Abstract
Introduction:
Little is known about the burden of chronic pain after major head and neck tumors’ therapy. In this study, we aimed to estimate the prevalence of chronic pain, explore the factors associated with the presence of chronic pain, and assess the consequences of chronic pain on the patients’ quality of life.
Methodology:
This was a cross-sectional survey among patients who had completed their therapy (e.g., surgery, radiotherapy, and chemotherapy) for major head and neck (larynx, nasopharynx, oropharynx, hypopharynx, oral cavity, tongue, and sinuses) tumors after at least 3 months. We collected relevant demographic and clinical data and administered the Brief Pain Inventory-Short Form, Neuropathic Pain Questionnaire-Short Form, and Pain Catastrophizing Scale questionnaires. Possible risk factors were explored using a classification tree model.
Results:
A total of 102 patients (59 men, 42 women) were enrolled in this study between 3 and 72 months after tumor treatment. 30% of the patients reported having chronic pain after their major head and neck tumors’ therapy. The average pain score in the last 24-hr was 3.4 (standard deviation = 2.7). The prevalence of patients with chronic pain was higher (42%) among those who had surgery. Factors associated with chronic pain were female sex, older age, surgery, advanced cancer stage, and radiotherapy. Patients who reported having chronic pain also reported having a lower quality of life manifested by impairments in general activity, mood, walking ability, normal work, and sleeping. Patients who reported having chronic pain had higher Pain Catastrophizing Scale scores.
Conclusion:
Our study highlighted the high burden of chronic pain after therapy for major head and neck tumors. We identified demographic and clinical factors that are associated with the presence of chronic pain. Further studies are required to better understand the risk factors to implement strategies to prevent, alleviate, and treat chronic pain associated with major head and neck tumor therapies.
Keywords: Anesthesia, cancer, chronic pain, head and neck, radiotherapy, surgery
Introduction
Head and neck tumors include benign and cancerous tumors in the larynx, throat, lips, mouth, nose, and salivary glands. They are relatively uncommon, for instance, head and neck cancers account for about 3% of all cancers in the United States in 2016. Common head and neck cancer global prevalence in 2015, in thousands, are as follows: nasopharynx cancer 732.7 (580.5–883.9), which has increased by 18.0% (0.2%–39.8%) from 2005, laryngeal cancer 1412.6 (1340.0–1499.9), which has increased by 26.2% (20.1%–33.0%) from 2005, and lip and oral cavity cancer 2425.1 (2278.7–2582.3), which has increased by 38.6% (30.3%–47.1%) from 2005.[1]
Although head and neck cancers are relatively uncommon malignancies, treatment usually requires invasive surgeries, radiotherapy, and chemotherapy, which may lead to lifelong disabilities. Nonetheless, we know little about the burden of chronic pain after therapy in this population. Understanding the burden of chronic pain after major head and neck tumor therapy is important for multiple reasons. First, by recognizing the possibility of chronic pain, physicians (e.g., surgeons and oncologists) can prepare patients and their family members in advance, and develop strategies (e.g., pain and behavioral therapy) to help patients cope with persistent pain after treatment. Second, management plans can be better tailored for patients, especially for tumors with poor prognostic outcomes. Finally, as anesthesiologists become more cognizant of risk factors associated with this issue, perioperative measures may be implemented to reduce the probabilities of chronic pain. For instance, lidocaine infusion and paravertebral nerve block have been shown to reduce the incidence of chronic postmastectomy pain.[2,3]
The primary aim of this study was to estimate the prevalence of chronic pain after major head and neck tumors’ therapy. The secondary aims were to identify the factors that are associated with the presence of chronic pain and assess the impact of chronic pain on patients’ quality of life.
Methodology
This was a cross-sectional survey conducted between May 2015 and December 2016 in King Fahad Medical City, Riyadh, Saudi Arabia (Institutional Review Board Approval No. 15-230). Eligible patients were those who had completed their therapy (e.g., surgery, radiotherapy, and chemotherapy) for major head and neck tumor for at least 3 months, regardless of age, gender, or ethnicity. Head and neck tumors that are eligible are as follows: (1) larynx, (2) nasopharynx, (3) oropharynx, (4) hypopharynx, (5) oral cavity, (6) tongue, (7) sinuses, and (8) salivary glands. Exclusion criteria were patient refusal, patient unable to understand the questionnaires, and those with thyroid gland cancer. An electronic data-capturing template was made to standardize data collection and maintain quality.
