Abstract
Background:
Ambient aerosol fine particulate matter (PM2.5) is associated with male reproductive toxicity in experiments and may have adverse effects in the female. However, studies evaluating the protective effects and precise mechanisms of aspirin, Vitamin C, Vitamin E, or ozone against toxic effects of PM2.5 are sparse. This study was conducted to investigate the possible protective effects and mechanisms of aspirin, Vitamin C, Vitamin E, or ozone on fertility in female mice treated with PM2.5.
Methods:
Eighty-four ICR mice were divided into six groups: control group, PM2.5 group, PM2.5 + aspirin group, PM2.5 + Vitamin C group, PM2.5 + Vitamin E group, and PM2.5 + ozone group. PM2.5 was given by intratracheal instillation every 2 days for 3 weeks. Aspirin, Vitamin C, and Vitamin E were given once a day by oral gavage for 3 weeks, and ozone was administered by intraperitoneal injection once a day for 3 weeks. The levels of anti-Müllerian hormone (AMH), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and 8-hydroxy-2’-deoxyguanosine (8-OHdG) were measured using enzyme-linked immunosorbent assay. Western blotting analysis was used to analyze the expressions of Bcl-2, Bax, and caspase-3 in ovaries. Changes in histological structure were examined by light microscope and electron microscopy was used to detect ultramicrostructure.
Results:
The results demonstrated that PM2.5 decreased AMH levels (P < 0.001); however, aspirin (P < 0.001), Vitamin C (P < 0.001), Vitamin E (P = 0.001), and ozone (P = 0.002) alleviated the decrease. Changes of IL-6, TNF-α, 8-OHdG, Bax/Bcl-2, and caspase-3 in PM2.5 group were increased compared to control group (P < 0.001), while in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups, they were statistically decreased compared to PM2.5 group (P < 0.001 or P < 0.05).
Conclusions:
PM2.5 cause the damage of ovaries, and aspirin, Vitamin C, Vitamin E, and ozone antagonizes the damage. The protective mechanism is probably due to its ability to blunt the inflammatory and oxidative stress caused by PM2.5, which subsequently suppressing the expression of apoptotic regulatory protein and reducing the incidence of ovary apoptosis.
Keywords: Aspirin, Fine Particulate Matter, Ozone, Reproductive Toxicity, Vitamin C, Vitamin E
Introduction
Ambient aerosol fine particulate matter (PM2.5) is ubiquitous and persistent in real life. Numerous epidemiological studies have documented that PM2.5 has potential adverse effects on humans and animals including cardiovascular events,[1,2] lung cancer,[3] and chronic obstructive pulmonary disease.[4] In addition to the adverse effects of PM2.5 on respiratory and cardiovascular systems, recent studies have shown that PM exposure is a risk factor for degraded semen quality in males.[5,6] However, a few studies have investigated the influence of PM-induced reproductive toxicity in females.
PM2.5-induced inflammatory and oxidative stress responses have been considered as an important part in the triggering of cellular pathological process.[7,8] Aspirin is a classic nonsteroidal anti-inflammatory drug and has obvious anti-inflammatory effects as well as antioxidant effects demonstrated experimentally in both animals and humans.[9,10] Vitamin C (ascorbic acid) is an essential water-soluble vitamin in the human body, which is not only an effective antioxidant[11] but also can both prevent histamine release and increase the detoxification of histamine,[12] thus essentially acting as an anti-inflammatory agent. Vitamin E is an essential lipid-soluble vitamin in the human body, which is an effective antioxidant.[13] In addition, Vitamin E has been shown to be cardioprotective against peroxidative damage induced by tobacco smoke.[14,15] Ozone is a very reactive gas and is well known as a potent oxidant.[16] However, recent studies have clearly shown that a small and precisely ozone dose can induce upregulation of antioxidant enzymes.[17,18] Further, other results have confirmed that neither structural nor enzymatic cell damage occurs if an appropriate ozone therapeutic window is used.[19] Finally, ozone therapy has proven successful in treating a variety of illnesses.[18,20]
Despite these previous studies, little information is available about the effects of aspirin, Vitamin C, Vitamin E, and ozone on female reproductive endocrine function and ovarian structure in PM2.5-treated mice.
The aim of the present study was to explore the effects of aspirin, Vitamin C, Vitamin E, and ozone on female reproductive performance and ovarian structure in PM2.5-treated mice. The results of this investigation could provide an approach for preventing or alleviating the toxicity produced by aerosolized PM2.5 pollution.
Methods
Animals
Eighty-four, 4-week-old, specific-pathogen-free, ICR female mice, weighing 18–22 g, were purchased from Beijing Weitonglihua Laboratory Animal Co., Ltd., (Beijing, China). The mice were acclimatized to the laboratory environment for 7 days before starting the experiment. They were housed in plastic cages and allowed free access to standard mice chow and tap water. Animals were maintained under standard conditions with a 12 h light-dark cycle at 24 ± 2°C and 50 ± 5% humidity. All protocols used in this experiment were approved by the Animal Care and Use Committee (Beijing, China).
Fine particulate matter suspension preparation
All of the particulate matter (PM) used in this study had a diameter of <2.5 μm. It was provided by the Chinese Research Academy of Environmental Science in Beijing, China. The atmospheric PM2.5 sampling location was on the roof of the academy, during March 2016. The daily PM2.5 samples were collected on membrane filters for 24 h/day using a PM2.5 air sampler (Tianhong, China). After sampling, the membrane filters were packed in clean aluminum foil and stored at −20°C until use.
Each time before use, the PM2.5 membrane filters were weighed, and then the weighed membrane filter was placed in normal saline (NS). A dose of 10 mg of particles was suspended in 1.5 ml of NS and sonicated for 30 min to scatter the particles. After 30 min of ultrasonic oscillation, the supernatant fluid was obtained. The instillation method was a nonsurgical intratracheal instillation adapted from Bai et al.'s method.[21] Specific procedures are as follows: using the left hand to catch mice under conditions of no anesthesia, make the head of mice tilt 15°, with the right hand to hold Eppendorf and absorb the PM2.5 particles suspension that has been prepared, dripped into the nasal cavity of mice dropwise, and then waiting for the automatic suction. In addition, the process of drip should be soft, and observe the response of mice, and meanwhile, the velocity should not be too fast. The exposure groups were treated every 2 days for up to 11 times.
Drug preparation
Aspirin and Vitamin C were dissolved in 0.9% NS. Vitamin E was dissolved in corn oil. Aspirin was prepared as a solution of 1.5 mg/ml, Vitamin C as a solution of 4.5 mg/ml, and Vitamin E as a solution of 40 mg/ml; each was administered by oral gavage depending on randomization. All drugs were prepared freshly immediately before use. The ozone was obtained from an ozone therapeutic apparatus (Kastner, Germany), with the concentration of the ozone at 30 μg/ml, and administered by intraperitoneal injection.
