Abstract
Objective:
This study was designed to compare the severity of gastrointestinal (GI) side effects in Type 2 diabetes mellitus (DM) patients receiving tablet or capsule forms of metformin.
Methods:
In this prospective interventional study, patients were evaluated from June to November 2016 at DM clinics affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. Adult patients with Type 2 DM who were eligible for inclusion criteria switched from metformin tablet to metformin capsule. Hemoglobin A1c (HbA1c), GI side effects, and patient satisfaction based on visual analog scale (VAS) were assessed during a 6-week follow-up of receiving metformin capsule.
Findings:
One hundred and three patients were evaluated, and 75 patients participated in this study. At the baseline, 40 patients (53.3%) had GI side effects due to metformin tablet which was reduced to 16 patients (21.3%) after switching to metformin capsule (P = 0.001). There was also an improvement in HbA1c (from 7 to 6.8,P < 0.0001). The results of patients’ satisfaction based on VAS and numeric rating scale indicated that in 59 patients (78.67%), GI side effects were reduced after switching to metformin capsule (mean score: 7.2 with the range of 6–9) while 16 patients stated no treatment preference.
Conclusion:
Switching to metformin capsule may result in less GI side effects, with no further side effect complications.
KEYWORDS: Gastrointestinal side effects, Metformin capsule, Metformin tablet, type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (DM) is a chronic and progressive disease, which needs strict monitoring and control from diagnosis and also through different stages of the disease.[1,2] High prevalence of this disorder imposes a significant burden to the global health, and thus, numerous efforts have been utilized for both prevention and management of this worldwide concern.[3,4]
Metformin, a biguanide antihyperglycemic medication,[5] is still considered as the best medication and the first line of treatment either as monotherapy or in combination with other drugs for Type 2 DM due to its efficacy, very low risk of hypoglycemia and weight gain, and low cost.[1,6,7,8,9,10,11] It also has very low risk for including lactic acidosis as a side effect.[12]
Gastrointestinal (GI) side effects are the main complaint of patients receiving metformin tablet. The most common GI side effects associated with metformin are diarrhea, nausea, and vomiting (with a prevalence of 2%–63% according to the recent literature)[6,10,11] which occur more than all other oral antidiabetic agents. Although these GI side effects often diminish over time and can be minimized by careful dose adjustment and taking metformin at mealtimes,[6,10] they may impair compliance and cause approximately 5% of patients to discontinue therapy.[10,11]
In this study, we aimed to compare the patients’ satisfaction and GI side effects of oral metformin in either dosage forms of tablet or capsule.
METHODS
This prospective interventional study was conducted from June to November 2016 at DM clinics affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. The study obtained approval from the Research Council and Ethics Committee of Isfahan University of Medical Sciences (No. 395013).
At first, patients diagnosed with Type 2 DM who were treated by metformin tablet alone or in combination with other oral antidiabetic drugs were invited to participate in the study. Then, the participants were selected by the following criteria.
Components if the criteria by which the patients were selected consist of adult (age >18 years), Type 2 DM patients, with 6.5%< hemoglobin A1c (HbA1c) <9.5%. Criteria of noninclusion are patients being treated with insulin, had received systemic corticosteroids during recent 3 months, chronic gastroparesis or chronic severe GI symptoms, history of gastric or duodenal ulcers, any inadequately controlled or untreated cardiovascular, hepatic, pulmonary, renal, or neurologic conditions, glomerular filtration rate <50 ml/min, pregnancy or plan of pregnancy.
After obtaining written informed consent from the selected patients, the data were recorded in a checklist including demographic information (date of birth, gender), body weight and height, laboratory test results (HbA1c, fasting blood sugar, creatinine), medical and surgical history, and relevant medication history. Furthermore, GI side effects before the study related to metformin tablet including diarrhea, nausea, vomiting, abdominal pain, and bloating were asked and mentioned in the checklist. All included patients received metformin capsule with the same amount of metformin tablet, previously which was kindly provided by Farabi Pharmaceuticals, Isfahan, Iran, on our request which contained 500 mg metformin powder in addition to 5 mg magnesium acetate inside the hard gelatin capsule (HGC) in conventional release formulation.