Data collection
Demographics: Age, sex, weight, and height (at the time of the interview)
Clinical characteristics: Complained of pain at the time of the diagnosis, tumor stage, site and size, tumor histopathologic diagnosis, date of diagnosis, whether the patient has tracheostomy, as well as tumor recurrence and metastasis
Treatment: Treatment modality, including type, dosage, and duration
-
Interview questions:
- Have you had pain at the time of the initial cancer diagnosis?
- Was pain your main complaint at the time of the diagnosis?
- Do you currently have pain at the site of surgery/cancer?
- How severe is your pain (from 0 = no pain to 10 = pain as bad as you can imagine)?
- What is the duration of pain?
- What is the characteristic of the pain? Brief, intermittent, or continuous
- How would you describe the pain? Throbbing, stabbing, sharp, aching, heavy, tender, and hot burning (adopted from the Arabic version of Short-Form McGill Pain Questionnaire)[4]
- What are the factors that increase your pain? Swallowing, speaking, others (specify)
- Are you currently taking any medication for pain?
- Has the cancer treatment worsened your pain?
- If yes, what do you think is the cause of the pain?
- If the patient had graft, do you feel pain at the site of graft?
- How severe is your pain (from 0 = no pain to 10 = pain as bad as you can imagine)?
All patients completed the following questionnaires:
Brief Pain Inventory-Short Form Arabic version
The Brief Pain Inventory-Short Form (BPI-SF) is commonly used to assess patients’ pain in clinical settings.[5] Two domains of pain are assessed with the BPI – pain severity and pain interference. Pain severity is measured with four items, assessing pain at its “worst,” “least,” “average,” and “now” (current pain). The intensity of pain is rated from 0 (no pain) to 10 (pain as bad as you can imagine). Pain interference is measured with seven items, assessing the extent to which pain has interfered with seven daily activities (general activity, walking, work, mood, enjoyment of life, relations with others, and sleep). Patients rate, from 0 (does not interfere) to 10 (completely interferes), how pain has interfered with their functioning. In the present study, Cronbach's alphas (α) were 0.91 and 0.89 for pain severity and pain interference at the primary site and 0.76 and 0.65 for pain severity and pain interference at the graft site, respectively.
Neuropathic Pain Questionnaire-Short Form Arabic version
The Neuropathic Pain Questionnaire-Short Form (NPQ-SF) consists of three items assessing tingling pain, numbness, and increased pain due to touch. Patients rate each item on a scale from 0 (no pain) to 100 (worst imaginable pain/greatest increase). After multiplying each item score by a discriminant function coefficient (tingling: 0.017, numbness: 0.015, increased pain due to touch: 0.011), the scores are summed and incorporated with a set constant value (−1.302) to create a discriminant function score. A score of 0 or above indicate neuropathic pain.[6] Cronbach's αs for the NPQ-SF were 0.63 and 0.52 for the primary and graft sites, respectively.
Pain Catastrophizing Scale Arabic version
The Pain Catastrophizing Scale (PCS) consists of 13 items assessing the thoughts and feelings associated with pain. The PCS assesses three dimensions of pain catastrophizing: rumination (four items), magnification (three items), and helplessness (six items). Patients rate on a 5-point Likert-type scale (0 = not at all, 1 = to a slight degree, 2 = to a moderate degree, 3 = to a great degree, 4 = all the time), the degree to which they have the described thoughts and feelings when they are experiencing pain. The total PCS score is computed by summing the score on all the items, with a higher score indicating increased tendency of pain catastrophizing. The scores for the three PCS subscales are obtained by summing the corresponding items.[7] In the present study, Cronbach's αs were 0.93, 0.84, 0.85, and 0.86 for the PCS total, rumination, magnification, and helplessness scales, respectively.
Data analysis
All data analyses were performed in R version 3.3.2 (2016-10-31). Descriptive statistics (mean, standard deviation [SD], minimum, maximum) were presented for continuous variables, whereas number (n) and proportion (%) were presented for categorical variables. Differences between chronic pain and nonchronic pain patients were examined using Chi-square test, Fisher's exact test, or linear regression models when appropriate. Associations between the pain measures, chronic pain, demographic and clinical characteristics were examined using multiple regression models.
