Abstract
Stereotactic radiosurgery (SRS), typically administered in a single session, is widely employed to safely, efficiently, and effectively treat small intracranial lesions. However, for large lesions or those in close proximity to critical structures, it can be difficult to obtain an acceptable balance of tumor control while avoiding damage to normal tissue when single-fraction SRS is utilized. Treating a lesion in 2 to 5 fractions of SRS (termed “hypofractionated SRS” [HF-SRS]) potentially provides the ability to treat a lesion with a total dose of radiation that provides both adequate tumor control and acceptable toxicity. Indeed, studies of HF-SRS in large brain metastases, vestibular schwannomas, meningiomas, and gliomas suggest that a superior balance of tumor control and toxicity is observed compared with single-fraction SRS. Nonetheless, a great deal of effort remains to understand radiobiologic mechanisms for HF-SRS driving the dose-volume response relationship for tumors and normal tissues and to utilize this fundamental knowledge and the results of clinic studies to optimize HF-SRS. In particular, the application of HF-SRS in the setting of immunomodulatory cancer therapies offers special challenges and opportunities.
Keywords: brain metastases, hypofractionated stereotactic radiosurgery, normal tissue injury, radiobiology, stereotactic radiosurgery
Stereotactic radiosurgery (SRS) has established a major role in the treatment of brain tumors based on its ability to precisely and accurately deliver a high dose of radiation to a target, effectively ablating all viable tumor while minimizing dose and preventing damage in surrounding normal tissue. Initially, precise, accurate delivery of the radiation required the use of a stereotactic headframe fixed to the patient’s skull, and thus radiosurgical treatments were most often delivered in a single fraction. While single-fraction treatment of small lesions was both effective at controlling tumors and sparing of normal tissue, the ability to safely deliver an adequate dose in a single fraction to larger tumors was limited at tumor diameters above 2–3 cm, as shown in the Radiation Therapy Oncology Group (RTOG) trial 9005.1 In that study of SRS delivered to either brain metastases following whole-brain radiotherapy (WBRT) or recurrent gliomas after partial brain irradiation, dose limits of 24 Gy, 18 Gy, and 15 Gy were established for lesions <2, 2–3, and 3–4 cm in maximum dimension. Paradoxically, as tumor diameter increases, tumor volume and the number of tumor cells increases dramatically, and one would prefer to administer higher doses to the larger tumor volumes to kill equivalent proportions of tumor cells. Indeed, a study from the Cleveland Clinic found that the dose limit imposed by increasing size resulted in much lower rates of local control for brain metastases larger than 2 cm in diameter.2
In contrast, “conventional” radiation therapy minimizes damage by utilizing multiple small fractions, typically delivered to much larger target volumes consisting of the tumor and the surrounding tissue at risk for tumor involvement or extension. Normal tissue repair between fractions permits the administration of high total doses of radiation to the tumor while maintaining acceptable toxicity in the surrounding normal tissue. In this setting, a course of radiation therapy, delivered to a brain tumor with definitive intent, often spans several weeks. In addition, the early radiotherapy systems employed relocatable immobilization devices that resulted in larger day-to-day variations in patient positioning than the fixed headframes. Furthermore, high-resolution on-machine image guidance to correct the position at the time of treatment only became widely available in the past 10 years. This technological advance allows for tighter, or smaller, volume expansion to account for setup error and patient motion during the administration of radiation therapy.3,4
The introduction of image-guided radiosurgery systems offers highly accurate, precise, and reproducible patient positioning and target localization, facilitating multifraction treatments with radiosurgical quality. Potentially, the use of a hypofractionated (nominally 2–5 sessions) stereotactic radiosurgery (HF-SRS) may provide an improved balance of tumor control and normal tissue toxicity over single-fraction SRS, particularly in larger tumors and those located next to or within critical structures. This paper discusses the radiobiologic rationale underlying HF-SRS, presents data on clinical outcomes for HF-SRS in the treatment of intracranial lesions, and reviews current clinical trials employing HF-SRS.