Animal experiments
Mice were divided randomly into six equal groups of 14 animals each: (1) control group, (2) PM2.5 group, (3) PM2.5 + aspirin group, (4) PM2.5 + Vitamin C group, (5) PM2.5 + Vitamin E group, and (6) PM2.5 + ozone group.
After one week of acclimation, each mouse in the five exposed groups was instilled with a PM2.5 suspension at the same concentration, and the exposed concentration used in this study was 10 mg/kg. Each PM2.5 solution was freshly prepared with NS. For the control group, each mouse was instilled with the suspension using the same method and dosage as that used for the other five exposure groups.
Mice in PM2.5 + aspirin group (n = 14), PM2.5 + Vitamin C group (n = 14), and PM2.5 + Vitamin E group (n = 14) were each administered either aspirin (15 mg/kg), or Vitamin C (45 mg/kg), or Vitamin E (100 mg/kg) once a day by oral gavage according to their respective group assignment. Mice in PM2.5 + ozone group (n = 14) were administered ozone (0.4 mg/kg) once a day by intraperitoneal injection. The mice in the control group (n = 14) and PM2.5 group (n = 14) were administered NS once a day by oral gavage using the same method and dosage as that used for other intervention groups. The interventions were repeated daily for 22 days.
Serum sampling and processing
After finishing the last administration, all mice were sacrificed one day later. Blood samples were collected from the hearts of all mice and then centrifuged at 3000 rpm for 30 min to obtain the serum. The serum samples were quickly removed and kept at −80°C for further analysis of the anti-Müllerian hormone (AMH) levels.
Measurement of anti-Müllerian hormone in the serum of female mice
The levels of AMH were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Jiamay Biotech Co., Ltd., Beijing, China), as per the manufacturer's protocols.
Ovarian inflammatory cytokines measurement
The levels of the inflammatory cytokines, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) in mice ovaries were assayed using a TNF-α and IL-6 ELISA kit (Jiamay Biotech Co., Ltd., Beijing, China), as per the manufacturer's instructions.
Ovarian oxidative stress measurement
The ovarian concentrations of 8-hydroxy-2’-deoxyguanosine (8-OHdG) were performed through ELISA with the use of 8-OHdG kits (Jiamay Biotech Co., Ltd., Beijing, China). All procedures followed the manufacturers’ recommendation.
Protein extraction and Western blotting
The ovarian tissues were lysed with ice-cold Pro-PREPTM buffer (Jiamay Biotech Co., Ltd., Beijing, China). Then, the tissue lysates were clarified by centrifugation at 12,000 rpm for 10 min at 4°C and the supernatants were used for the assays. Concentrations of the proteins were determined using a BCA Protein Assay Kit (Thermo Pierce, Rockford, IL, USA). The proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene difluoride membranes. The membranes were then blocked for 1 h at 37°C by the use of 5% nonfat milk and incubated overnight at 4°C with the rabbit polyclonal anti-Bcl-2 antibody (Jiamay Biotech Co., Ltd., Beijing, China), rabbit polyclonal anti-Bax antibody (Jiamay Biotech Co., Ltd., Beijing, China), rabbit polyclonalanti-caspase-3 antibody (Jiamay Biotech Co., Ltd., Beijing, China), and rabbit polyclonal anti-β-actin antibody (Jiamay Biotech Co., Ltd., Beijing, China) in the Primary Antibody Dilution Buffer and subsequently with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody for 1 h at 37°C. Immunoreactive bands were detected using the Enhanced chemiluminescence system (Jiamay Biotech Co., Ltd., Beijing, China). After washing four times with TBS-T, the band intensity was quantified by densitometry.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed using the in situ Cell Death Detection Kit (Jiamay Biotech Co., Ltd., Beijing, China), to identify ovarian apoptosis in the 5 μm-thick paraffined sections. TUNEL assay was performed as per the manufacturer's instructions. Briefly, the ovarian paraffin sections were deparaffinized by heating at 60°C for 4 min, then washed twice in xylene and rehydrated through a graded series of ethanol and double-distilled water. The sections were incubated with 20 μg/ml of proteinase K for 15 min at room temperature and then washed four times with phosphate-buffered saline (PBS). Endogenous peroxidase was blocked with 2% H2O2 in PBS for 5 min at room temperature and washing twice in PBS for a total of 10 min. Then, 50 μl terminal deoxynucleotidyl transferase (TdT) reaction mix was added to the tissue, and the sections were incubated in a humidified atmosphere for 1 h at 37°C in the dark. Meanwhile, for negative control, sections were incubated with 50 μl reaction buffer without TdT treatment. All sections were then incubated with stop/wash buffer for 30 min, and then added with anti-digoxigenin conjugate for 30 min at room temperature, after which the sections were stained with 4’,6-diamidino-2-phenylindole. TUNEL images were obtained under a light microscope.
Histopathological examination
Mice were deeply anesthetized and then euthanized 24 h after the last treatment. The ovaries were removed and then fixed in 10% formalin at room temperature. Ovaries were dehydrated and embedded in paraffin. Next, tissue samples were sectioned serially every 5 μm and the sections then stained with hematoxylin and eosin and examined under a light microscope.
Electron microscopy
Two mice were taken from each group for the examination of electron microscope (EM) 24 h after the last treatment. Animals were anesthetized and perfused through the aorta with warm saline followed by a mixed solution of 4% paraformaldehyde and 2% glutaraldehyde (Sigma, St. Louis, MO, USA). The ovaries were removed after 1 h at 4°C. The tissues were fixed in 3% glutaraldehyde overnight and then rinsed with 0.1 M phosphate buffer (PB) three times. After postfixed with 1% osmium tetroxide (Sigma, St. Louis, MO, USA) in sodium PB for 2 h at 4°C, tissues were dehydrated with a graded series of ethanol washes, and then the dehydrated specimens were embedded in araldite for 24 h. Ultrathin sections (1 μm) were cut from the embedded tissue and then stained by 3% uranyl acetate-lead citrate. Ovarian ultrastructures were observed using an EM.
Statistical analysis
All values were analyzed by the statistical software package SPSS version 22.0 (IBM Co., Ltd., New York, USA). Data were expressed as mean ± standard deviation (SD). Comparisons between groups were analyzed using single-factor analysis of variance, followed by the least significant difference test. Moreover, differences were considered statistically significant if the P < 0.05.
Results
Anti-Müllerian hormone levels in the serum of female mice
Concentrations of AMH in the serum of mice are presented in Figure 1. AMH levels in PM2.5 group were statistically decreased when compared to the levels in the control group (P < 0.001). The concentrations of AMH in PM2.5 + aspirin (P < 0.001), PM2.5 + Vitamin C (P < 0.001), PM2.5 + Vitamin E (P = 0.001), and PM2.5 + ozone (P = 0.002) groups were significantly increased compared to the levels in PM2.5 group. Moreover, interestingly, the changes of AMH concentrations in PM2.5 + aspirin (P = 0.407) and PM2.5 + Vitamin C (P = 0.051) groups were not significant relative to the control, whereas statistically significant differences were identified in PM2.5 + Vitamin E (P = 0.002) and PM2.5 + ozone (P = 0.002) groups when compared to the control group.