Classification tree model, a nonparametric statistical method used to formulate decision rules, was used to explore the relative importance of demographic and clinical characteristics in identifying patients reported having chronic pain and those who did not. There is no prior assumption regarding the distribution of the predictor variables, classification tree model is particularly applicable considering that most of the clinical variables are categorical. The classification tree model was fitted by recursively partitioning the data into increasingly homogeneous subgroups until no further improvements can be made.[8]
The classification tree model was constructed using the “rpart” package in R,[9] which implemented the classification and regression tree method.[10] The optimum tree model was obtained using 10-fold cross-validation and twenty minimum cases in parent node. Gini index was used to determine which variable gives the best split (i.e., the primary splitter). After the data are separated, the process is applied to each subgroup and continued recursively until no further improvement can be made. In addition to the primary splitters (i.e., the variable that provides the best split at the node), candidate splitters (i.e., variables that give the second and third splits at the node) are also identified. These candidate splitters are considered important variables, but are not used in the actual splitting of the classification tree. Only the variable that provided the best split at a node was presented in the present study.
The relative importance of each variable included in the fitted tree models was evaluated using varImp() in the “caret” package.[11] The measure of variable importance is computed as the sum of the improvement measure attributable to each predictor variable in each split for which it was a primary or candidate (i.e., important but not used in a split) splitter. The variable importance measure is scaled to be between 0 (the least important) and 100 (the most important).
Results
A total of 102 patients (59 men, 42 women) were enrolled in this study. The average age was 49.6 years (SD = 14.8), with average body mass index (BMI) of 28.3 (SD = 6.2). The duration after therapy at the time of interview ranged between 3 and 72 months (mean = 21.6, SD = 17.6). 93 (91%) patients had malignant cancer and 9 (9%) had benign tumors. 11 (12%) patients had cancer recurrence. 82 (80%) patients were interviewed at the clinic, while 20 (20%) had phone interview.
Among the 102 patients, 30 (30%) reported chronic persistent pain after therapy at the primary tumor/surgery site. 39 patients (38%) reported pain as a primary presentation at the time of diagnosis, among which 16 patients (41%) continue to have pain after therapy (i.e., at the time of interview). When asked “what you think is the cause of your current chronic pain?,” 58.3% (n = 14/24) of the patients answered “surgery,” 25% (n = 6/24) answered “radiotherapy,” 12.5% (n = 3/24) answered “both radiotherapy and chemotherapy,” and 4% (only one patient) answered “cancer recurrence.” In response to the question, “whether the treatment that you had worsen your baseline pain?,” 23 (22.5%) patients thought that cancer treatment has worsened their pain.
Sixty-one (60%) patients had surgery for their tumor, 59 for the primary tumor, and two for recurrent tumor. The prevalence of chronic postsurgical pain (CPSP) was 42% (26 out of 61 patients). Among these 26 patients, 16 (61%) reported having pain as the primary presenting symptom at the time of the diagnosis. 35% (n = 6/17) of the patients who had flap surgery also reported chronic pain at the flap site. Among patients who had combined chemo-radiotherapy (no surgery), 8.5% (n = 3/35) reported having chronic pain, whereas 25% (n = 2/8) of the patients who had radiotherapy only reported having chronic pain.
Other demographic and clinical characteristics of patients separated by chronic pain are shown in Tables 1 and 2. The characteristics of this pain are presented in Table 3.
Table 1.
Descriptive statistics of patients with and without chronic pain
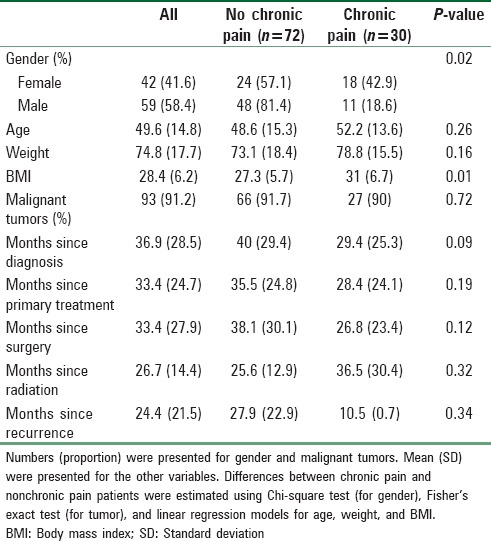
Table 2.
