Abstract
We previously identified phenylquinoxalinone CFTRact-J027 (4) as a cystic fibrosis transmembrane conductance regulator (CFTR) activator with an EC50 of ∼200 nM and demonstrated its therapeutic efficacy in mouse models of constipation. Here, structure v–activity studies were done on 36 synthesized phenylquinoxalinone analogs to identify compounds with improved potency and altered metabolic stability. Synthesis of the phenylquinoxalinone core was generally accomplished by condensation of 1,2-phenylenediamines with substituted phenyloxoacetates. Structure–activity studies established, among other features, the privileged nature of a properly positioned nitro moiety on the 3-aryl group. Synthesized analogs showed improved CFTR activation potency compared to 4 with EC50 down to 21 nM and with greater metabolic stability. CFTR activators have potential therapeutic indications in constipation, dry eye, cholestatic liver diseases, and inflammatory lung disorders.
Graphical abstract
INTRODUCTION
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-regulated chloride channel expressed in mammalian epithelia in the respiratory, gastrointestinal, and reproductive systems, as well as in exocrine glands and other tissues.1 Loss-of-function mutations in CFTR cause cystic fibrosis, and CFTR overactivation causes certain secretory diarrheas including cholera and Travelers’ diarrhea.2 CFTR is considered an important drug target, with activators of CFTR of potential benefit for constipation,3,4 dry eye,5 inflammatory lung disorders,6 and cholestatic liver disease; inhibitors of wild-type CFTR may be useful for treatment of certain secretory diarrheas and polycystic kidney disease;7,8 and correctors and potentiators of mutant CFTRs have been shown to be useful for treatment of cystic fibrosis.9
We previously identified by high-throughput screening the phenylquinoxalinone CFTRact-J027 (4, Figure 1) as a CFTR activator and demonstrated its efficacy in normalizing stool output, hydration, and intestinal transit in a mouse model of opioid-induced constipation.3 Phenylquinoxalinone 4 activated CFTR chloride conductance with an EC50 of ∼200 nM and showed no apparent off-target actions or toxicity following chronic administration in mice. In a follow-up study,4 4 was shown by patch-clamp and biochemical studies to target CFTR directly and was demonstrated to activate CFTR in human enterocytes and normalize stool parameters in mouse models of acute and chronic constipation. Side-by-side comparisons of intestinal fluid secretion and stool output in constipation models showed greater efficacy of 4 than supramaximal doses of the FDA-approved drugs lubiprostone and linaclotide.
Figure 1.
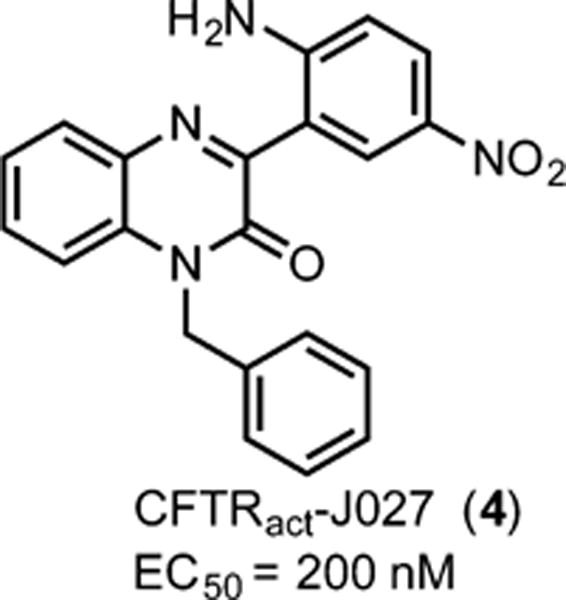
A phenylquinoxalinone CFTR activator identified by high-throughput screening.
Here, motivated by the potential therapeutic utility of phenylquinoxalinone-based CFTR activators in constipation and other diseases, we synthesized 36 analogs of phenylquinoxalinone 4 in order to establish structure–activity relationships and to identify compounds with greater potency. Also, while the rapid hepatic metabolism of 4 results in minimal systemic exposure following oral administration in mice, which is desirable for treatment of constipation, we also sought phenylquinoxalinone CFTR activators with greater metabolic stability for treatment of lung and liver disorders where systemic exposure is necessary.
RESULTS AND DISCUSSION
Chemistry
General Synthesis of Phenylquinoxalinones
Most of the phenylquinoxalinones in this study were expediently synthesized in four steps starting from acetophenones (Scheme 1). We generated the phenylquinoxalinone core by condensing o-phenylenediamines with substituted phenyloxoacetates (6), which were synthesized following literature methods.10
Scheme 1. Synthesis of Phenylquinoxalinones 1–3a.
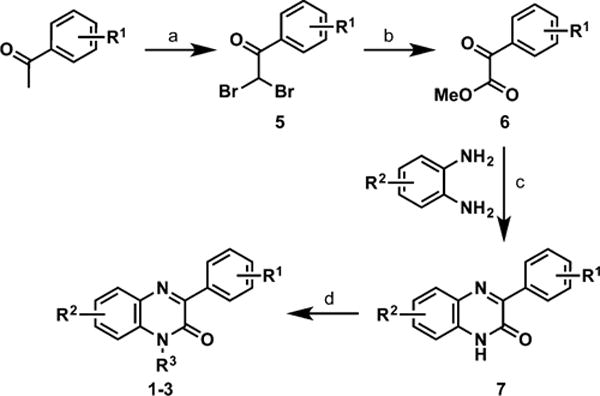
a(a) Br2, 1,4-dioxane; (b) DMSO, Δ; MeOH; (c) toluene, Δ; (d) R3-Br, K2CO3, DMF.
Briefly, substituted acetophenone was doubly brominated with bromine in 1,4-dioxane to give 5, then heated in DMSO followed by addition of methanol to give 6.10 Phenylquinoxalinone 7 was N1-alkylated using K2CO3 and R3−X in DMF,11 and pure products (1–3) were obtained via column chromatography.
Phenylquinoxalinone 1a was prepared as outlined in Scheme 2. Treatment of methyl 3-fluoro-4-nitrobenzoate (8) with methyl 2-cyanoacetate under basic conditions delivered intermediate 9.12 Subsequent copper(I) iodide-catalyzed aerobic oxidation12 delivered methyl 2-oxo-2-phenylacetate 10, and from here, target 1a was prepared in parallel to the chemistry employed in Scheme 1. With 1a in hand, saponification and nitro reduction were accomplished as outlined in Scheme 3 to deliver analogs 1b, 1j, and 1k.
Scheme 2. Synthesis of Phenylquinoxalinone 1aa.
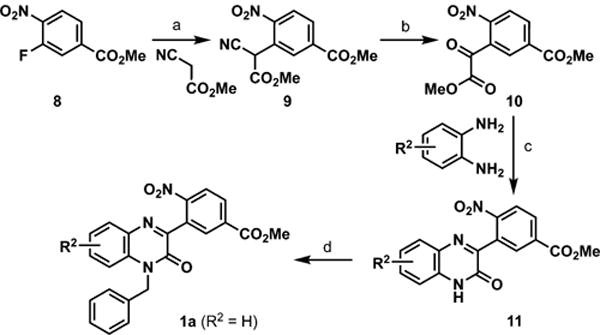
a(a) Cs2CO3, DMSO; (b) CuI, 1,10-phen, ACN, O2; (c) toluene, Δ; (d) BnBr, K2CO3, DMF.
Scheme 3. Diversification from Phenylquinoxalinone 1aa.
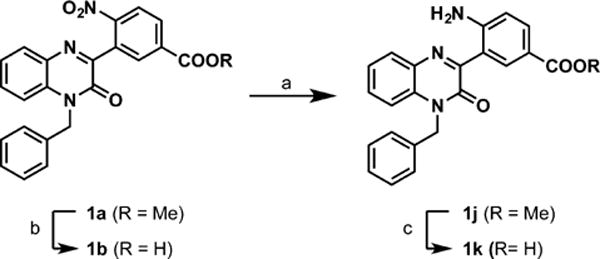
a(a) H2, Pd/C, MeOH; (b) NaOH, EtOH; (c) KOH, MeOH.
Constrained ring phenylquinoxalinone analogs 16 and 20 were prepared as outlined in Scheme 4. 2-Amino-3-nitrophenol was N- and O-alkylated with 1,2-dibromoethane to deliver intermediate 14, and subsequent nitro reduction and condensation of the resulting diamine with 1-acetyl-5-nitroindoline-2,3-dione led smoothly to analog 16.13 Employing 2-bromo-1-phenylethan-1-one in place of 1,2-dibromoethane and 5-nitroindoline-2,3-dione in place of 1-acetyl-5-nitroindoline-2,3-dione delivered analog 20.14 Interestingly, the reaction of N1-benzyl-4-(trifluoromethyl)benzene-1,2-diamine with 5-fluoroisation (in analogy with the protocol employed to prepare compounds 16, 20, and 4) led to 22 and the attempted deacylation of 21 (X = NO2) led to 23.
Scheme 4. Synthesis of Constrained Ring Phenylquinoxalinone Analogsa.
