microwell plates at a density of 1×104 cells/0.33 cm2 and were exposed to curcumin as mentioned. Immediately after irradiation cells were transferred to the incubator microscope unit. Morphological properties were monitored, analysed and photodocumented every 2h for 16h with the incubator microscope unit IncuCyte (EssenBioScience, Hertfordshire, UK).
Cell proliferation
Cells were cultivated in microwell plates at a density of 2×104 cells/0.33 cm2. Cells were exposed to curcumin as mentioned. Immediately after irradiation cells were pulsed for 16h with 5-bromo-2'-deoxyuridine (BrdU). The incorporation rate of BrdU was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Roche, Mannheim, Germany) as previously described 28. Briefly, cells were fixed and immune complexes were formed using peroxidase-coupled BrdU-antibodies. A colorimetric reaction with tetramethylbenzidine as a substrate gave rise to a reaction product measured at 450nm in a scanning multiwell spectrophotometer (ELISA reader, MR 5000; Dynatech, Guernsey, UK).
Membrane integrity
Cell lysis was quantified using the cytotoxicity detection kit (Roche), which is based on the release of lactate dehydrogenase (LDH) from damaged cells. Briefly, HN cells were seeded in microwell plates as described above and were treated with curcumin and UVA or VIS. As a positive control (maximal cell damage), cells were treated with 1% Triton X-100 (Merck, Darmstadt, Germany). Subsequently, the cell-free supernatants were incubated with NAD+, which is reduced by LDH to NADH/H+. In a second step, NADH/H+ reduces a yellow tetrazolium salt to a red-coloured formazan salt. The amount of red colour is proportional to the number of lysed cells. For quantitation, the absorbance of the reaction product was measured at 490nm using a multiwell spectrophotometer.
Reactive oxygen species generation
Oxidation of dihydrorhodamine 123, an oxidation-sensitive indicator, which can be converted to the fluorescent derivative rhodamine 123, namely by superoxide and hydrogen peroxide 29, 30 was used as indicator for free radical generation as previously described 24. HN cells were pre-incubated with the respective curcumin media and 10mM dihydrorhodamine 123 for 1h. Thereafter the cells were washed with PBS and irradiated for 5min with VIS. Rhodamine 123 fluorescence was consecutively measured every 10min (excitation 485nm, emission at 530nm) using a CytoFluor (series 4000, PerSeptive Biosystems, Framingham, USA).
Apoptosis
DNA fragmentation was chosen as indicator of apoptosis. Quantification was performed with the Cell Death Detection ELISA (CDD; Roche, Mannheim, Germany) according to the manufacturer`s specifications. Cells were seeded in 96-well microtiter plates with a density of approximately 2×104 cells/0.33 cm2. On the following day cells were treated as described above. 15h after the treatment culture medium was discarded and the adherent cells were lysed and further processed as described 23.
Immunhistochemical analysis of cleaved caspase-3
HN cells were seeded in a cell density of 2x 104 cells/ml on glass cover slides. On the following day the cultures were treated as described above or treated with 1µM or 10µM camptothecin (Sigma-Aldrich) as positive control. All cultures were fixed after 4h in 4% paraformaldehyd (Carl Roth) for 15min at room temperature. Thereafter the cultures were washed three times for 10min with PBS before blocking with PBS containing 5% (v/v) bovine serum albumin (Carl Roth) and 0.3% Triton X-100 for 60min. Subsequently cells were stained overnight at 4°C with 1:500 diluted polyclonal rabbit anti-human cleaved caspase-3 (ASP-175, Cell Signaling, Frankfurt, Germany). Before incubating with 1:500 diluted polyclonal goat anti-rabbit IgG (H+L), Alexa Fluor 546 conjugated (Thermo Fisher Scientific, Rockford, USA) for 2h at room temperature, cells were washed three times with PBS. After the secondary antibody incubation cells were washed again three times for 10min in PBS. Cell nuclei were counterstained with DAPI (Thermo Fisher Scientific) for 10min at room temperature and washed briefly in PBS and demineralised water. Coverslips were covered with Aqua Poly Mount (Polysciences, Hirschberg an der Bergstrasse, Germany) and fixed on microscope slides. Photographs were taken with a digital camera (Sony Cyber Shot 3.3, Sony, Cologne, Germany) connected to an Olympus BX-50 (Olympus, Hamburg, Germany) and processed with ImageJ 31.
Presentation of data and statistical analysis
All data are presented as mean values ± standard deviation. Statistical significance of the data was evaluated by the Wilcoxon-Mann-Whitney U-test (BIAS, Frankfurt, Germany). Each set of data was related to the referring untreated control. Differences were considered significant at p≤0.05: *, p≤0.01: **, ##, p≤0.001: ***, ###.