Clinical characteristics of patients with and without chronic pain
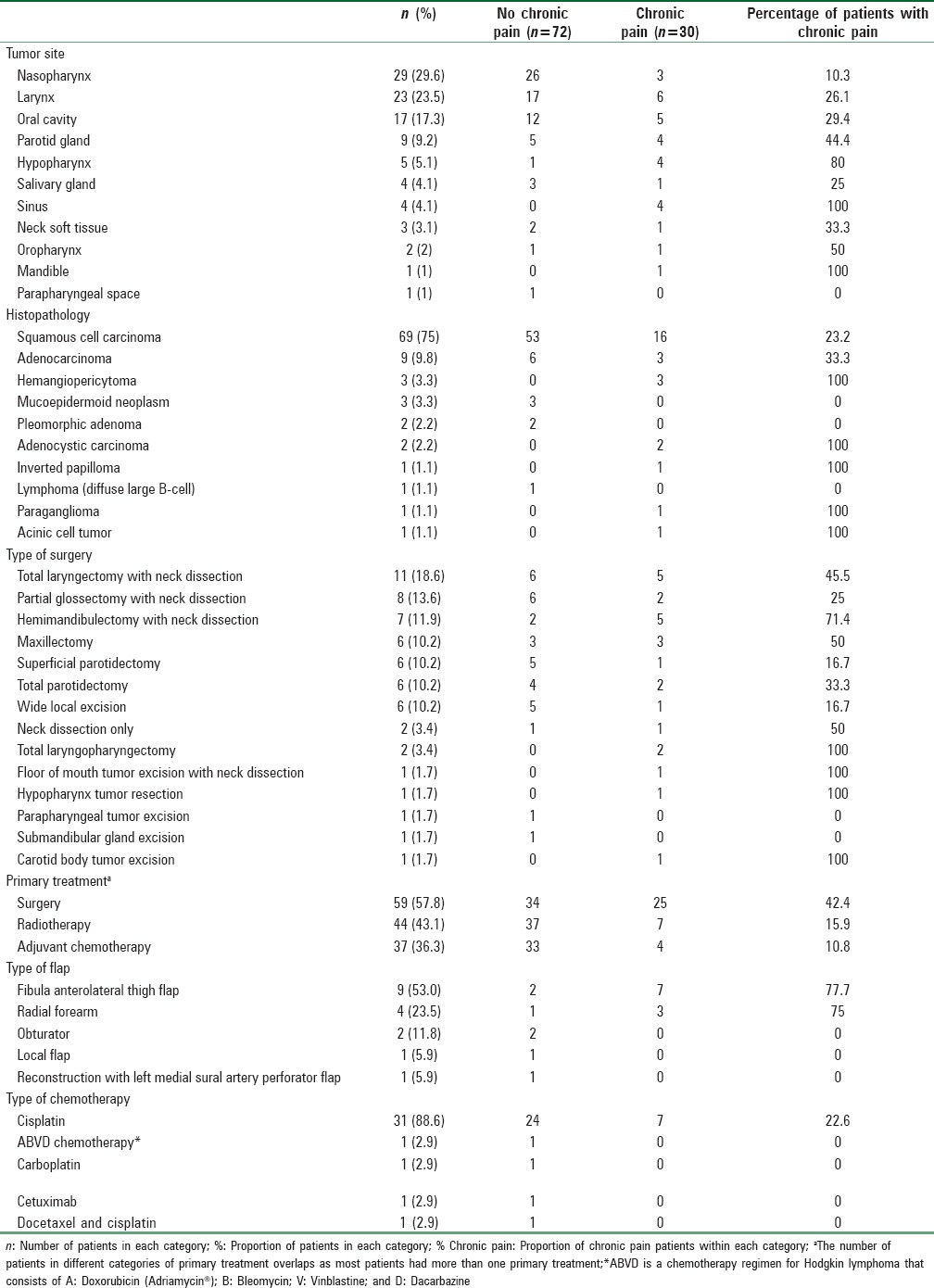
Table 3.
Pain characteristics (at the primary tumor site)
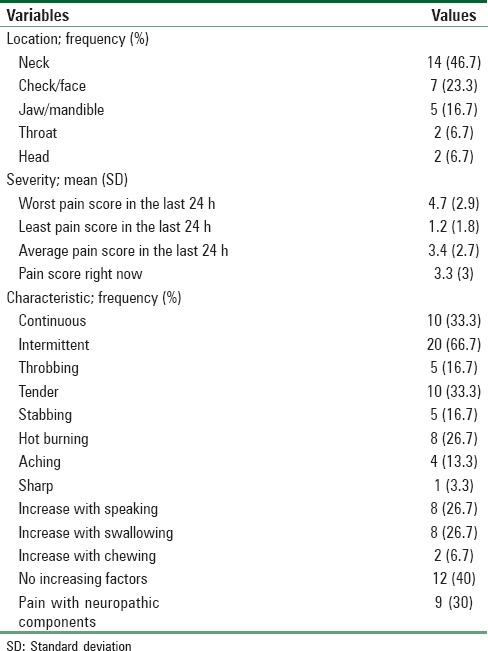
Associations between demographic and clinical characteristics with chronic pain
We constructed a classification tree model to identify demographic and clinical characteristics associated with the presence of chronic pain. The classification model can serve as a decision tree to evaluate the importance of candidate variables when identifying patients who reported chronic pain versus those who did not. Demographic features included were patient's age and gender; clinical characteristics included were TNM staging, cancer stage (benign, I, II, III, IV), surgery (yes/no), radiotherapy (yes/no), chemotherapy (yes/no), recurrence (yes/no), and tracheostomy (yes/no).
