Abstract
Introduction:
The Short-Form McGill Pain Questionnaire (SF-MPQ) is a widely used tool for qualitative and quantitative pain assessment. Our aim was to translate, culturally adapt, and validate the SF-MPQ in Arabic.
Methods:
A systematic translation process was used to translate the original English SF-MPQ into Arabic. After the pilot study, we validated our version in patients with chronic pain at two tertiary care centers. We tested the reliability of our version using internal consistency and test-retest reliability. We examined the validity by assessing construct validity, concurrent validity (by investigating the associations between SF-MPQ, Brief Pain Inventory [BPI], and Self-completed Leeds Assessment of Neuropathic Symptoms and Signs [S-LANSS]), and face validity. The questionnaire was administered twice to examine responsiveness.
Results:
A total of 142 participants (68 men and 74 women) were included in this study. Cronbach's α was 0.85 (95% confidence interval: 0.81 – 0.89), and interclass correlation coefficients were 0.71 (0.62–0.79) for the whole scale. SF-MPQ was moderately associated with patients’ present pain (r = 0.55, P < 0.001) and the numerical rating scale (r = 0.42, P < 0.001). The total pain score was moderately correlated with pain severity and interference assessed with the BPI (rs = 0.39 to 0.49, all Ps < 0.001). SF-MPQ total pain score was weakly associated with neuropathic pain assessed with S-LANSS (r = 0.26, P < 0.01). Most patients found the SF-MPQ questions to be clear and easy to understand and thought the questionnaire items covered all their problem areas regarding their pain.
Conclusion:
Our translated version of SF-MPQ was reliable and valid for use among Arabic-speaking patients. The SF-MPQ is a good qualitative and quantitative assessment tool for pain but is only weakly associated with neuropathic pain.
Keywords: Anesthesia, Arabic, chronic pain, reliability, Short-Form McGill Pain Questionnaire, validity
Introduction
Pain is perhaps the most common chief complaint. When sufficiently severe, pain can impair life quality and worsen overall function. For example, the incidence of chronic pain, especially chronic back and neck pain, is increasing worldwide. In fact, chronic low back and neck pain is now the fourth most common cause of disability-adjusted life years worldwide across all ages and the first among people between 40 and 44 years old.[1]
The prevalence of chronic pain, particularly chronic pain associated with musculoskeletal disorders, is increasing in the Eastern Mediterranean region where most of the world's Arabic-speaking population resides. Musculoskeletal disorders include back, neck, knee, and shoulder chronic pain conditions. Low back pain and neck pain have the highest burden in Eastern Mediterranean regions countries and have the highest number of years lived with disability among other disorders. For example, the reported prevalence of low back pain ranges from 32/1000 in Kuwait to 159/1000 in Egypt.[2]
An individual's pain experience is influenced by various ethnic and cultural factors, which affect level of distress, coping style, and treatment preferences. Pain reporting is, therefore, a complex cognitive process that is influenced by specific cultural and environmental factors. Compounded by its subjective nature, accurate pain assessment is challenging. Various instruments are available to evaluate pain, such as the Brief Pain Inventory (BPI) and the McGill Pain Questionnaire (MPQ). The MPQ, introduced by Melzack and Torgerson in 1971, is among the most widely used instruments to evaluate pain. The original long-form of the questionnaire was modified to a shorter one (Short-Form MPQ [SF-MPQ]) in 1987.[3] The SF-MPQ is suitable to assess sensory, affective, and evaluative dimensions of pain. It is easy to administer and usually takes about 5 min to complete.
Arabic-speaking communities, estimated with a total population of almost 400 million as of 2015, are culturally diverse despite sharing a common language. Although the SF-MPQ has been translated into many languages and validated in many populations around the world, an Arabic version of the SF-MPQ is not yet available. Our goal was thus to translate, culturally adapt, and validate the SF-MPQ questionnaire in Arabic.
Methods
A repeated measures study was conducted between September 2014 and December 2016 in two tertiary hospitals in Riyadh – Saudi Arabia: King Faisal Specialized Hospital (KFSH) (Institutional Review Board [IRB] approval No. 2141 101) and King Fahad Medical City (KFMC) (IRB approval No. 14-107). Data were captured electronically to standardize the data collection process and maintain quality.