Radiobiologic Rationale for Hypofractionated versus Single-Fraction Radiosurgery
The relationship between radiation dose and tumor cell survival may be represented by the linear quadratic model, at least below 10 Gy per fraction.5 In the linear-quadratic model, a plot of surviving cell fraction (SCF) versus radiation dose shows that the log of the SCF is initially linearly proportional to dose (D, units Gy) with a slope of −α (ie, SCF = exp[−αD]). As dose increases, SCF decreases even more rapidly, and at moderate doses, SCF depends on dose and dose squared (ie, SCF = exp[−αD − βD2]). Tissue response to radiation is often characterized by the α/β ratio, which tends to be on the order of 2–3 Gy for brain tissue and 10 Gy for many rapidly proliferating tumors. Of course, the response to radiation is also influenced by many other factors, including the microenvironment (eg, oxygen content) and the capacity of cells to repair, repopulate, and redistribute in the cell cycle.6,7
The concept of using multiple small fractions of radiation, delivered over successive days, rather than a single large fraction to minimize normal tissue toxicity is well supported by preclinical data and clinical experience.5 Using the linear-quadratic model, one can calculate a biologically effective dose (BED) for a particular α/β ratio (units Gy), total dose (D), and dose/fraction (d, Gy):
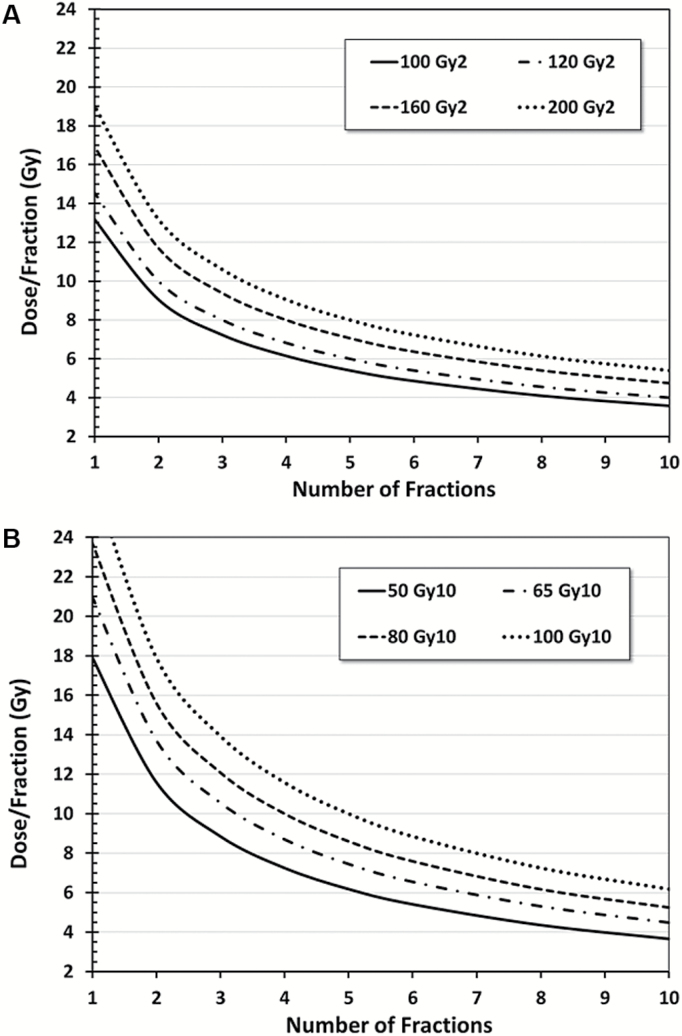
Thus, the BED for a low α/β tissue will increase much more rapidly with increasing dose per fraction than the BED for a high α/β tissue. Consequently, one could potentially exploit the difference in α/β ratio between tumor and normal tissue by fractionating the dose and thereby improving the therapeutic ratio. For example, consider the case of a tumor in a normal tissue with an α/β ratio of 10 and 2 Gy, respectively. For 16 Gy delivered in a single fraction, the BED will be 41.6 Gy10 and 144 Gy2 for tumor and normal tissue, respectively. However, for a course of 8 fractions delivered at 5.08 Gy/fraction, BED for the normal tissue remains at 144 Gy2, but the BED for the tumor is 61.3 Gy10, an increase of 47%. Alternatively, treating in 5 fractions at 5.4 Gy/fraction yields the same BED for the tumor (41.6 Gy10), though the BED for the normal tissue is reduced by 31% (99.9 Gy2 vs 144 Gy2). Representative isoeffect plots are presented in Fig. 1.
