Abstract
Background
Circulating cytokines, chemokines, and soluble cytokine receptors can serve as biomarkers of inflammation and immune dysregulation. Good reliability of multiplex platforms, which allow for simultaneous, comprehensive biomarker assessment, is critical for their utility in epidemiologic studies. We examined the reliability of the Meso-Scale Discovery (MSD) platform to simultaneously quantitate 15 cytokines and chemokines and the Luminex platform (R&D Systems) to quantitate 5 soluble receptors and 2 chemokines and cytokines and evaluated long-term within-person correlation of these biomarkers.
Methods
The detectability and reliability of these assay systems were assessed using the same external controls across plates and archived sera from 250 HIV− men in the Multicenter AIDS Cohort Study. Using up to four visits per person from 1984 – 2009, age-adjusted intraclass correlation coefficients (ICC) of biomarkers with > 80% detectability (CCL11, CXCL8, CXCL10, CCL2, CCL4, CCL13, CCL17, CXCL13, IL-10, IL-12p70, IL-6, TNF-α, BAFF, sCD14, sCD27, sgp130, sIL-2Rα, and sTNF-R2) were obtained using linear mixed models.
Results
Most biomarkers were detectable in 80% of control samples; IFN-γ, GM-CSF, and IL-2 were undetectable in > 20% of samples. Among the HIV-uninfected men, most biomarkers showed fair to strong within-person correlation (ICC > 0.40) up to 15 years. The ICC for CXCL8 was good in the short term but decreased with increasing time between visits, becoming lower (ICC < 0.40) after 8 years.
Conclusions
These multiplexed assays showed acceptable reliability for use in epidemiologic research, despite some technical variability and limitations in cytokine quantitation. Most biomarkers displayed moderate-to-excellent intra-individual variability over the long term, suggesting their utility in prospective studies investigating etiologic associations with diverse chronic conditions.
Keywords: Inflammatory biomarkers, multiplex assay reliability, intraclass correlation, reliability
1. Introduction
Inflammation and immune activation are associated with chronic health conditions, including malignancies [1, 2], cardiovascular disease [3], kidney and liver dysfunction [4, 5], and AIDS [6]. Chronic inflammation is characterized by persistent activation of the innate and adaptive immune systems. Circulating serologic biomarkers, such as chemokines, cytokines, soluble cytokine receptors, and acute phase proteins are commonly used in epidemiologic studies to understand underlying inflammatory mechanisms associated with disease risk and progression. Most prior work has focused on single or small numbers of biomarkers, such as TNF-α, IL-6, and C-reactive protein (CRP). While informative, this approach offers an incomplete picture of the complex inflammatory response comprised of multiple interacting circulating mediators. Multiplex technologies permit concurrent testing of large numbers of analytes using minimal sample volume, allowing for rapid, cost-effective quantitation of a more comprehensive panel of biomarkers. While it is important to capitalize on these emerging methods, formal assessment of assay reliability is warranted. Prior studies of multiplex reliability have been restricted by small sample sizes or a limited number of biomarkers.[7, 8].
Furthermore, studies often use a single blood sample to quantitate circulating concentrations of inflammatory biomarkers and characterize a participant’s risk, assuming that a single measurement represents the individual’s long-term state of inflammation. High within-person variability can result in measurement error, biasing risk estimates towards the null and attenuating the likelihood of identifying valid exposure-disease associations. The intraclass correlation coefficient (ICC) assesses inter-individual (between people) variability relative to total variability (between and within individuals) and provides a measure of the extent to which a biomarker tracks within a person over time. Low ICCs necessitate multiple measurements over time to more accurately capture the inflammatory state, while low variability within a person, or high tracking, improves the precision of estimates from longitudinal studies. [9] Biomarkers exhibiting constant within-person correlation over time may also suggest a lack of immunological response to transient or acute exposures, potentially offering an important insight into the relationship between biomarkers and disease. [10] Previous studies of within- person biomarker variability have been limited by small sample sizes, a narrow range of biomarkers, or relatively short periods of time between biomarker measurements (≤ 5 years). [11–20]
This study had two aims: 1) to determine the detectability and reliability of the Meso-Scale Discovery (MSD) and Luminex platforms; and 2) to evaluate the long-term within-person correlation of 22 biomarkers of inflammation in a well-characterized, long-standing prospective cohort study. Results from this study will aid in designing future epidemiologic studies on the role of inflammatory biomarkers in disease etiology.
2. Materials and Methods
2.1 Study design and population
This analysis was conducted within the Multicenter AIDS Cohort Study (MACS), a prospective cohort study of men who sex with men (MSM) enrolled at four U.S. locations (Baltimore/Washington D.C., Chicago, Los Angeles, and Pittsburgh) to examine the natural and treated histories of HIV-1 infection. Since 1984, 6,972 participants have been enrolled: 4,954 in 1984–1985, 668 in 1987–1991, and 1,350 in 2001–2003. Institutional review boards at each center approved the MACS protocols and informed consent was obtained from all participants. Descriptions of the MACS protocol have been published previously. [21, 22] Study highlights, including data collection forms, may be found at https://statepi.jhsph.edu/macs/macs.html. Briefly, study participants are evaluated every six months with standardized interviews, physical examinations, and laboratory analysis of collected blood. Serum, plasma, and peripheral blood mononuclear cells are frozen and stored in local and national repositories. Serum samples used in this study were previously unthawed.
Control plasma samples isolated from an HIV-uninfected donor and a HIV-infected donor during a single blood draw were aliquoted and frozen. These external control plasma samples were obtained from Thomas N. Denny at Duke Human Vaccine Institute, Immunology and Virology Quality Assessment Center. Duke University IRB approvals were in place for these activities. To evaluate the reliability of the MSD platform, previously unthawed control plasma samples were run in duplicate on each plate over the course of the study.
To assess the ICCs, the concentrations of 22 inflammatory biomarkers were measured in serum samples from 250 HIV-uninfected men. Four study visits per individual were selected to represent the age and race distributions of the underlying cohort population from 1984 – 2009. All HIV-uninfected MACS men who were hepatitis C (HCV)-infected were included, to provide sufficient numbers for comparative analyses. All longitudinal specimens per individual were run on the same plate.