Translation and cultural adaptation
Initial translation (forward translation)
Five bilingual translators from five Arabic countries (Syria, Saudi Arabia, Yemen, Sudan, and Egypt) with different dialects were assigned. All translators were native Arabic speakers; two were nonmedical. Each translator produced a written report of the translation that they completed, after which all the translators met to discuss the translation and came to a consensus of the translated version of the instrument.
Backward translation
Two translators who were totally blind to the original (English) questionnaire were assigned to translate the final Arabic version back into the English language. This is a process of validity check to make sure that the translated version reflects the same item content as the original version. English (the source language) was the mother tongue for these two translators, and they were not aware of the concepts being explored.
An expert committee
Composed of a methodologist, health professionals, and language professionals consolidated various versions of the questionnaire and developed the prefinal version of the questionnaire for field testing. The committee eventually reviewed all the translations and reached consensus on any discrepancy.
Measures
Short-Form McGill Pain Questionnaire
The SF-MPQ is a 15-item checklist assessing the sensory dimension (throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender, and splitting) and affective dimension (tiring-exhausting, sickening, fearful, and punishing-cruel) of the pain experience. The 15 items are rated on a four-point pain intensity scale: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. The sum of the intensity values for the corresponding descriptors yields the sensory (11 items), affective (4 items), and total (15 items) pain scores. In addition to the 15-item checklist, two items are included to assess the overall pain experience. Patients were asked to rate the level of their present pain on the present pain intensity (PPI) index: 1 = no pain, 2 = mild, 3 = discomfort, 4 = distressing, 5 = horrible, and 6 = excruciating. Patients also described whether their pain was brief, intermittent, or continuous. The SF-MPQ also includes a visual analog scale.[3]
Numerical rating scale
Numerical rating scale (NRS) is an 11-point pain intensity score used to assess current overall pain intensity (from 0= “no pain” to 10= “pain as bad as you can imagine”).[4]
Brief Pain Inventory
The BPI is used to assess patients’ pain in clinical settings. Two domains of pain are assessed with the BPI – pain severity and pain interference. Pain severity is measured with four items, assessing pain at its “worst,” “least,” “average,” and “now” (current pain). The intensity of pain is rated from 0 (no pain) to 10 (pain as bad as you can imagine). Pain interference is measured with seven items, assessing the extent to which pain has interfered with seven daily activities (general activity, walking, work, mood, enjoyment of life, relations with others, and sleep). Patients rated, from 0 (does not interfere) to 10 (completely interferes), how pain has interfered with their functioning.[5] We used the MD Anderson Cancer Center Arabic BPI-SF version, a previously translated and validated version.[6] In the current study, Cronbach's α was 0.82 and 0.87 for pain severity and pain interference, respectively.
Self-completed Leeds Assessment of Neuropathic Symptoms and Signs
The Self-completed Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) consists of seven items assessing pain of primarily neuropathic origin. It comprises a 7-item pain scale, including the sensory descriptors and items for sensory examination. A score of 12 or above with the S-LANSS suggests pain of predominantly neuropathic origin.[7] We used a previously translated and validated Arabic version of the questionnaire.[8] Cronbach's α was 0.59 in the current sample.
Study protocol
An Arabic version of the SF-MPQ questionnaire was administered twice to chronic pain patients in the pain clinic. This questionnaire was part of a package that contained other questionnaires (BPI and S-LANSS) as validating questionnaires (all in Arabic). Eligible patients were between 17 and 80 years old and reported chronic pain of at least 3 months’ duration. Exclusion criteria included psychosis, significant visual impairment, physical disability, or patient's refusal to participate in the study. The patients completed the questionnaire for the first time (Time 1) in the clinic, after the researcher explained the purpose of the study, obtained verbal consent, and answered all queries. The questionnaire was completed the second time (Time 2) by telephone interview after at least 3 days.