Results
Influence of curcumin on cell morphology
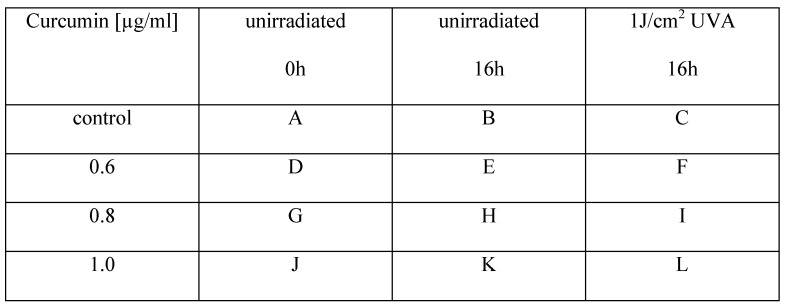
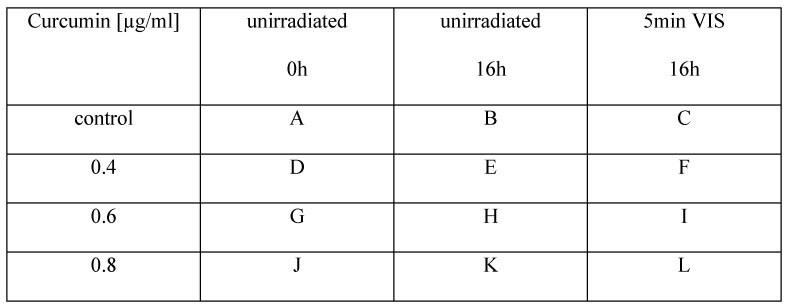
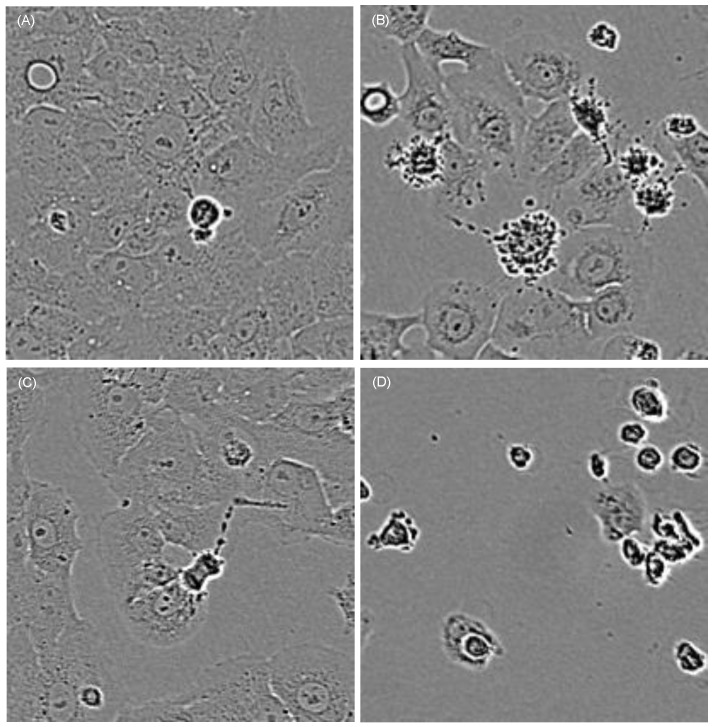
First of all we investigated whether cell morphology was influenced by curcumin and/or the applied combination treatment. HN cells were pre-incubated with curcumin (0.01µg/ml-1µg/ml). Subsequently, they were either left un-irradiated, were irradiated with 1J/cm2 UVA or were irradiated for 5min with VIS. Immediately after the treatment the cultures were monitored every second hour for 16h with an incubator microscope unit. Cell morphology did not alter in cultures treated solely by the specified curcumin concentrations. In cultures treated with curcumin concentrations of 0.8µg/ml and 1µg/ml in combination with UVA (Fig. 1) as well as in cultures treated with 0.6µg/ml and 0.8µg/ml curcumin in combination with VIS (Fig. 2) cell retraction and dynamic plasma membrane blebbing were evident. Clear discrimination between mitotic and apoptotic cells was possible (Fig. 3).
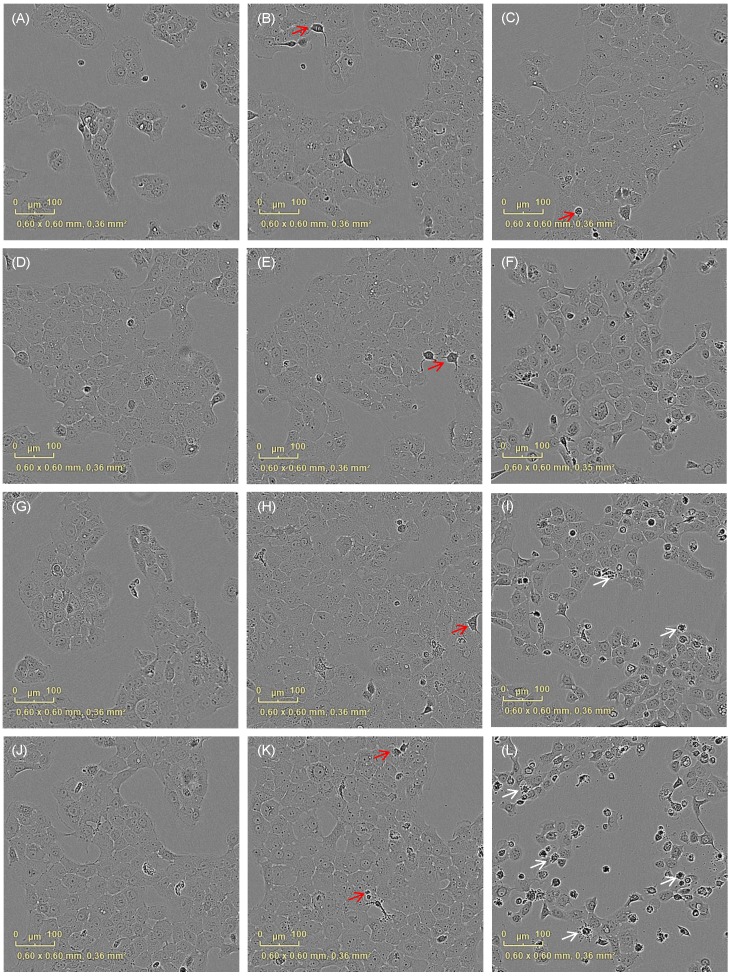
Figure 1.
Cell morphology after irradiation with UVA changes depending on the curcumin concentrations. Cell integrity was monitored with an incubator microscope unit immediately after the treatment and after 16h. Morphological criteria of mitotic cells (red arrows) as well as apoptotic cells (white arrows) were observable and exemplarily marked.