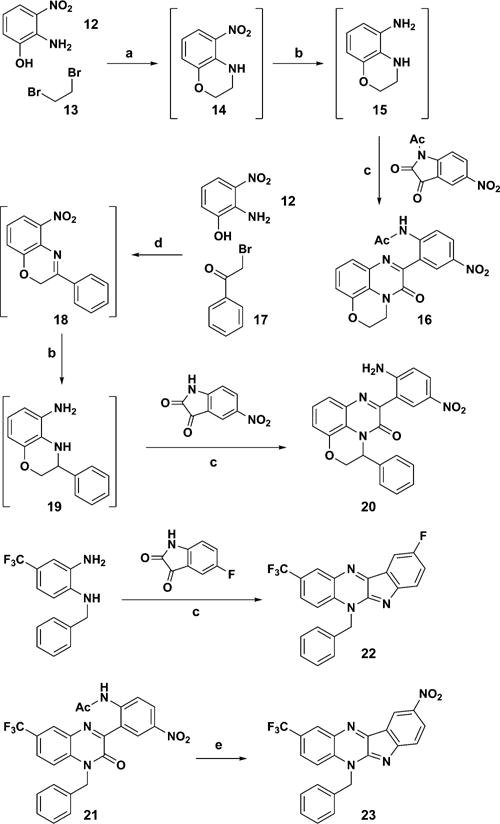
a(a) KOH, DMF; (b) H2, Pd/C, MeOH; (c) HOAc, toluene; (d) K2CO3, ACN; (e) HCl, MeOH.
Modifications of the 3-Aryl Ring
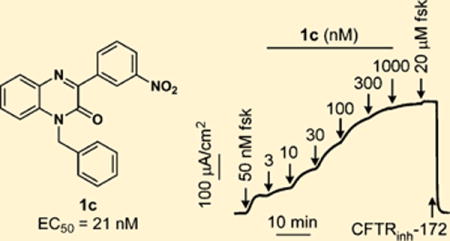
Compound 4 contains a 2-amino-5-nitro phenyl ring at the 3-position of the quinoxalinone core (Figure 1), and our first effort was to modify this ring. Several compounds (Table 1) were rationally synthesized, and their activities were determined using a plate reader assay. The most active compound was CFTRact-J125 (1c), which only lacks the 2-amino group at C2 of the 3-aryl ring compared to 4, and it showed an ∼10-fold increased potency when compared to 4. We then synthesized a series of analogs retaining this amino group deletion. In contrast to the high activity of 1c, compounds without the 3-nitro group had significantly lower activity (1h), indicating the privileged nature of the 3-nitro group.
Table 1.
CFTR Activation with Variation in the 3-Aryl Ringa
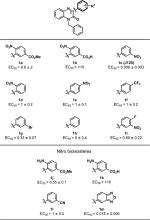
|
EC50 reported in μM ± SEM.
Bioisosteric compounds containing −COOR replacements of the −NO2 moiety in the 3-aryl group were synthesized according to Schemes 2 and 3. It was found that carboxylic acid analog 1k was inactive, while methyl ester analog 1j was moderately potent. Combining a 2-NO2 with either a 5-COOMe or a 5-COOH (1a and 1b, respectively) resulted in very low activity. Positional deviation of the nitro group also resulted in reduced activity, as with 2-nitro or 4-nitro analogs 1d and 1e, respectively. Introduction of other functional groups, such as 4-CF3 or 3-Br, in place of the 3-NO2 of 1c also showed low activity (1f and 1g, respectively). Introducing a fluorine atom into 1c (i.e., 1i) reduced activity. Replacements of the nitro group with a bioisosteric nitrile (1l) greatly reduced activity, but interestingly, the bioisosteric benzoxadiazole (1m) showed comparable potency to the nitro analog (1c).
Modifications of the Quinoxalinone Core
Moving forward with 1c as the lead, quinoxalinone backbone modifications were undertaken (Table 2). Halogen substitution at the 6-position, such as 6-Cl (2b) or 6-Br (2f), showed good activity, albeit less than that of 1c. Substitution at the 5-, 7-, or 8-positions generally resulted in lower activity. Disubstitution at the 6- and 7-positions (dichloro, 2d; difluoro, 2g; dimethyl, 2i) reduced activity.
Table 2.
CFTR Activation with Variation in the Quinoxalinone Corea
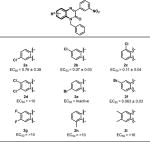
|
EC50 reported in μM ± SEM.
Modifications of the N1-Substituent
We next modified the N1-benzyl group (Table 3). We reported previously that placing substituents on the benzyl ring was not tolerated.4 However, heterocycle analogs such as thiophenyl (3j), furanyl (3i), and pyridyl (3h) showed comparable activity. Among short chains, allyl and ethyl groups showed moderate activity, but methyl and propyl groups had poor activity (3a–e). Replacing the phenyl with a larger aromatic group, such as naphthyl, significantly reduced activity (3f and 3g).
Table 3.
CFTR Activation with Variation in the N1-Substituenta
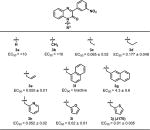
|
EC50 reported in μM ± SEM.
Compounds with Constrained Rings
Motivated by our prior findings that constrained rings can enhance the activity of a CFTR corrector,15 we next examined two different types of constrained analogs (Scheme 4) with hindered rotation of the N1-alkyl group (16 and 20) or phenyl ring (22 and 23). As previously shown, the N-acetylated version of 4 has very low activity;4 we therefore attempted to synthesize 16 without an N-acyl group, but both deacylation of 16 and condensation with 5-nitroisatin failed. The activities of neither 20 (EC50 = >10 μM) nor 16 (EC50 = 0.82 μM) were greater than that of 4 (see Table 4). Phenyl-ring constrained compounds 22 and 23 were unexpected byproducts of the deacylation reaction (see Scheme 4 and Table 4). These compounds are purple and red, respectively, a consequence of their extended aromatic systems, and those colors are different from the bright yellow color of most derivatives of 4. Intramolecular heterocycle formation in this system might have been facilitated by the presence of the electron withdrawing CF3 group in the quinoxalinone backbone under these acidic deacylation conditions. With other substituents in the quinoxalinone backbone, only small amounts of uncharacterized reddish byproduct formed during the deacylation step, suggesting a minimal amount of byproduct formation.
Table 4.
CFTR Activation with Constrained Ring Analogsa
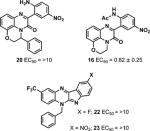
|
EC50 reported in μM ± SEM.
Biology
In Vitro Characterization of Phenylquinoxalinones
Phenylquinoxalinone CFTR activators with the highest potency as determined by plate-reader assay, 1c and CFTRact-J170 (3j, Table 3), were further characterized. Short-circuit current measurements were done using CFTR-expressing FRT cells in the presence of a transepithelial chloride gradient and with permeabilization of the cell basolateral membrane; consequently, current is a direct, linear measure of CFTR chloride conductance. Compounds 1c and 3j were added to the apical side of the monolayer. Representative data in Figure 2 for 1c and 3j shows a small increase in current following addition of a low concentration of forskolin, followed by concentration-dependent increases in current following activator additions. EC50 values were determined to be 21 ± 4 and 70 ± 11 nM for 1c and 3j, respectively. The potency for CFTR inhibition by 1c was similar in nonpermeabilized and permeabilized CFTR-expressing FRT cells (data not shown).
Figure 2.

Short-circuit current measurement of CFTR activation by 1c and 3j. (A) Measurements done in FRT cells expressing human wild-type CFTR showing responses to indicated concentrations of forskolin, 1c, or 3j, and 10 μM CFTR inhibitor CFTRinh-172. (B) Concentration-dependent activation of CFTR (mean ± SEM, n = 3).
The CFTR specificity of the most potent compound, 1c, was further studied. At 10 μM, 1c did not affect the cellular cAMP level (Figure 3A), nor did it elevate cytoplasmic calcium or inhibit the ATP-stimulated elevation in cytoplasmic calcium (Figure 3B). In addition, 1c at 10 μM neither inhibited nor activated calcium-activated chloride channels in HT-29 cells (Figure 3C) or in FRT cells expressing TMEM16A (Figure 3D).
Figure 3.
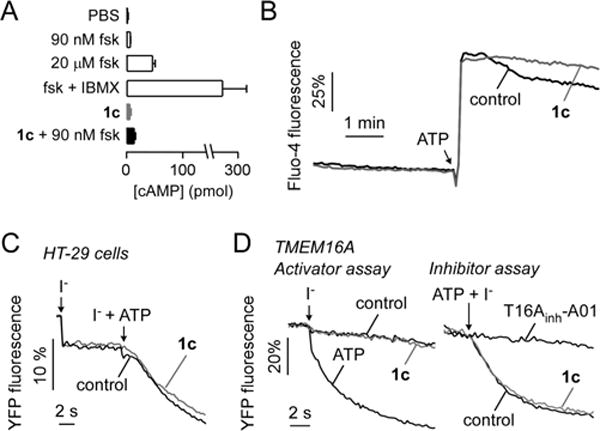
In vitro characterization of 1c. (A) Cellular cAMP in FRT cells in response to incubation for 10 min with 10 μM 1c without or with 90 nM forskolin (fsk). Positive controls included fsk (100 nM and 20 μM) and fsk + IBMX (20 μM + 100 μM) (mean ± SEM, n = 4). (B) Cytoplasmic calcium measured by Fluo-4 fluorescence. FRT cells were pretreated for 5 min with 10 μM 1c (or control) with 100 μM ATP added as a calcium agonist as indicated. (C) CaCC activity measured in HT-29 cells expressing YFP showing no activation (iodide addition) or inhibition (iodide + ATP addition) by 10 μM 1c. (D) TMEM16A activity measured in FRT cells expressing YFP showing no activation (iodide addition) or inhibition (iodide + ATP addition) by 10 μM 1c.