Figure 1.

Effects of different medications and ozone on the levels of serum AMH in PM2.5-treated female mice. Values are mean ± SD (n = 6/group). Using one-way ANOVA, followed by LSD multiple range test, comparing with control group, significant difference is indicated by *P < 0.001 and §P < 0.01. Comparing with PM2.5 group, significant difference is indicated by †P < 0.001 and ‡P < 0.01. PM2.5: Fine particulate matter; SD: Standard deviation; ANOVA: Analysis of variance; AMH: Anti-Müllerian hormone; LSD: Least significant difference.
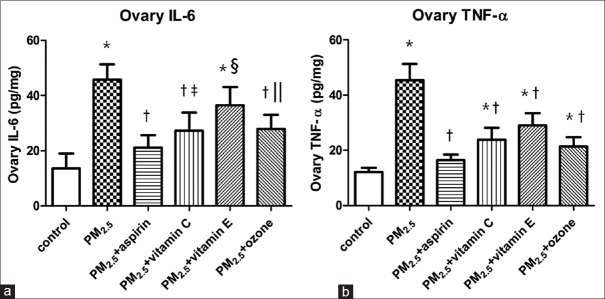
Changes in inflammatory cytokines in ovarian tissue
To observe the changes in ovarian inflammation, we measured IL-6 and TNF-α. As shown in Figure 2, we found significant increases in IL-6 and TNF-α levels in the PM2.5 group when compared to the levels in the control group (P < 0.001). The IL-6 and TNF-α levels in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups were statistically decreased when compared to the levels in PM2.5 group (P < 0.001 or P < 0.05). In addition, there were no statistically significant differences of IL-6 and TNF-α concentrations between PM2.5 + aspirin group and control group (P = 0.086 and P = 0.061, respectively). However, significant differences were observed in PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups when compared to the control group (P < 0.001 or P < 0.05).
Figure 2.
Effects of different medications and ozone on the levels of ovarian inflammation in PM2.5-treated mice. (a) The concentrations of IL-6 in ovarian tissue of female mice. (b) The concentrations of TNF-α in ovarian tissue of female mice. Values are mean ± SD (n = 6/group). Using one-way ANOVA, followed by LSD multiple range test, comparing with control group, significant difference is indicated by *P < 0.001, ‖P < 0.01 and ‡P < 0.05. Comparing with PM2.5 group, significant difference is indicated by †P < 0.001 and §P < 0.05. SD: Standard deviation; ANOVA: Analysis of variance; PM2.5: Fine particulate matter; TNF-α: Tumor necrosis factor-α; IL: Interleukin; LSD: Least significant difference.
Changes of oxidative stress in ovarian tissue
The concentrations of 8-OHdG, a marker of oxidative stress, were determined to observe the changes of oxidative stress in ovarian. 8-OHdG concentrations are presented in Figure 3. As shown in Figure 3, 8-OHdG concentrations were significantly higher in PM2.5 group than that of the control group (P < 0.001). In addition, the 8-OHdG levels in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups were statistically lower as compared to the levels in PM2.5 group (P < 0.001). In addition, compared to the control group, significant differences were observed in PM2.5 + Vitamin E (P = 0.001) and PM2.5 + ozone (P < 0.001) groups, while no statistically significant differences were observed in PM2.5 + aspirin (P = 0.087) and PM2.5 + Vitamin C (P = 0.058) groups.
Figure 3.
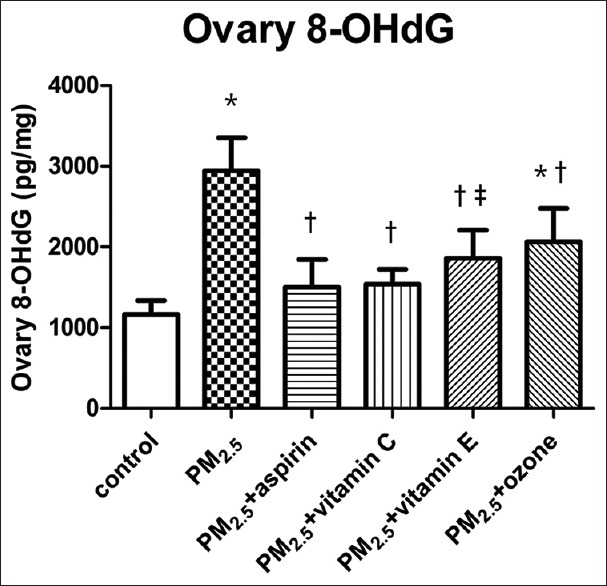
Effects of different medications and ozone on the levels of ovarian 8-OHdG in PM2.5-treated mice. Values are mean ± SD (n = 6/group). Using one-way ANOVA, followed by LSD multiple range test, comparing with control group, significant difference is indicated by *P < 0.001 and ‡P < 0.01. Comparing with PM2.5 group, significant difference is indicated by †P < 0.001. SD: Standard deviation; ANOVA: Analysis of variance; 8-OHdG: 8-hydroxy-2’-deoxyguanosine; PM2.5: Fine particulate matter; LSD: Least significant difference.
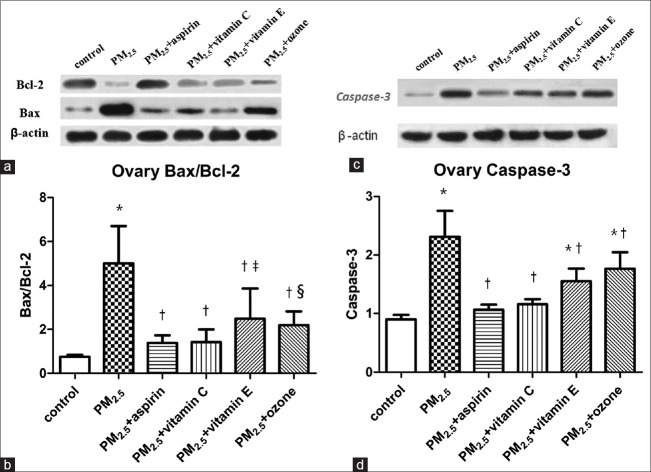
Changes of apoptotic regulatory protein expression in ovaries
Western blotting analysis was used to analyze the expressions of antiapoptotic protein Bcl-2 and apoptosis-promoting protein Bax and caspase-3 in ovarian tissues of both groups. As the results shown in Figure 4, proapoptotic Bax protein increased, and antiapoptotic Bcl-2 protein decreased significantly in PM2.5 group compared to control group while the increases of Bax protein and the decreases of Bcl-2 in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups were slightly [Figure 4a]. The Bax/Bcl-2 ratio showed an significant increase in PM2.5 group (P < 0.001) as compared to control group and the statistically significance decreases were observed in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups compared to PM2.5 group (P < 0.001). Moreover, the ratios of Bax/Bcl-2 in PM2.5 + aspirin (P = 0.256) and PM2.5 + Vitamin C (P = 0.242) groups were not significant relative to the control, while statistically significant differences were identified in PM2.5 + Vitamin E (P = 0.004) and PM2.5 + ozone (P = 0.016) groups when compared to the control group [Figure 4b]. In addition, as shown in [Figure 4c] and [Figure 4d], the expression of apoptosis-promoting protein caspase-3 in PM2.5 group showed a significant increase as compared to control group (P < 0.001), and comparing with PM2.5 group, significant decreases were identified in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups (P < 0.001). Nevertheless, no significant differences were found between PM2.5 + aspirin (P = 0.24), PM2.5 + Vitamin C (P = 0.069), and control group.