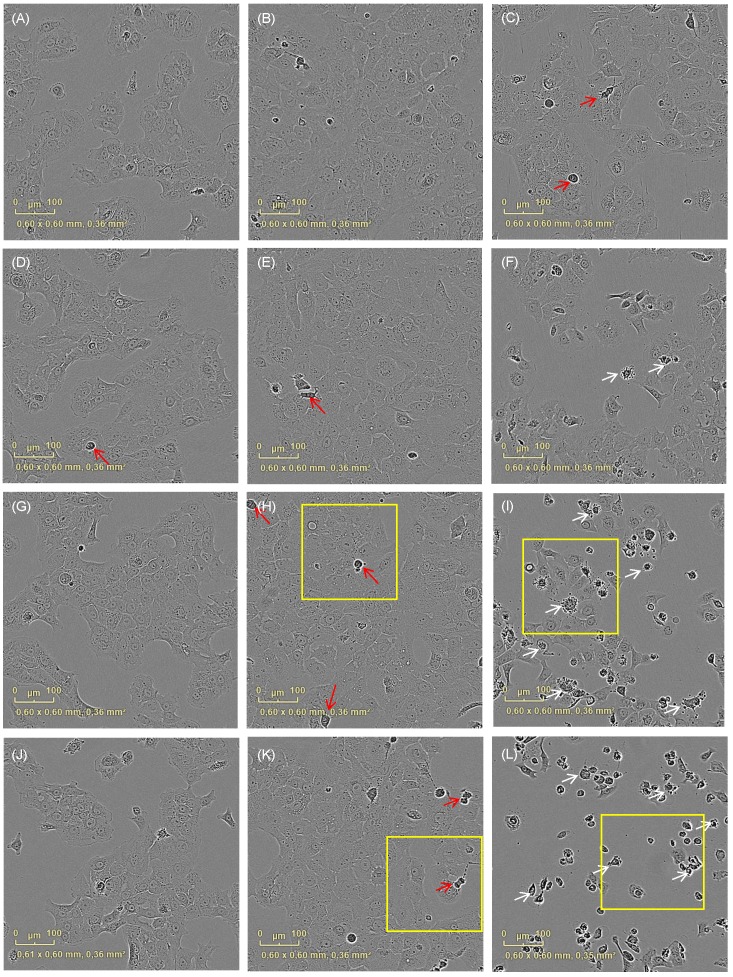
Figure 2.
Cell morphology changes after irradiation with VIS depending on the applied curcumin concentrations. Cell integrity was monitored with an incubator microscope unit immediately after the treatment and after 16h. Morphological criteria of apoptotic cells (white arrows) were observable after co-treatment with 0.4µg/ml (F), 0.6µg/ml (I) and 0.8µg/ml (L) curcumin 16h after VIS irradiation in contrast to criteria of mitotic cell (red arrows) that were less observable in the co-treated cultures. Characteristic culture areas (yellow squares) are enlarged in Fig. 3.
Figure 3.
Cells treated with curcumin show mitotic as well as apoptotic morphological criteria depending on the applied curcumin concentration and co-treatment with light. Characteristic cell areas marked by yellow squares in Fig. 2 are herein shown in a higher magnification. Cells were treated for 1h with 0.6µg/ml (A, B) or 0.8µg/ml (C, D) curcumin. Cells that were kept light protected (A, C) showed clear mitotic activity after 16h whereas cells irradiated with VIS (B, D) showed membrane blebbing and complete cell retraction.
Influence of curcumin on membrane integrity
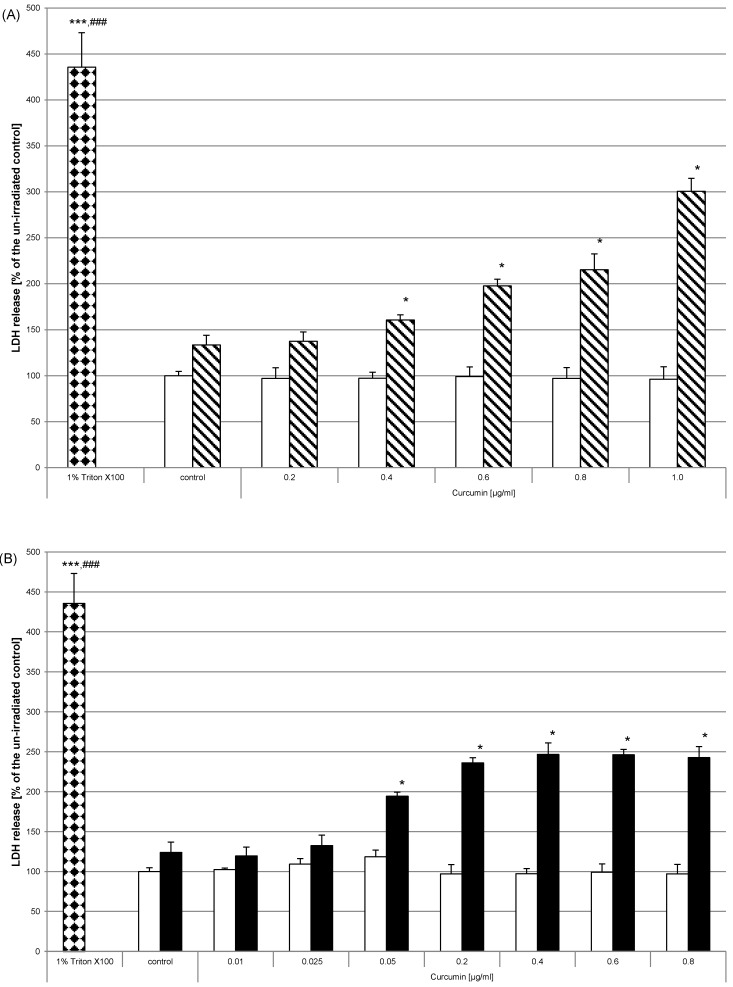
After showing irradiation and curcumin concentration dependent morphological changes of HN cells we ascertained whether cell integrity was influenced by curcumin and/or the applied combination treatment. HN cells were pre-incubated and irradiated as described before. After 24h the enzyme activity of lactate dehydrogenase in cell free supernatants was analysed. Curcumin without light treatment had no effect on lactate dehydrogenase liberalisation (Fig. 4, white bars). After irradiation with either UVA (Fig. 4A, striped bars) or VIS (Fig. 4B, black bars) we observed that the enzyme activity increased depending on the applied curcumin concentrations. Lactate dehydrogenase activity in supernatants increased after UVA irradiation when applying 0.4µg/ml - 1µg/ml curcumin. After VIS irradiation significant lactate dehydrogenase enzyme activity increase was observed for curcumin concentration of 0.05µg/ml. Maximal activity increase was reached when applying 0.4µg/ml curcumin after VIS irradiation. After irradiation with UVA a steady increase was observed for the tested curcumin concentrations. As positive control HN cells were treated with 1% Triton X-100 (bricked bars). Comparison of these cultures to the controls or to the curcumin treated cultures revealed a highly significant activity increase caused by Triton X-100.