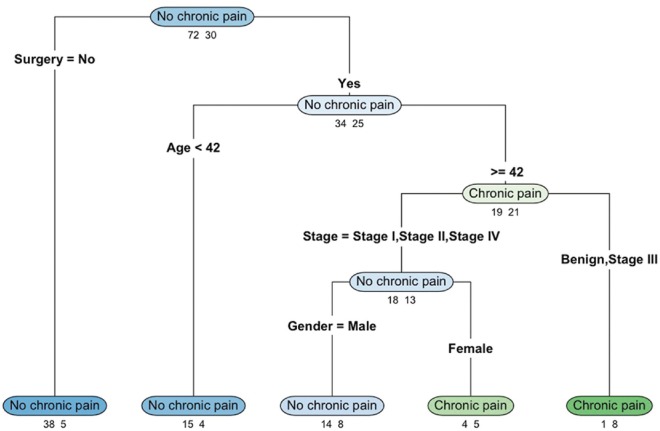
A four-level classification tree was constructed and illustrated using the “rpart.plot” package [Figure 1].[12] Each node on the classification tree indicates the most likely outcome in the observations (chronic pain vs. no chronic pain). The numbers below each node represent the number of observations in the nonchronic pain (left) and chronic pain (right) groups. The sum of the numbers below each node refers to the number of patients who are in the corresponding branch. At the top of the figure, the node “no chronic pain” indicates that the majority of the patients were classified as having no chronic pain. The numbers below the node indicate that, among the 102 patients (72 + 30) in the study, 72 patients were classified as having no chronic pain and thirty as having chronic pain.
Figure 1.
Classification tree categorizing chronic pain and no chronic pain using demographic and clinical characteristics. The numbers below each classification outcome indicate the number of observations in the no chronic pain (left) and chronic pain (right) groups
Surgery was the variable used for the first split, separating patients into those who did not have surgery (left branch) and those who had surgery (right branch). This is the first level of the classification tree. The “no chronic pain” node (left branch) indicates that 43 patients (38 + 5) did not have surgery. Among them, the majority (38 out of 43) was classified as having no chronic pain and five were classified as having chronic pain. The “no chronic pain” node (right branch) indicates that 59 patients (34 + 25) had surgery. Among these patients, 34 were classified as having no chronic pain and 25 were classified as having chronic pain.
Focusing on the patients who had surgery (right branch), age was used for the second split, further separating patients who had surgery into those younger than 42 years old (left branch) and those 42 years old or older (right branch). Among the 19 patients (15 + 4) who were younger than 42 years old (and had surgery), 15 were classified as having no chronic pain and four having chronic pain. As for the 40 patients (19 + 21) who were at least 42 years old (and had surgery), 19 were classified as having no chronic pain and 21 having chronic pain. This group of patients was further split using the cancer stage (the third split): patients with cancer at Stages I, II, or IV (left branch) versus those with benign tumor or Stage III cancer (right branch). Among the 9 patients (1 + 8) with either benign tumor or Stage III cancer (and had surgery and were at least 42 years old), only one was classified as having no chronic pain whereas eight were classified as having chronic pain. With respect to the 31 patients (18 + 13) who had Stage I, II, or IV cancer (had surgery and were at least 42 years old), 18 were classified as having no chronic pain and 13 were classified as having chronic pain.
A fourth split was made for this group, separating them by gender. Of the 22 male patients (14 + 8; left branch), 14 were classified as having no chronic pain and eight were classified as having chronic pain. As for the nine female patients (4 + 5; right branch), four were classified as having no chronic pain and five were classified as having chronic pain.
Overall, this model showed that patients who had surgery were more likely to have chronic pain than those who did not have surgery. Among patients who had surgery, younger patients (<42 years) were more likely to have no chronic pain than older patients. As for older patients (≥42 years), those with Stage III cancer were more likely to have chronic pain. Among those with Stages I, II, or IV cancer, male patients were less likely to have chronic pain than female patients. The misclassification rate of this classification tree model is 21.57%, indicating that more than three-quarters of the patients were correctly classified into chronic pain versus nonchronic pain groups using only their demographic and clinical characteristics.