Figure 4.
Effects of medications and ozone on the expression of ovarian apoptotic regulatory proteins in PM2.5-treated mice. (a) Images of Western blot for Bcl-2 and Bax. (b) Comparisons of Bax/Bcl-2 ratio. (c) Images of Western blot for caspase-3. (d) Comparisons of the expression of caspase-3. Values are mean ± SD. Using one-way ANOVA, followed by LSD multiple range test, comparing with control group, significant difference is indicated by *P < 0.001, ‡P < 0.01 and §P < 0.05. Comparing with PM2.5 group, significant difference is indicated by †P < 0.001. SD: Standard deviation; ANOVA: Analysis of variance; LSD: Least significant difference; PM2.5: Fine particulate matter.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Apoptosis in ovarian follicles was assessed in histological sections by TUNEL assay. TUNEL-positive staining was observed in the ovarian tissue of both groups. As shown in Figure 5, ovarian tissue in groups of control, PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E and PM2.5 + ozone were stained with light brown color. However, a deeper brown color appeared in PM2.5 group, which suggested that the number of apoptotic cells in ovarian tissue from the PM2.5 group was higher than that of other groups.
Figure 5.
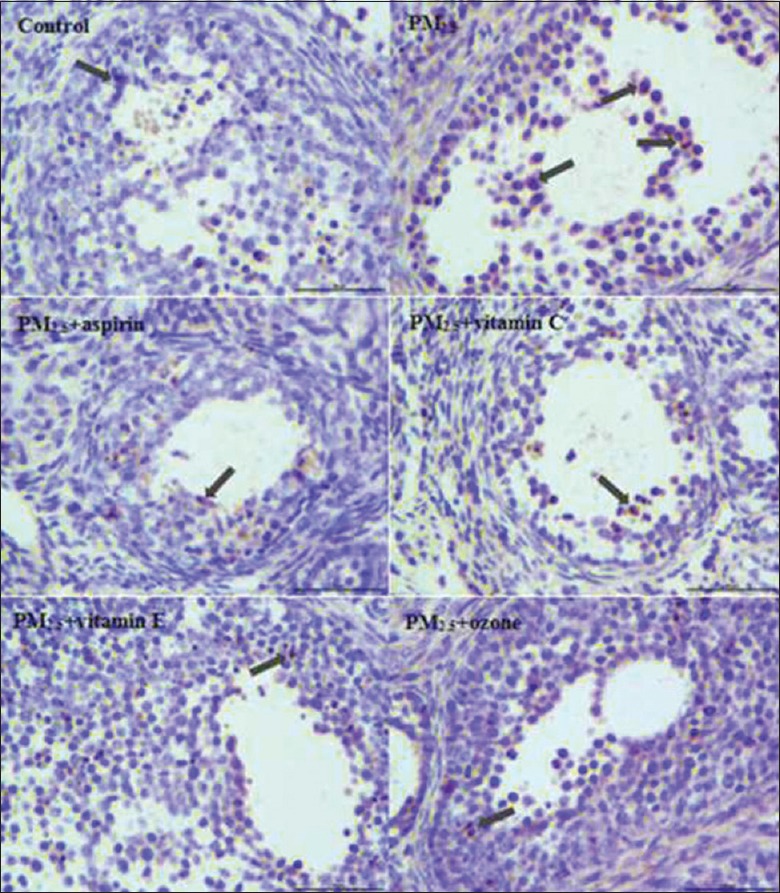
Apoptotic cells were determined by TUNEL staining in the ovarian tissues (original magnification, ×400). TUNEL-positive cells were found in the granulosa cells (black arrows). TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
Changes of ovarian histopathologic and follicle counting
To understand the effects of aspirin, Vitamin C, Vitamin E, and ozone after exposure to PM2.5, changes in the histological structure of the ovary were examined. As shown in Figure 6, ovarian histological structures were damaged after exposure to PM2.5 when compared to the control group that can be seen as hemorrhage and vascular congestion in ovarian structures. However, after treatment with aspirin, Vitamin C, Vitamin E, and ozone, the damage in ovarian histological structures was statistically decreased.
Figure 6.

Comparison of the ovarian appearance changes in mice of different groups under light microscopy (hematoxylin and eosin staining, original magnification, ×100). The normal ovarian histological structures were observed in control group. Hemorrhage (thin arrow) and vascular congestion (star) were found in PM2.5 group. However, in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups, the changes in hemorrhage (thin arrow) and vascular congestion (star) were decreased compared to the PM2.5 group. PM2.5: Fine particulate matter.
Changes in ovarian ultrastructure
In all groups, EM was used to detect the ovarian tissue ultramicrostructure to observe the damage to mitochondria in the ovarian tissue. Comparisons of the ultrastructural changes of mitochondria in mice ovarian tissue in different groups are depicted in Figure 7. Control groups displayed normal ultrastructural features of mitochondria. As shown in Figure 7 (control), the individual mitochondria morphology was intact and the outer membrane was smooth, and the mitochondrial cristae were arranged regularly. In the PM2.5 group, the mitochondria swelled, mitochondrial membrane appeared impaired, mitochondrial cristae were arranged in a chaotic manner and occasionally disappeared, and mitochondria showed vacuolization. However, after treatment with aspirin, Vitamin C, Vitamin E, or ozone, the mitochondrial membranes maintained their integrity, mitochondrial cristae could still be seen and demonstrated relatively regular arrangement, although the mitochondria exhibited slight swelling. And meanwhile, the level of vacuolization in the mitochondria was less than that in PM2.5 group.
Figure 7.