During the study, patients were excluded from the study if they needed insulin, had GI adverse event, could not tolerate metformin capsule, or wanted to leave the study voluntarily. Patients receiving metformin capsule were followed and evaluated every 1–2 weeks for a total of 6 weeks. Evaluations for each visit included self-monitoring blood glucose (SMBG) results, vital signs, severity of GI side effects and adverse events by completing the researcher-designed form which was taken from visual analog scale (VAS) and numeric rating scale (NRS),[13] including the severity of GI side effect rating from 1 (the least) to 10 (the most). HbA1c, SMBG results, GI tolerability, and adverse events were assessed at baseline and on completion of the study to compare with each other. Related forms were filled by interview and also appropriate laboratory measures. At the end of the study, patients’ satisfaction forms, the researcher-designed form which was retrieved from VAS and NRS[13] including rating from 1 (the worst) to 10 (the best), were completed to evaluate the acceptability of metformin capsule consumption over metformin tablet.
The data were analyzed using SPSS Corp. Released 2011. IBM Statistics for Windows, version 20.0. Armonk, NY, USA: IBM Corp. Normality of quantitative variables was evaluated by Shapiro–Wilk test; normal data were presented as mean ± standard deviation and non normal ones as median (interquartile range). Changes in HbA1c and GI side effects between two steps were evaluated by Wilcoxon-signed ranks and McNemar's test, respectively.
RESULTS
One hundred and three patients were evaluated for possible enrollment, of which 87 patients met the inclusion criteria. Over 6 weeks of follow-up, 12 patients withdrew the study voluntarily, 5 patients in the 2nd week, and 7 in the 4th week; the reasons were mostly social and economic ones arising from repeated attendance at the clinic. Data for 75 eligible patients were analyzed. Demographic characteristics of patients are summarized in Table 1. Sixty-four patients (85.33%) were on metformin alone and the others (14.67%) were on combination of metformin with other antihyperglycemic medications. The mean daily dose of metformin intake was 1000 mg (range, 500–3000 mg). The most common concurrent controlled illnesses were dyslipidemia (29.33%), hypertension (16%), hypothyroidism (16%), cardiovascular diseases (12%), osteoporosis (4%), and fatty liver (3%).
Table 1.
Characteristics and demographic view in type 2 diabetes mellitus patients before receiving Metformin capsule (n =75)

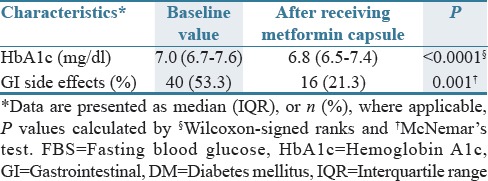
As seen in Table 2, 40 patients (53.3%) had GI side effects with persistent use of metformin tablet for 7.8 years in average (range, 1–30 years), including epigastric and abdominal pain, nausea, vomiting, diarrhea, and bloating. After receiving metformin capsule, about 16 patients who also had GI side effects with metformin tablet (21.3%, P = 0.001) reported GI side effects on metformin capsule, of whom none discontinued therapy. There was an improvement in HbA1c (from 7 to 6.8, P < 0.0001) after switching to metformin capsule. The result of patients’ satisfaction based on VAS and NRS[13] indicated that in 59 patients (78.67%), GI side effects were reduced after switching to metformin capsule (mean score: 7.2, with the range of 6–9) while 16 patients stated no treatment preference. None experienced worsening of GI side effects by metformin capsule.
Table 2.
Comparison of fasting blood glucose, hemoglobin A1c, and gastrointestinal symptoms in Type 2 diabetes mellitus patients at baseline and after switching to metformin capsule (n =75)
DISCUSSION
In this study, we observed significant reduction in GI side effects of the patients after changing metformin tablet to metformin capsule. To the best of our knowledge, this is the first study that evaluated the GI effects of metformin capsule dosage form. Patients received metformin capsule for 6 weeks without any changes in the previous dosage (mean dosage was 1000 mg/day). Our results indicated that switching to metformin capsule led to a greater patient satisfaction (78.67%). A significant 21.3% reduction in GI side effects was observed after metformin capsule therapy. Improvement of GI side effects may be due to delay in release of metformin in the stomach, resulting a reduction in stomach irritation and GI side effects. HbA1c was checked to evaluate the effect of metformin capsule on controlling blood sugar. We have shown that switching from metformin tablets to capsules was associated with a 0.96% reduction in HbA1c (7–6.8). This degree of decrement although statistically significant is not clinically important.