Fig. 1.
Biologically effective dose (BED) isoeffect plots for dose/fraction and number of fractions administered for (A) α/β = 2 Gy and (B) α/β = 10 Gy calculated using the linear-quadratic (LQ) model.5
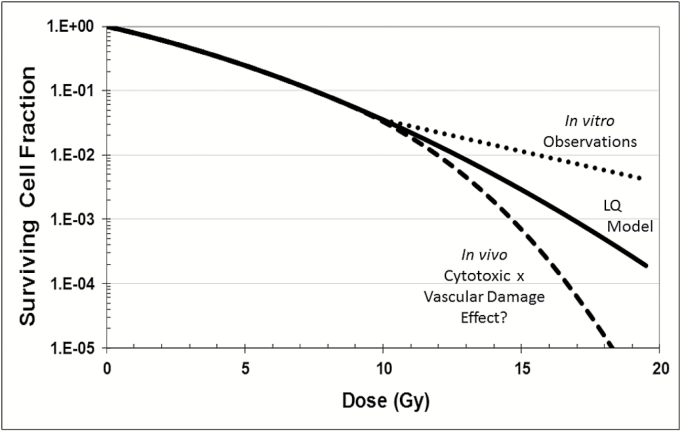
The shape of the dose-response curve above 10 Gy is controversial.8–10 Some argue that the linear-quadratic model provides an adequate representation of the dose-response relationship at high doses and that observed clinical outcomes are entirely consistent with the predictions of this model.11–13 Others assert that radiobiologic mechanisms, such as profound vascular damage14,15 and antigen expression, different from classic DNA damage, are evoked above a threshold dose of 8–12 Gy and that the high levels of tumor control observed in radiosurgery reflect this “new radiobiology” and enhanced dose-response13,16–18 (see Fig. 2). If there is indeed a step change in response above some threshold dose—and there is no fundamental reason that this threshold should be the same for tumor versus normal tissue—then it would seem appropriate to design treatment plans and select dose regimens such that the dose in the tumor always exceeds this threshold. Conversely, the plan should be designed such that the dose in the surrounding normal tissue rarely goes above the threshold. In any case, an improved understanding of the in vivo dose-response curves and underlying radiobiologic mechanisms for tumors and normal tissues (which likely differ) not only would help with rational plan design but could open new avenues for increasing the therapeutic ratio.
Fig. 2.
Speculative surviving cell fraction (SCF) versus single-dose irradiation response curves for the linear-quadratic (LQ) model, in vitro cell cultures and in vivo tumors with SCF determined by the product of direct cell kill and indirect vascular damage.
The above issue of dose-response does not include the other critical element in assessing toxicity—the volume of normal tissue irradiated. As discussed by Marks et al in the QUANTEC series of papers,19–21 normal tissue complications increase as the volume of tissue receiving some minimum dose, and this phenomenon is observed in a wide variety of tissues. For example, the volume of brain tissue receiving 12 Gy or more in radiosurgery appears to be correlated with the risk of radionecrosis, particularly when this volume exceeds 10–15 mL. Note, however, that this limitation appears overly restrictive, as it appears that virtually every single-fraction radiosurgery plan would exceed this limit when large lesions were treated to accepted doses.22 While the linear-quadratic model may be employed in converting doses to some uniform basis, the most relevant method for doing so remains unclear.23 Recognizing these limitations, the fundamental principles of SRS—highly conformal treatment plans, minimal margin around the target, accurate and precise target localization, minimization of position deviation, robust quality assurance—should aid in minimizing the irradiated volume and should always be employed.