Pilot study
The prefinal version was pilot tested on a group of 34 patients (19 males and 15 females, data not shown). Both interviews (Time 1 and Time 2) were completed in person, after which the participants were asked about their experience and thoughts about the current version. No specific constructive feedback was received. The committee met at this point and approved the prefinal version as final [the final Arabic version is presented in Appendix 1]. No changes were implemented to the prefinal version.
Assessing face validity
After completing the SF-MPQ at Time 1, patients responded to five statements regarding the SF-MPQ items on a 5-point Likert-type scale: 1 = totally disagree, 2 = disagree, 3 = undecided, 4 = agree, and 5 = strongly agree. The five statements were: (1) questions were clear and easy; (2) questions covered all your problem areas regarding your pain; (3) you would like the use of this questionnaire for future assessments; (4) the questionnaire lacks important questions regarding your pain; and (5) some of the questions violate your privacy.
Statistical analysis
All data analyses were performed in R version 3.3.2 (2016-10-31). Descriptive statistics (mean, standard deviation [SD], minimum, and maximum) were presented for all the SF-MPQ items, SF-MPQ composite scores (sensory, affective, and total pain scores), BPI items, and the S-LANSS composite score.
Reliability
The internal consistency of the SF-MPQ was examined using Cronbach's α. Cronbach's α ranges from 0 (no internal consistency; none of the items are correlated with each other) to 1 (perfect internal consistency; all of the items are perfectly correlated with each other). αs were computed for all the 15 items (total pain scores) as well as the sensory (11 items) and affective pain (4 items) subscales. An instrument with α ≥70 is typically considered to have adequate internal consistency.[9] As α is a function of the questionnaire's length, α is expected to be lower for the subscales than for the total scale.
Test-retest reliability was assessed by a second administration (T2) of the SF-MPQ, after at least 48 h of the first administration (T1). The stability of the individuals’ responses was estimated using the Pearson's correlation coefficients (r) and intraclass correlation coefficients (ICCs) between their responses in the two administrations. rs and ICCs between the two assessments were computed for the SF-MPQ sensory, affective, and total pain scores. Test-retest reliability was considered to be weak if r <0.3, moderate if 0.3 ≤ r <0.5, and strong if r ≥0.5. ICC ≥0.70 was considered to indicate good test-retest reliability.[10]
Validity
Construct validity of the SF-MPQ was examined by investigating the associations between the SF-MPQ composite scores (total, sensory, and affective pain), patients’ present pain and pain description in the SF-MPQ, and the NRS. To establish concurrent validity of the SF-MPQ, the extent to which the SF-MPQ is correlated with two other validated measures of pain, the BPI and the S-LANSS, was examined. Pearson's correlation coefficient (r) was used to evaluate the strength of the associations between continuous variables; r <0.3 was considered to be weak, moderate if 0.3 ≤ r<0.5, and strong if r ≥0.5. Given the ordinal nature of the SF-MPQ pain description (brief, intermittent, and continuous), linear regression models were used to examine the extent to which SF-MPQ scores differ among patients who described their pain as brief, intermittent, or continuous.
Results
A total of 142 participants (68 men and 74 women) participated in the validation study of the SF-MPQ questionnaire. The average age was 51 years (SD = 16), with average body mass index of 32 kg/m2 (SD = 8). Most of the patients had university-level education (42%), with fewer having received some high school (31%), less than high school (12%), or no education (15%). The majority of our patients were married (84%); 9% were single, 3% were divorced, and 4% were widowed. Twenty-eight percent were rated as American Society of Anesthesiologists Physical Status 1, 55% were rated as 2, 17% were rated as 3, and < 1% were rated as 4. One hundred and nine (727%) patients were from KFSH and 33 (23%) from KFMC. Most of the patients (92%) reported having current pain.