Efficacy of 1c in a Loperamide-Induced Mouse Model of Acute Constipation
We previously demonstrated the efficacy of 4 in a loperamide-induced mouse model of constipation.3,4 Here, the efficacy of 1c was tested. Phenylquinoxalinone 1c was administered orally to mice 1 h prior to loperamide and 3-h stool samples were collected after loperamide. Figure 4 shows that orally administered 1c fully normalized stool weight, pellet number, and hydration with half-maximal effective dose (ED50) < 1 mg/kg.
Figure 4.
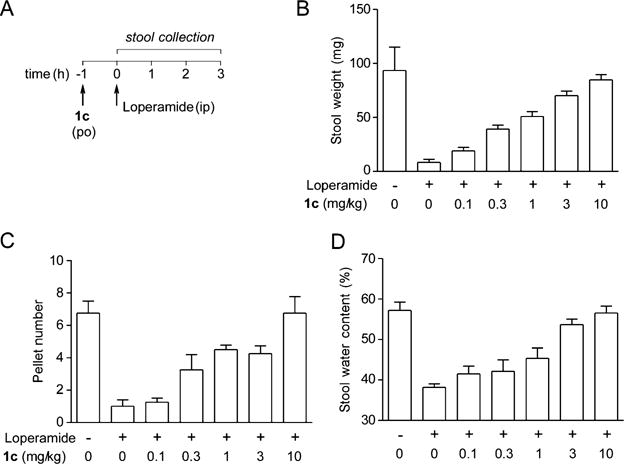
Efficacy of 1c in a mouse model of acute constipation. (A) Experimental protocol. Mice were treated with 1c (orally) or vehicle 1 h before loperamide (0.3 mg/kg, intraperitoneal). Weight, pellet number, and water content were determined for stool collected at 3 h. (B−D) Stool weight, pellet number, and water content after treatment with 0, 0.1, 0.3, 1, 3, or 10 mg/kg of 1c (mean ± SEM, 4 mice per group). The control was without treatment with loperamide and 1c.
Phenylquinoxalinone 4 was shown previously to have minimal oral bioavailability and rapid metabolism.3,4 Though these properties are favorable for “topical” applications in the treatment of constipation and dry eye in which systemic exposure is not needed, they are not favorable for treatment of liver and lung diseases where systemic exposure and organ accumulation are desired. Figure 5 shows substantially slower in vitro hepatic microsomal metabolism of 1c compared with 4, with nearly 80% of 4 metabolized at 60 min compared with <40% metabolism of 1c.
Figure 5.

In vitro metabolic stability of 1c. Remaining 1c following incubation with human hepatic microsomes in the presence of NADPH after incubation for specified times (0, 15, 60 min), compared with reference compound 4 (mean ± SEM, n = 3).
Indeed, a number of important structure–activity relationships have been revealed in the present study. Previously, the amino group on the 3-aryl ring of phenylquinaxolinones, present in all of the 175 analogs reported in our preliminary study, was assumed to be a central structural feature (see 4 with an EC50 = 200 nM).4 Through the series of compounds synthesized and assayed in this work, we determined that deletion of this amino group generally improves the potency of this class of CFTR activators (compare 1c, the amino-deleted analog of 4, with an EC50 = 21 nM). Additional amino-deleted analogs further established that an unsubstituted quinoxalinone core affords the best potency (compare 1c with the quinoxalinone core analogs depicted in Table 2). Indeed, there appears to be a delicate balance between electronic and steric effects, especially considering that the highest performing analog with a substituted core (2f) underperforms by nearly an order of magnitude compared to unsubstituted 1c. Quinoxalinone N-substituent effects were also thoroughly explored in this work (see Table 3), and in general, N-benzyl or N-heteroaromatic groups provide optimal potency. Finally, structure–activity results reported here establish that in vitro hepatic microsomal metabolism is also dramatically variable, see Figure 5 comparing the 60 min metabolic results for 1c (<40% metabolized) with that for 4 (nearly 80% metabolized).
Small molecule CFTR activators have potential utility in various diseases involving epithelial fluid secretion. Compounds with minimal systemic absorption are of interest for treatment of dry eye disorders5 with topical (eye drop) compound administration, and for constipation3 with oral administration, as CFTR is expressed in surface-exposed epithelial cells in these tissues. However, compounds that are absorbed and accumulate in relevant tissues are needed to treat disorders of cholestasis16 (by increasing bile flow) and inflammatory lung diseases (by increasing airway surface liquid volume).17
CONCLUSIONS
In conclusion, synthesis of 36 phenylquinoxalinones established structure–activity relationships and identified compounds with ∼10-fold improved potency and greater metabolic stability than reference compound 4. The most potent analog, 1c, showed CFTR selectivity and efficacy in a mouse model of acute opioid-induced constipation. CFTR activation by phenylquinoxalinones may have utility in constipation and dry eye, as supported by prior experimental animal data,3,4 as well as in inflammatory lung disorders and hepatic cholestasis.
EXPERIMENTAL SECTION
General Experimental
All compounds described in this manuscript have ≥95% purity. The analytical method used to determine purity was 1H NMR (see the accompanying Supporting Information file, which provides the 1H and 13C NMR for the 36 compounds assayed) and HPLC/HRMS. For HRMS analysis, samples were analyzed by flow-injection analysis into a Thermo Fisher Scientific LTQ Orbitrap (San Jose, CA) operated in the centroided mode. Samples were injected into a mixture of 50% MeOH/H2O and 0.1% formic acid at a flow of 0.2 mL/min. Source parameters were 5.5 kV spray voltage, capillary temperature of 275 °C, and sheath gas setting of 20. Spectral data were acquired at a resolution setting of 100,000 fwhm with the lockmass feature, which typically results in a mass accuracy <2 ppm.
CFTR Functional Assays
Fischer rat thyroid (FRT) cells stably coexpressing human wild-type CFTR and the halide-sensitive yellow fluorescent protein (YFP)-H148Q were cultured as described.18 Fluorescence plate reader assays of CFTR function were done as described in ref 18, in which 96-well plates containing near-confluent cell cultures were washed with phosphate-buffered saline (PBS) and incubated for 10 min with PBS containing test compound and 125 nM forskolin. Assays of iodide influx into cells were done in single wells by continuous measurement of YFP fluorescence just for 2 s before (for baseline) and 12 s after addition of an iodide containing solution (final 140 mM iodide). TMEM16A activity assay was done similarly, as described in ref 19, using FRT cells coexpressing YFP and TMEM16A. Non-TMEM16A CaCC activity was assayed as described in ref 20 in HT-29 cells expressing YFP. In each assay, iodide influx rate and concentration-dependent curves were computed as described.18–20 For short-circuit current measurement, cells were cultured on porous filters, and current was measured in the presence of a transepithelial chloride gradient and following permeabilization of the basolateral membrane, as described.21 Cyclic AMP and cytoplasmic calcium measurement were done as described.19,22
Loperamide Model of Acute Constipation in Mice
All mouse experiments were approved by the UCSF Institutional Animal Care and Use Committee (approval number: AN108711-02A) and were conducted in accordance with the NIH guidelines for the care and use of animals. As described in ref 3, CD1 mice (age 8–10 weeks) were administered 0.3 mg/kg loperamide intraperitoneally (ip) and placed in metabolic cages with free access to food and water. Stool samples were collected for 3 h for determination of total stool weight, number of fecal pellets, and stool water content (by wet and dry weight measurements). Compound 1c (or vehicle control) was administered orally 1 h prior to loperamide. The vehicle consisted of saline containing 5% DMSO and 10% Kolliphor HS 15.
In Vitro Metabolic Stability
Test compound (at 5 μM) was incubated for specified times at 37 °C with mouse liver microsomes (1 mg protein/mL; Sigma-Aldrich, St. Louis, MO) in potassium phosphate buffer containing 1 mM NADPH, as described.3 Following ethyl acetate extraction, nonmetabolized parent compound was assayed by LC/MS.
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between two groups were performed using the unpaired Student’s t-test. P < 0.05 was considered statistically significant.
General Procedure for Synthesis of Dibromophenylpropa-nedione Derivatives (5)
A flask was charged with 1,4-dioxane (15 mL) and bubbled with N2 for 10 min with stirring. Br2 (2 mL, 39 mmol) was added, and the solution was stirred for 30 min with slow N2 bubbling. Substituted acetophenone (12 mmol) was dissolved in 1,4-dioxane (20 mL) and added. The mixed solution was stirred for 3 h, poured in water, and extracted with ethyl acetate. The organic layer was washed with water (3×) and brine and then dried over magnesium sulfate. Solvent was removed in vacuo to yield reddish oil of 5, which was used for next step without further purification.
General Procedure for Synthesis of Oxophenylacetate Derivatives (6)
Anhydrous DMSO (15 mL) was added to the oily product of 5 and heated at 75 °C overnight. The solution was cooled to RT, methanol (10 mL) was added, and the solution was stirred overnight. The solution was poured in water and extracted with ethyl acetate. The organic layer was washed with water (3×) and brine and dried over magnesium sulfate. The brown oily product was used in the next step without purification.