2.2 Laboratory methods
2.2.1. MSD platform
Serum concentrations of 9 cytokines and 7 chemokines were determined using the Meso-Scale Discovery (MSD) platform (Meso-Scale Diagnostics, LLC, Rockville, MD). The MSD system is an electrochemiluminesence-based, 96-well format solid-phase multiplex assay. Two separate kits, the Human Proinflammatory 9-Plex Ultra-Sensitive kit and Human Chemokine 7-Plex Ultra-Sensitive kit, were used to determine concentrations of IL-1β, IL-2, IL-6, IL-10, IL-12p70, IFN-γ, GMCSF, TNF-α, and CCL11, CXCL10 , CCL2, CCL13, CCL4, and CCL17 , respectively. CXCL8 was included in both kits. MSD assays were performed according to the manufacturer’s instructions. All MSD testing was conducted in a single laboratory by a single technician at the Johns Hopkins Bloomberg School of Public Health.
Cytokine limits of detection ranged from 0.8 – 1.2 pg/ml, while those for chemokines ranged from 39 – 158 pg/ml. Standard curves were done on each plate in duplicate. The lower limit of detection (LLOD) for each plate-specific analyte was the concentration 2.5 standard deviations above the background.
2.2.2. Luminex platform
Serum concentrations of the soluble receptors were determined using the multiplexed Luminex xMAP system (Fluorokine® MAP) using assays produced by R & D systems (Minneapolis, MN) following the manufacturer’s instructions, and a Bio-Plex 200 Luminex instrument and Bio-Plex software (Bio-Rad, Hercules, CA). Concentrations of four soluble receptors (sCD14, sgp130, sIL-2Rα, sTNF-R2), plus a cytokine (BAFF) and the chemokine CXCL13, were measured in a single panel (Human Biomarker Custom Premix Kit A). All testing for these markers was conducted in a single laboratory at the University of California, Los Angeles. One external serum control from a normal donor (no spiked values) was run in duplicate on each assay. This control sample was from a single blood draw that was aliquoted multiply and stored at − 80°C. For each plate tested, a biomarker- and plate-specific LLOD was defined. All the samples tested in the multiplex Kit A had concentrations above the lowest standard of the standard curve; therefore, the LLOD was defined as the observed concentration of the lowest standard.
2.3 Statistical Methods
Biomarker concentrations were natural-logarithm-transformed prior to analysis to account for non-normally distributed residuals. The detectability and reliability of each platform were calculated using the external control samples. Detectability was defined as the proportion of samples above the assay’s LLOD. The plate-specific intra-assay coefficient of variation (CV) for each marker was calculated as follows: (standard deviation (SD) of the duplicate values/mean of the duplicate values) x 100. The median and IQR (25%, 75% percentiles) intra-assay CV were obtained using all the plate-specific intra-assay CVs from both controls. Each control’s inter-assay CV (across plates) was obtained by calculating the SD of all the means across the plates and dividing it by the overall mean value for each analyte. An overall inter-assay CV for each marker was calculated by averaging inter-assay CVs for both controls. For markers with > 20% of samples below the LLOD, Cohen’s kappa was used to assess detectability agreement between the duplicate control concentrations per plate. [23]
2.3.1. Within-person temporal reliability
ICCs (the proportion of total variability due to between-person variability) were calculated for different intervals of time between visits. Between-person and within-person variance components and 95% confidence intervals (CI) were determined using linear mixed models, adjusting for age at visit. Age was kept as a continuous variable and centered at the median age across all person-visits. Categories of time between visits were: 0–2 years, 2.1–4 years, 4.1–8 years, 8.1–12 years, 12.1–15 years, and > 15 years. All combinations of visit pairings were used in the analysis, i.e. consecutive and non-consecutive pairs for the same individual. For example, if time between the first and second visits for ID X was 3.9 years, the time contributed by the visit-pair was allocated to the 2.1–4 year interval; if time between the first and third visits was 16.0 years, it was allocated to the > 15 year interval, etc. Parameters were estimated using the maximum likelihood estimate (MLE) method in Stata 13. We established a priori that ICCs would be calculated only for cytokines that were detectable in more than 80% of samples from the 250 HIV-uninfected men. Rosner’s interpretation of ICCs was utilized: ICCs between 0.40 and 0.75 reflect fair to good reliability and ICCs ≥ 0.75 reflect strong reliability. [24]
All analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Inc., Cary, North Carolina) and Stata, version 13 (College Station, Texas).
3. Results
3.1. Multiplexed Assay Reliability – MSD platform
3.1.1. Proportion Detectable in Control Samples
For the MSD platform, the 2 external control samples were tested in duplicate across 146 assay runs from September 2010 – April 2012. Table 1 provides the proportion detectable, median (IQR) of the detectable concentrations, the proportion of plates with intra-assay CVs ≥ 15%, control-specific inter-assay CVs, and the median intra- and overall inter-assay CVs across all 292 samples. On the chemokine plates, all analytes were 100% detectable, as were two analytes on the cytokine plates (TNF-α and CXCL8). IL-10, IL-12p70, IL-1β, and IL-6 were detectable in ≥ 85% of the samples, with IL-10 and IL-6 detectable in nearly all samples.
TABLE 1.