To identify and select the optimal dose regimen that maximizes tumor kill and minimizes normal tissue damage, one should also consider time. Decreasing the time between fractions and the total length of the treatment course should decrease tumor cell repopulation and, thus, enhance the efficacy of the regimen. In particular, this should be more beneficial in the more rapidly growing malignant tumors (eg, metastases, high-grade gliomas) than in the indolent benign tumors (eg, World Health Organization grade I schwannomas and meningiomas). However, too short an interval between treatments could result in less complete repair of normal tissues and more pronounced late effects. While a minimum interval of 8 hours between treatments has generally been considered adequate to permit repair of normal tissues, the QUANTEC analysis of daily versus twice-daily brain treatments called this into question. Lawrence et al20 showed that hyperfractionated treatment was associated with increased radionecrosis compared with once-daily treatment at equivalent BED. In hypofractionated SRS, treatment may be delivered once daily on consecutive days or as infrequently as twice a week. In this case, the issue still revolves around the optimum timing that permits adequate repair of normal tissues while minimizing the adverse impact of tumor cell repopulation.
Finally, intriguing evidence is emerging that treatment of tumors may also release antigens, stimulating the immune system and leading to improved local control and, perhaps more importantly, decreased appearance of new, distant disease in the brain and body.24 While the high dose per fraction observed in single-fraction SRS may be quite effective at damaging the vasculature and enhancing local control, the resulting impaired perfusion could limit transport of antigens and immune cells, inhibiting the global immunomodulatory effect of radiation.25 Thus, it has been suggested that a hypofractionated regimen could still generate antigens without impairing transport and that this treatment strategy would produce a more robust immune response.24,26 Such an approach might have even greater impact when combined with one or more of the immune modulating drugs that have entered and profoundly altered clinical practice, though a great deal remains to be understood about this relationship. A more detailed discussion is beyond the scope of this paper, but practitioners should carefully approach patients who will receive radiosurgery in the setting of concurrent, adjuvant, or post-adjuvant immunomodulatory therapy.
In the next section, relevant clinical trials are reviewed to help elucidate the clinical radiobiology and lead to an informed decision on the most appropriate regimen.
Clinical Outcomes
Brain Metastases
Brain metastases occur in approximately 20%–40% of patients with advanced cancer and have become more prevalent over the past decade given advancements in systemic therapies for certain cancers, such as trastuzumab for human epidermal growth factor receptor 2–amplified breast cancer, targeted tyrosine kinase inhibitors for epidermal growth factor receptor mutated non–small cell lung cancer, and immunotherapy for melanoma. Given these systemic therapy advancements, not only do patients live longer in general, providing more time to develop brain metastases, but patients with brain metastases live longer post treatment (due to increased survival from targeted systemic drugs), thus leading to the rising prevalence of brain metastases. Given the longer survival of many cancer patients with brain metastases, local control and potential long-term toxicity of each treated lesion are increasingly important clinical considerations that may influence not only survival, but neurocognitive function and quality of life.
The management of brain metastases is multifaceted and more complicated in many ways than the management of primary brain tumors. This is because there are a number of treatment options for brain metastases and because brain metastases behavior and outcomes are influenced by histology and the extent of extracranial disease as well. Brain metastases management options include observation, surgery, systemic therapy, and radiation therapy.27 Observation may be appropriate for patients with small, asymptomatic brain metastases, particularly if they have a short life expectancy.28 Neurosurgical resection of a brain metastasis may be advised for larger, symptomatic brain metastases, or if there are no other easily biopsied lesions for pathologic confirmation in patients. Systemic therapy can be considered for small, asymptomatic brain metastases if the systemic therapy in consideration has some known activity in the brain. Radiation therapy remains the mainstay of treatment for brain metastases, delivered either as WBRT, depending on the histology and number of metastases, or as SRS, or possibly HF-SRS, which is the subject of discussion here.
