Abstract
Objective:
This study was conducted to evaluate the combination of oral supplements with 2% minoxidil solution in four groups of women with hair loss.
Methods:
A prospective, randomized controlled trial was conducted from July to December 2016 in dermatology clinics affiliated to Isfahan University of Medical Sciences. A total of 73, 15–45-year-old, women with hair loss participated in this 4-month study. Simple randomization using Random Allocation Software was done to put the participants in four groups to receive coadministration of zinc sulfate and calcium pantothenate, zinc sulfate, calcium pantothenate, and 2% minoxidil solution. The primary endpoint was the change in hair density and diameter measured by dermatoscope. Secondary endpoints included the researcher's evaluation, dermatologist's opinion - which was blinded to the study - from comparing the participants’ photographs before and after treatment and finally, overall changes in hair density measured by participants’ self-assessment.
Findings:
Seventy-three women participated in this study. Primary hair count and thickness were 118.5 ± 10 hairs/cm2 and 58.8 ± 5.8 μ that changed to 124 ± 11 hairs/cm2 and 62.3 ± 4.3 μ respectively (P < 0.001) which in the zinc plus pantothenate group these changes were from 118.6 ± 9.9 hairs/cm2 to 121.9 ± 11.1 hairs/cm2 (P = 0.042) and from 62.2 ± 6.6 μ to 64.0 ± 5.0 μ (P = 0.126), respectively. Hair density increments were more obvious in the minoxidil group, and hair thickness increments were more obvious in pantothenate group. Participants’ satisfaction was 85% in the combination therapy which was more than other groups. Participants’ satisfaction, author's and blind dermatologist's opinion showed a significant correlation (P = 0.0001).
Conclusion:
Based on the participants’ satisfaction, the combination of zinc sulfate and calcium pantothenate when administered in a pulse therapy way could be a good choice for hair loss controlling in initial stages.
KEYWORDS: Androgenic alopecia, calcium pantothenate, female pattern baldness, Minoxidil, zinc sulfate
INTRODUCTION
Female pattern hair loss is described as a diffuse reduction in hair density which mainly affects crown and frontal scalp.[1,2,3] It is a distressing situation and affects self-esteem and self-image negatively.[4,5,6] Known common causes which lead to hair loss include medical conditions, such as hypothyroidism, polycystic ovarian disease, nutritional deficiencies, some medications, and physiological and emotional stresses.[7,8]
There are some medications which can improve hair growth; the best known and clinically validated medication approved for increasing hair growth is minoxidil solution which is used worldwide as a therapeutic option.[3,9,10,11] Although it is generally well-tolerated, patients may experience pruritus, dandruff and feeling greasy in their hair using this medication.[3,9] Minoxidil should be used lifelong, as its effects disappear by discontinuation.[12]
Certainly, nutrition affects hair condition, for instance, many studies have shown that administration of zinc sulfate and other multivitamins could improve hair growth.[11,13,14] However, daily administration of zinc sulfate, inhibit absorption of copper which is also needed for hair growth.[15] Pantothenic acid (Vitamin B5) is another nutrient that has been mentioned to influence hair growth and diameter.[16]
So far, the concomitant use of these drugs (zinc sulfate and pantothenic acid) as a pulse therapy to control hair loss has not been studied yet. In this way of administration, the patients could benefit advantages of both medications and copper, as well.[15]
We conducted a prospective, comparative, randomized controlled trial study with 4 months follow-up to evaluate participants’ satisfaction and efficacy of this treatment regimen in women with hair loss. It was aimed to determine the participants’ self-satisfaction and the efficacy of administration of both zinc sulfate and pantothenate as a pulse therapy regimen on hair loss in comparison with three other groups who received daily administration of zinc sulfate, pantothenate in the available form of calcium pantothenate and 2% minoxidil solution, respectively.
METHODS
This was a 4-month, prospective, randomized controlled trial conducted from July to December 2016 in Isfahan. All medications and hair analysis were provided free of charge to enrolled participants. All participants were recruited after providing written informed consent.
Female volunteers aged 15–45 years old, complaining about hair loss were recruited after providing written informed consent. Participants were excluded if they suffered from other dermatological disorders or other form of alopecia such as alopecia areata. Patients were also excluded if they did not take medications regularly. Other exclusion criteria were other known causes of hair loss such as recently diagnosed, uncontrolled anemia, hypothyroidism, and polycystic ovarian disease; patients who received topical scalp treatment during last month or systemic treatment for the past 3 months before the study; and pregnancy or desire to become pregnant. Participants were asked to maintain their usual shampooing frequency and color of their hair and also not to change their diet or medications during the study.