The causes of chronic pain were radiculopathy 63 (44.3%), mechanical neck pain 11 (7.7%), musculoskeletal 10 (7%), osteoarthritis 10 (7%), nerve injury 8 (5.6%), failed back surgery syndrome 7 (5%), sacroiliitis 5 (3.5%), unknown cause 4 (2.8%), spinal stenosis 3 (2.1%), spinal cord injury 2 (1.4%), carpal tunnel syndrome 2 (1.4%), complex regional pain syndrome 2 (1.4%), postamputation 2 (1.4%), spondylolisthesis 2 (1.4%), trigeminal neuralgia 2 (1.4%), chronic headache 1 (0.7%), diabetic neuropathy 1 (0.7%), fasciculopathy 1 (0.7%), fibromyalgia 1 (0.7%), meralgia paresthetica 1 (0.7%), occipital neuralgia 1 (0.7%), rotator cuff tear 1 (0.7%), spondylosis 1 (0.7%), and thoracic outlet syndrome 1 (0.7%).
Patients were contacted for the second interview an average of 9 days (SD = 8) after their initial participation. The majority of the patients (92%) completed the second interview within 10 days after the initial interview.
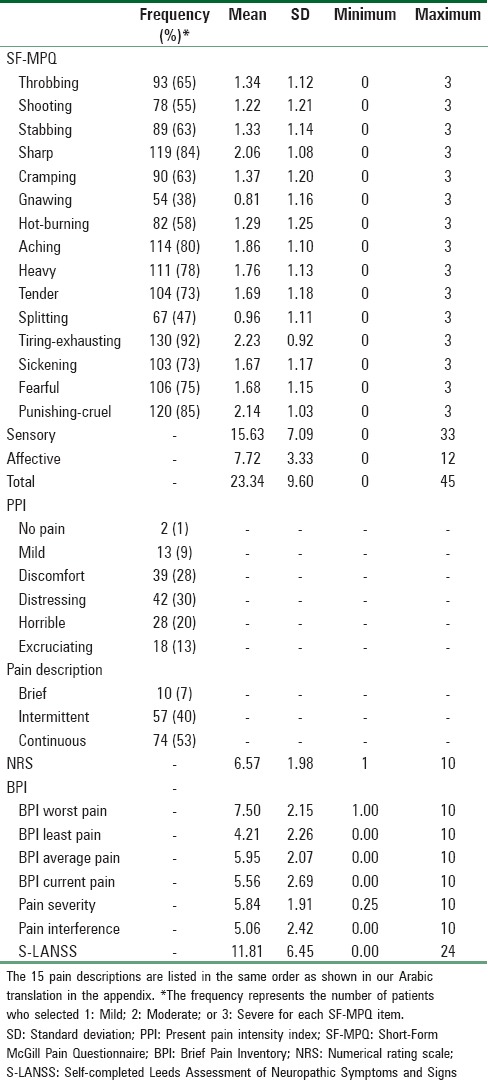
The descriptive statistics of the SF-MPQ, NRS, BPI, and S-LANSS at Time 1 and Time 2 are presented in Tables 1 and 2, respectively.
Table 1.
Descriptive statistics of the Short-Form McGill Pain Questionnaire, numerical rating scale, Brief Pain Inventory, and Self-completed Leeds Assessment of Neuropathic Symptoms and Signs at Time 1 for chronic pain patients
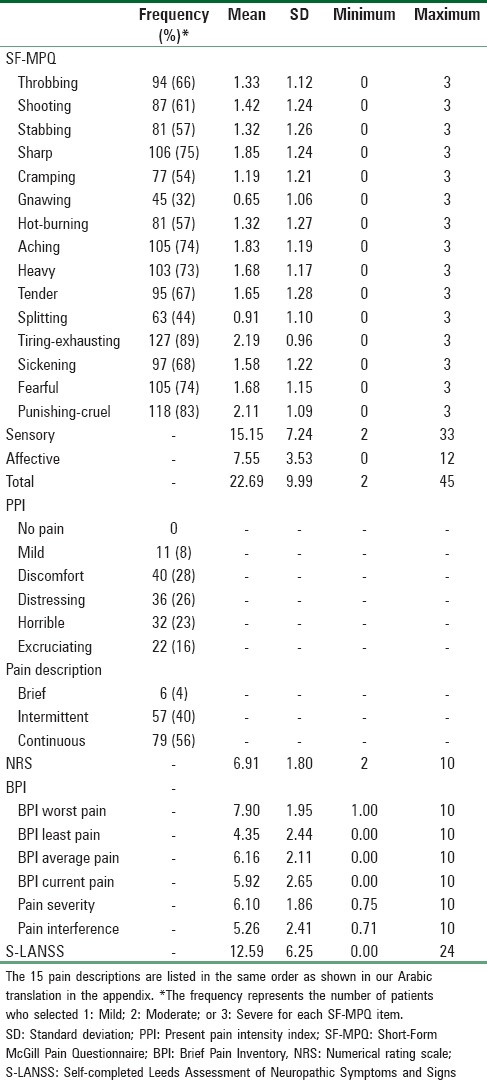
Table 2.