The use of SRS and HF-SRS, as opposed to WBRT, for brain metastases is increasing for several reasons. One reason is the rising incidence and prevalence of brain metastases, which have led to a heightened experience, more studies and publications, and, thus, justified interest in the use of SRS. Another reason is increasing evidence that WBRT impairs neurocognition with limited positive impact on survival for some histologies.29,30 Finally, recent advances in the technology and delivery of both SRS and HF-SRS have led to wider availability and use of this technology.
With respect to SRS and HF-SRS for brain metastases, there are a number of physical, biologic, and clinical factors to consider. Physical considerations include the size of the target, whether or not margin is needed and to what extent31 (eg, none vs 1 mm, 2 mm, 3 mm, or 5 mm), the optimal dose to both the target and surrounding normal tissue, and treatment planning stipulations such as dose fall-off and conformality. In addition, confidence in immobilization of a patient, image guidance and verification pretreatment, and patient motion monitoring during treatment all influence treatment design and delivery. Biologic considerations for SRS and HF-SRS include the histology of metastases (eg, radiosensitive vs radioresistant), possible use of systemic agents that penetrate or affect the CNS around the time of SRS, and, of course, fractionation. For any lesion, depending on size, histology, and possible concurrent therapies, there may be an advantage of either a single-fraction or hypofractionated radiation schedule. Patient-specific clinical considerations for SRS and HF-SRS include life expectancy of a given patient, urgency of other needed systemic treatments for the patient, and whether those therapies, such as chemotherapy, immunotherapy, antibody therapies, or even antibiotics, may be delivered concurrently. Other patient-specific clinical considerations are inherent factors such as hypertension, diabetes, smoking history, vasculitis, and other medical comorbidities that may make a patient more susceptible to toxicity, such as radiation necrosis,32,33 following SRS.
The physical, biologic, and clinical considerations for SRS and HF-SRS are not limited to the list above, which simply highlights the many considerations in managing and treating a patient with brain metastases. Fortunately, the outcome of many patients with brain metastases continues to improve, and this is due in part to the precise and powerful tool of SRS. With this surge in the use of SRS, more studies on outcomes following SRS have been published recently with mounting evidence that HF-SRS is an effective approach in terms of both local control and reduced toxicity, particularly for larger brain metastases.
Local control following SRS is excellent, particularly for smaller brain metastases. Unfortunately, larger brain metastases are best managed with lower doses of SRS necessitated by the increased volume of normal brain tissue exposed to radiation associated with a larger target volume. However, these lower doses lead to lower rates of long-term local control of the tumor, as discussed above. These dose-volume data for brain metastases SRS is best exemplified by the RTOG 90-05 dose escalation study,1 which is the basis of dosing guidelines for brain metastases SRS still today. Despite this paradox of dose limitations for larger brain metastases, single-fraction SRS remained the standard of care for many years for larger brain metastases as original SRS treatment systems utilized a fixed frame system to immobilize patients, and that fixed frame system was not amenable for multiple fractions.