The relative importance of the predictor variables was evaluated. As shown in Figure 2, gender was the most important variable, followed by age, cancer stage, and surgery (yes/no). On the other hand, metastasis (M = 0/1) and recurrence (yes/no) were relatively less important.
Figure 2.
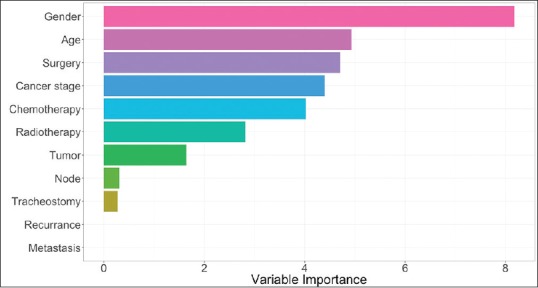
Variable importance estimated for the classification tree model. The variable importance measure is scaled to be between 0 (the least important) and 100 (the most important)
Associations between pain measures (nature, severity, and interference) with chronic pain and patients’ characteristics
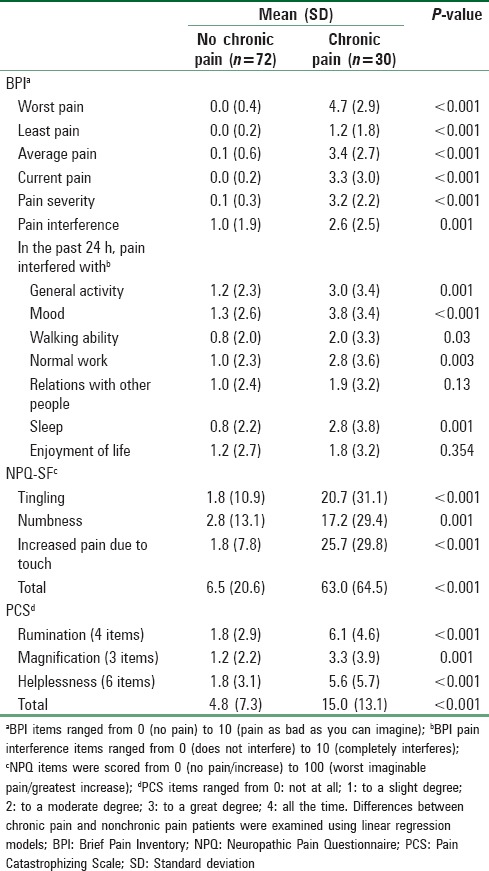
The descriptive statistics of the BPI and NPQ-SF regarding the patients’ primary sites, as well as patients’ scores on the PCS total scale and the rumination, magnification, and helplessness subscales are shown in Table 4. Results from linear regression models showed that chronic pain patients reported more pain on the BPI and NPQ-SF scales than nonchronic pain patients (all P ≤ 0.001). With the exception of relations with other people and enjoyment of life, patients with chronic pain reported having increased interference in the aspects of their daily activities than patients without chronic pain (all P < 0.05). Chronic pain patients also scored higher on the PCS total scale and the three subscales than nonchronic pain patients (all P ≤ 0.001).
Table 4.
Descriptive statistics of Brief Pain Inventory, Neuropathic Pain Questionnaire, and Pain Catastrophizing Scales between patients with and without chronic pain
Multiple regression models were used to examine the associations between the pain measures (BPI, NPQ-SF, and PCS) and patients’ demographic (age, gender) and clinical characteristics (cancer stage, surgery, radiotherapy, chemotherapy, recurrence, and tracheostomy). Results showed that male patients scored statistically significantly lower on the BPI severity scale regarding the primary site than female patients (β = −1.44, standard error [SE] =0.47, t = −3.04, P = 0.003). None of the other demographic or clinical characteristics were associated with patients’ BPI severity scores regarding the primary site.
Similarly, male patients scored statistically significantly lower on the total NPQ-SF regarding the primary site than female patients (β = −30.95, SE = 11.07, t = −2.8, P = 0.01). Compared with patients with less advanced stages of cancer, those with more advanced stages of cancer reported statistically significantly higher scores on the total NPQ-SF (β =24.77, SE = 11.69, t = 2.12, P = 0.04).
None of the demographic or clinical characteristics were associated with the PCS total, rumination, magnification, or helplessness subscales (all P > 0.05).