Descriptive statistics of the Short-Form McGill Pain Questionnaire, numerical rating scale, Brief Pain Inventory, and Self-completed Leeds Assessment of Neuropathic Symptoms and Signs at Time 2 for chronic pain patients
Reliability
Cronbach's α for the SF-MPQ is shown in Table 3. Results showed good internal consistency for the overall 15 SF-MPQ items. The internal consistencies for the sensory and affective subscales were slightly lower as to be expected given the subscales consist of fewer items.
Table 3.
Cronbach's alpha for the Short-Form McGill Pain Questionnaire
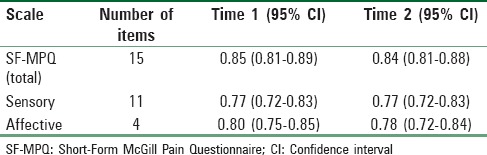
Test-retest reliability was estimated using patients (n = 129) with complete SF-MPQ data for both interviews; patients with missing data on the SF-MPQ were omitted. rs between the two interviews was 0.71 (95% confidence interval [CI]: 0.62–0.79), 0.72 (0.63–0.79), and 0.66 (0.55–0.74) for the total, sensory, and affective pain scores, respectively.
ICCs were 0.71 (95% CI: 0.62–0.79), 0.72 (0.63–0.8), and 0.66 (0.56–0.75) for the total, sensory, and affective pain scores, respectively. Results suggested good test-retest reliability for the SF-MPQ total and sensory pain scores and moderate test-retest reliability for the affective pain dimension.
The correlations between the two assessments were also examined for patients’ responses on the NRS (r = 0.51, 95% CI: 0.38–0.62), PPI (r = 0.73, 95% CI: 0.64–0.8), and the description of their pain (r = 0.61, 95% CI: 0.5–0.71). Results showed moderate to strong associations between the two assessments, indicating good test-retest reliability for these three individual items on the SF-MPQ.
Validity
Construct validity
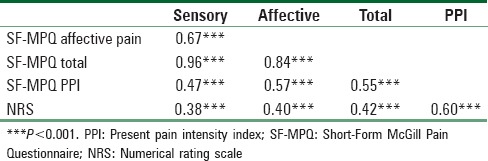
The construct validity of the SF-MPQ was assessed by examining the correlations between the SF-MPQ, PPI, and the NRS at Time 1. Results in Table 4 showed that the SF-MPQ sensory, affective, and total pain scores were moderately associated with PPI (rs = 0.47–0.57, all Ps < 0.001) and the NRS (rs = 0.38–0.42, all Ps < 0.001). Patients who reported more pain on the SF-MPQ were more likely to report more pain on the PPI and scored higher on the NRS than those who reported less pain on the SF-MPQ.
Table 4.
Pearson correlation coefficients between Short-Form McGill Pain Questionnaire, present pain, and numerical rating scale at Time 1
The average SF-MPQ pain scores among patients who reported their pain as brief, intermittent, or continuous at Time 1 are illustrated in Figure 1. Linear regression models were used to examine the average differences on the SF-MPQ sensory, affective, and total pain scores among patients who described their pain as brief, intermittent, or continuous at Time 1. Results showed significant variation for SF-MPQ sensory (F(2, 133) = 4.77, P = 0.01), affective (F(2, 134) = 9.09, P <0.0001), and total pain scores (F(2, 132) = 6.96, P = 0.001). Post-hoc Tukey tests showed that patients who described their pain as continuous had statistically significantly higher sensory (t = 2.81, P = 0.01), affective (t = 3.41, P = 0.002), and total pain scores (t = 3.25, P = 0.004) than those who described their pain as intermittent. Results from post hoc Tukey tests also showed that patients who described their pain as continuous had statistically significantly higher affective (t = 3.09, P = 0.006) and total pain scores (t = 2.35, P = 0.047) than those who described their pain as brief.