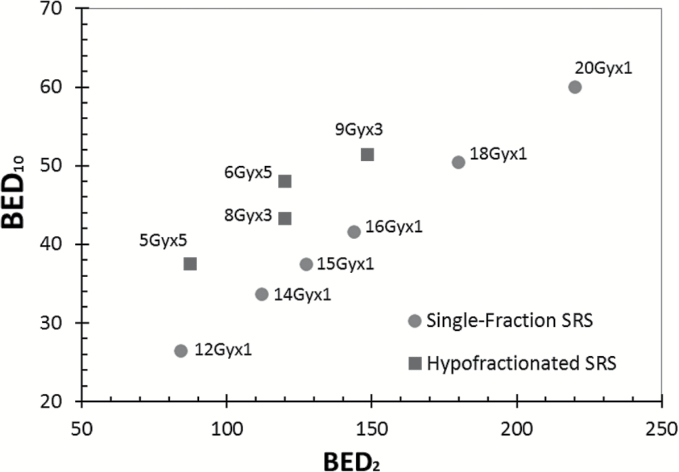
More recently, nonfixed SRS immobilization systems have been developed and are used routinely for SRS in many centers worldwide now. These nonfixed immobilization systems are effective at holding patients in a reproducible, precise position that can be used for several fractions, particularly with both on-board imaging to verify accurate setup and patient motion monitoring systems during radiation therapy.3,4 Given these advancements, there has been more interest in treating larger brain metastases with multiple fractions of SRS, or HF-SRS. There have been numerous retrospective studies published about the outcomes of intact and resected larger brain metastases following HF-SRS.34–48 Although retrospective analyses have inherent selection bias and other limitations, the data from these studies suggest improved local control of treated lesions and perhaps decreased radiation necrosis for larger lesions with HF-SRS in comparison to historical controls treated with SRS. The calculated BED2 (associated with normal tissue toxicity) and BED10 (associated with control of rapidly proliferating tumors) for typical single-fraction SRS and HF-SRS regimens are shown in Fig. 3. Note an improved balance of lower BED2 and higher BED10, which should yield decreased toxicity and improved tumor control for the hypofractionated versus single-fraction schemes.
Fig. 3.
BED2 and BED10 (the biologically effective doses calculated for an α/β ratio of 2 and 10 Gy, respectively) for typical single-fraction SRS and hypofractionated SRS. Note that BED2 is associated with response of normal tissue to radiation, and increasing BED2 results in more damage to normal tissue. The response of rapidly proliferating tissue, such as many brain metastases and gliomas, to radiation is better represented by BED10, and a higher BED10 suggests better local control.
For example, a recently published Italian study45 compared the results of single-fraction SRS and HF-SRS, 3 × 9 Gy, in brain metastases >2.0 cm and found a significant improvement in local control and less radiation necrosis with HF-SRS. The study included 289 patients, with 151 treated with single-fraction SRS and 138 treated with HF-SRS. All metastases were treated with linear accelerator–based SRS using the iPlan treatment planning system (Brainlab), and there was a 2-mm or 1-mm margin expansion on the gross tumor volume (GTV) to planning target volume (clinical target volume was considered equivalent to GTV). The single-fraction dosing SRS followed RTOG 90-05 guidelines. Patients were treated with HF-SRS over SRS if the target volumes were close to critical structures, or if the metastases were ≥3 cm in size. Although the study is retrospective, the results are encouraging, as patients were treated in a homogeneous manner with clear stipulations with respect to treatment approach and the series is relatively large with excellent follow-up. The same group published their experience using HF-SRS to treat postoperative resection cavities of large brain metastases.44
Benign Brain Tumors
Meningiomas and vestibular schwannomas are 2 of the most common benign brain tumors in adults. They are both extra-axial and have sharp margins of demarcation, lending themselves very well to highly conformal radiation therapy techniques, especially single-fraction SRS and HF-SRS.49 Controversies exist with regard to the appropriate fractionation for stereotactic radiation delivery.
Meningiomas
There are abundant data on the use of SRS for the treatment of meningiomas. The most common prescribed dose ranged from 12 to 13 Gy in a single fraction, and an excellent local control of 90%–98% has been achieved with toxicity rate of <10% and a median follow-up of 31–89 months. Tumors in non–skull-base locations tend to carry a higher risk of adverse radiation reaction.50 In a recent series of SRS for parafalcine and parasagittal meningiomas (n = 212) from the University of Virginia, 38.2% of the patients developed progressive peritumoral edema, with 5.2% of them having further progression of edema. Tumor volume >10 cc and venous sinus invasion/compression were factors predicting post-SRS edema.51 Two large retrospective series on gamma knife–based SRS for cavernous sinus (n = 159) and petroclival meningiomas (n = 168) from the University of Pittsburgh Medical Center (UPMC) showed long-term local control in excess of 90% in 10 years with complication rates of 7%–8%.52,53 The median dose used for those 2 series was 13 Gy in a single fraction. In a recent multicenter study of SRS for sellar/parasellar meningiomas in which 763 patients were included for analysis with a median follow-up of 66.7 months, the 5- and 10-year progression-free survival rates were 95% and 82%, respectively, with new or worsening cranial nerve deficits observed in 9.6% of patients.54 The dose cutoff for optimal tumor control was 13 Gy in 1 fraction.