Some studies have shown taking metformin tablets at mealtimes, or using careful dose adjustment to minimize poor compliance with immediate release form of metformin[6,8] or switching to extended release form of metformin can reduce GI symptoms.[10,14] However, it was reported that some patients may discontinue therapy due to the higher medication costs. The disintegration time for HGCs is about 15 min.[15] Since metformin capsule used in this study provided efficacy and patient satisfaction, it may be considered a good alternative to the previous formulations, bearing in mind that the cost of therapy with this dosage form will not be much different from that with conventional tablets.
Our study had some limitations which included open-label design with no comparator, leaving a number of patients during the study due to repeated attendance at clinic, using simple questioning techniques rather than validated quality of life measures to record GI side effects and patients’ satisfaction, not evaluating GI side effects after switching the patients back to metformin tablet, and seeing if the time passing can improve GI compliance.
Further studies are therefore required to resolve these limitations with a large double-blinded study and to specify the exact time of drug release from capsules in GI tract to rationalize the better GI tolerability of metformin capsules.
Capsular form of metformin may result in decrement of GI side effects and intolerability in diabetic patients and may be an option for those that metformin is an appropriate medication for them but cannot tolerate it due to the mentioned side effects.
AUTHORS’ CONTRIBUTION
Mansour Siavash contributed in the project idea, study design, supervision, data analysis and manuscript preparation. Majid Tabbakhian contributed in the study design, drug evaluation, and manuscript preparation. Ali Mohammad Sabzghabaee contributed in the study design, drug evaluation, and manuscript preparation. Niloufar Razavi contributed in the study design, conduct, data analysis and manuscript preparation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes – 2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschner PM, Muñoz OM, Girón D, García OM, Fernández-Ávila DG, Casas LÁ, et al. Clinical practice guideline for the prevention, early detection, diagnosis, management and follow up of type 2 diabetes mellitus in adults. Colomb Med (Cali) 2016;47:109–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Paulweber B, Valensi P, Lindström J, Lalic NM, Greaves CJ, McKee M, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res. 2010;42(Suppl 1):S3–36. doi: 10.1055/s-0029-1240928. [DOI] [PubMed] [Google Scholar]
- 4.Velasco-Contreras ME. Evolution of the type 2 diabetes mellitus epidemia in insured population at the IMSS. Rev Med Inst Mex Seguro Soc. 2016;54:490–03. [PubMed] [Google Scholar]
- 5.Napolitano A, Miller S, Nicholls AW, Baker D, Van Horn S, Thomas E, et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e100778. doi: 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab. 2016 doi: 10.1111/dom.12854. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Burton JH, Johnson M, Johnson J, Hsia DS, Greenway FL, Heiman ML. Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and fasting glucose levels. J Diabetes Sci Technol. 2015;9:808–14. doi: 10.1177/1932296815577425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015;75:1071–94. doi: 10.1007/s40265-015-0416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes, 2015: A patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–42. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Han KA, Oh HJ, Tan KE, Sothiratnam R, Tjokroprawiro A, et al. Safety, tolerability, and efficacy of metformin extended-release oral antidiabetic therapy in patients with type 2 diabetes: An observational trial in Asia. J Diabetes. 2012;4:395–406. doi: 10.1111/j.1753-0407.2012.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–35. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haloob I, de Zoysa JR. Metformin associated lactic acidosis in Auckland City Hospital 2005 to 2009. World J Nephrol. 2016;5:367–71. doi: 10.5527/wjn.v5.i4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for pain (VAS pain), Numeric Rating Scale for pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–52. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med. 2011;123:15–23. doi: 10.3810/pgm.2011.01.2241. [DOI] [PubMed] [Google Scholar]
- 15.Stegemann S, Connolly P, Matthews W, Barnett R, Aylott M, Schrooten K, et al. Application of QbD principles for the evaluation of empty hard capsules as an input parameter in formulation development and manufacturing. AAPS PharmSciTech. 2014;15:542–9. doi: 10.1208/s12249-014-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