Figure 1.
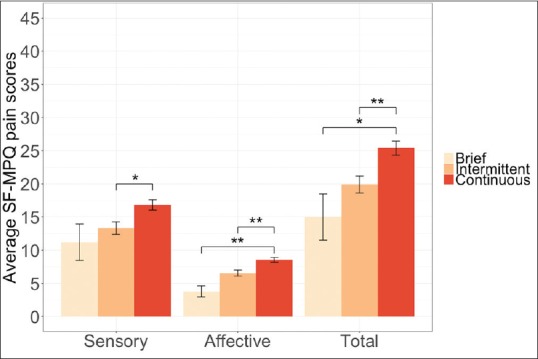
Average Short-Form McGill Pain Questionnaire sensory, affective, and total pain scores by pain description at Time 1. Error bars represent standard errors (*P < 0.05; **P < 0.01). The maximum scores for total SF-MPQ pain scores are 45
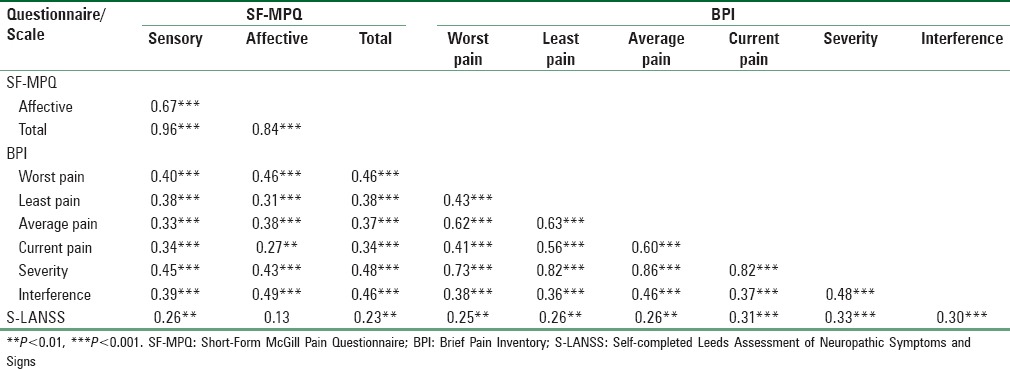
To investigate the concurrent validity of the SF-MPQ, the extent to which the SF-MPQ was associated with the BPI and S-LANSS was examined. As shown in Table 5, the SF-MPQ sensory, affective, and total pain scores were moderately correlated with pain severity and interference assessed with the BPI (rs = 0.39–0.49, all Ps < 0.001). The SF-MPQ sensory, affective, and total pain scores were also positively correlated with the four BPI items assessing the worst, least, average, and current pain (rs = 0.27–0.46, all Ps < 0.01). The SF-MPQ sensory and total pain scores were weakly associated with neuropathic pain assessed with S-LANSS (rs = 0.23–0.26, all Ps < 0.01), whereas the association between SF-MPQ affective pain score and S-LANSS was not statistically significant.
Table 5.
Pearson correlation coefficients between Short-Form McGill Pain Questionnaire, Brief Pain Inventory, and Self-completed Leeds Assessment of Neuropathic Symptoms and Signs at Time 1
Face validity
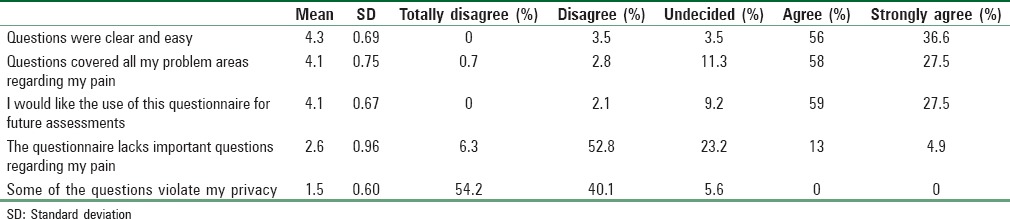
Patients’ responses to the five questions assessing the face validity of the SF-MPQ are presented in Table 6. The majority of the patients endorsed agree or strongly agree for the first three questions assessing face validity. Results showed that most patients found the SF-MPQ questions to be clear and easy to understand, the questionnaire items covered all their problem areas regarding their pain, and most would like to use the SF-MPQ for their long-term follow-up assessment. Most patients disagreed that the SF-MPQ lacks important questions regarding their pain, suggesting that the SF-MPQ addressed most, if not all, of the important issues associated with their pain. Finally, none of the patients felt that the SF-MPQ questions violated their privacy.