For patients who have meningiomas which are located close to critical structures and/or are larger in size, situations where single-fraction SRS is perceived to carry a higher risk of complications, HF-SRS has been utilized. However, the data on the use of HF-SRS for meningiomas in this setting are admittedly limited. A study from Stanford University included 27 patients with meningiomas situated within 2 mm of a “short segment” of the optic apparatus and treated with HF-SRS to an average marginal dose of 20.3 Gy in 2–5 fractions.55 A local control and vision preservation rate of 94% was observed for the entire cohort of 49 patients, including those with lesions other than meningiomas. Bria et al56 from UPMC treated 73 patients with meningiomas in skull-base and non–skull-base locations with HF-SRS to a median dose of 17.5 Gy (range, 6–27 Gy) in 3 fractions (range, 1–5). They found a local control rate of 95% at a median follow-up of 16.1 months. Subjective improvement in tumor-related symptoms was observed in 60% of the patients. Other studies have yielded similar results.57
SRS and HF-SRS appear to have equivalent local control and toxicity rates.58 However, SRS series have much longer follow-up intervals compared with HF-SRS series, whose long-term efficacy and toxicities are less known. As most patients with meningiomas treated with HF-SRS either had tumors close to critical structures or larger tumors, it is difficult to eliminate the inherent bias when comparing SRS and HF-SRS. In general, most patients with meningiomas, especially those with tumors in skull-base locations, are most suitable for SRS only when the tumor is <3 cm diameter50 and is not close to critical structures, such as the optic apparatus. When a tumor does not meet these criteria, HF-SRS may be a more appropriate treatment. Of course, conventionally fractionated stereotactic radiotherapy, typically delivered in 1.8–2.0 Gy daily fractions to a total dose of 45 to 60 Gy, remains an option for lesions that are very large and/or are intimately associated with critical structures.59
Vestibular Schwannomas
For patients who are not suitable for microsurgical treatment or who refuse surgery, SRS constitutes the standard treatment for vestibular schwannoma. An early report of 162 patients with unilateral vestibular schwannomas treated with single-fraction SRS to an average marginal dose of 16 Gy showed an excellent local control rate of 98%.60 However, the hearing preservation rate was only 51% and the rates of trigeminal and facial nerve injury were 21% and 27%, respectively. Given these findings, the prescribed dose was reduced to 12–13 Gy single-fraction SRS at most centers, resulting in improved hearing preservation and much lower rates of trigeminal and facial nerve injury while maintaining excellent local control61 (Table 1). Although very good local control and relatively acceptable rates of preservation of functions of the trigeminal, facial, and vestibulocochlear nerves have also been achieved in patients with larger (>3–4 cm3) tumors treated with SRS,62 in general HF-SRS is preferred in this setting. There have been some studies examining the outcomes of patients with larger vestibular schwannomas treated with HF-SRS. The regimens ranged from 18–21 Gy in 3 fractions to 25 Gy in 5 fractions, resulting in similar local control, hearing preservation, and preservation of trigeminal and facial nerve functions,63–68 as shown in Table 1. However, the follow-up times are typically much shorter than those for single-fraction SRS.
Table 1.