General Procedure for Synthesis of N-H Phenylquinoxali-none Derivatives (7)
Substituted phenyloxoacetate (6, 1 mmol) was mixed with o-phenylenediamine (1 mmol) in toluene (20 mL) and heated at 70 °C overnight. The precipitate that formed was collected by filtration, triturated with toluene and hexane, and used in the next step without purification.
General Procedure for N-Alkylation of Phenylquinaxolinone (1–3)
Compound 7 (0.5 mmol) was dissolved in DMF (20 mL), benzyl bromide (0.6 mmol) and K2CO3 (1 mmol) were added, and the mixture was stirred overnight. The solution was diluted with water and extracted with ethyl acetate. The organic layer was washed with water three times. The organic layer was washed with brine and dried over magnesium sulfate. The final product obtained after solvent evaporation was purified by flash column chromatography.
Methyl 3-(4-Benzyl-3-oxo-3,4-dihydroquinoxalin-2-yl)-4-nitrobenzoate (1a)
Methyl 3-fluoro-4-nitrobenzoate (8, 1.0 g, 5 mmol) was mixed with Cs2CO3 (3.3 g, 10 mmol) in DMSO (20 mL). Methyl cyanoacetate (1.0 g, 10 mmol) was added, and the solution was heated at 130 °C for 4 h and then maintained at 90 °C overnight. Upon cooling, the reaction mixture was extracted with ethyl acetate, and the organic layer was washed with 1 N HCl, water (3×), and brine. After drying over magnesium sulfate and filtration, the solvent was removed in vacuo to yield 9 as a purple oil, which was used without purification in the next step.
Crude intermediate 9 (1.8 g, 6.5 mmol) was dissolved in acetonitrile (20 mL), and CuI (1 g, 5.3 mmol) and 1,10-phenanthroline (0.23 g, 1.3 mmol) were added. The mixture was reacted at 50 °C overnight with an O2 balloon. After cooling, the solution was filtered through Celite and concentrated in vacuo. The product was purified by flash column chromatography to yield colorless 10 after solvent evaporation. Yield = 0.66 g (38%).
Intermediate 10 (163 mg, 0.61 mmol) was mixed with o-phenylenediamine (78 mg, 0.72 mmol) in toluene (30 mL) and heated at 70 °C overnight. The resulting tan precipitate of 11 was collected by filtration, triturated with toluene and hexane, and airdried; it was used in the next step without purification. Yield = 185 mg (93%).
Intermediate 11 (185 mg, 0.57 mmol) was mixed with benzyl bromide (150 mg, 0.88 mmol) and K2CO3 (170 mg, 1.2 mmol) in DMF (10 mL) and stirred overnight at RT. After dilution with water, the solution was extracted with ethyl acetate, washed with water (3×) and brine and dried over MgSO4. Solvent removal and purification by column chromatography gave 1a. Yield = 149 mg (63%). 1H NMR (400 MHz, CDCl3) δ 8.40 (d, J = 1.8 Hz, 1H), 8.22 (dd, J = 8.5, 1.9 Hz, 1H), 8.13 (d, J = 8.4 Hz, 1H), 7.89 (dd, J = 8.0, 1.6 Hz, 1H), 7.50–7.37 (m, 1H), 7.36–7.06 (m, 7H), 5.44 (s, 2H), 3.92 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.83, 154.65, 154.19, 151.57, 134.82, 134.73, 133.30, 133.17, 132.98, 131.80, 131.61, 131.28, 130.79, 129.05, 127.83, 126.89, 124.22, 124.20, 114.82, 52.91, 46.25. HRMS [C23H17N3O5 + H]+: calcd 416.1247/found 416.1253.
3-(4-Benzyl-3-oxo-3,4-dihydroquinoxalin-2-yl)-4-nitrobenzoic Acid (1b)
Compound 1a (10 mg, 0.024 mmol) was dissolved in hot ethanol (30 mL). Sodium hydroxide (0.1 g, 2.5 mmol) dissolved in water (5 mL) was added, and the mixture stirred for 1 h. The cooled solution was acidified with 1 N HCl and extracted with ethyl acetate. The organic layer was washed with water and brine, then dried over MgSO4. Solvent was removed in vacuo, and product was purified by flash column chromatography. Yield = 9 mg (93%). 1H NMR (400 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.30 (d, J = 1.4 Hz, 2H), 7.98 (dd, J = 8.0, 1.4 Hz, 1H), 7.62 (ddd, J = 8.6, 7.2, 1.5 Hz, 1H), 7.56–7.39 (m, 2H), 7.39–7.09 (m, 5H), 5.53 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 165.89, 154.75, 154.16, 151.48, 135.90, 133.43, 133.14, 132.95, 132.23, 131.82, 131.49, 130.53, 129.19, 127.93, 127.19, 124.88, 124.65, 115.88, 45.52. HRMS [C22H15N3O5 + H]+: calcd 402.1090/found 402.1085.
1-Benzyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (1c)
Yield = 150 mg (70%). 1H NMR (400 MHz, CDCl3) δ 9.37 (t, J = 2.0 Hz, 1H), 8.88 (ddd, J = 7.9, 1.7, 1.1 Hz, 1H), 8.37 (ddd, J = 8.3, 2.3, 1.1 Hz, 1H), 8.03 (dd, J = 8.0, 1.5 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.55 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.47–7.30 (m, 7H), 5.63 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 154.51, 151.07, 148.25, 137.44, 135.48, 135.02, 133.08, 132.97, 131.27, 130.92, 129.00, 128.93, 127.82, 126.92, 124.70, 124.13, 114.50, 109.99, 46.23. HRMS [C21H15N3O3 + H]+: calcd 358.1192/found 358.1188.
1-Benzyl-3-(2-nitrophenyl)quinoxalin-2(1H)-one (1d)
Yield = 108 mg (69%). 1H NMR (400 MHz, CDCl3) δ 8.18 (dd, J = 8.1, 1.1 Hz, 1H), 7.97 (dd, J = 8.0, 1.5 Hz, 1H), 7.87–7.73 (m, 2H), 7.65 (ddd, J = 8.7, 7.0, 2.0 Hz, 1H), 7.48 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.42–7.24 (m, 7H), 5.52 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 155.76, 154.35, 149.16, 135.01, 133.82, 133.30, 132.94, 131.72, 131.66, 131.00, 130.58, 129.04, 127.78, 126.96, 126.92, 124.09, 124.08, 114.83, 46.19. HRMS [C21H15N3O3 + H]+: calcd 358.1192/found 358.1187.
1-Benzyl-3-(4-nitrophenyl)quinoxalin-2(1H)-one (1e)
Yield = 94 mg (49%). 1H NMR (400 MHz, CDCl3) δ 8.76–8.56 (m, 2H), 8.42− 8.23 (m, 2H), 8.01 (dd, J = 8.0, 1.5 Hz, 1H), 7.56 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.47–7.29 (m, 7H), 5.62 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.55, 151.50, 148.67, 141.76, 134.97, 133.20, 133.02, 131.58, 131.08, 130.67, 129.05, 127.90, 126.93, 124.25, 123.14, 114.58, 46.29. HRMS [C21H15N3O3 + H]+: calcd 358.1192/found 358.1187.
1-Benzyl-3-(4-(trifluoromethyl)phenyl)quinoxalin-2(1H)-one (1f)
Yield = 120 mg (83%). 1H NMR (600 MHz, CDCl3) δ 8.53 (d, J = 8.2 Hz, 2H), 7.98 (dd, J = 8.0, 1.6 Hz, 1H), 7.75 (d, J = 8.2 Hz, 2H), 7.50 (ddd, J = 8.6, 7.2, 1.6 Hz, 1H), 7.42–7.26 (m, 7H), 5.59 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 154.61, 152.55, 139.23, 135.16, 133.25, 132.96, 131.91 (q, JC−F = 33 Hz), 130.90, 130.85, 129.97, 128.93, 127.74, 126.92, 124.88 (br), 124.23 (q, JC−F = 325 Hz), 123.92, 114.40, 46.18. HRMS [C22H15F3N2O + H]+: calcd 381.1215/found 381.1206.
1-Benzyl-3-(3-bromophenyl)quinoxalin-2(1H)-one (1g)
Yield = 295 mg (75%). 1H NMR (400 MHz, CDCl3) δ 8.62 (t, J = 1.8 Hz, 1H), 8.42 (dt, J = 7.9, 1.3 Hz, 1H), 7.99 (dd, J = 8.0, 1.5 Hz, 1H), 7.65 (ddd, J = 8.0, 2.1, 1.0 Hz, 1H), 7.50 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.44–7.29 (m, 8H), 5.60 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.58, 152.33, 137.85, 135.18, 133.32, 133.20, 132.83, 132.49, 130.82, 130.76, 129.60, 129.00, 128.35, 127.78, 126.93, 123.99, 122.29, 114.42, 46.20. HRMS [C21H15BrN2O + H]+: calcd 391.0446/found 391.0442.
1-Benzyl-3-phenylquinoxalin-2(1H)-one (1h)
Yield = 62 mg (44%). 1H NMR (400 MHz, CDCl3) δ 8.40 (ddd, J = 6.3, 2.9, 1.5 Hz, 2H), 7.99 (dd, J = 8.0, 1.5 Hz, 1H), 7.53 (tt, J = 3.9, 2.4 Hz, 3H), 7.48 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.40–7.18 (m, 7H), 5.61 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.80, 154.23, 136.01, 135.37, 133.38, 132.76, 130.60, 130.45, 130.32, 129.66, 128.95, 128.13, 127.71, 126.99, 123.81, 114.36, 46.15. HRMS [C21H16N2O + H]+: calcd 313.1341/found 313.1341.