During baseline visit, a medical history which consisted participants’ profile, medical history, concomitants medications, and medical treatment for hair loss were obtained. The scalp was also examined to rule out the presence of other disorders. Then, the participants were referred to a skin care and esthetic clinic for hair analysis. A digital photograph for analyzing hair density and thickness was taken using video dermatoscope multicam 1000 (Bomtech, Electronics Co, Seoul, South Korea).
Following the baseline visit, participants returned for evaluation. In the second visit, they were asked about the probable side effects of medications and rate of their hair loss after treatment (less, without change, more or much more).
Finally, in the last visit, physical examination, asking about side effects, rate of hair loss, and hair analysis was repeated. In addition, at that time, participants’ self-satisfaction and specialists’ opinion were asked by grading the change in hair density using a seven-point scale (3: Greatly increased, 2: Moderately increased, 1: Slightly increased, 0: No change, −1: Slightly decreased, −2: Moderately decreased, or − 3: Greatly decreased). After that, participants’ photos (before and after treatment) were shown to a dermatologist who was blinded to the study and based on the same scale, her opinion about the participants’ hair volume was also added to the study.
Eligible participants were allocated into four groups by Random Allocation Software and in simple randomization method to receive coadministration of zinc sulfate and calcium pantothenate; zinc sulfate capsules 220 mg (equivalent to 50 mg zinc) from Alhavi Company; pantothenic acid in available form of calcium pantothenate tablets 100 mg from Mehr Darou Company; and 2% minoxidil solution from Pak Darou Company.
Participants in the first group were instructed to take simultaneously a zinc sulfate capsule and a calcium pantothenate tablet twice a week. Participants of the second and third group were instructed to take one capsule/tablet of their assigned treatment with water once a day. And finally, participants of the last group were instructed to apply 1 ml of 2% minoxidil solution twice daily in diverse parts of the scalp and softly massage with the help of their fingertips and let it dry. They were also instructed not to wash their hair for the next 4 h.[9] The study obtained approval from the Research Council and Ethics Committee of Isfahan University of Medical Sciences (No. 394675).
The primary endpoint was the change in hair density and diameter measured by dermatoscope. Secondary endpoints included the author's evaluation, dermatologist's opinion - which was blinded to the study - from comparing the patients’ photographs before and after treatment and finally, overall changes in hair density measured by participants’ self-assessments.
The data were analyzed by SPSS ver. 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Comparison of density change and thickness change within and among groups were evaluated by one-way ANOVA. The correlation among participants’ satisfaction, author's and blind dermatologist's opinion was analyzed by Pearson correlation.
RESULTS
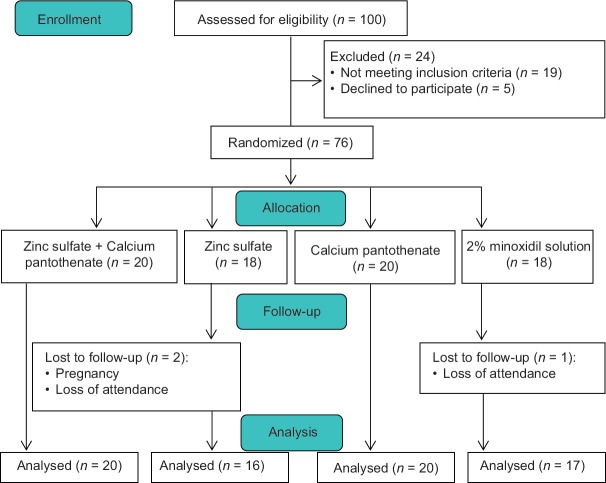
A total of 100 women aged between 15 and 45 years old volunteered to participate in this study. Seventy-six were eligible for enrollment. Over 120 days of follow-up, three participants withdrew the study for personal reasons. Data for 73 eligible participants were analyzed. Participants’ disposition is shown in Figure 1. Demographic and baseline characteristics of the participants are summarized in Table 1.
Figure 1.
CONSORT diagram of the study
Table 1.
Demographic and baseline characteristics of the patients
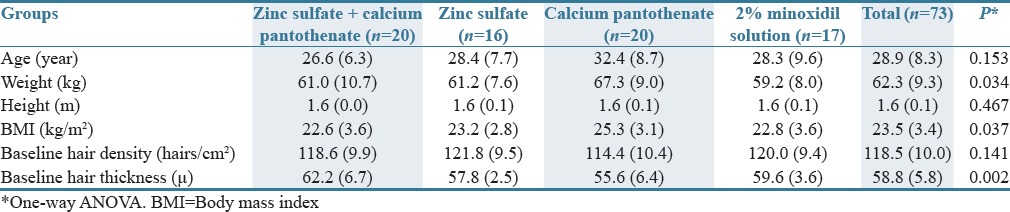
There was not a significant difference in marital and educational status among four groups (P > 0.01). Furthermore, the difference in shampooing frequency (per week), involved region of the scalp, medical and drug history, family history of hair loss, and previous treatment for hair loss was not significant among the groups.