Reliability characteristics of the Meso-Scale Discovery platform using the external control plasma samples
| Control 1 (n = 146 duplicates) | Control 2 (n = 146 duplicates) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Detectable (%) |
Median, pg/mL |
25% | 75% | CVs ≥ 15% (%)a |
Inter- assay CV (%) |
Detectable (%) |
Median, pg/mL |
25% | 75% | CVs ≥ 15% (%)a |
Inter- assay CV (%) |
Median intra- assay CV (%) |
Overall inter- assay CV (%)b |
| Chemokinesd | ||||||||||||||
| CCL11 CXCL8-chemoc | 100 | 3,033 | 2,640 | 3,356 | 24.7 | 16.6 | 100 | 846 | 724 | 951 | 27.4 | 16.2 | 7.6 | 16.4 |
| CXCL10 | 100 | 474 | 418 | 536 | 5.5 | 17.4 | 100 | 88.0 | 78.0 | 103 | 9.6 | 19.4 | 3.7 | 18.4 |
| CCL4 | 100 | 149 | 140 | 159 | 4.1 | 9.8 | 100 | 36.6 | 33.3 | 39.2 | 7.5 | 11.1 | 4.0 | 10.4 |
| CCL2 | 100 | 318 | 287 | 337 | 5.5 | 11.2 | 100 | 165 | 149 | 178 | 6.9 | 12.2 | 4.2 | 11.7 |
| CCL13 | 100 | 798 | 726 | 867 | 13.0 | 12.5 | 100 | 969 | 892 | 1,056 | 8.9 | 11.7 | 4.2 | 12.1 |
| CCL17 | 100 | 733 | 674 | 810 | 9.6 | 12.6 | 100 | 706 | 648 | 761 | 8.2 | 12.0 | 3.3 | 12.3 |
| Cytokinese | ||||||||||||||
| IL-10 | 97.9 | 1.7 | 1.2 | 2.2 | 47.9 | 35.1 | 97.6 | 1.4 | 1.0 | 1.8 | 49.3 | 34.9 | 13.3 | 35.0 |
| IL-12p70 | 87.3 | 1.4 | 1.1 | 1.9 | 61.6 | 33.0 | 95.2 | 1.7 | 1.3 | 2.4 | 49.3 | 36.8 | 14.5 | 34.9 |
| IL-6 | 97.6 | 0.6 | 0.5 | 0.7 | 30.8 | 20.0 | 98.3 | 0.8 | 0.7 | 1.0 | 18.5 | 21.5 | 8.3 | 20.7 |
| CXCL8-proc | 100 | 7.6 | 6.9 | 8.3 | 8.9 | 13.0 | 100 | 5.2 | 4.7 | 5.8 | 9.6 | 16.4 | 4.9 | 14.7 |
| TNF-α | 100 | 6.9 | 5.9 | 7.9 | 11.6 | 19.8 | 100 | 5.6 | 4.7 | 6.2 | 18.5 | 20.3 | 5.0 | 20.1 |
| IL-1β | 87.3 | 0.6 | 0.5 | 0.7 | 50.7 | 67.3 | 87.3 | 0.8 | 0.6 | 1.0 | 52.7 | 119.9 | 13.2 | 93.6 |
Percent of plates with intra-assay CVs ≥ 15%.
Mean of Control 1 and Control 2 inter-assay CVs.
CXCL8 was included in both the chemokine and cytokine kits.
14 of the samples were missing chemokine measurements (total number of measurements per control = 286).
2 of the samples were missing cytokine measurements (total number of measurements per control = 290).
NOTE: CXCL8-pro denotes CXCL8 tested on the MSD proinflammatory cytokine kit; CXCL8-chemo denotes CXCL8 tested on the MSD chemokine kit.
The proinflammatory cytokines GM-CSF, IFN-γ, and IL-2 were detectable in < 80% of control samples (Table 2).
TABLE 2.
Agreement among cytokines with >20% undetectable among the external controls tested on the Meso-Scale Discovery platform
| Control 1
|
Control 2
|
|||||
|---|---|---|---|---|---|---|
| Cytokines | % undetectable | Κa | Κb | % undetectable | Κa | Κb |
| GM-CSF | 50.3 | 0.40 | 0.40 | 55.1 | 0.30 | 0.30 |
| IFN-γ | 53.1 | 0.30 | 0.30 | 61.3 | 0.30 | 0.30 |
| IL-2 | 38.7 | 0.40 | 0.40 | 60.6 | 0.30 | 0.30 |
Kappa statistic. No adjustment to the lower limit of detection.
Kappa statistic. Modified cutoff by 0.5*SD of marker distribution.
3.1.2. Coefficients of Variation
The estimated median intra-assay CVs for the 7 chemokines ranged from 3.3% (CCL17) to 7.6% (CCL11) and for the 5 cytokines from 5.0% (TNF-α) to 14.5% (IL-12p70) (Table 1). The median intra-assay CVs for CXCL8, run on both the chemokine and cytokine kits, were 4.3% and 4.9%, respectively. The inter-assay CVs were higher for the cytokines compared to the chemokines.
There was poor agreement (kappas ≤ 0.4) between control duplicates for the 3 cytokines with < 80% detectability (Table 2). To account for the possibility this resulted from low values lying close to the LLOD, we created new cutoffs by adding 0.5 x SD of the detectable samples to the LLOD, and assigning all values between the LLOD and the new cutoff as undetectable. This modification did not change the kappa statistics.
3.2. Multiplexed Assay Reliability – Luminex platform
Table 3 shows the median (IQR) concentrations and median intra- and inter-assay CVs for the 7 biomarkers run on the Luminex platform. For this analysis, one external control was run in duplicate on 153 plates, resulting in 306 biomarker measurements. All 7 biomarkers showed acceptable intra-assay reliability (median CVs < 10%). With the exception of CXCL13 and sIL-2Rα, all inter-assay CVs were less than 15%.
TABLE 3.
Reliability characteristics of the Luminex platform using the external control samples
| Biomarkers | Median, pg/mL | 25% | 75% | Plates with CVs ≥15% (%)b | Median intra-assay CV (%) | Inter- assay CV (%) |
|---|---|---|---|---|---|---|
| BAFF | 2,425 | 2,192 | 2,717 | 5.2 | 4.5 | 14.7 |
| CXCL13 | 570 | 447 | 662 | 7.2 | 3.9 | 26.1 |
| sCD14a | 1,480 | 1,387 | 1,574 | 9.2 | 5.2 | 10.5 |
| sCD27 | 9,304 | 8,514 | 10,290 | 3.9 | 4.7 | 13.9 |
| sgp130a | 253 | 239 | 266 | 4.6 | 4.1 | 8.8 |
| sIL-2Rα | 1,402 | 1,262 | 1,540 | 4.6 | 4.5 | 29.0 |
| sTNF-R2 | 2,624 | 2,479 | 2,821 | 6.5 | 4.0 | 9.7 |
Percent of plates with intra-assay CVs ≥ 15%.