Figure 4.
Curcumin influences membrane integrity after irradiation with UVA or VIS. Cell integrity was monitored by assaying LDH activity in supernatants 24h after treating HN cells with curcumin and successive UVA (A) or VIS (B) irradiation. Neither the applied curcumin concentrations (white bars) nor one of the light entities alone influenced the membrane integrity of HN cells. Co-stimulation with curcumin and either of the light sources increased the LDH activity (striped bars, black bars). The positive controls 1% Triton-X100 (bricked bars) caused maximal LDH activity. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from sextuplicate replicates per experimental condition were calculated. * p ≤ 0.05; *** p≤0.001 versus the un-irradiated control; ### p≤0.001 versus any sample.
DNA synthesis was impaired after curcumin and light treatment
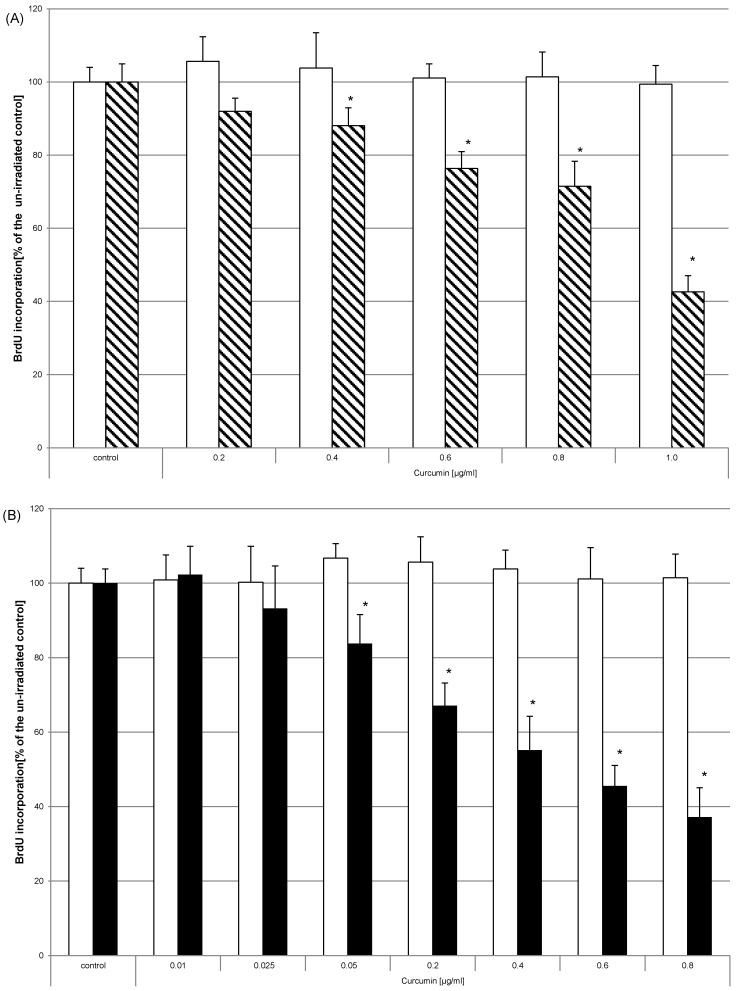
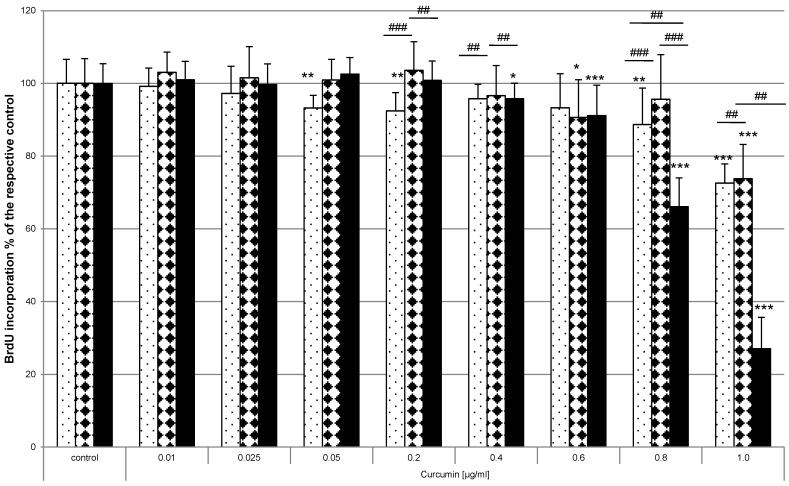
HN cells were pre-exposed to curcumin before irradiation. The impact on proliferation was analysed by monitoring the incorporation of BrdU as thymidine base analogue during the S-phase of mitosis. Curcumin alone or irradiation alone did not influence BrdU incorporation in any way (Fig. 5 white bars). Analysis after UVA (Fig. 5A, striped bars) or VIS (Fig. 5B, black bars) irradiation showed that the amount of incorporated BrdU and therefore the proliferation potential was significantly reduced in the samples that had been exposed to curcumin. Administering 0.8µg/ml curcumin before UVA treatment reduced BrdU incorporation to 71% of the irradiated control. The same curcumin concentration and VIS irradiation reduced the BrdU incorporation to 37% of the respective irradiated control. Even low concentrations of 0.05µg/ml after VIS irradiation revealed a 17% lower BrdU incorporation rate than in irradiated control cultures. To classify whether HN cells are more sensitive to the described photodynamic treatment than other cells direct comparison to the spontaneously immortalized keratinocyte cell line HaCaT and the epidermoid cancer cell line A431 was performed (Fig. 6). In all three cell lines curcumin concentration dependent BrdU incorporation reduction after VIS irradiation was evident. Furthermore it could be shown that a highly significant lower BrdU incorporation after treatment with 0.6µg/ml and 0.8µg/ml curcumin in combination with VIS could be observed in HN (black bars) cells than in A431 (bricked bars) or HaCaT (pointed bars) cultures.