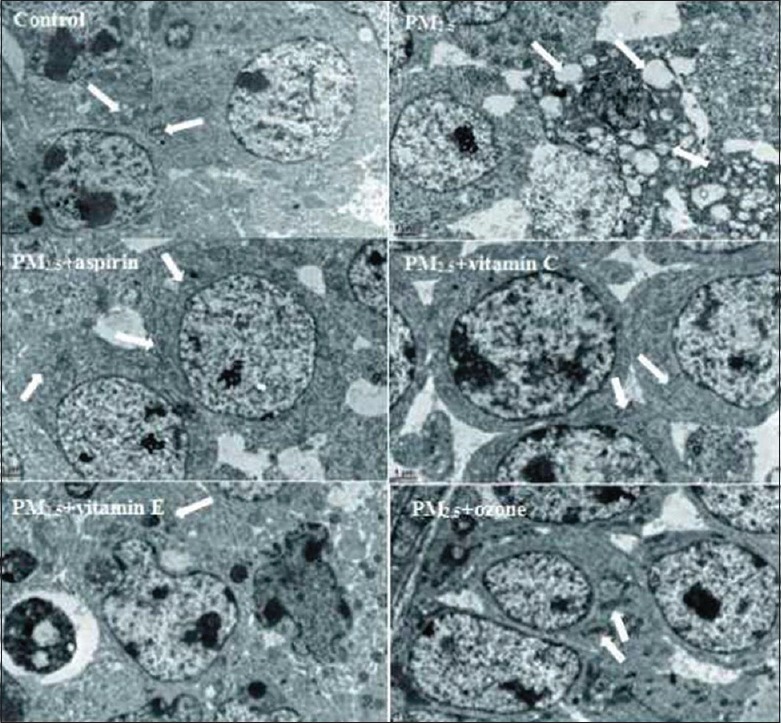
Comparison of the ultrastructural alterations in mitochondria in ovarian tissues in mice of different groups (original magnification ×10,000). The normal ovarian ultramicrostructures were observed in control group. In PM2.5 group, arrows indicate the abnormal mitochondria with swollen mitochondria, absence of cristae and vacuolization. In addition, in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups, although mild mitochondrial swelling and irregularly arrayed cristae were still observed, the alterations were decreased compared to the PM2.5 group. PM2.5: Fine particulate matter.
Discussion
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian inflammation in mice treated with fine particulate matter
Previous studies reported that PM2.5 could induce acute systemic inflammatory responses.[22] Aspirin is a classical anti-inflammatory drug, and previous studies have shown that ozone has obvious anti-inflammatory effects.[23] Furthermore, a study by Dhalla et al.[24] reported that oxidative stress could initiate the inflammatory process to cause tissue damage. Since both Vitamins C and E exhibit antioxidant properties, we speculated that they might blunt the inflammation caused by PM2.5 through their antioxidant abilities. To observe the protective effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian inflammation after the exposure of PM2.5, we measured inflammatory cytokines IL-6 and TNF-α in ovarian tissue. IL-6 might stimulate the production of neutrophils and the proliferation of B-lymphocytes, while TNF-α is a multifunctional cytokine that might alter the normal barrier function of the endothelium and thereby increase the permeability of the same.[25]
The results of the present experiments showed that PM2.5 exposure increased the levels of IL-6 and TNF-α in ovarian tissue in mice, and aspirin, Vitamin C, Vitamin E, and ozone all alleviated the inflammatory response. In addition, the morphological changes caused by inflammation, in the PM2.5 group, we observed hemorrhage, vascular congestion, and mitochondrial structural changes, such as swelling, membrane impairment, disordered arrangement of cristae, and vacuolization. However, after the administration of any one of aspirin, Vitamin C, Vitamin E, or ozone, the pathological changes were ameliorated. All results demonstrated that the significant ovarian inflammation caused by PM2.5 was reduced through the use of experimental anti-inflammatory agents.
This suggests that exposure to PM2.5 results in an inflammatory response that is related to ovarian injury and appears to be the most likely mechanism for ovarian injury. Meanwhile, the anti-inflammatory effects of aspirin, Vitamin C, Vitamin E, or ozone may be involved in the protection from this exposure.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian oxidative stress in mice treated with fine particulate matter
Dhalla et al.[24] reported that oxidative stress may play important roles in PM2.5-induced injures. Exposure to PM2.5 leads to an imbalance of prooxidants and antioxidants in the cellular environment, which in turn causes an increase in the generation of reactive oxygen species. These oxygen free radicals initiate the lipid peroxidation process by attacking membranes resulting in cellular injury, including apoptosis and increased membrane permeability in cells and mitochondria.[26] Therefore, interventions that enhance antioxidative properties may contribute to the protection of ovarian tissue, by reducing ovarian apoptosis and thereby improving the preservation of ovarian reserve.
Vitamins C and E are widely used as antioxidative agents and are known to have minimal side effects. The protective effects of Vitamins C and E on oxidative damages have been reported in a previous study.[27] Similarly, aspirin and ozone also have antioxidant properties in addition to their anti-inflammatory effects.[10,23,28] However, their protective function against PM2.5-induced damages has never been clarified. Moreover, aspirin, Vitamin C, Vitamin E, and ozone are attractive from the clinical point of view because of their low cost and minimal side effects.
Reactive oxygen species give rise to the formation of DNA-protein crosslinks, strand breakage, and alteration of the purine and pyrimidine bases.[29] 8-OHdG is an excision repair product of oxidative DNA damage and is one of the most common biomarkers of oxidative damage in DNA.[30] Therefore, ovarian tissue 8-OHdG concentrations were measured as an indicator of oxidative stress in the present study.
Our data showed that aspirin, Vitamin C, Vitamin E, and ozone were able to restrict the oxidative stress caused by PM2.5. This study suggested that increased 8-OHdG levels were consistently relieved by aspirin, Vitamin C, Vitamin E, and ozone treatment. In addition, after treatment with aspirin and Vitamin C, the levels of 8-OHdG reach the same levels as the control, which indicates that the antioxidant effect of aspirin and Vitamin C is better than Vitamin E and ozone in PM2.5-induced oxidative damage. Meanwhile, the number of apoptotic cells in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, and PM2.5 + ozone groups decreased significantly compared to PM2.5 group, which revealed that Vitamin C, Vitamin E, aspirin, and ozone were able to reduce the apoptosis of ovarian cells presumably by restricting the oxidative stress following exposure to PM2.5. Moreover, we demonstrated that antioxidative protection translates to better preservation in ovarian reserve, measured as smaller decreases in AMH concentrations.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on serum anti-Müllerian hormone in mice treated with fine particulate matter
AMH is a dimeric glycoprotein of the transforming growth factor-superfamily and exclusively produced by granulosa cells of small follicles in the ovary.[31] Several studies have validated AMH as an early, reliable, and clinically useful biomarker of ovarian reserve.[32,33,34] Compared to other endocrine markers of ovarian reserve such as serum concentrations of follicle-stimulating hormone (FSH), inhibin B, and estradiol, the serum AMH concentration seems to be a more accurate predictor as it offers several advantages;[35] it is the only marker that was thought to be stable throughout the phases of the menstrual cycle and it is relatively unaffected following hormonal therapy;[36,37,38] however, FSH, inhibin B, and estradiol are more dependent on cycle stage. In addition, AMH is detectable in the peripheral circulation;[39] therefore, it is considered a noninvasive method of evaluating ovarian reserve. Thus, in the present study, serum AMH concentrations are used as a hormonal indicator of ovarian reserve.