3.3. Within-person temporal reliability
For this analysis, we used 971 samples contributed by 250 HIV-uninfected MACS participants from June 1984 to September 2009; 89.6% of the participants (n = 224) had 4 samples, 9.2% (n = 23) had 3 samples, and 3 had 2 samples. The median (IQR) age across all person-visits was 45.6 years (39.7, 52.5), ranging from 18.7 – 74.5 years. Black men comprised 38.7% of the sample, and approximately 24% of the men had chronic HCV (RNA positive) at the first available measurement. Across all person-visits, nearly 55.0% had a BMI classified as either overweight (25 – 29.9 kg/m2) or obese ( ≥ 30 kg/m2).
The median time from first to last sample was 18.3 years (IQR: 5.5 – 19.8). Across all possible pairs of visits contributed by the same person, the median time between visits was 4.5 years (IQR: 2.6, 12.8). The median time from first to second sample was 2.5 years (IQR: 1.5 – 4.0), from second to third was 4.5 years (IQR: 2.0 – 12.4), and from third to fourth was 4.0 years (IQR: 2.0 – 4.5). Table 4 shows the proportion of detectable samples and the median (IQR) concentrations for the 250 participants. Over 25% of the samples had undetectable GM-CSF, IFN-γ, IL-2, and IL-1β concentrations. The remaining analytes were detectable in > 98% of the samples, except for IL-12p70 (93.5%). Initially, we anticipated calculating ICCs for 6 separate time intervals between blood draws: 0 – 2, 2.1 – 4, 4.1 – 8, 8.1 – 12, 12.1 – 15, and > 15 years. Due to sparse numbers of visit-pairs in 8.1 – 12 and 12.1 – 15 years, we combined these into one interval, 8.1 – 15 years. The numbers of visit-pairs contributing to the intervals were 223, 355, 358, 183, and 337, respectively.
TABLE 4.
Proportion detectable and median (IQR) of the detectable concentrations of chemokines, cytokines, and soluble cytokine receptors for the 250 HIV-uninfected men (number of person-visits = 971), Multicenter AIDS Cohort Study
| N | % | Median, pg/mL | 25% | 75% | |
|---|---|---|---|---|---|
|
|
|||||
| Chemokines | |||||
| CCL11 | 971 | 100 | 1,696 | 1,219 | 2,419 |
| CXCL8 | 971 | 100 | 13.3 | 8.9 | 22.9 |
| CXCL10 | 971 | 100 | 140 | 92.0 | 234 |
| CCL2 | 971 | 100 | 508 | 362 | 657 |
| CCL4 | 967 | 99.6 | 146 | 98.4 | 205 |
| CCL13 | 971 | 100 | 749 | 580 | 991 |
| CCL17 | 970 | 99.9 | 532 | 368 | 836 |
| CXCL13 | 960 | 98.9 | 298 | 246 | 349 |
| Cytokines | |||||
| GM-CSF | 620 | 63.9 | 1.1 | 0.7 | 2.1 |
| IFN-γ | 587 | 60.5 | 1.3 | 0.9 | 2.1 |
| IL-10 | 969 | 99.8 | 3.2 | 2.1 | 6.3 |
| IL-12p70 | 908 | 93.5 | 2.6 | 1.4 | 7.4 |
| IL-1β | 541 | 55.7 | 0.5 | 0.3 | 0.9 |
| IL-2 | 697 | 71.8 | 0.7 | 0.5 | 1.4 |
| IL-6 | 970 | 99.9 | 1.0 | 0.7 | 1.5 |
| TNF-α | 970 | 99.9 | 8.5 | 6.9 | 10.6 |
| BAFF | 971 | 100 | 1,970 | 1,731 | 2,263 |
| Soluble Cytokine Receptors | |||||
| sCD14a | 970 | 99.9 | 2,100 | 1,800 | 2,500 |
| sCD27 | 971 | 100 | 9,067 | 7,491 | 11,442 |
| sgp130a | 971 | 100 | 255 | 230 | 289 |
| sIL-2Rα | 971 | 100 | 1,382 | 1,139 | 1,739 |
| sTNF-R2 | 971 | 100 | 2,307 | 1,880 | 2,917 |
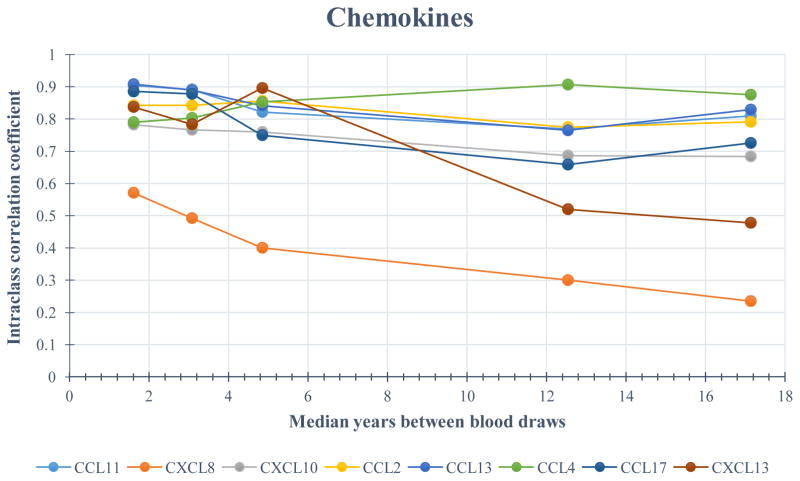
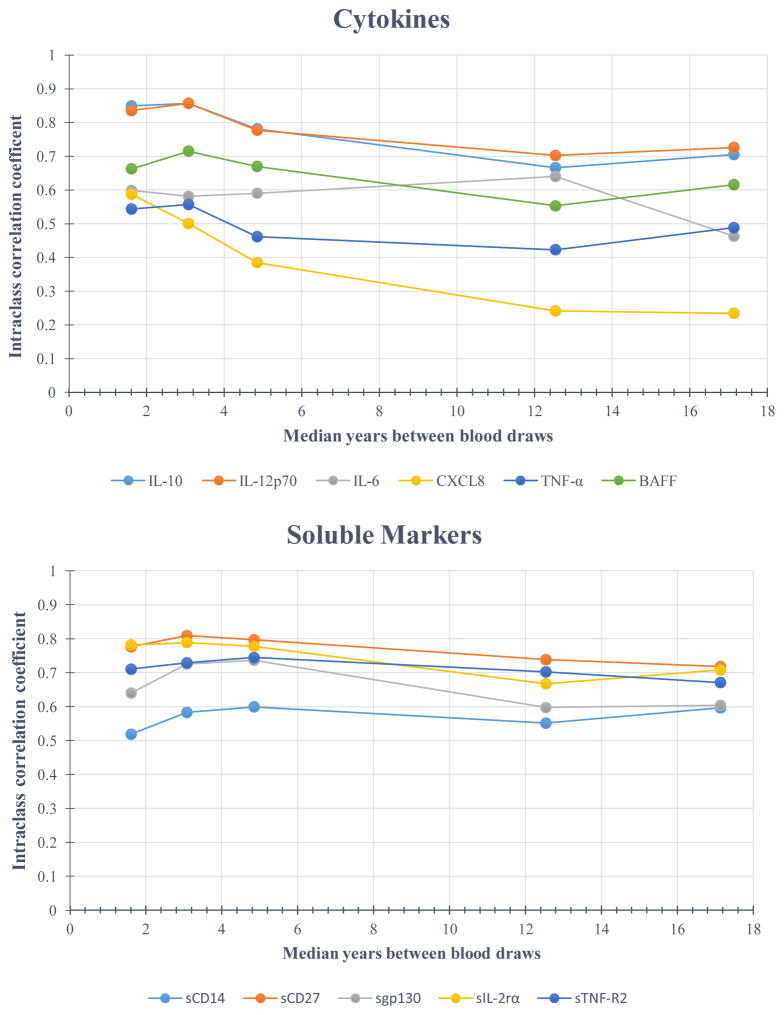
Figure 1 illustrates the ICCs for each analyte by time between measurements, with estimates plotted at the median of each visit-pair interval. All ICCs were significantly (p < 0.05) greater than 0. (See Table 5 for ICC estimates with 95% confidence intervals.) Tracking was strong (ICC ≥ 0.75) for the majority of chemokines measured in samples spanning 2 years or less; only CXCL8 exhibited good tracking (> 0.55), with statistically equivalent ICCs on both the chemokine and cytokine kits (0.57 and 0.59, respectively). Among the cytokines, IL-10 and IL-12p70 had strong tracking (ICC = 0.85 and 0.84, respectively), with IL-6, TNF-α, and BAFF exhibiting good ICCs (0.60, 0.54, and 0.66, respectively) during this shortest interval. The tracking of the soluble receptors was also good to strong, with the exception of sCD14, which exhibited moderate within-person correlation (ICC = 0.52).
Figure 1.
Age-adjusted intraclass correlation coefficients (ICC) for each analyte across 5 visit-pair time intervals (0 – 2, 2.1 – 4, 4.1 – 8, 8.1 – 15, and > 15 years) between blood draws for 250 HIV-uninfected men in the Multicenter AIDS Cohort Study (MACS). Estimates are plotted at the median of each visit-pair interval.
TABLE 5.
Age-adjusted intraclass correlation coefficients (ICC) and 95% confidence intervals for each biomarker across five categories of time between blood draws for 250 HIV-uninfected men, Multicenter AIDS Cohort Study (MACS), 1984 – 2009
| Interval 1
|
Interval 2
|
Interval 3
|
Interval 4
|
Interval 5
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 – 2 years | 2.1 – 4 years | 4.1 – 8 years | 8.1 – 15 years | > 15 years | ||||||
| n = 223a | n = 355 | n = 358 | n = 183 | n = 297 | ||||||
| Biomarkers b | ICCc | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI |
| Chemokines | ||||||||||