Figure 5.
Curcumin in combination with UVA or VIS inhibits proliferation. HN cells were pre-incubated with curcumin and thereafter irradiated with UVA (A) or VIS (B). The proliferative potential was monitored by BrdU incorporation for 16h after the treatment. BrdU incorporation was not influenced by administering curcumin in the used concentrations (white bars). Combining these concentrations with either UVA (striped bars) or VIS (black bars) significantly reduced the BrdU concentration. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from sextuplicate replicates per experimental condition were calculated. * p ≤ 0.05 versus the respective un-irradiated control.
Figure 6.
Curcumin in combination with VIS inhibits proliferation in different cell lines. HaCaT (pointed bars), A431 (bricked bars) and HN cells (black bars) were pre-incubated with curcumin and thereafter irradiated with VIS. The proliferative potential was monitored by BrdU incorporation for 16h after the treatment. BrdU incorporation was reduced depending on the applied curcumin concentration and investigated cell specie. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from sextuplicate replicates per experimental condition were calculated. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, versus the respective control and ### p ≤ 0.01, ###p ≤ 0.001, versus another cell line treated with the same curcumin concentration.
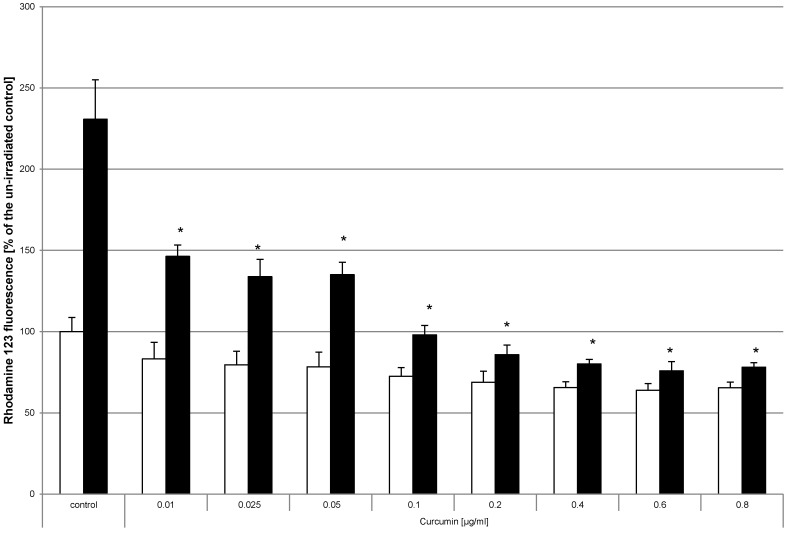
The concentration of VIS induced reactive oxygen species was reduced by curcumin
Generation of reactive oxygen species (ROS) is a known parameter for evaluation of the phototoxic potential of photosensitizing compounds 32-34. Monitoring the generation of ROS after curcumin and light treatment was chosen to study whether curcumin utilizes a similar mode of action. No significant induction of ROS was detected by treatment with curcumin alone (Fig. 7, white bars). To induce ROS, cell cultures were irradiated with VIS for 5min (Fig. 7, black bars) achieving a 130% higher ROS level than under control conditions. Analysis of cultures that had been pre-incubated with curcumin before VIS irradiation showed a significant reduction of ROS generation. Pre-incubation with curcumin concentrations of 0.1µg/ml to 1µg/ml completely reversed the ROS inducing influence of VIS irradiation. Likewise ROS savaging effects were observed when using 50mM H2O2 as ROS inducer (data not shown).
Figure 7.
Curcumin reduces VIS related oxidative stress HN cells were pre-incubated with dihydrorhodamine and curcumin. Thereafter the cultures were irradiated with VIS for 5min (black bars) or were kept light protected (white bars). Fluorescence measured after 60 minutes is shown. The values of the un-irradiated controls were set to 100%. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from sextuplicate replicates per experimental condition were calculated. * p ≤ 0.05 versus the irradiated control.