Table 6.
Descriptive statistics for face validity
Discussion
A common limitation of pain assessment tools is their sociocultural dependence, which limits their use in different populations. A simple translation of a given instrument is of little value due to the difficulty of matching the conceptual semantics and metric equivalencies of the items between different languages. The adaptation process overcomes this problem by creating a translated version with a better cultural fit to the intended population.
Our results indicated good internal consistency and good test-retest reliability for the Arabic version of the SF-MPQ. SF-MPQ sensory, affective, and total pain scores were moderately associated with NRS. The SF-MPQ sensory, affective, and total pain scores were moderately correlated with pain severity and interference assessed with the BPI and weakly associated with S-LANSS. As in previous studies, the SF-MPQ showed only weak correlation with neuropathic pain.[11] The weak correlation between the SF-MPQ and S-LANSS suggests that the SF-MPQ is not a good predictor of neuropathic pain as the SF-MPQ does not include symptoms specific to neuropathic pain, such as numbness.
Our findings indicate that our translated version of the SF-MPQ is reliable and valid for use in Arabic-speaking patients. All selected descriptive pain words in our questionnaire were used at least by one-third of the sample. This cutoff was used as a requirement for deciding if the selected word is appropriate to describe the pain state in the group studied.[3] The most commonly used description was “tiring-exhausting” (89%–92%), and the one used least was “gnawing” (32%–37%). The descriptions “punishing-cruel” and “tiring-exhausting” were associated with highest pain intensity scores.
Translation of a preexisting questionnaire into a different language and its subsequent validation is important to understand the psychometric properties of a given scale and enable cross-cultural comparisons. The SF-MPQ was previously translated and validated into multiple languages including Spanish,[12] Brazilian–Portuguese,[13] Greek,[14] Japanese,[15] Thai,[16] and Turkish.[17] The introduction of an Arabic version of the SF-MPQ will allow clinicians and researchers to examine the similarities and differences in pain among patients from different countries.
Although none of the participants thought any of the questions violate their privacy, about 5% were undecided. About 18% of the participants thought that the questionnaire lacks important questions about their pain. As none of the participants provided additional comments with respect to their response choices, it is unclear whether specific revisions can be made for improvement for the current translated version. Future studies should consider examining whether additional items need to be included to further address issues related to pain that may have been overlooked in the SF-MPQ.
Conclusion
Our translated version of SF-MPQ was reliable and valid for use among Arabic-speaking patients. Furthermore, we provided a validated translation for commonly used pain descriptions that can be used in various clinical situations for pain assessment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Dr. Daniel I. Sessler for his critical review and manuscript editing. We would like to thank Doctors Bjarni Valtysson, Hani Alghamd, and Mamdouh Haddara from the Department of Anesthesiology and Pain Management in King Faisal Specialist Hospital, Riyadh, Saudi Arabia, and Doctors Shehu Yousef, Ali Al Shoaiby, and Sarfaraz Khan from the Department of Anesthesiology and Pain Management in King Fahad Medical City, Riyadh, Saudi Arabia, for their help in facilitating patients recruitments.