1-Benzyl-3-(4-fluoro-3-nitrophenyl)quinoxalin-2(1H)-one (1i)
Yield = 127 mg (71%). 1H NMR (600 MHz, CDCl3) δ 9.31 (dd, J = 7.5, 2.3 Hz, 1H), 8.87 (ddd, J = 8.8, 4.3, 2.3 Hz, 1H), 7.98 (dd, J = 8.0, 1.5 Hz, 1H), 7.52 (ddd, J = 8.6, 7.2, 1.5 Hz, 1H), 7.45–7.36 (m, 2H), 7.36–7.31 (m, 3H), 7.28 (dt, J = 9.7, 3.1 Hz, 3H), 5.59 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 158.19, 154.54 (d, JC−F = 12 Hz), 149.65, 136.75, 136.63, 134.89, 132.92 (br), 132.79 (br), 131.41, 130.86, 129.03, 127.88, 127.67 (br), 126.87, 124.28, 118.06, 117.79, 114.56. 46.25. HRMS [C21H14FN3O3 + H]+: calcd 376.1098/found 376.1092.
Methyl 4-Amino-3-(4-benzyl-3-oxo-3,4-dihydroquinoxalin-2-yl)-benzoate (1j)
Compound 1a (120 mg, 0.29 mmol) was dissolved in hot ethanol (50 mL). After cooling, a saturated NH4Cl solution (30 mL) and Zn dust (1 g) were added, and the mixture stirred for 3 h. The solution was filtered through Celite, concentrated, and purified by flash column chromatography. Yield = 100 mg (90%). 1H NMR (600 MHz, CDCl3) δ 9.26–8.97 (m, 1H), 7.91 (ddd, J = 8.6, 2.1, 0.9 Hz, 1H), 7.83 (dt, J = 8.0, 1.2 Hz, 1H), 7.45 (ddt, J = 8.5, 7.2, 1.2 Hz, 1H), 7.38–7.11 (m, 7H), 6.77 (dd, J = 8.6, 0.8 Hz, 1H), 6.18 (s, 2H), 5.59 (s, 2H), 3.87 (d, J = 0.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.68, 154.23, 151.69, 135.24, 134.67, 132.68, 132.44, 132.17, 130.20, 129.51, 128.90, 127.67, 126.94, 123.79, 118.49, 117.26, 116.47, 114.44, 51.61, 46.44. HRMS [C23H19N3O3 + H]+: calcd 386.1505/found 386.1509.
4-Amino-3-(4-benzyl-3-oxo-3,4-dihydroquinoxalin-2-yl)benzoic Acid (1k)
Compound 1j (20 mg, 0.052 mmol) was dissolved in methanol (100 mL) at 85 °C. KOH (0.2 g, 3.6 mmol) and water (30 mL) were added, and the solution was refluxed overnight. Solvent was removed in vacuo and acidified with 1 N HCl. The product was extracted with dichloromethane. Yield = 13 mg (67%). 1H NMR (600 MHz, acetone-d6) δ 9.20 (d, J = 2.1 Hz, 1H), 7.89 (dd, J = 8.0, 1.5 Hz, 1H), 7.84 (dd, J = 8.6, 2.1 Hz, 1H), 7.51 (ddd, J = 8.5, 7.1, 1.5 Hz, 1H), 7.47 (dd, J = 8.5, 1.4 Hz, 1H), 7.43–7.30 (m, 5H), 7.30–7.19 (m, 1H), 7.08 (s, 2H), 6.93 (d, J = 8.7 Hz, 1H), 5.67 (s, 2H). 13C NMR (151 MHz, acetone-d6) δ 166.89, 154.55, 154.34, 152.99, 136.23, 135.27, 132.57, 132.28, 132.11, 130.02, 129.31, 128.70, 127.32, 126.98, 123.52, 116.81, 116.56, 115.85, 114.84, 45.73. HRMS [C22H17N3O3 + H]+: calcd 372.1348/found 372.1351.
3-(4-Benzyl-3-oxo-3,4-dihydroquinoxalin-2-yl)benzonitrile (1l)
Yield = 27 mg (62%). 1H NMR (400 MHz, CDCl3) δ 8.81 (t, J = 1.7 Hz, 1H), 8.72 (dt, J = 8.1, 1.5 Hz, 1H), 7.97 (dd, J = 8.0, 1.6 Hz, 1H), 7.76 (dq, J = 7.7, 1.4 Hz, 1H), 7.60 (td, J = 7.9, 1.8 Hz, 1H), 7.51 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.41–7.26 (m, 7H), 5.58 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.54, 151.35, 137.00, 135.02, 133.85, 133.45, 133.13, 132.93, 131.27, 130.90, 129.04, 128.92, 127.88, 126.94, 124.20, 118.73, 114.53, 112.46, 46.26. HRMS [C22H15N3O + H]+: calcd 338.1293/found 338.1290.
3-(Benzo[c][1,2,5]oxadiazol-5-yl)-1-benzylquinoxalin-2(1H)-one (1m)
Yield = 30 mg (75%). 1H NMR (400 MHz, CDCl3) δ 9.37− 9.25 (m, 1H), 8.83 (ddd, J = 7.8, 1.7, 1.1 Hz, 1H), 8.34 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.00 (dd, J = 8.0, 1.4 Hz, 1H), 7.71–7.55 (m, 3H), 7.42 (ddd, J = 8.3, 7.0, 1.4 Hz, 1H), 7.25 (dd, J = 5.2, 1.3 Hz, 2H), 7.20 (dt, J = 3.5, 1.0 Hz, 1H), 6.97 (dd, J = 5.1, 3.5 Hz, 1H), 5.70 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.04, 151.18, 148.25, 137.33, 136.88, 135.52, 133.15, 132.49, 131.35, 131.18, 129.04, 127.58, 126.85, 126.01, 124.84, 124.70, 124.31, 113.96, 41.24. HRMS [C21H14N4O2 + H]+: calcd 355.1195/found 355.1207.
1-Benzyl-7-chloro-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2a)
Yield = 31 mg (15%). 1H NMR (400 MHz, CDCl3) δ 9.35 (t, J = 2.0 Hz, 1H), 8.86 (dt, J = 7.9, 1.3 Hz, 1H), 8.36 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.99–7.85 (m, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.44–7.30 (m, 7H), 5.56 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.27, 151.03, 148.24, 137.44, 137.07, 135.52, 134.44, 133.77, 131.97, 131.58, 129.19, 129.09, 128.12, 126.95, 125.02, 124.79, 124.71, 114.47, 46.42. HRMS [C21H14ClN3O3 + H]+: calcd 392.0802/found 392.0810.
1-Benzyl-6-chloro-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2b)
Yield = 56 mg (31%). 1H NMR (400 MHz, CDCl3) δ 9.37 (t, J = 2.0 Hz, 1H), 8.87 (dt, J = 7.9, 1.3 Hz, 1H), 8.39 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.02 (d, J = 2.4 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.49 (dd, J = 9.0, 2.4 Hz, 1H), 7.42–7.20 (m, 6H), 5.60 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.22, 152.30, 148.24, 137.00, 135.59, 134.61, 133.57, 131.59, 131.31, 130.09, 129.60, 129.15, 129.11, 128.06, 126.85, 125.19, 124.86, 115.71, 46.44. HRMS [C21H14ClN3O3 + H]+: calcd 392.0802/found 392.0819.
1-Benzyl-5-chloro-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2c)
Yield = 8 mg (8%). 1H NMR (400 MHz, CDCl3) δ 9.51 (t, J = 2.0 Hz, 1H), 8.96 (dt, J = 7.9, 1.4 Hz, 1H), 8.39 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.71 (t, J = 8.1 Hz, 1H), 7.57–7.40 (m, 2H), 7.40–7.18 (m, 6H), 5.63 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.25, 150.92, 148.29, 137.14, 135.84, 135.73, 134.66, 134.45, 131.23, 129.73, 129.16, 129.12, 128.01, 126.80, 125.26, 125.10, 125.03, 113.41, 46.67. HRMS [C21H14ClN3O3 + H]+: calcd 392.0802/found 392.0813.
1-Benzyl-6,7-dichloro-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2d)
Yield = 13 mg (31%). 1H NMR (400 MHz, CDCl3) δ 9.35 (t, J =2.0 Hz, 1H), 8.86 (ddt, J = 7.9, 2.8, 1.3 Hz, 1H), 8.46–8.28 (m, 1H), 8.11 (s, 1H), 7.70 (td, J = 8.1, 3.8 Hz, 1H), 7.46 (s, 1H), 7.43–7.28 (m, 5H), 5.56 (d, J = 6.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 154.00, 152.23, 148.25, 136.73, 135.57, 134.16, 132.21, 132.08, 131.98, 131.53, 129.28, 129.18, 128.27, 126.90, 125.35, 124.85, 124.73, 115.94, 46.56. HRMS [C21H13Cl2N3O3 + H]+: calcd 426.0412/found 426.0405.