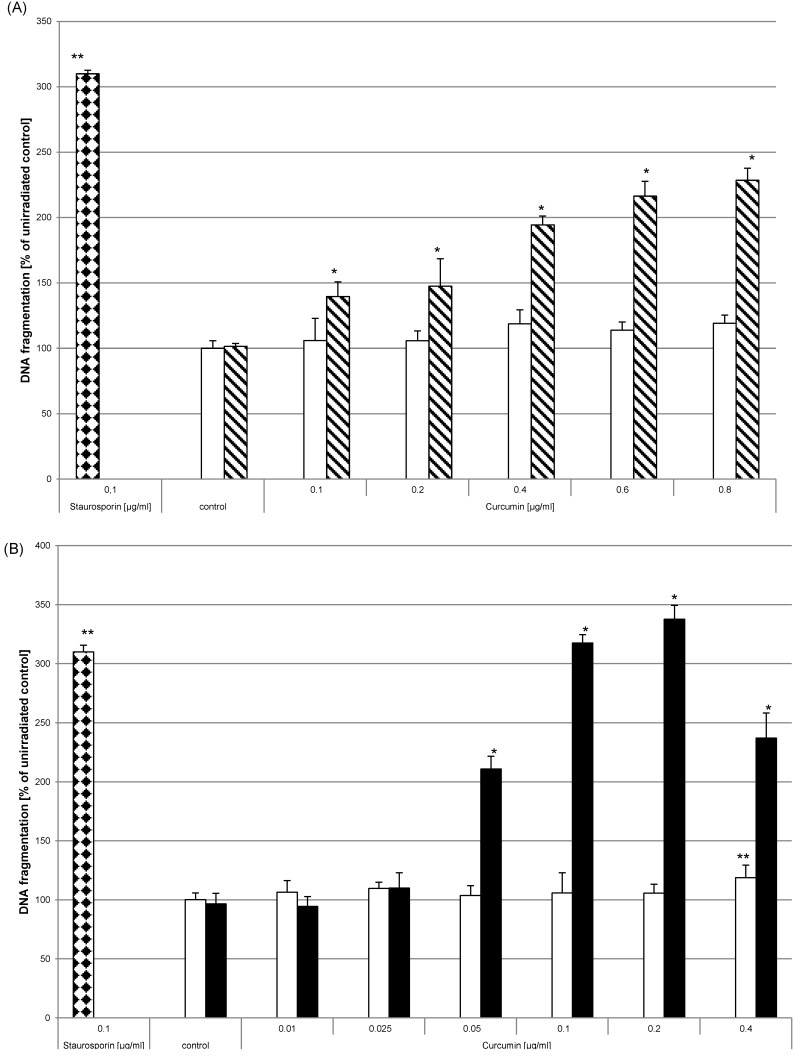
Curcumin and light induced DNA fragmentation
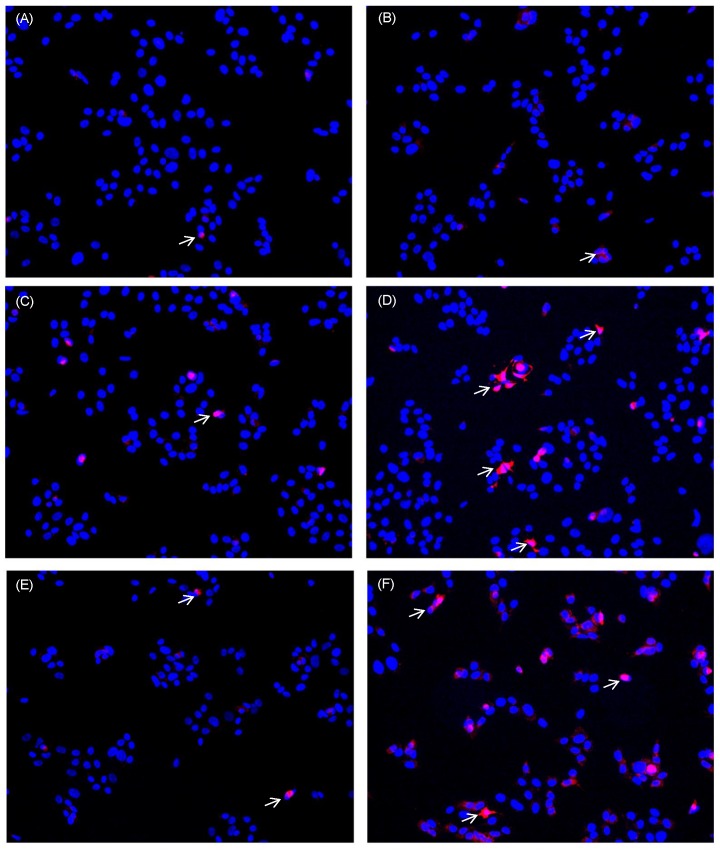
Apart from reducing proliferation the induction of apoptosis is also a desired effect to target tumour cells. As analytical parameter for apoptotic processes we monitored DNA fragmentation. HN cells were seeded in multiwell plates. After adherence they were either left untreated, were treated with 0.1µg/ml staurosporine (bricked bars) or were pre-incubated with curcumin (0.01µg/ml - 0.8µg/ml) prior to irradiation. Curcumin alone did not induce DNA fragmentation (Fig. 8, white bars). DNA fragmentation of cell cultures treated with curcumin and light was significantly increased. After UVA irradiation (Fig. 8A, striped bars) an increase of DNA fragmentation in comparison to the irradiated control of 40% (0.1µg/ml curcumin) to 129% (0.8µg/ml curcumin) was observed. Analysing DNA fragmentation of curcumin/VIS treated cultures showed a 110% increase after pre-incubation with 0.05µg/ml curcumin. The DNA fragmentation after VIS irradiation (Fig. 8B, black bars) increased depending on the applied curcumin concentration up to a final concentration of 0.2µg/ml curcumin. Further increasing the curcumin concentration to 0.4µg/ml resulted in a decreased DNA fragmentation in comparison to the cultures treated with 0.2µg/ml curcumin. Immunhistochemical staining of cleaved caspase-3 (Fig. 9) showed a concentration dependent increase of cleaved caspase-3 positive cells 4h after treatment with 1µg/ml (Fig. 9E) and 10µg/ml camptothecin (Fig. 9F). Furthermore it could be shown that incubation with 1µg/ml Curcumin (Fig. 9C) alone induced a slight induction of caspase-3 cleavage. Irradiation with VIS (Fig. 9B) induced no caspase-3 cleavage. After the combination treatment of curcumin and VIS (Fig. 9D) an increased number of cleaved caspase-3 positive cells as well as an increase of fluorescence intensity could be observed.
Figure 8.
Apoptosis is induced by curcumin-UVA or curcumin-VIS treatment. HN cells were pre-incubated with curcumin and thereafter irradiated with UVA (A) or VIS (B). DNA fragmentation was evaluated after 15h. The applied curcumin concentrations had no effect on DNA fragmentation (white bars). Combining curcumin with UVA (striped bars) or VIS (black bars) induced concentration dependent increase of DNA fragmentation comparable to a treatment with 0.1µg/ml staurosporine (bricked bars). The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from sextuplicate replicates per experimental condition were calculated. * p ≤ 0.05 versus the respective un-irradiated control.