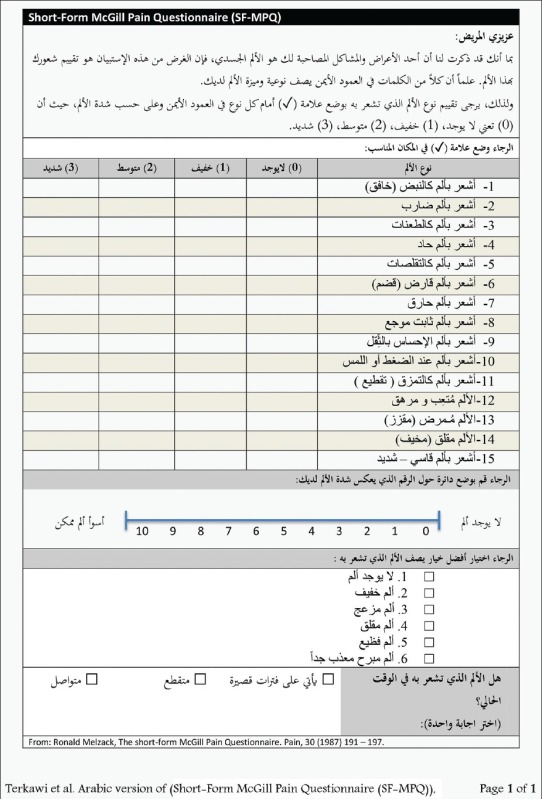
Appendix 1: Arabic Short-Form McGill Pain Questionnaire version
References
- 1.GBD DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–58. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moradi-Lakeh MH, Vollset SE, El Bcheraoui C, Daoud F, Afshin A, Charara R, et al. Burden of musculoskeletal disorders in the Eastern Mediterranean Region, 1990–2013: Findings from the Global Burden of Disease Study 2013. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210146. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 4.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: The Guilford Press; 2001. pp. 15–34. [Google Scholar]
- 5.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 6.Brief Pain Inventory – Arabic Version. [Last accessed on 2017 Jan 07]. Available from: https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI-SF_English-24h_Original_SAMPLE.pdf .
- 7.Bennett M. The LANSS Pain Scale: The Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–57. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 8.Elzahaf RA, Tashani OA, Unsworth BA, Johnson MI. Translation and linguistic validation of the Self-completed Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) Scale for use in a Libyan population. Pain Pract. 2013;13:198–205. doi: 10.1111/j.1533-2500.2012.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Nunnally J. Psychometric Theory. New York: McGraw-Hill; 1978. [Google Scholar]
- 10.Nunnally JC, Bernstein IH. Psychometric Theory. 3rd ed. New York: McGraw-Hill; 1994. [Google Scholar]
- 11.Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill pain questionnaire. J Pain. 2012;13:1250–7. doi: 10.1016/j.jpain.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lázaro C, Caseras X, Whizar-Lugo VM, Wenk R, Baldioceda F, Bernal R, et al. Psychometric properties of a Spanish version of the McGill Pain Questionnaire in several Spanish-speaking countries. Clin J Pain. 2001;17:365–74. doi: 10.1097/00002508-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira KA, de Andrade DC, Teixeira MJ. Development and validation of a Brazilian version of the short-form McGill pain questionnaire (SF-MPQ) Pain Manag Nurs. 2013;14:210–9. doi: 10.1016/j.pmn.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Georgoudis G, Watson PJ, Oldham JA. The development and validation of a Greek version of the short-form McGill pain questionnaire. Eur J Pain. 2000;4:275–81. doi: 10.1053/eujp.2000.0186. [DOI] [PubMed] [Google Scholar]
- 15.Arimura T, Hosoi M, Tsukiyama Y, Yoshida T, Fujiwara D, Tanaka M, et al. Pain questionnaire development focusing on cross-cultural equivalence to the original questionnaire: The Japanese version of the Short-Form McGill pain questionnaire. Pain Med. 2012;13:541–51. doi: 10.1111/j.1526-4637.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 16.Kitisomprayoonkul W, Klaphajone J, Kovindha A. Thai short-form McGill pain questionnaire. J Med Assoc Thai. 2006;89:846–53. [PubMed] [Google Scholar]
- 17.Yakut Y, Yakut E, Bayar K, Uygur F. Reliability and validity of the Turkish version short-form McGill pain questionnaire in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1083–7. doi: 10.1007/s10067-006-0452-6. [DOI] [PubMed] [Google Scholar]