The results of our study showed that serum concentrations of AMH in the PM2.5 group were significantly lower than control group. However, after treatment with aspirin, Vitamin C, Vitamin E, or ozone, the levels of AMH significantly increased compared to the PM2.5 group, with no significant differences found between PM2.5 + aspirin, PM2.5 + Vitamin C, and the control group. These results indicated that PM2.5 can cause a decrease in ovarian reserve, and that treatment with aspirin, Vitamin C, Vitamin E, or ozone could ameliorate this damage. As reported previously, smoking is associated with a more rapid decline in AMH levels compared to nonsmokers.[40,41] These results indicate that aspirin, Vitamin C, Vitamin E, and ozone are able to improve the ovarian reserve to different degrees. Further, the mechanism may be related to either or both the anti-inflammatory and antioxidant effects of these agents.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian apoptosis in mice treated with fine particulate matter
Apoptosis is a biologically functional model of cell suicide essential for both development and maintenance of tissue homeostasis, and that is regulated by the dynamic balance between proapoptotic factors and antiapoptotic factors. To investigate the protective mechanism of these interventions on PM2.5-induced lesions, we stained the ovarian cells by TUNEL to measure apoptosis. The results showed that TUNEL-positive cell numbers in PM2.5 treatment groups were higher than that in the control group and PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, or PM2.5 + ozone groups.
In addition, these findings correlated well with the morphological changes observed under the microscope. Mice treated with aspirin, Vitamin C, Vitamin E, or ozone, when exposed to PM2.5, had reduced numbers of apoptotic cells compared to the mice exposed to PM2.5. This suggests that apoptosis might contribute to the lesion in ovaries following exposure to PM2.5 and antiapoptosis might be the protective mechanism displayed by aspirin, Vitamin C, Vitamin E, or ozone. Moreover, previous studies have demonstrated that oxidative stress can induce apoptosis by a mitochondrial pathway.[42] Therefore, the apoptosis of ovarian cells observed in the PM2.5 group in this study may be related to the oxidative damage.
It is well known that caspases are a family of intracellular cysteine proteases that act as proapoptotic factors that play an important role in the initiation and execution of apoptosis in mammalian cells. Among the caspases, caspase-3 is ultimately responsible for most apoptotic processes.[43] To examine whether caspase-3 is involved in PM2.5-induced apoptosis, the expression of caspase-3 in ovarian cells was measured. The present results showed that PM2.5 markedly increased the expression of caspase-3 in a manner that might be relevant to PM2.5-induced apoptosis in ovarian cells. And interestingly, we have also observed that the expression of caspase-3 in PM2.5 + aspirin, PM2.5 + Vitamin C, PM2.5 + Vitamin E, or PM2.5 + ozone group was significantly decreased compared to the PM2.5 group, which indicates that aspirin, Vitamin C, Vitamin E, or ozone might have protective effects on PM2.5-induced apoptosis in ovarian cells. These results also suggest that PM2.5-induced apoptosis was dependent on caspase-3 activation, and aspirin, Vitamin C, Vitamin E, or ozone can decrease PM2.5-induced apoptosis of ovarian cells by reducing the activity of caspase-3.
Many studies have demonstrated that the mitochondrial pathway is the most common mechanism of apoptosis and constitutes the core part of the apoptotic pathway in mammalian cells.[44] However, the integrity of the mitochondrial membranes is regulated by the Bcl-2 family of proteins.[45] The Bcl-2 family of proteins can be separated into antiapoptotic and proapoptotic members, including the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax. In this study, we found that PM2.5 enhanced the expression of the proapoptotic protein Bax and significantly decreased that of the antiapoptotic Bcl-2, resulting in a marked increase of the Bax/Bcl-2 ratio. Moreover, after treatment with aspirin, Vitamin C, Vitamin E, or ozone, the expression levels of Bax were reduced when compared to the PM2.5 group, while levels of Bcl-2 were increased which resulted in a decrease in the ratio of Bax/Bcl-2. The imbalance of Bax and Bcl-2 expressions and increased Bax/Bcl-2 ratios observed in these experiments are consistent with enhanced apoptosis in the PM2.5 group. In addition, these results suggest that aspirin, Vitamin C, Vitamin E, or ozone can reduce the apoptosis of ovarian cells by decreasing the ratio of Bax/Bcl-2. Obviously, a reduced apoptosis rate of ovarian cells could decrease the reproductive toxicity caused by PM2.5.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian histopathology in mice treated with fine particulate matter
The protective effects of aspirin, Vitamin C, Vitamin E, and ozone on PM2.5 injury to the ovary were also demonstrated by histopathology. Our histological findings support our biochemical and molecular findings. Ovarian damage following exposure to PM2.5 was demonstrated by hemorrhage, vascular congestion, and follicular degeneration. These results provided a strong pathological basis for the lower concentration of serum AMH in the PM2.5 group. However, the administration of aspirin, Vitamin C, Vitamin E, or ozone protected ovaries from injuries induced by PM2.5, as shown in our histopathological results. The comparison of the protective effects of aspirin, Vitamin C, Vitamin E, or ozone demonstrated that the administration of aspirin and Vitamin C provided better protective effects and resulted in lesser histopathological damage to the ovary.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian ultrastructure in mice treated with fine particulate matter
Results of the present study showed that some abnormal ultrastructural alterations in the ovarian mitochondria such as swelling, cristae disorder, and vacuolation occurred after exposure of PM2.5. However, the ovarian mitochondria of the mice treated with aspirin, Vitamin C, Vitamin E, or ozone appeared to be normal. This protective effect was demonstrated by the mitochondrial cristae reappearing and the internal structure of the microtubules maintaining their integrity, indicating that treatment with aspirin, Vitamin C, Vitamin E, or ozone ameliorated the damages of PM2.5. These ultrastructural damage results hint that PM2.5 exposure induces strong ultrastructural changes in mitochondria, which subsequently leads to mitochondrial dysfunction. As it is reported in the literature, PM exposure could induce significant decreases in mitochondrial membrane potential and impaired oxidative phosphorylation.[46] Further, this also suggests that PM2.5 exposure causes mitochondrial damage linked to the mitochondrial dysfunction and the abnormal alterations in mitochondrial morphology. Aspirin, Vitamin C, Vitamin E, and ozone protected the structural integrity of mitochondria so that they could maintain their normal function and ultimately preventing reproductive toxicity caused by PM2.5.