1-Benzyl-7-bromo-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2e)
Yield = 47 mg (15%). 1H NMR (400 MHz, CDCl3) δ 9.35 (t, J = 2.0 Hz, 1H), 8.86 (dt, J = 7.9, 1.3 Hz, 1H), 8.37 (dd, J = 8.4, 2.3 Hz, 1H), 7.86 (d, J = 8.3 Hz, 1H), 7.69 (t, J = 8.1 Hz, 1H), 7.52 (d, J = 8.5 Hz, 2H), 7.46–7.29 (m, 5H), 5.56 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.22, 151.28, 148.25, 137.08, 135.52, 134.44, 133.89, 132.07, 131.89, 129.20, 129.11, 128.13, 127.65, 126.97, 125.69, 125.05, 124.73, 117.46, 46.40. HRMS [C21H14BrN3O3 + H]+: calcd 436.0297/found 436.0291.
1-Benzyl-6,7-difluoro-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2g)
Yield = 13 mg (10%). 1H NMR (400 MHz, CDCl3) δ 9.35 (t, J = 2.0 Hz, 1H), 8.85 (dt, J = 7.9, 1.4 Hz, 1H), 8.38 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.82 (dd, J = 10.0, 8.2 Hz, 1H), 7.70 (t, J = 8.1 Hz, 1H), 7.47–7.22 (m, 5H), 7.15 (dd, J = 11.3, 7.0 Hz, 1H), 5.55 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.16, 152.20 (dd, JC−F = 257, 15 Hz), 151.47, 148.25, 147.15 (dd, JC−F= 250, 15 Hz), 136.87, 135.49, 134.17, 130.27 (br), 129.41 (br), 129.28, 129.15, 128.26, 126.85, 125.17, 124.73, 118.26 (dd, JC−F = 18, 2 Hz), 103.22 (d, JC−F = 23 Hz), 46.87. HRMS [C21H13F2N3O3 + H]+: calcd 394.1003/found 394.0996.
1-Benzyl-8-methyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2h)
Yield = 36 mg (28%). 1H NMR (600 MHz, CDCl3) δ 9.41 (s, 1H), 8.91 (dt, J = 7.9, 1.3 Hz, 1H), 8.43–8.10 (m, 1H), 7.66 (t, J = 8.0 Hz, 1H), 7.50–7.20 (m, 8H), 5.60 (s, 2H), 2.79 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 154.44, 148.96, 148.33, 139.84, 137.88, 135.41, 135.20, 133.19, 131.65, 131.10, 128.92, 128.87, 127.70, 126.84, 125.30, 124.69, 124.54, 112.42, 46.31, 17.59. HRMS [C22H17N3O3 + H]+: calcd 372.1348 found 372.1343
1-Benzyl-6,7-dimethyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2i)
Yield = 13 mg (31%). 1H NMR (400 MHz, CDCl3) δ 9.35 (t, J = 2.0 Hz, 1H), 8.86 (dt, J = 7.8, 1.4 Hz, 1H), 8.33 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.77 (s, 1H), 7.66 (t, J = 8.0 Hz, 1H), 7.43–7.22 (m, 5H), 7.13 (s, 1H), 5.59 (s, 2H), 2.38 (d, J = 1.2 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 154.59, 149.83, 148.21, 141.67, 137.78, 135.44, 135.23, 133.39, 131.66, 131.12, 130.88, 129.00, 128.93, 127.76, 126.91, 124.59, 124.47, 114.91, 46.11, 20.84, 19.21. HRMS [C23H19N3O3 + H]+: calcd 386.1505/found 386.1499.
1-Benzyl-6-bromo-3-(3-nitrophenyl)quinoxalin-2(1H)-one (2f)
Yield = 106 mg (34%). 1H NMR (400 MHz, CDCl3) δ 9.44–9.22 (m, 1H), 8.87 (dt, J = 7.9, 1.4 Hz, 1H), 8.38 (ddd, J = 8.2, 2.4, 1.2 Hz, 1H), 8.18 (d, J = 2.3 Hz, 1H), 7.70 (t, J = 8.1 Hz, 1H), 7.61 (dd, J = 8.9, 2.3 Hz, 1H), 7.44–7.26 (m, 5H), 7.23 (d, J = 9.0 Hz, 1H), 5.59 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.22, 152.22, 148.24, 136.98, 135.59, 134.58, 134.02, 133.86, 133.18, 132.02, 129.14, 128.07, 126.97, 126.85, 125.20, 124.86, 116.82, 115.98, 46.41. HRMS [C21H14BrN3O3 + H]+: calcd 436.0297/found 436.0290.
3-(3-Nitrophenyl)quinoxalin-2(1H)-one (3a)
Yield = 220 mg (75%). 1H NMR (400 MHz, DMSO-d6) δ 12.76 (s, 1H), 9.31–9.12 (m, 1H), 8.78 (ddd, J = 7.9, 1.7, 1.1 Hz, 1H), 8.38 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 7.96–7.88 (m, 1H), 7.82 (t, J = 8.0 Hz, 1H), 7.71–7.48 (m, 1H), 7.47–7.25 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 155.02, 152.12, 147.99, 137.43, 135.81, 132.85, 132.34, 131.58, 130.08, 129.52, 125.19, 124.22, 124.15, 115.77. HRMS [C14H9N3O3 + H]+: calcd 268.0722/found 268.0713. HRMS [C14H9N3O3 + H]+: calcd 268.0722/found 268.0713.
1-Methyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3b)
Yield = 39 mg (74%). 1H NMR (600 MHz, CDCl3) δ 9.29 (s, 1H), 8.79 (dt, J = 7.9, 1.4 Hz, 1H), 8.33 (ddd, J = 8.2, 2.3, 1.1 Hz, 1H), 7.99 (dd, J = 8.0, 1.5 Hz, 1H), 7.74–7.54 (m, 2H), 7.54–7.32 (m, 2H), 3.81 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 154.43, 151.10, 148.27, 137.51, 135.36, 133.59, 132.86, 131.25, 130.83, 128.89, 124.65, 124.64, 124.05, 113.68, 29.34. HRMS [C15H11N3O3 + H]+: calcd 282.0879/found 282.0870.
1-Ethyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3c)
Yield = 54 mg (70%). 1H NMR (400 MHz, CDCl3) δ 9.44–9.14 (m, 1H), 8.83 (ddd, J = 7.9, 1.7, 1.1 Hz, 1H), 8.34 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.09–7.88 (m, 1H), 7.77–7.58 (m, 2H), 7.53–7.36 (m, 2H), 4.44 (q, J = 7.2 Hz, 2H), 1.47 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 153.96, 151.05, 148.19, 137.50, 135.49, 133.16, 132.53, 131.32, 131.10, 128.97, 124.71, 123.96, 113.61, 37.80, 12.44. HRMS [C16H13N3O3 + H]+: calcd 296.1035/found 296.1037.
1-Propyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3d)
Yield = 13 mg (23%). 1H NMR (600 MHz, CDCl3) δ 9.30 (t, J = 1.9 Hz, 1H), 8.80 (dt, J = 7.9, 1.3 Hz, 1H), 8.32 (ddd, J = 8.1, 2.3, 1.1 Hz, 1H), 7.99 (dd, J = 8.0, 1.4 Hz, 1H), 7.73–7.55 (m, 2H), 7.52–7.32 (m, 2H), 4.38–4.17 (m, 2H), 1.87 (hept, J = 7.5 Hz, 2H), 1.10 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 154.18, 151.04, 148.25, 137.55, 135.40, 133.11, 132.82, 131.14, 131.04, 128.86, 124.67, 124.61, 123.85, 113.72, 44.19, 20.67, 11.34. HRMS [C17H15N3O3 + H]+: calcd 310.1192/found 310.1182.
1-Allyl-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3e)
Yield = 91 mg (79%). 1H NMR (400 MHz, CDCl3) δ 9.34 (t, J = 2.0 Hz, 1H), 8.92− 8.73 (m, 1H), 8.35 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.02 (dd, J = 8.0, 1.5 Hz, 1H), 7.77–7.54 (m, 2H), 7.51–7.32 (m, 2H), 6.02 (ddt, J = 17.3, 10.4, 5.2 Hz, 1H), 5.42–5.15 (m, 2H), 5.03 (dt, J = 5.2, 1.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 154.02, 151.05, 148.20, 137.41, 135.51, 133.01, 132.81, 131.28, 130.92, 130.36, 128.99, 124.77, 124.71, 124.15, 118.44, 114.30, 44.88. HRMS [C17H13N3O3 + H]+: calcd 308.1035/found 308.1036.
1-(Naphthalen-1-ylmethyl)-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3f)
Yield = 54 mg (35%). 1H NMR (600 MHz, CDCl3) δ 9.39 (t, J = 2.0 Hz, 1H), 8.89 (dt, J = 7.9, 1.4 Hz, 1H), 8.32 (ddd, J = 8.3, 2.4, 1.1 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.08–8.01 (m, 1H), 7.94 (dd, J = 8.3, 1.2 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.71–7.62 (m, 2H), 7.59 (ddd, J = 8.1, 6.9, 1.1 Hz, 1H), 7.46–7.33 (m, 2H), 7.32–7.22 (m, 1H), 7.08 (dd, J = 8.0, 1.6 Hz, 1H), 6.83 (dd, J = 7.3, 1.2 Hz, 1H), 6.04 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 154.50, 150.92, 148.33, 137.43, 135.48, 133.92, 133.15, 133.12, 131.33, 130.85, 130.52, 129.24, 129.17, 128.91, 128.19, 128.17, 126.71, 126.12, 125.38, 124.73, 124.20, 122.30, 122.07, 114.77, 44.04. HRMS [C25H17N3O3 + H]+: calcd 408.1348/found 408.1342.