Discussion
In this study, 30% of the patients reported having chronic pain after therapy for major head and neck tumors. This number was even higher (42%) among patients who had surgery. This is quite a big percentage knowing that some of these patients were interviewed 6 years after their primary treatment. Patients with chronic pain reported an average pain score in the last 24 h of 3.4 (SD = 2.7). Factors that were found to be associated with chronic pain are sex (more on female), age (more in older patients), BMI (more with obesity), surgery, radiotherapy, and advanced cancer stages (Stage III), which indicate more aggressive cancer and subsequently more extensive surgery. Patients who reported having chronic pain subsequently had worse quality of life, manifested by general activity, mood, walking ability, normal work, and sleeping. Patients who reported having chronic pain also had statistically significantly higher pain scores on the BPI, NPQ-SF, and higher scores on the PCS, as compared to those who did not report having chronic pain.
CPSP is defined as pain persisting for at least 3 months after surgery. The incidence rate of CPSP was estimated to be about 30% after hernia surgery, and as high as 50% after thoracic surgery. Five risk factor domains; demographic, pain, clinical, surgery related, and psychological, are associated with the development of persistent incisional pain. CPSP was found to affect patients’ outcome in four domains: pain, physical functioning, psychological functioning, and global ratings of outcome.[13] We therefore considered these factors when conducted our study.
There is a paucity of research on this important topic. Chua et al.[14] examined the incidence of pain associated with different types of head and neck cancers among forty patients in 1999. 52% of the patients reported severe pain near sites of the tumor origin. Pain was caused by tumor recurrence in 35% of the patients, treatment sequelae (mostly surgery) in 30%, multiple etiologies in 25%, and unrelated causes in 10%. Pain was identified as mixed nociceptive and neuropathic pain in 37.5% of the patients, nociceptive pain in 32.5%, myofascial in 13.0%, neuropathic in 7.5%, and other mixed types in 7.5%. In addition, the presence of skull base or mandibular bone involvement was found to have significant influence on the severity of pain. These results suggested that cancer treatment (surgery, radiotherapy, and chemotherapy) might be associated with chronic pain among head and neck cancer patients. More recently in 2009, Scharpf et al.[15] examined 339 patients who had head and neck cancer treatment (e.g., postsurgery, postradiotherapy, postchemotherapy, or combination). They evaluated the relationship between pain and health-related quality of life during the 1st year, cancer recurrence, and 5-year disease-specific survival rates. Pain was associated with younger age, worse general, physical, and mental health conditions, more depressive symptoms, lower survival rate, and higher recurrence within the 1st year. The average pain scores decreased from 2.7 at 3 months after discharge to 1.6 at 12 months. Posttreatment pain and tumor site were independent predictors of recurrence. Five-year survival rate was 82% among patients with less posttreatment pain (scored between 1 and 3 out of 10) and 65% among those with more pain (scored between 7 and 10 out of 10). Pain intensity, age, and treatment modality were independent predictors of 5-year survival.
Among head and neck cancer patients, pain was reported to be present in the majority of patients before, during, and after treatment (50%, 81%, and 70%, respectively),[16] which showed that pain persisted for more than 6 months posttreatment for 36% of the patients, among which a third of the patients reported worse pain after treatment than before treatment. Acute pain associated with surgery, radiotherapy, chemotherapy, or a combination of treatment modalities is common, and management of such acute pain has been well studied. Chronic pain that persists for more than 6 months after treatment is often overlooked by patients, family members, and physicians, as the risk of recurrence decreases over time. However, chronic pain can be devastating to patients and their family members without proper treatment, as chronic pain will interfere with patients’ daily activities and overall physical and mental health.
In this study of head and neck tumor patients, the proportion of patients who reported having chronic pain was higher among females than males. This finding is consistent with the previous research.[17,18] Patients who reported chronic pain had, on average, higher BMI than those who reported having no chronic pain. Around 60% of our patients underwent surgery as part of their treatment modality; among which 42% reported having chronic pain. In comparison, smaller proportions of patients who had radiotherapy and/or chemotherapy reported having chronic pain. Our results showed that chronic pain is more common among patients who had surgery for their major head and neck tumor.
Our study has some limitations. First, the small sample size restricted our ability to more accurately estimate the prevalence rate of chronic pain among each type of head and neck cancer. Second, we were unable to estimate the onset or changes in chronic pain over time as patients were interviewed at different time points after their initial diagnoses and subsequent treatment. To better understand the prevalence of chronic pain and identify the associated risk factors among head and neck cancer patients, longitudinal studies among large representative samples are much needed.