Figure 9.
Caspase-3 cleavage is induced by curcumin-VIS treatment and camptothecin. HN cells were either cultivated in control medium (A, B) or were pre-incubated with 1µg/ml curcumin (C, D). Thereafter they were irradiated with VIS (B, D) or were kept light protected (A, C). As apoptotic inducer camptothecin was used in two concentrations; 1µg/ml (E) and 10µg/ml (F). Caspase-3 cleavage was analysed after 4h. Cleaved caspase-3 positive cells are indicated by white arrows exemplarily.
Discussion
Oral squamous cell carcinomas have a high incidence and recurrence rate 35 as well as a low 5 year survival rate 4, 5. Amending therapeutic options is therefore highly desired. During the last decades a re-orientation to natural compounds that have been used for centuries in traditional medicine is observed. Among those is the phytochemical curcumin. It is qualified as a cancer therapeutic due to its low risk of adverse events 36, 37 in comparison to other cytostatics. Another beneficial factor is that curcumin targets a broad range of signalling pathways 38 that are involved in cancer and inflammatory diseases 39, 40. To overcome the obstacle of the low bioavailability of curcumin 14 we have established a photodynamic treatment combining low curcumin concentrations and radiation with either UVA or VIS. Differences of efficiency of the two light entities were observed. The effects induced after VIS treatments were more potent than after UVA treatments. This correlates to the absorption maximum of curcumin at 420nm 42. The spectrum of VIS (380-780nm) overlaps this absorption peak whereas UVA (315-400nm) can only peripherally activate curcumin which has an absorption range of 300 to 500nm. We previously showed that cell proliferation was reduced and apoptosis induced after photodynamic treatment of cancerous and non-cancerous cell lines 23, 24 as well as in a mouse xenograft tumour model 25.
In this study we analysed whether curcumin can also be used for oral squamous cell carcinoma. In this context we particularly focused on tumour-relevant parameters such as proliferation and apoptosis. Of great interest was that HN cells were more sensitive to the photodynamic treatment than epidermoid carcinoma cells or the keratinocyte cell line HaCaT. Clear cell line specific reduction of the proliferative potential after photodynamic treatment with curcumin was evident. Treating primary gingiva fibroblasts and human submandibular cancer cells using curcumin doses of up to 10µM in combination with or without successive irradiation with a dental lamp 41, 42 revealed a concentration dependent viability reduction. In contrast to our results these effects were first observed after application of concentrations higher than 1µM curcumin. Reduction of cell proliferation was already observed after combination treatment of 0.05µM curcumin and VIS. Even though the enzyme activity of lactate dehydrogenase 24h after curcumin-light treatment was increased in comparison to the untreated control, there was still a highly significant difference to the enzyme activity of cultures whose membranes had been lysed with Triton X-100. Lactate dehydrogenase enzyme activity is classically used as indicator for cell membrane damage 34 and therefore indicates necrosis. In absence of phagocytosis it has been described that apoptosis can lead to processes that are called secondary necrosis 43 due to membrane disintegration. It is very likely that the lactate dehydrogenase enzyme activity increase in this study is related to secondary necrosis. The irradiation dependent induction of DNA fragmentation and caspase activation are positive indicators for apoptosis 44-46. In some cases cytotoxicity and apoptosis are induced by ROS release 32, 41. The reduced ROS secretion after the photodynamic treatment can be considered as another indicator that cytotoxicity in the used concentrations is not likely and that apoptosis is induced in a ROS independent fashion. Tumour progression is a very complex process. Oxidative stress is one factor that promotes tumour progression 47. Therefore inducing apoptosis, reducing proliferation and oxidative stress seems a promising therapeutic strategy. Compared to the sole oral application of curcumin the application of curcumin and light irradiation has many advantages in clinical use. The combined treatment of light irradiation and curcumin requires significantly lower concentrations of curcumin to achieve the desired effects 48. Moreover, the anti-tumorigenic effects are limited to the irradiated area protecting the surrounding tissue. The combined curcumin/irradiation treatment represents a reasonable addition to the established anti-cancer therapies and can be applied in combination with the approved methods of tumour excision, radiatio and chemotherapy. Nearly all areas in the buccal cavity can be treated with curcumin, e.g. in the form of a cream. Irradiation through an optical fibre is easy to realize with standard equipment. The photodynamic treatment with curcumin and light represents a reasonable addition to the established treatment regimens of oral tumours.
Conclusion
Curcumin in low concentrations is a potential candidate for photodynamic therapy of oral squamous cell carcinoma due to its anti-proliferative and pro-apoptotic potential. Further studies utilising tissue and animal models will evaluate this innovative treatment.
Acknowledgments
The authors are grateful to Dr. Adrian Sewell for comments and discussion and Stefanie Hoffmann and Monika Doll for technical support.