Effects of aspirin, Vitamin C, Vitamin E, and ozone on ovarian function in mice treated with fine particulate matter
In animals, PM2.5 exposure caused ovarian tissues inflammation and oxidative stress reactions while the protective effects of aspirin, Vitamin C, Vitamin E, and ozone are clear; however, whether the results of the present study can be applied to humans is not clear. Furthermore, our experiment is the only study at the animal level, and therefore, there is a need to strengthen the epidemiological investigations to confirm our findings.
In this experimental study, compared to Vitamin C, Vitamin E, and ozone, the protective effect of aspirin is better than other interventional measures. Reasons may be that aspirin is not only a very good anti-inflammatory agent but also has a strong antioxidant effect – the two main mechanisms for damage caused by PM2.5. In addition, aspirin is a drug with a history of more than two thousand years and has proved to have anti-inflammatory, anticancer,[47] anticoagulation,[48] and antirheumatic[49] effects. This study represents the first demonstration that aspirin can significantly reduce ovarian tissue damages caused by PM2.5.
Vitamins C and E are two of the essential vitamins for humans, and Vitamin E is often used in the protection of the reproductive system. However, in this experiment, its protective effect on reproductive toxicity caused by PM2.5 was inferior to aspirin. The reason may be that Vitamin E appears to only work through the mechanism of resistance to oxidative stress.
Ozone as a new treatment method has several studies that confirmed its obvious anti-inflammatory and antioxidant effects, but in this study, the protective effects in PM2.5 + aspirin group and PM2.5 + Vitamin C group were significantly better. This may indicate that we did not use the optimum concentration of ozone to protect fromPM2.5-induced injuries; and of course, there is a need for further research.
One limitation of this study was the exposure mode of PM2.5. In the present study, we used intratracheal instillation to perform the PM2.5 exposure, which is clearly not a physiological route of delivery for humans. However, intratracheal instillation can get more accurate exposure dose of PM2.5. Another limitation of this study was the short exposure durations and large particle concentrations, while in real life in chronic exposure, there would be lower concentrations for a longer period. This likely does not represent the spatial and temporal variability of the process during daily human contact. Furthermore, we also did related research of PM2.5 impact on reproductive axis, but unsuccessful, of course further researches are needed. In addition, in the present study, we only examined the mechanisms of inflammation and oxidative stress, and there may be alternative existing mechanisms yet to be elucidated. Despite these limitations, our study included a carefully standardized experimental condition and the instilled dose of particles in our study was strictly calculated per previous studies.
In conclusion, evidence from this study provides strong biochemical and histopathological support that PM2.5 could cause the damage of ovaries, and aspirin, Vitamin C, Vitamin E, and ozone reduced the damage and ameliorated the changes in ovarian structure caused by PM2.5 and may be useful in protecting the ovaries from PM2.5-induced damage in humans. Moreover, aspirin and Vitamin C have shown to be more effective in reducing tissue damage compared to treatment with Vitamin E and ozone. The protective effect of aspirin, Vitamin C, Vitamin E, and ozone is probably due to its ability to blunt the inflammatory and oxidative stress caused by ambient PM2.5, which subsequently suppressing the expression of apoptotic regulatory protein in ovary and reduce the incidence of ovary apoptosis. These results indicate that moderate intake of aspirin, Vitamin C, Vitamin E, or ozone is warranted when exposure to PM2.5 is unavoidable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet. 2016;388:696–704. doi: 10.1016/S0140-6736(16)00378-0. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58. doi: 10.1056/NEJMoa054409. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 3.Hamra GB, Guha N, Cohen A, Laden F, Raaschou-Nielsen O, Samet JM, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect. 2014;122:906–11. doi: 10.1289/ehp/1408092. doi: 10.1289/ehp.1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samoli E, Stafoggia M, Rodopoulou S, Ostro B, Alessandrini E, Basagaña X, et al. Which specific causes of death are associated with short term exposure to fine and coarse particles in Southern Europe? Results from the MED-PARTICLES project. Environ Int. 2014;67:54–61. doi: 10.1016/j.envint.2014.02.013. doi: 10.1016/j.envint.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Hammoud A, Carrell DT, Gibson M, Sanderson M, Parker-Jones K, Peterson CM. Decreased sperm motility is associated with air pollution in Salt Lake City. Fertil Steril. 2010;93:1875–9. doi: 10.1016/j.fertnstert.2008.12.089. doi: 10.1016/j.fertnstert.2008.12.089. [DOI] [PubMed] [Google Scholar]
- 6.Zhou N, Cui Z, Yang S, Han X, Chen G, Zhou Z, et al. Air pollution and decreased semen quality: A comparative study of Chongqing urban and rural areas. Environ Pollut. 2014;187:145–52. doi: 10.1016/j.envpol.2013.12.030. doi: 10.1016/j.envpol.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Grahame TJ, Schlesinger RB. Oxidative stress-induced telomeric erosion as a mechanism underlying airborne particulate matter-related cardiovascular disease. Part Fibre Toxicol. 2012;9:21. doi: 10.1186/1743-8977-9-21. doi: 10.1186/1743-8977-9- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, Zin WA. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. 2010;26:481–98. doi: 10.1007/s10565-010-9158-2. doi: 10.1007/s10565-010-9158-2. [DOI] [PubMed] [Google Scholar]
- 9.Grosser N, Schröder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol. 2003;23:1345–51. doi: 10.1161/01.ATV.0000083296.57581.AE. doi: 10.1161/01.ATV.0000083296.57581.AE. [DOI] [PubMed] [Google Scholar]
- 10.Wu R, Lamontagne D, de Champlain J. Antioxidative properties of acetylsalicylic acid on vascular tissues from normotensive and spontaneously hypertensive rats. Circulation. 2002;105:387–92. doi: 10.1161/hc0302.102609. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich M, Block G, Hudes M, Morrow JD, Norkus EP, Traber MG, et al. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:7–13. [PubMed] [Google Scholar]
- 12.Johnston CS, Martin LJ, Cai X. Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. J Am Coll Nutr. 1992;11:172–6. [PubMed] [Google Scholar]
- 13.Moriguchi S, Muraga M. Vitamin E and immunity. Vitam Horm. 2000;59:305–36. doi: 10.1016/s0083-6729(00)59011-6. [DOI] [PubMed] [Google Scholar]
- 14.Al-Malki AL, Moselhy SS. Protective effect of Vitamin E and epicatechin against nicotine-induced oxidative stress in rats. Toxicol Ind Health. 2013;29:202–8. doi: 10.1177/0748233711430976. doi: 10.1177/0748233711430976. [DOI] [PubMed] [Google Scholar]