| CCL11 | 0.90 | (0.87 – 0.93) | 0.89 | (0.87 – 0.91) | 0.82 | (0.78 – 0.85) | 0.77 | (0.70 – 0.82) | 0.81 | (0.76 – 0.85) |
| CXCL8–chemo | 0.57 | (0.48 – 0.66) | 0.49 | (0.42 – 0.57) | 0.40 | (0.33 – 0.48) | 0.30 | (0.20 – 0.42) | 0.24 | (0.16 – 0.33) |
| CXCL10 | 0.78 | (0.72 – 0.83) | 0.77 | (0.72 – 0.81) | 0.76 | (0.71 – 0.80) | 0.69 | (0.60 – 0.76) | 0.68 | (0.62 – 0.74) |
| CCL2 | 0.84 | (0.80 – 0.88) | 0.84 | (0.81 – 0.87) | 0.86 | (0.82 – 0.88) | 0.77 | (0.71 – 0.83) | 0.79 | (0.74 – 0.83) |
| CCL13 | 0.91 | (0.88 – 0.93) | 0.89 | (0.86 – 0.91) | 0.84 | (0.81 – 0.87) | 0.76 | (0.70 – 0.82) | 0.83 | (0.79 – 0.86) |
| CCL4 | 0.79 | (0.73 – 0.84) | 0.80 | (0.76 – 0.84) | 0.85 | (0.82 – 0.88) | 0.91 | (0.88 – 0.93) | 0.88 | (0.84 – 0.90) |
| CCL17 | 0.89 | (0.85 – 0.91) | 0.88 | (0.85 – 0.90) | 0.75 | (0.70 – 0.79) | 0.66 | (0.57 – 0.74) | 0.73 | (0.67 – 0.78) |
| CXCL13 | 0.84 | (0.79 – 0.88) | 0.78 | (0.74 – 0.82) | 0.90 | (0.87 – 0.92) | 0.52 | (0.42 – 0.62) | 0.48 | (0.40 – 0.56) |
| Cytokines | ||||||||||
| IL-10 | 0.85 | (0.81 – 0.88) | 0.86 | (0.82 – 0.89) | 0.78 | (0.74 – 0.82) | 0.67 | (0.58 – 0.74) | 0.71 | (0.64 – 0.76) |
| IL-12p70 | 0.84 | (0.79 – 0.87) | 0.86 | (0.82 – 0.88) | 0.78 | (0.73 – 0.82) | 0.70 | (0.62 – 0.77) | 0.73 | (0.67 – 0.78) |
| IL-6 | 0.60 | (0.51 – 0.68) | 0.58 | (0.51 – 0.65) | 0.59 | (0.52 – 0.65) | 0.64 | (0.55 – 0.72) | 0.46 | (0.38 – 0.55) |
| CXCL8-pro | 0.59 | (0.50 – 0.67) | 0.50 | (0.43 – 0.57) | 0.39 | (0.31 – 0.47) | 0.24 | (0.15 – 0.37) | 0.23 | (0.16 – 0.33) |
| TNF-α | 0.54 | (0.46 – 0.63) | 0.56 | (0.49 – 0.63) | 0.46 | (0.39 – 0.54) | 0.42 | (0.32 – 0.53) | 0.49 | (0.40 – 0.57) |
| BAFF | 0.66 | (0.58 – 0.73) | 0.72 | (0.66 – 0.77) | 0.67 | (0.61 – 0.73) | 0.55 | (0.45 – 0.65) | 0.62 | (0.54 – 0.68) |
| Soluble Cytokine Receptors | ||||||||||
| sCD14d | 0.52 | (0.43 – 0.61) | 0.58 | (0.51 – 0.65) | 0.60 | (0.53 – 0.66) | 0.55 | (0.45 – 0.65) | 0.60 | (0.52 – 0.67) |
| sCD27 | 0.78 | (0.72 – 0.83) | 0.81 | (0.77 – 0.84) | 0.80 | (0.75 – 0.83) | 0.74 | (0.67 – 0.80) | 0.72 | (0.66 – 0.77) |
| sgp130 | 0.64 | (0.56 – 0.72) | 0.73 | (0.67 – 0.77) | 0.74 | (0.68 –0.78) | 0.60 | (0.51 – 0.68) | 0.60 | (0.53 – 0.68) |
| sIL-2Rα | 0.78 | (0.72 – 0.83) | 0.79 | (0.74 – 0.83) | 0.78 | (0.73 – 0.82) | 0.67 | (0.58 – 0.74) | 0.71 | (0.65 – 0.76) |
| sTNF-R2 | 0.71 | (0.64 – 0.77) | 0.73 | (0.67 – 0.78) | 0.74 | (0.69 – 0.79) | 0.70 | (0.62 – 0.77) | 0.67 | (0.60 – 0.73) |
Number of visit-pairs contributing to interval of time between blood draws.
Undetectable values were assigned a value equal to one-half the plate-specific lower limit of detection.
Age-adjusted. Age at visit was modeled continuously and centered at the median age across all 971 HIV-uninfected person-visits.
One observation with an implausible value for sCD14 was excluded only from analyses of sCD14 (n = 970).
NOTE: CXCL8-pro denotes CXCL8 tested on the MSD proinflammatory cytokine kit; CXCL8-chemo denotes CXCL8 tested on the MSD chemokine kit
Although there was some degradation in the ICCs as time between measurements increased, most remained moderate to high, with the exception of CXCL8 and CXCL13. For example, with measurements 8.1 to 15 years apart, the ICCs for all the soluble receptors decreased to 0.50 – 0.74. Similarly, the ICCs for the cytokines decreased to < 0.75; only those for IL-10, IL-12p70, and IL-6 remained above 0.60. Even when the time between sample measurements was > 15 years, several markers remained highly correlated within a person, including CCL11, CCL2, CCL13, and CCL4 (ICCs > 0.75), and CXCL10, CCL17, IL-10, IL-12p70, BAFF, sCD14, sCD27, sgp130, sIL-2Rα, and sTNF-R2 (ICCs > 0.50). In contrast, CXCL8 exhibited good tracking for measurements taken within 4 years but was poorly correlated within a person for longer intervals between measurements. The ICC for CXCL13 was strong (≥ 0.78) up to 8 years between visits but was fair to good after 8 years, with ICCs of 0.52 (8.1 – 15 years) and 0.48 (> 15 years).
4. Discussion
Recent advances in multiplexing technologies have led to a wide array of commercially available platforms capable of quantifying an increasing number of soluble proteins in smaller sample volumes with greater sensitivity. These advancements are transforming biomarker discovery research, allowing for the exploration of the biologic underpinnings of disease pathogenesis in large population-based cohort studies. Along with unique analytic issues inherent to large-scale biomarker studies are questions of assay reliability over time. Here, we assessed several important methodological aspects of biomarker measurement including detectability, assay reliability, and within-person variability. Methodological concerns (e.g., inter- and intra-plate variation, inter- and intra-laboratory variation, assay drift over time, assay reliability across different manufactured lots) can be compounded as the number of samples and/or analytes increases, resulting in attenuated effect estimates and reduced statistical power. [25]