After 4 months (120 ± 7 days) of treatment, hair count increased from 118.5 ± 10 hairs/cm2 to 124 ± 11 hairs/cm2 (P = 0.001). Hair thickness also increased from 58.8 ± 5.8 μ to 62.3 ± 4.3 μ (P = 0.001). In the zinc plus pantothenate group, these changes were from 118.6 ± 9.9 hairs/cm2 to 121.9 ± 11.1 hairs/cm2 (P = 0.042) and from 62.2 ± 6.6 μ to 64.0 ± 5.0 μ (P = 0.126), respectively. These changes for the minoxidil group were from 120.1 ± 9.7 hairs/cm2 to 132.3 ± 10.5 hairs/cm2 (P = 0.001) and from 60 ± 3.1 μ to 63 ± 3.0 μ (P = 0.001), respectively. Hair density increments were more obvious in the minoxidil, then pantothenate, after that combination and finally zinc group, respectively. On the other hand, hair thickness increment was more obvious in the pantothenate, then zinc, minoxidil, and finally combination group, respectively [Figure 2].
Figure 2.
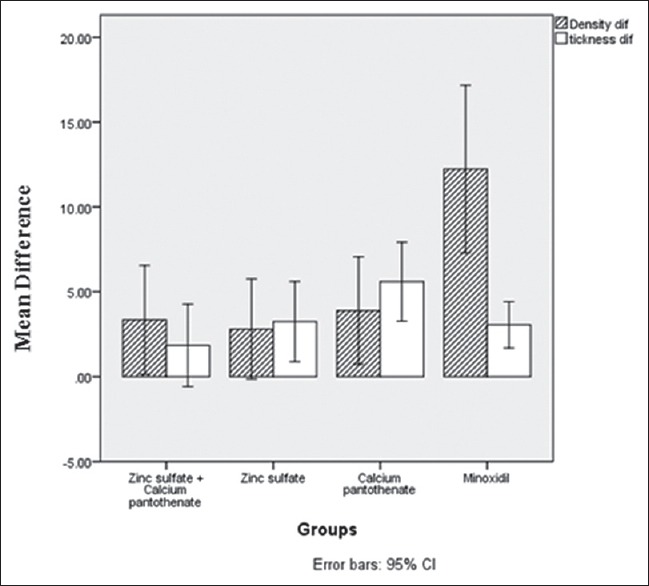
Change in hair density (hairs/cm2) and hair thickness (micron) during the study
The first group received combination therapy had a significant change in hair density (P = 0.042), while the change in hair thickness was significant in zinc group (P = 0.01). For pantothenate and minoxidil groups, it was significant for both hair density and hair thickness (P = 0.01).
Findings of the experts’ impressions of change in hair volume based on a comparison between the participants’ photographs before and after treatment, showed a 95% (19 participants) of the combination group claim slight to great improvement in hair volume which is 44.4% (8 participants), 50% (10 participants), and 94.4% (17 participants) for zinc, pantothenate, and minoxidil groups, respectively.
The results of participant satisfaction showed 85% (17 participants) of the combination group claim slight to moderately improvement in controlling hair loss which is 55.5% (10 participants), 80% (16 participants), and 88.8% (16 participants) for zinc, pantothenate, and minoxidil groups, respectively.
Participants’ satisfaction has shown a correlation with researcher's and blind dermatologist's opinion (0.585 and 0.514 respectively, and P < 0.001 for both).
No adverse effects of oral medications were reported. Only in minoxidil Group, 1 participant experienced dandruff, and two of them experienced pruritus alone, and two others experienced both pruritus and dandruff while using 2% minoxidil solution.
DISCUSSION
Hair loss may have an important impact on quality of life and self-image, especially in women.[17] This prospective, randomized study compared the therapeutic effects of the combination of zinc sulfate and calcium pantothenate, daily administration of zinc sulfate, daily administration of calcium pantothenate with daily administration of minoxidil solution 2% in women with hair loss. Different aspects were analyzed; changes of hair density and thickness measured by dermatoscope was an important tool to estimate the efficacy of the treatments. All participants and the blinded dermatologist separately evaluated the clinical outcome based on a seven-point scale questionnaire. Interestingly, the calcium pantothenate group participants’ assessments tended to be higher compared with experts’ assessments. It may be because of improvements occurred after 4 months of treatment including an increase in hair thickness and controlling hair loss which resulted in participant contentment and may lead to overestimation of clinical effects of calcium pantothenate.