- 15.Gumustekin K, Taysi S, Alp HH, Aktas O, Oztasan N, Akcay F, et al. Vitamin E and Hippophea rhamnoides L. extract reduce nicotine-induced oxidative stress in rat heart. Cell Biochem Funct. 2010;28:329–33. doi: 10.1002/cbf.1663. doi: 10.1002/cbf.1663. [DOI] [PubMed] [Google Scholar]
- 16.Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol Appl Pharmacol. 2006;216:493–504. doi: 10.1016/j.taap.2006.06.009. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Bocci V, Borrelli E, Travagli V, Zanardi I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29:646–82. doi: 10.1002/med.20150. doi: 10.1002/med.20150. [DOI] [PubMed] [Google Scholar]
- 18.Hernández F, Menéndez S, Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radic Biol Med. 1995;19:115–9. doi: 10.1016/0891-5849(94)00201-t. [DOI] [PubMed] [Google Scholar]
- 19.Travagli V, Zanardi I, Silvietti A, Bocci V. A physicochemical investigation on the effects of ozone on blood. Int J Biol Macromol. 2007;41:504–11. doi: 10.1016/j.ijbiomac.2007.06.010. doi: 10.1016/j.ijbiomac.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Bocci V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine. Br J Biomed Sci. 1999;56:270–9. [PubMed] [Google Scholar]
- 21.Bai R, Zhang L, Liu Y, Meng L, Wang L, Wu Y, et al. Pulmonary responses to printer toner particles in mice after intratracheal instillation. Toxicol Lett. 2010;199:288–300. doi: 10.1016/j.toxlet.2010.09.011. doi: 10.1016/j.toxlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Behbod B, Urch B, Speck M, Scott JA, Liu L, Poon R, et al. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup Environ Med. 2013;70:761–7. doi: 10.1136/oemed-2013-101498. doi: 10.1136/oemed-2013-101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamora ZB, Borrego A, López OY, Delgado R, González R, Menéndez S, et al. Effects of ozone oxidative preconditioning on TNF-alpha release and antioxidant-prooxidant intracellular balance in mice during endotoxic shock. Mediators Inflamm. 2005;2005:16–22. doi: 10.1155/MI.2005.16. doi: 10.1155/MI.2005.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 25.Brett J, Gerlach H, Nawroth P, Steinberg S, Godman G, Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989;169:1977–91. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–64. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özkaya D, Naziroglu M, Armagan A, Demirel A, Köroglu BK, Çolakoglu N, et al. Dietary Vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct. 2011;29:287–93. doi: 10.1002/cbf.1749. doi: 10.1002/cbf.1749. [DOI] [PubMed] [Google Scholar]
- 28.Ayyadevara S, Bharill P, Dandapat A, Hu C, Khaidakov M, Mitra S, et al. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal. 2013;18:481–90. doi: 10.1089/ars.2011.4151. doi: 10.1089/ars.2011.4151. [DOI] [PubMed] [Google Scholar]
- 29.Nagai R, Watanabe K, Wakatsuki A, Hamada F, Shinohara K, Hayashi Y, et al. Melatonin preserves fetal growth in rats by protecting against ischemia/reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J Pineal Res. 2008;45:271–6. doi: 10.1111/j.1600-079X.2008.00586.x. doi: 10.1111/j.1600-079X.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakae D, Kobayashi Y, Akai H, Andoh N, Satoh H, Ohashi K, et al. Involvement of 8-hydroxyguanine formation in the initiation of rat liver carcinogenesis by low dose levels of N-nitrosodiethylamine. Cancer Res. 1997;57:1281–7. [PubMed] [Google Scholar]
- 31.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RA, Nelson SM, Wallace WH. Measuring anti-Müllerian hormone for the assessment of ovarian reserve: When and for whom is it indicated? Maturitas. 2012;71:28–33. doi: 10.1016/j.maturitas.2011.11.008. doi: 10.1016/j.maturitas.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 34.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85. doi: 10.1093/humupd/dmt062. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 35.Hale GE, Burger HG. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract Res Clin Obstet Gynaecol. 2009;23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001. doi: 10.1016/j.bpobgyn.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM. Biomarkers of ovarian response: Current and future applications. Fertil Steril. 2013;99:963–9. doi: 10.1016/j.fertnstert.2012.11.051. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 37.Toner JP, Seifer DB. Why we may abandon basal follicle-stimulating hormone testing: A sea change in determining ovarian reserve using antimüllerian hormone. Fertil Steril. 2013;99:1825–30. doi: 10.1016/j.fertnstert.2013.03.001. doi: 10.1016/j.fertnstert.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Shah DK, Mejia RB, Lebovic DI. Effect of surgery for endometrioma on ovarian function. J Minim Invasive Gynecol. 2014;21:203–9. doi: 10.1016/j.jmig.2013.09.012. doi: 10.1016/j.jmig.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WH. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87. doi: 10.1093/molehr/gar059. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 40.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause. 2010;17:571–6. doi: 10.1097/gme.0b013e3181c7deba. doi: 10.1097/gme.0b013e3181c7deba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowers MR, McConnell D, Yosef M, Jannausch ML, Harlow SD, Randolph JF., Jr Relating smoking, obesity, insulin resistance, and ovarian biomarker changes to the final menstrual period. Ann N Y Acad Sci. 2010;1204:95–103. doi: 10.1111/j.1749-6632.2010.05523.x. doi: 10.1111/j.1749-6632.2010.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22. doi: 10.1007/s10495-007-0756-2. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 43.Tang XL, Yang XY, Jung HJ, Kim SY, Jung SY, Choi DY, et al. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull. 2009;32:1399–405. doi: 10.1248/bpb.32.1399. [DOI] [PubMed] [Google Scholar]
- 44.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13. doi: 10.1016/j.bbamcr.2003.10.016. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Marchini T, Magnani N, D’Annunzio V, Tasat D, Gelpi RJ, Alvarez S, et al. Impaired cardiac mitochondrial function and contractile reserve following an acute exposure to environmental particulate matter. Biochim Biophys Acta. 2013;1830:2545–52. doi: 10.1016/j.bbagen.2012.11.012. doi: 10.1016/j.bbagen.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Zong M, Fan DD, Lin S, Song YP, Wang ZY, Ma XL, et al. Anti-cancer activity and potential mechanism of a novel aspirin derivative. Eur J Pharmacol. 2016;791:137–46. doi: 10.1016/j.ejphar.2016.07.050. doi: 10.1016/j.ejphar.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 48.Massimi I, Lotti LV, Temperilli F, Mancone M, Sardella G, Calcagno S, et al. Enhanced platelet MRP4 expression and correlation with platelet function in patients under chronic aspirin treatment. Thromb Haemost. 2016;116:1100–10. doi: 10.1160/TH16-04-0316. doi: 10.1160/TH16-04-0316. [DOI] [PubMed] [Google Scholar]
- 49.Pasero G, Marson P. A short history of anti-rheumatic therapy. II. Aspirin. Reumatismo. 2010;62:148–56. doi: 10.4081/reumatismo.2010.148. [DOI] [PubMed] [Google Scholar]