Our results suggest acceptable reliability of the MSD platform for the chemokines, as measured by high levels of detectability and low assay CVs using the external control samples. Reliability was less consistent for the proinflammatory cytokines. IL-10, IL-12p70, IL-6, and TNF-α exhibited acceptable detectability and reliability; however, IL-2, IL-1β, IFN-γ, and GM-CSF demonstrated inconsistent detectability. Possible reasons for this could be low sensitivity of the assay, potential degradation of the analyte due to long-term storage, or low cytokine concentrations in these control sera. Modifying the lower cutoff did not change the agreement, arguing against this last explanation. These analytes have low endogenous concentrations in the peripheral circulation, which may result from tissue utilization of cytokines or the distribution of cytokine-expressing cells. Likewise, some analytes may be less reliable due to the antibody pairs used in the assay. [26] The occurrence of variable plate-specific CVs underscored the need to run all longitudinal samples for an individual on the same plate, as well as samples from comparative groups of interest on each plate, in order to avoid bias or the attenuation of parameter estimates. [27, 28]
The ICC provides another measure of assay reliability, particularly with longitudinal specimens collected closely in time, and individual biomarker tracking over longer time periods. With poor within-person correlation, a biomarker-disease association may be missed if based only a single measure, particularly if not captured in the most biologically relevant time period. While moderate to large associations may still be detected, effect sizes will be attenuated. [18] ICCs can be used to estimate the extent of measurement error associated with a single measurement and then employed to obtain corrected estimates of the “true” association, under the assumption of non-differential measurement error. This is done by multiplying the natural logarithm of the hypothesized true measure of association by the ICC and then exponentiating the result (RRob = exp[ICC x ln RRtrue] where RR is the relative risk). [29, 30] ICCs > 0.80, as we observed, would result in only a moderate downward bias of exposure-disease associations. [31] In addition, smaller sample sizes may be adequate in studies of repeated biomarker measurements of markers that have high within-person correlation. [32]
Yet, while assay reliability is important, particularly for planning studies, an assay does not need to exhibit perfect reliability in order to have epidemiologic utility. Variability in biomarker concentrations should be considered in the context of the biological question. For example, significant associations may be identified in the face of large technical variability, if the biological variability is larger than the technical variability. Biomarkers with many undetectable values may still prove informative if expressed disproportionately according to disease. [7] For example, prior MACS studies looking at the association between B-cell inflammatory markers and HIV-associated non-Hodgkin B-cell lymphoma identified significant biomarker-disease associations by comparing the fractions detectable versus undetectable.[33, 34]
Using an ICC ≥ 0.75 as a criterion for strong within-person correlation, 11 of the 22 biomarkers in our study exhibited strong reliability over the shortest time between measurements (0 – 2 years). The remaining 8 showed fair to good reliability. The short-term variability of IL-6, CXCL8, and TNF-α, which comprise the classic inflammatory cascade, has been the subject of several previous reliability analyses using other assay platforms. Studies of IL-6 have reported short-term ICCs comparable to [15, 35, 36] or higher [11, 16, 19] than what we observed. Similarly, previous studies of CXCL8 showed short-term ICCs comparable to [12] [16], lower [36] [11], or higher [15, 19] than in the present study. The moderate ICCs observed for TNF-α were similar to some studies [16, 17] but lower than others. [11, 12, 15, 19, 36] These differences may be due to differences in study populations or sample handling. The current study comprised exclusively male participants, mostly middle-aged, in a population at risk for HIV acquisition, unlike most previous studies. While uninfected with HIV, they were more likely to be exposed to HIV-related risk factors, such as chronic HCV infection and recreational drug use, potentially predisposing them to transient and variable inflammatory stimuli. In addition, we have previously reported that discrepancies may arise from different assay technologies or lack of a centralized laboratory.[37]