1-(Naphthalen-2-ylmethyl)-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3g)
Yield = 80 mg (75%). 1H NMR (400 MHz, CDCl3) δ 9.40 (t, J = 2.0 Hz, 1H), 8.91 (ddd, J = 7.9, 1.7, 1.1 Hz, 1H), 8.38 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.09–7.98 (m, 1H), 7.91–7.80 (m, 2H), 7.78 (dd, J = 6.2, 3.4 Hz, 1H), 7.74–7.65 (m, 2H), 7.57–7.44 (m, 4H), 7.41 (ddd, J = 8.3, 7.6, 1.2 Hz, 2H), 5.79 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.64, 151.22, 148.26, 137.47, 135.59, 133.33, 133.16, 132.99, 132.85, 132.47, 131.39, 130.98, 129.06, 127.75, 126.53, 126.24, 125.72, 124.86, 124.79, 124.72, 124.27, 114.63, 46.52. HRMS [C25H17N3O3 + H]+: calcd 408.1348 found 408.1339.
3-(3-Nitrophenyl)-1-(pyridin-2-ylmethyl)quinoxalin-2(1H)-one (3h)
Yield = 82 mg (90%). 1H NMR (400 MHz, CDCl3) δ 9.37 (t, J = 2.0 Hz, 1H), 8.86 (dt, J = 7.9, 1.4 Hz, 1H), 8.61 (dt, J = 4.8, 1.4 Hz, 1H), 8.36 (ddd, J = 8.2, 2.3, 1.1 Hz, 1H), 8.01 (dd, J = 8.1, 1.3 Hz, 1H), 7.75–7.62 (m, 2H), 7.62–7.51 (m, 2H), 7.41 (ddd, J = 8.2, 6.5, 2.0 Hz, 1H), 7.34 (d, J = 7.9 Hz, 1H), 7.24 (ddd, J = 7.5, 4.9, 1.1 Hz, 1H), 5.73 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 155.11, 154.53, 151.10, 149.49, 148.23, 137.43, 137.27, 135.52, 133.10, 131.43, 130.79, 129.03, 124.82, 124.74, 124.31, 122.98, 122.23, 115.06, 48.25. HRMS [C20H14N4O3 + H]+: calcd 359.1144/found 359.1138.
1-(Furan-2-ylmethyl)-3-(3-nitrophenyl)quinoxalin-2(1H)-one (3i)
Yield = 238 mg (67%) 1H NMR (400 MHz, CDCl3) δ 9.32 (t, J = 2.0 Hz, 1H), 8.83 (dt, J = 7.8, 1.4 Hz, 1H), 8.34 (ddd, J = 8.2, 2.3, 1.1 Hz, 1H), 8.00 (dd, J = 8.0, 1.5 Hz, 1H), 7.78–7.51 (m, 3H), 7.44 (ddd, J = 8.3, 7.0, 1.5 Hz, 1H), 7.39 (s, 1H), 6.49 (d, J = 3.2 Hz, 1H), 6.36 (dd, J = 3.3, 1.9 Hz, 1H), 5.56 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.03, 151.07, 148.57, 148.19, 142.62, 137.34, 135.51, 133.01, 132.82, 131.33, 130.92, 129.00, 124.79, 124.69, 124.26, 114.38, 110.74, 109.85, 39.18. HRMS [C19H13N3O4 + H]+: calcd 348.0985/found 348.0984.
3-(3-Nitrophenyl)-1-(thiophen-2-ylmethyl)quinoxalin-2(1H)-one (3j)
Yield = 48 mg (35%). 1H NMR (400 MHz, CDCl3) δ 9.36–9.28 (m, 1H), 8.83 (ddd, J = 7.8, 1.7, 1.1 Hz, 1H), 8.34 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 8.06–7.95 (m, 1H), 7.72–7.55 (m, 3H), 7.42 (ddd, J = 8.3, 7.0, 1.4 Hz, 1H), 7.25–7.23 (m, 1H), 7.20 (dq, J = 3.5, 0.9 Hz, 1H), 6.97 (dd, J = 5.1, 3.5 Hz, 1H), 5.70 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 154.05, 151.18, 148.25, 137.33, 136.88, 135.53, 133.15, 132.49, 131.35, 131.18, 129.05, 127.58, 126.86, 126.02, 124.85, 124.70, 124.32, 113.96, 41.24. HRMS [C19H13N3O3S + H]+: calcd 364.0756/found 364.0749.
N-(4-nitro-2-(5-oxo-2,3-dihydro-5H-[1,4]oxazino[4,3,2-de]-quinoxalin-6-yl)phenyl)acetamide (16)
2-Amino-3-nitrophenol (1 g, 6.5 mmol) was dissolved in DMF (15 mL). 1,2-Dibromoethane (0.7 mL, 8.1 mmol) and KOH (0.3 g, 5.3 mmol) were added, and the mixture was refluxed at 160 °C for 3 d. After cooling, the solution was poured into water and extracted with ethyl acetate. The ethyl acetate solution was washed with water and brine and then dried with magnesium sulfate. After filtration and concentration in vacuo, the product was purified by column chromatography with a 30:70 mixture of ethyl acetate/hexane to yield intermediate 14 as a red crystalline product. This red product was dissolved in methanol (20 mL), and Pd/C (0.1 g) was added. H2 was bubbled for 2 h until the solution turned nearly colorless. The solution was filtered through Celite, and solvent was removed in vacuo to yield 3,4-dihydro-2H-benzo[b][1,4]-oxazin-5-amine (intermediate 15) as a light brown oil (0.102 g, 10%), which was used directly in the next step.
This 3,4-dihydro-2H-benzo[b][1,4]oxazin-5-amine (15, 0.102 g, 0.68 mmol) was dissolved in a mixture of 20 mL of acetic acid and 20 mL of toluene. N-Acyl-5-nitroisatin (0.22 g, 0.94 mmol) was added, and the mixture was refluxed at 90 °C overnight. Upon cooling, solvent was removed in vacuo, and the residue was washed with ethanol to yield 16 as a dark tan oil. Yield = 0.246 g (99%). 1H NMR (400 MHz, DMSO-d6) δ 10.19 (s, 1H), 8.55 (s, 1H), 8.34 (s, 2H), 7.52 (d, J = 8.0 Hz, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.24 (d, J = 8.0 Hz, 1H), 4.46 (t, J = 4.9 Hz, 2H), 4.19 (t, J = 4.8 Hz, 2H), 2.04 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 169.41, 154.38, 152.13, 143.50, 143.22, 142.31, 132.85, 127.02, 126.93, 125.65, 123.98, 122.47, 122.07, 121.23, 117.02, 63.92, 24.63. HRMS [C22H16N4O4 + H]+: calcd 401.1250/found 401.1237.
6-(2-Amino-5-nitrophenyl)-3-phenyl-2,3-dihydro-5H-[1,4]-oxazino[4,3,2-de]quinoxalin-5-one (20)
2-Amino-3-nitrophenol (0.83 g, 5.4 mmol) was mixed with K2CO3 (1.13 g, 8.2 mmol) in acetonitrile (100 mL). 2-Bromoacetophenone (1.3 g, 6.5 mmol) was added portion-wise and stirred overnight. Ethyl acetate (100 mL) was added, and the solution was filtered and washed with water, 1 N HCl, and brine. The solution was dried over magnesium sulfate and filtered, and solvent was removed in vacuo. The resulting crude product (18) was partly dissolved in hot methanol (100 mL). After cooling, Pd/C (0.2 g) was added, and H2 was bubbled until the starting material was consumed as monitored by TLC. The solution was filtered through Celite, and the solvent was removed in vacuo. The resulting diamine was purified by column chromatography with a 30:70 mixture of ethyl acetate/hexane. An orange-brown oil of intermediate 19 was obtained. Yield = 0.6 g (50%).
This 3-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-5-amine (19: 0.2 g, 0.9 mmol) was dissolved in a mixture of acetic acid (20 mL) and toluene (40 mL). 5-Nitroisatin (0.17 g, 0.88 mmol) was added, and the mixture refluxed at 100 °C for 2 h. Upon cooling, solvent was removed in vacuo, and the product was purified by column chromatography with a 30:70 mixture of ethyl acetate/hexane to yield 20. Yield = 16 mg (5%). 1H NMR (600 MHz, DMSO-d6) δ 9.21 (d, J = 2.8 Hz, 1H), 8.08–7.87 (m, 3H), 7.64 (dd, J = 8.1, 1.2 Hz, 1H), 7.39–7.21 (m, 4H), 7.21–7.09 (m, 3H), 6.90 (d, J = 9.2 Hz, 1H), 5.98 (t, J = 1.6 Hz, 1H), 4.74 (dd, J = 11.8, 1.3 Hz, 1H), 4.45 (dd, J = 11.7, 2.8 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 155.09, 153.39, 151.79, 142.99, 138.01, 135.39, 131.91, 129.46, 129.04, 128.08, 126.93, 126.72, 124.09, 122.12, 120.45, 116.57, 116.25, 115.20, 109.99, 69.48, 53.31. HRMS [C18H14N4O5 + H]+: calcd 367.1043/found 367.1038.