Conclusion
Our study highlighted the presence of high burden of chronic pain after major head and neck tumor (mainly cancer) therapy that might be underestimated in clinical practice. This pain affects the patients’ quality of life in many aspects. Our results suggest that patients’ sex, age, cancer stage, and treatment modality are candidate factors associated with chronic pain after treatment of head and neck tumors. We hope our results encourage other researchers to further study this important aspect of patients’ battle.
Financial support and sponsorship
Dr. Siny Tsang received support by the research-training grant 5-T32-MH 13043 from the National Institute of Mental Health.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Dr. Daniel I. Sessler for his critical review and manuscript editing. We would like to thank Dr. Mohammed Aleid from the Department of Urology, Security Forces Hospital, Riyadh – Saudi Arabia, and Dr. Khalid H. Al-Qahtani from the Department of Otolaryngology, King Fahad Medical City, Riyadh – Saudi Arabia, for their help in patient recruitments.
References
- 1.GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: A double-blind, placebo-controlled randomized trial. Pain Physician. 2015;18:E139–46. [PubMed] [Google Scholar]
- 3.Terkawi AS, Tsang S, Sessler DI, Terkawi RS, Nunemaker MS, Durieux ME, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: A mixed-effects meta-analysis. Pain Physician. 2015;18:E757–80. [PubMed] [Google Scholar]
- 4.Terkawi AS, Tsang S, Abolkhair A, Alsharif M, Alswiti M, Alsadoun A, et al. Development and validation of Arabic version of the short-form Mcgill pain questionnaire. Saudi J Anesth. 2017;11(Suppl 1):S2–10. doi: 10.4103/sja.SJA_42_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 6.Terkawi AS, Backonja MM, Abolkhair A, Al-Zhahrani T, Almaharbi S, Joy J, et al. Development and validation of Arabic version of the neuropathic pain questionnaire-short form. Saudi J Anesth. 2017;11(Suppl 1):S53–62. doi: 10.4103/sja.SJA_86_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terkawi AS, Sullivan M, Abolkhair A, Al-Zhahrani T, Terkawi RS, Alasfar EM, et al. Development and validation of Arabic version of the pain catastrophizing scale. Saudi J Anesth. 2017;11(Suppl 1):S63–70. doi: 10.4103/sja.SJA_130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark LA, Pregibon D. Tree-based models. In: Chambers JM, Hastie TJ, editors. Statistical Models in S. Pacific. Grove, CA: Wadsworth and Brooks; 1992. [Google Scholar]
- 9.Therneau T, Atkinson B, Ripley B. rpart: Recursive Partitioning and Regression Trees. (Version R package version 4.1-10) 2015. [Last accessed on 2017 Feb 2]. Available from: https://www.cran.r-project.org/package=rpart .
- 10.Breiman L, Friedman J, Stone C, Olshen R. Classification and Regression Trees. Belmont, CA: Chapman & Hall/CRC; 1984. [Google Scholar]
- 11.Knuh M. caret: Classification and Regression Training. (Version R package version 6.0-73) 2016. [Last accessed on 2017 Feb 2]. Available from: https://www.cran.r-project.org/package=caret .
- 12.Milborrow S. rpart.plot: Plot rpart Models. An Enhanced Version of plot.rpart. 2016. [Last accessed on 2017 Feb 2]. Available from: http://www.CRAN.R-project.org/package=rpart.plot .
- 13.VanDenKerkhof EG, Peters ML, Bruce J. Chronic pain after surgery: Time for standardization. A framework to establish core risk factor and outcome domains for epidemiological studies? Clin J Pain. 2013;29:2–8. doi: 10.1097/AJP.0b013e31824730c2. [DOI] [PubMed] [Google Scholar]
- 14.Chua KS, Reddy SK, Lee MC, Patt RB. Pain and loss of function in head and neck cancer survivors. J Pain Symptom Manage. 1999;18:193–202. doi: 10.1016/s0885-3924(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 15.Scharpf J, Karnell LH, Christensen AJ, Funk GF. The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Head Neck Surg. 2009;135:789–94. doi: 10.1001/archoto.2009.107. [DOI] [PubMed] [Google Scholar]
- 16.Mirabile A, Airoldi M, Ripamonti C, Bolner A, Murphy B, Russi E, et al. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit Rev Oncol Hematol. 2016;99:100–6. doi: 10.1016/j.critrevonc.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Fillingim RB, Gear RW. Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, et al. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–24. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]