Many other studies investigating minoxidil in women with hair loss have confirmed a good treatment response;[9,12] however, it should be considered that positive effects of minoxidil will disappear after discontinuation of drug administration.[12] Furthermore, patients usually have low compliance to use topical medications rather than oral supplements which have fewer side effects, too.
The study had some limitations including; the participants were not blinded to treatment, because of differences in medications; the stage of hair loss and the effect of treatment regimens based on this stage were not determined. Further studies are therefore required to resolve these limitations with a large double-blinded study.
According to participants’ satisfaction, the coadministration of zinc sulfate capsules and calcium pantothenate tablets when administered in a pulse therapy may result in hair growth as it provides basic nutrients needed for hair growth. Because of availability, affordability, no serious side effects and patients’ desire to use oral medications instead of topical ones; this combination could be a good choice for controlling hair loss, especially in initial stages.
AUTHORS’ CONTRIBUTION
Prof. Mansour Siavash contributed in the project idea, study design, supervision, data analysis and manuscript preparation; Dr. Fereshteh Tavakoli contributed in study design and conduct, data analysis and manuscript preparation; Dr. Fatemeh Mokhtari contributed in study conduct and manuscript preparation.
Financial support and sponsorship
Research Council and Ethics Committee of School of Medicine of Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are indebted to Research Council and Ethics Committee of School of Medicine of Isfahan University of Medical Sciences for its financial and spiritual supports, and also the participants who take part in the study. We would like to thank staff members of Arman skin care and esthetic clinic especially Dr. Ali Assilian and Mrs. Maryam Aboutalebi and Dr. Masoom Shahbazi for their cooperation.
REFERENCES
- 1.Le Floc’h C, Cheniti A, Connétable S, Piccardi N, Vincenzi C, Tosti A. Effect of a nutritional supplement on hair loss in women. J Cosmet Dermatol. 2015;14:76–82. doi: 10.1111/jocd.12127. [DOI] [PubMed] [Google Scholar]
- 2.Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860. doi: 10.5812/ijem.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Zuuren EJ, Fedorowicz Z. Interventions for female pattern hair loss. JAMA Dermatol. 2017;153:329–30. doi: 10.1001/jamadermatol.2016.5790. [DOI] [PubMed] [Google Scholar]
- 4.Dlova NC, Fabbrocini G, Lauro C, Spano M, Tosti A, Hift RH. Quality of life in South African black women with alopecia: A pilot study. Int J Dermatol. 2016;55:875–81. doi: 10.1111/ijd.13042. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Donk J, Hunfeld JA, Passchier J, Knegt-Junk KJ, Nieboer C. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Soc Sci Med. 1994;38:159–63. doi: 10.1016/0277-9536(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 6.van der Donk J, Passchier J, Knegt-Junk C, van der Wegen-Keijser MH, Nieboer C, Stolz E, et al. Psychological characteristics of women with androgenetic alopecia: A controlled study. Br J Dermatol. 1991;125:248–52. doi: 10.1111/j.1365-2133.1991.tb14749.x. [DOI] [PubMed] [Google Scholar]
- 7.Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28–34. [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn M, Shinkai K, Pasch L, Kuzmich L, Cedars M, Huddleston H. Prevalence of androgenic alopecia in patients with polycystic ovary syndrome and characterization of associated clinical and biochemical features. Fertil Steril. 2014;101:1129–34. doi: 10.1016/j.fertnstert.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65:1126–34.e2. doi: 10.1016/j.jaad.2010.09.724. [DOI] [PubMed] [Google Scholar]
- 10.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy LL, Emer JJ. Female pattern alopecia: Current perspectives. Int J Womens Health. 2013;5:541–56. doi: 10.2147/IJWH.S49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130–6. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 13.Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: A review. Dermatol Res Pract 2014. 2014 doi: 10.1155/2014/709152. 709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lourith N, Kanlayavattanakul M. Hair loss and herbs for treatment. J Cosmet Dermatol. 2013;12:210–22. doi: 10.1111/jocd.12051. [DOI] [PubMed] [Google Scholar]
- 15.Bremner I, Beattie JH. Copper and zinc metabolism in health and disease: Speciation and interactions. Proc Nutr Soc. 1995;54:489–99. doi: 10.1079/pns19950017. [DOI] [PubMed] [Google Scholar]
- 16.Brzezinska-Wcislo L. Evaluation of Vitamin B6 and calcium pantothenate effectiveness on hair growth from clinical and trichographic aspects for treatment of diffuse alopecia in women. Wiad Lek. 2001;54:11–8. [PubMed] [Google Scholar]
- 17.Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28:611–8. doi: 10.1016/j.det.2010.03.011. [DOI] [PubMed] [Google Scholar]