5-Benzyl-9-fluoro-2-(trifluoromethyl)-5a,10a-dihydro-5H-indolo[2,3-b]quinoxaline (22)
Yield = 98 mg (15%). 1H NMR (600 MHz, CDCl3) δ 8.57 (s, 1H), 7.96 (dd, J = 7.6, 2.6 Hz, 1H), 7.83 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 8.9 Hz, 1H), 7.63 (dd, J = 8.6, 4.2 Hz, 1H), 7.41 (td, J = 9.0, 2.7 Hz, 1H), 7.35–7.26 (m, 5H), 6.06 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 158.71 (d, JC−F = 240 Hz), 154.95, 147.04, 134.09, 133.93, 131.33, 129.19, 128.76 (br), 128.27, 126.80, 126.32 (br), 125.80 (d, JC−F = 33 Hz), 123.77, 123.73 (q, JC−F = 272 Hz), 120.58 (q, JC−F = 24 Hz), 119.80, 119.75, 115.35, 109.43 (d, JC−F = 24 Hz), 49.35. HRMS [C22H13F4N3 + H]+: calcd 396.1124/found 396.1105.
5-Benzyl-9-nitro-2-(trifluoromethyl)-5a,10a-dihydro-5H-indolo[2,3-b]quinoxaline (23)
Yield = 17 mg (22%). 1H NMR (800 MHz, DMSO-d6) δ 9.01 (d, J = 2.4 Hz, 1H), 8.71 (d, J = 2.0 Hz, 1H), 8.59 (dd, J = 8.7, 2.4 Hz, 1H), 8.22 (d, J = 8.9 Hz, 1H), 8.17 (dd, J = 9.0, 2.2 Hz, 1H), 7.82 (d, J = 8.7 Hz, 1H), 7.45–7.37 (m, 2H), 7.35–7.31 (m, 2H), 7.31–7.23 (m, 1H), 6.25 (s, 2H). 13C NMR (201 MHz, DMSO-d6) δ 163.95, 154.04, 150.17, 141.98, 135.07, 134.96, 132.05, 129.30, 128.91, 128.50 (br), 128.33, 127.48, 127.44 (br), 125.48 (q, JC−F = 26 Hz), 124.29 (q, JC−F = 204 Hz), 123.34, 119.35, 118.91, 118.21, 49.67. HRMS [C22H13F3N4O2 + H]+: calcd 423.1069/found 423.1062.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (Grants DK072517, DK099803, DK075302, EY13574, DK101373, and EB00415) and the UC Davis Tara K. Telford CF Fund (fellowship to J.S.Z.).
ABBREVIATIONS USED
- CFTR
cystic fibrosis transmembrane conductance regulator
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- FRT
Fischer rat thyroid
- YFP
yellow fluorescent protein
- PBS
phosphate-buffered saline
- RT
room temperature
- TLC
thin layer chromatography
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.6b01759.
1H and 13C NMR data for all assayed compounds (PDF) Molecular formula strings (CSV)
ORCID
Mark J. Kurth: 0000-0001-8496-6125
Author Contributions
The manuscript was written by J.-H.S., J.S.Z., P.-W.P., A.S.V., and M.J.K. Synthesis of substrates was performed by J.-H.S., J.S.Z., A.P.T, and C.K.K. Assays and biological data collection were performed by O.C., P.-W.P., and S.L. All authors have given approval to the final version of the manuscript.
Notes
The authors declare the following competing financial interest(s): O. Cil and A. S. Verkman are named inventors on provisional patent filings, with rights owned by the University of California, San Francisco.
References
- 1.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discovery. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. Activation of intestinal CFTR Cl-channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 1994;13:1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Moon C, Zhang W, Sundaram N, Yarlagadda S, Reddy VS, Arora K, Helmrath MA, Naren AP. Drug-induced secretory diarrhea: A role for CFTR. Pharmacol Res. 2015;102:107–112. doi: 10.1016/j.phrs.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Field M. Mechanisms of action of cholera and Escherichia coli enterotoxins. Am J Clin Nutr. 1979;32:189–196. doi: 10.1093/ajcn/32.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Cil O, Phuan PW, Lee S, Tan J, Haggie PM, Levin MH, Sun L, Thiagarajah JR, Ma T, Verkman AS. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol Gastroenterol Hepatol. 2016;2:317–327. doi: 10.1016/j.jcmgh.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cil O, Phuan PW, Son JH, Zhu JS, Ku CK, Tabib NA, Teuthorn AP, Ferrera L, Zachos NC, Lin R, Galietta LJ, Donowitz M, Kurth MJ, Verkman AS. Phenylquinoxalinone CFTR activator as potential prosecretory therapy for constipation. Transl Res. 2016 doi: 10.1016/j.trsl.2016.10.003. 10.1016/j.trsl.2016.10.003. [DOI] [PMC free article] [PubMed]
- 5.(a) Flores AM, Casey SD, Felix CM, Phuan PW, Verkman AS, Levin MH. Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease. FASEB J. 2016;30:1789–1797. doi: 10.1096/fj.201500180. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee S, Phuan PW, Felix CM, Tan JA, Levin MH, Verkman AS. Nanomolar-potency aminophenyl-1,3,5-triazine activators of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel for pro-secretory therapy of dry eye diseases. J Med Chem. 2017;60:1210–1218. doi: 10.1021/acs.jmedchem.6b01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon GM, Raju SV, Dransfield MT, Rowe SM. Therapeutic approaches to acquired Cystic Fibrosis Transmembrane Conductance Regulator dysfunction in chronic bronchitis. Ann Am Thorac Soc. 2016;13(Suppl 2):S169–176. doi: 10.1513/AnnalsATS.201509-601KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cil O, Phuan PW, Gillespie AM, Lee S, Tradtrantip L, Yin J, Tse M, Zachos NC, Lin R, Donowitz M, Verkman AS. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins. FASEB J. 2017;31:751–760. doi: 10.1096/fj.201600891R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: Cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol. 2015;50(S40):S3–S13. doi: 10.1002/ppul.23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghunadh A, Meruva SB, Kumar NA, Kumar GS, Rao LV, Syam Kumar U. An efficient and practical synthesis of aryl and hetaryl α-keto esters. Synthesis. 2012;2012:283–289. [Google Scholar]
- 11.Lawrence DS, Copper JE, Smith CD. Structure–activity studies of substituted quinoxalinones as multiple-drug-resistance antagonists. J Med Chem. 2001;44:594–601. doi: 10.1021/jm000282d. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Kim KH, Kim JN. Construction of 1,2,5-tricarbonyl compounds using methyl cyanoacetate as a glyoxylate anion synthon combined with copper(I) iodide-catalyzed aerobic oxidation. Adv Synth Catal. 2011;353:3335–3339. [Google Scholar]
- 13.Jean-Claude BJ, Just G. Synthesis of bi- and tri-cyclic tetrazepinones. J Chem Soc, Perkin Trans 1. 1991:2525–2529. [Google Scholar]
- 14.Fox BM, Sugimoto K, Iio K, Yoshida A, Zhang J, Li K, Hao X, Labelle M, Smith ML, Rubenstein SM, Ye G, McMinn D, Jackson S, Choi R, Shan B, Ma J, Miao S, Matsui T, Ogawa N, Suzuki M, Kobayashi A, Ozeki H, Okuma C, Ishii Y, Tomimoto D, Furakawa N, Tanaka M, Matsushita M, Takahashi M, Inaba T, Sagawa S, Kayser F. Discovery of 6-phenylpyrimido[4,5-b][1,4]oxazines as potent and selective acyl CoA:diacylglycerol acyltransferase 1 (DGAT1) inhibitors with in vivo efficacy in rodents. J Med Chem. 2014;57:3464–3483. doi: 10.1021/jm500135c. [DOI] [PubMed] [Google Scholar]
- 15.Coffman KC, Nguyen HN, Phuan PW, Hudson BM, Yu GJ, Bagdasarian AL, Montgomery D, Lodewyk MW, Yang B, Yoo CL, Verkman AS, Tantillo DJ, Kurth MJ. Constrained bithiazoles: small molecule correctors of defective ΔF508-CFTR protein trafficking. J Med Chem. 2014;57:6729–6738. doi: 10.1021/jm5007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2016 doi: 10.1002/hep.28991. [DOI] [PubMed] [Google Scholar]
- 17.Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016;71:284–287. doi: 10.1136/thoraxjnl-2015-207588. [DOI] [PubMed] [Google Scholar]
- 18.Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. High-affinity activators of CFTR chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- 19.Namkung W, Phuan P-W, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 21.Galietta LJ, Springsteel MF, Eda M, Niedzinski EJ, By K, Haddadin MJ, Kurth MJ, Nantz MH, Verkman AS. Novel CFTR chloride channel activators identified by screening of combinatorial libraries based on flavone and benzoquinolizinium lead compounds. J Biol Chem. 2001;276:19723–19728. doi: 10.1074/jbc.M101892200. [DOI] [PubMed] [Google Scholar]
- 22.Phuan PW, Yang B, Knapp JM, Wood AB, Lukacs GL, Kurth MJ, Verkman AS. Cyanoquinolines with independent corrector and potentiator activities restore ΔPhe508-cystic fibrosis transmembrane conductance regulator chloride channel function in cystic fibrosis. Mol Pharmacol. 2011;80:683–693. doi: 10.1124/mol.111.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