Although the ICCs were expected to diminish with increasing time between measurements, many exhibited considerable tracking over time. With few exceptions (CXCL8, IL-6, TNF-α, and CXCL13), all demonstrated good to strong within-person tracking up to 15 years apart, with some exhibiting ICCs ≥ 0.75 even up to 8 (CCL11, CXCL10, CCL2, CCL13, CCL4, CCL17, IL-10, IL-12p70, sCD27, sIL-2Rα, sTNF-R2) and 15 years (CCL11, CCL2, CCL13, CCL4, sCD27) between samples. Tracking strength may serve as an important characteristic of physiologic homeostasis and be informative for both clinical assessment and pathogenesis research. Characteristics which influence these biomarkers also may be fixed within a person but very heterogeneous in the population. The men in our study were all HIV-uninfected and therefore not subjected to the impact of HIV infection on the immune system. Nevertheless, because 25% were HCV-mono-infected, we considered the potential effect of HCV on the ICCs; however, adjustment for HCV did not alter our results (data not shown).
Our study has some limitations. First, the study population to assess the ICCs consisted of young to middle-age men who have sex with men in the United States, which may limit generalizability to other populations. Second, we were unable to evaluate the ICCs of 4 cytokines (IL-2, IL-1β, IFN-γ, and GM-CSF) because more than 20% of samples were undetectable. Also, the lack of a gold standard precluded assessing the validity of our biomarker measurements. In addition, concentrations in serum may not fully reflect biological processes. Moreover, because the intra-assay CVs are based on a small number of measurements per plate, estimates may not be representative of the full range of variability estimates given the relatively few data points. Finally, the estimated CVs are based on measurements from external samples and therefore may not be representative of the variability in measurements for the population used to estimate the ICCs.
Several strengths of our study should be noted. For evaluating the MSD assay reliability, we utilized the same two external control samples across 146 plates, allowing us to robustly determine assay reliability. In addition, each multiplex assay was conducted in a single laboratory with standardized protocols. The MSD testing was performed by a single technician, minimizing laboratory variability, and limited to a single assay lot. The centralized Luminex/R&D Systems assays also were done using a single assay lot. All of the samples tested underwent only one freeze-thaw cycle, limiting variation due to specimen degradation. In addition, all longitudinal specimens for an individual were run on the same plate, thus minimizing the effects of inter-assay variability. High retention from 1984 – 2009 in a well-characterized cohort with standardized data and specimen collection permitted the assessment of biomarker reliability over increasingly long periods of time, up to 15 years, which is, to our knowledge, otherwise unaccounted for in the literature and may be useful for diseases with long etiologic windows, such as cancer, or latency periods, such as HIV. Moreover, the large sample size allowed us to estimate ICCs with greater precision than many previous studies. As far as we know, only one other analysis, from the MACS, has reported on the temporal reliability of sgp130, BAFF, and CXCL13, assessed using the Luminex platform, but this was limited to a 2-year interval between sample collections. [36]
In conclusion, our data support the use of high-sensitivity multiplex assays in large-scale epidemiologic research. Despite some technical variability, most biomarkers exhibited acceptable detectability and reliability. Moreover, most yielded fair to strong within-person reliability in men over a period of up to 15 years, indicating low variability within individuals relative to between individuals and stability of these analytes over time when properly stored. Changes in these biomarkers over time may thus be efficiently studied, and evaluated for use as surrogates for endogenous and exogenous effects longitudinally. CXCL8, TNF-α, and CXCL13 may be less stable within a person over longer periods of time but were still reliable within short time periods. Our results may also be useful for statistically accounting for the attenuation of exposure-disease associations due to potential non-differential misclassification. Our findings highlight the utility of investigating inflammation and immune-related pathways through the multiplex measurement of a comprehensive panel of circulating biomarkers, which will be important for unraveling the complex profile of immune response and disease pathogenesis.
Highlights.
CCLL, CCL2, CCL13, and CCL4 exhibit strong within-person tracking up to 15 years apart.
CXCL13 exhibits strong tracking only up to 8 years between measurements.
CXCL10, CCL17, IL-10, IL-12p70, BAFF, sCD14, sCD27, sgp130, sIL-2Rα, and sTNF-R2 track moderately up to 15 years apart.
IL-6 and TNF-α exhibit fair tracking up to 15 years between measurements.
CXCL8 exhibits fair tracking only up to 4 years between measurements.