Abbreviations
- BrdU
5-bromo-2'-deoxyuridine
- DNA
deoxyribonucleic acid
- HN
human oral squamous cell carcinoma cell line
- LDH
lactate dehydrogenase
- ROS
reactive oxygen species
- UVA
ultraviolet-A
- VIS
visible light
References
- 1.Chen YJ, Chang JT, Liao CT, Wang HM, Yen TC, Chiu CC. et al. Head and neck cancer in the betel quid chewing area: recent advances in molecular carcinogenesis. Cancer Sci. 2008;99:1507–14. doi: 10.1111/j.1349-7006.2008.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawson RA, Odell EA. Cawson's Essentials Of Oral Pathology And Oral Medicine. Churchill Livingstone, Edinburgh, London, New York, Oxford, Philadelphia, St. Louis, Sydney, Toronto; 2002. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Sawair FA, Irwin CR, Gordon DJ, Leonard AG, Stephenson M, Napier SS. Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:1–9. doi: 10.1034/j.1600-0714.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 5.Sessions DG, Spector GJ, Lenox J, Haughey B, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112:616–25. doi: 10.1097/00005537-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bernier J. Current state-of-the-art for concurrent chemoradiation. Semin Radiat Oncol. 2009;19:3–10. doi: 10.1016/j.semradonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH. et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 8.Fan KH, Lin CY, Kang CJ, Huang SF, Wang HM, Chen EY. et al. Combined-modality treatment for advanced oral tongue squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:453–61. doi: 10.1016/j.ijrobp.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Kreppel M, Drebber U, Eich HT, Dreiseidler T, Zoller JE, Muller RP. et al. Combined-modality treatment in advanced oral squamous cell carcinoma: Primary surgery followed by adjuvant concomitant radiochemotherapy. Strahlenther Onkol. 2011;187:555–60. doi: 10.1007/s00066-010-2245-8. [DOI] [PubMed] [Google Scholar]
- 10.Kadioglu O, Cao J, Saeed ME, Greten HJ, Efferth T. Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals. Target Oncol. 2015;10:337–53. doi: 10.1007/s11523-014-0339-4. [DOI] [PubMed] [Google Scholar]
- 11.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 12.Xu YX, Pindolia KR, Janakiraman N, Noth CJ, Chapman RA, Gautam SC. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp Hematol. 1997;25:413–22. [PubMed] [Google Scholar]
- 13.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY. et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–76. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 14.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: potential role for STAT-3 and NF-kappaB signaling. The Journal of investigative dermatology. 2010;130:2110–9. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 16.Rashmi R, Kumar S, Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005;26:713–23. doi: 10.1093/carcin/bgi025. [DOI] [PubMed] [Google Scholar]
- 17.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 18.Singh DV, Godbole MM, Misra K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: simulation for next generation of P-gp inhibitors. J Mol Model. 2013;19:227–38. doi: 10.1007/s00894-012-1535-8. [DOI] [PubMed] [Google Scholar]
- 19.Schiborr C, Kocher A, Behnam D, Jandasek J, Toelstede S, Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Molecular nutrition & food research. 2014;58:516–27. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 20.Hegge AB, Bruzell E, Kristensen S, Tonnesen HH. Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin-effect of selected nanocarrier: studies on curcumin and curcuminoides XLVII. Eur J Pharm Sci. 2012;47:65–74. doi: 10.1016/j.ejps.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K. et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. International journal of nanomedicine. 2012;7:3667–77. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan H, Teh C, Sreejith S, Zhu L, Kwok A, Fang W. et al. Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew Chem Int Ed Engl. 2012;51:8373–7. doi: 10.1002/anie.201203993. [DOI] [PubMed] [Google Scholar]
- 23.Buss S, Dobra J, Goerg K, Hoffmann S, Kippenberger S, Kaufmann R. et al. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PloS one. 2013;8:e79748. doi: 10.1371/journal.pone.0079748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Diaz-Alperi J. et al. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. The Journal of investigative dermatology. 2007;127:1992–2000. doi: 10.1038/sj.jid.5700801. [DOI] [PubMed] [Google Scholar]
- 25.Dujic J, Kippenberger S, Ramirez-Bosca A, Diaz-Alperi J, Bereiter-Hahn J, Kaufmann R. et al. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. International journal of cancer Journal international du cancer. 2009;124:1422–8. doi: 10.1002/ijc.23997. [DOI] [PubMed] [Google Scholar]
- 26.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. The Journal of cell biology. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dujic J. Effect of curcumin and light on skin- and tumor cells. JW Goethe University Frankfurt; 2008. [Google Scholar]
- 28.Zöller N, Valesky E, Hofmann M, Bereiter-Hahn J, Bernd A, Kaufmann R. et al. Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells. Annals of dermatology. 2015;27:709–14. doi: 10.5021/ad.2015.27.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson LM, Chappell JB. Dihydrorhodamine 123: a fluorescent probe for superoxide generation? Eur J Biochem. 1993;217:973–80. doi: 10.1111/j.1432-1033.1993.tb18328.x. [DOI] [PubMed] [Google Scholar]
- 30.Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–55. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onoue S, Kawamura K, Igarashi N, Zhou Y, Fujikawa M, Yamada H. et al. Reactive oxygen species assay-based risk assessment of drug-induced phototoxicity: classification criteria and application to drug candidates. J Pharm Biomed Anal. 2008;47:967–72. doi: 10.1016/j.jpba.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Onoue S, Tsuda Y. Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharm Res. 2006;23:156–64. doi: 10.1007/s11095-005-8497-9. [DOI] [PubMed] [Google Scholar]
- 34.Yin JJ, Liu J, Ehrenshaft M, Roberts JE, Fu PP, Mason RP. et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes-generation of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 2012;263:81–8. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 36.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 38.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–92. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atsumi T, Tonosaki K, Fujisawa S. Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch Oral Biol. 2006;51:913–21. doi: 10.1016/j.archoralbio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Atsumi T, Tonosaki K, Fujisawa S. Comparative cytotoxicity and ROS generation by curcumin and tetrahydrocurcumin following visible-light irradiation or treatment with horseradish peroxidase. Anticancer Res. 2007;27:363–71. [PubMed] [Google Scholar]
- 43.Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–21. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC, Shieh TM. et al. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int J Oncol. 2013;43:1141–50. doi: 10.3892/ijo.2013.2050. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Man S, Qiu H, Liu Z, Zhang M, Ma L. et al. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ Toxicol Pharmacol. 2016;48:31–8. doi: 10.1016/j.etap.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y. et al. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol Med Rep. 2015;12:1151–6. doi: 10.3892/mmr.2015.3450. [DOI] [PubMed] [Google Scholar]
- 47.Meierjohann S. Oxidative stress in melanocyte senescence and melanoma transformation. Eur J Cell Biol. 2014;93:36–41. doi: 10.1016/j.ejcb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Bernd A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochemistry reviews: proceedings of the Phytochemical Society of Europe. 2014;13:183–9. doi: 10.1007/s11101-013-9296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]