Acknowledgments
Samples and data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with support from an American Recovery and Reinvestment Act (ARRA) supplement with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Donor identification and acquisition of external control samples from Duke was supported by the NIAID DAIDS IQA Program (HHSN27220054C). Cytokine profiling of pre-shipment material was performed in the Duke Human Vaccine Institute/Regional Biocontainment Laboratory Immunology Unit (Durham, NC) under the direction of Dr. Gregory D. Sempowski. The Regional Biocontainment Laboratory at Duke received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607).
The authors have no conflict of interest to report.
Abbreviations
- BAFF
B-cell activating factor
- BMI
body mass index
- CI
confidence interval
- CCL11
C-C motif ligand 11
- CV
coefficient of variation
- CXCL8
C-X-C motif ligand 8
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICC
intraclass correlation coefficient
- IFN
interferon
- IL
interleukin
- IQR
interquartile range
- LLOD
lower limit of detection
- sIL-2Rα
soluble IL-2 receptor alpha
- MACS
Multicenter AIDS Cohort Study
- sCD14
soluble cluster of differentiation 14
- sgp130
soluble glycoprotein 130
- TNF-α
tumor necrosis factor-alpha
- sTNF-R2
soluble tumor necrosis factor-receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moss SF, Blaser MJ. Mechanisms of disease: Inflammation and the origins of cancer. Nature clinical practice. Oncology. 2005;2(2):90–7. doi: 10.1038/ncponc0081. quiz 1 p following 113. [DOI] [PubMed] [Google Scholar]
- 2.Heikkila K, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer causes & control : CCC. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 3.Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. European heart journal. 2009;30(23):2838–44. doi: 10.1093/eurheartj/ehp477. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatric nephrology. 2009;24(8):1445–52. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Digestive diseases. 2010;28(1):179–85. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 6.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. The Journal of pathology. 2008;214(2):231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(9):1902–11. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agalliu I, et al. Detectability and reproducibility of plasma levels of chemokines and soluble receptors. Results in Immunology. 2013;3(0):79–84. doi: 10.1016/j.rinim.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken: John Wiley & Sons, Inc; 2004. p. 506. WIley Series in Probability and Statistics. [Google Scholar]
- 10.Galai N, et al. Tracking of markers and onset of disease among HIV-1 seroconverters. Statistics in medicine. 1993;12(22):2133–45. doi: 10.1002/sim.4780122207. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, et al. Reproducibility of serum cytokines and growth factors. Cytokine. 2009;45(1):44–9. doi: 10.1016/j.cyto.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann JN, et al. Intra-individual variability over time in serum cytokine levels among participants in the prostate, lung, colorectal, and ovarian cancer screening Trial. Cytokine. 2011;56(2):145–8. doi: 10.1016/j.cyto.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clendenen TV, et al. Temporal reliability of cytokines and growth factors in EDTA plasma. BMC research notes. 2010;3:302. doi: 10.1186/1756-0500-3-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linkov F, et al. Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. European cytokine network. 2009;20(1):21–6. doi: 10.1684/ecn.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro SL, et al. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(7):1167–70. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SA, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(11):2464–70. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RC, et al. Within-individual stability of obesity-related biomarkers among women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(6):1291–3. doi: 10.1158/1055-9965.EPI-06-1089. [DOI] [PubMed] [Google Scholar]
- 18.Platz EA, et al. Intra-individual variation in serum C-reactive protein over 4 years: an implication for epidemiologic studies. Cancer causes & control : CCC. 2010;21(6):847–51. doi: 10.1007/s10552-010-9511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong HL, et al. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(12):3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- 20.Epstein MM, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(11):2009–15. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow RA, et al. Infection with the human immunodeficiency virus: clinical manifestations and their relationship to immune deficiency. A report from the Multicenter AIDS Cohort Study. Annals of internal medicine. 1987;107(4):474–80. doi: 10.7326/0003-4819-107-4-474. [DOI] [PubMed] [Google Scholar]
- 22.Chmiel JS, et al. Factors associated with prevalent human immunodeficiency virus (HIV) infection in the Multicenter AIDS Cohort Study. American journal of epidemiology. 1987;126(4):568–77. doi: 10.1093/oxfordjournals.aje.a114696. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 24.Rosner BA. Fundamentals of Biostatistics. Vol. 6. Thomson: Brooks/Cole; 2006. [Google Scholar]
- 25.Whitney CW, Lind BK, Wahl PW. Quality assurance and quality control in longitudinal studies. Epidemiologic reviews. 1998;20(1):71–80. doi: 10.1093/oxfordjournals.epirev.a017973. [DOI] [PubMed] [Google Scholar]
- 26.Dabitao D, et al. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. Journal of immunological methods. 2011;372(1–2):71–7. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tworoger SS, Hankinson SE. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer causes & control : CCC. 2006;17(7):889–99. doi: 10.1007/s10552-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 28.Tworoger SS, Hankinson SE. Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(9):1578–81. doi: 10.1158/1055-9965.EPI-06-0629. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson SE, et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995;4(6):649–54. [PubMed] [Google Scholar]
- 30.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. American journal of epidemiology. 1992;136(11):1400–13. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 31.Hsing AW, et al. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(5):1004–8. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 32.Kirby AJ, Galai N, Munoz A. Sample size estimation using repeated measurements on biomarkers as outcomes. Controlled clinical trials. 1994;15(3):165–72. doi: 10.1016/0197-2456(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 33.Vendrame E, et al. Serum Levels of Cytokines, and Biomarkers for Inflammation and Immune Activation, and HIV-Associated Non-Hodgkin B cell Lymphoma Risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 doi: 10.1158/1055-9965.EPI-13-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breen EC, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(7):1303–14. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho GY, et al. Variability of serum levels of tumor necrosis factor-alpha, interleukin 6, and soluble interleukin 6 receptor over 2 years in young women. Cytokine. 2005;30(1):1–6. doi: 10.1016/j.cyto.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Epstein MM, et al. Temporal Stability of Serum Concentrations of Cytokines and Soluble Receptors Measured Across Two Years in Low-Risk HIV-Seronegative Men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breen EC, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clinical and vaccine immunology : CVI. 2011;18(8):1229–42. doi: 10.1128/CVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]