Comparison of single-fraction SRS and HF-SRS for vestibular schwannoma
| Single-Fraction SRS52 | HF-SRT54–59 | |
|---|---|---|
| Prescribed dose | 12–13 Gy in 1 fraction | 18–21 Gy in 3 fractions or 25 Gy in 5 fractions |
| Biologically effective dose to the brainstem assuming α/β = 2 Gy | 84.0–97.5 Gy2 | 3 fraction: 72.0–94.5 Gy2 5 fractions: 87.5 Gy2 |
| Biologically effective dose to the tumor assuming α/β = 3 Gy | 60.0–69.3 Gy3 | 3 fractions: 54.0–70.0 Gy3 5 fractions: 66.7 Gy3 |
| Biologically effective dose to the tumor assuming α/β = 10 Gy | 26.4–29.9 Gy10 | 3 fractions: 28.8–35.7 Gy10 5 fractions: 37.5 Gy10 |
| Follow-up intervals | 3.3–9.0 y | 0.5–7.3 y |
| Local tumor control | 91%–100% | 83%–100% |
| Hearing preservation | 13%–71% | 50%–81.5% |
| Incidence of CNV deficit | 0–8% | 0–7% |
| Incidence of CNVII deficit | 0–6% | 0–8% |
Abbreviations: CNV = cranial nerve 5; CNVII = cranial nerve 7.
Overall, SRS is still regarded as the standard nonsurgical treatment for smaller vestibular schwannomas given its long track record of excellent local control, the acceptable hearing preservation rate, and the excellent trigeminal and facial nerve preservation rates.61 Although HF-SRS appears to yield similar outcomes to SRS, it is still unclear whether local control will be equivalent as the HF-SRS data mature. For patients with larger vestibular schwannomas, SRS may still be appropriate in select cases, but HF-SRS should be considered to decrease the risk of complications, especially in patients with tumors abutting or compressing the brainstem.
Glioblastoma
For patients with newly diagnosed GBM who are young and exhibit a good performance status, the standard of care fractionation scheme is 60 Gy in 30 fractions.69 However, this 6-week treatment course may place a financial, psychosocial, and logistical burden on these often debilitated patients and their families, particularly since median life expectancy is under 16 months from the time of diagnosis. Consequently, a variety of clinical trials have explored 20,70 15,71–74 10,75–77 6,78 and 579 fractions for newly diagnosed GBM. For recurrent GBM, schedules range from 180 fraction to 1081 fractions.
In this section, we focus on differences in efficacy and toxicity between single- versus multiple-fraction radiosurgery treatments. More complete details are found in existing review articles.82,83
Newly Diagnosed GBM
Given that most GBM recurs near the initial site following treatment, early retrospective84 and prospective single arm85 trials studied the impact of SRS combined with traditional radiotherapy as a means of dose escalation.
RTOG 930586 studied a single-fraction SRS boost in patients with newly diagnosed GBM. This trial randomized 203 patients to treatment with or without SRS preceding standard 60 Gy external beam radiotherapy (EBRT) with BCNU chemotherapy. No differences in overall survival or patterns of failure were seen. Although controversy exists about the patient selection and study design, these phase III data effectively ended the routine use of an SRS boost in combination with EBRT in the pre-temozolomide era.
Late toxicity of EBRT with an SRS boost for newly diagnosed GBM was reported in 4% of patients on RTOG 930586 and in other studies ranged from 5%87 to 14%85 with SRS and 6%88 to 33%89 with HF-SRS. Although these and other prospective trials studied both SRS and HF-SRS,82,83 these nonrandomized data cannot be used to compare the outcomes of single-fraction versus hypofractionated SRS.
In addition to dose escalation, a goal of many phase I/II76,77,90,91 and randomized trials73,75,92 of hypofractionated radiotherapy was to shorten the treatment time for patients with limited life expectancy.93 The natural progression of this shortening is to complete treatment in 1 to 5 fractions.90,94,95 A retrospective report of SRS in a median of 5 fractions in newly diagnosed GBM94 noted a median survival of 16 months, similar to historical controls. In a prospective phase I trial of SRS for newly diagnosed GBM, 19 patients received 25–35 Gy in 5 fractions of HF-SRS.90 No radionecrosis was seen, but there was unacceptable toxicity on the 30 and 35 Gy arms, with no survival benefit at these higher doses. Although not considered HF-SRS, a similar concept was studied in 40 patients treated on a phase II trial of 36 Gy in 6 fractions with concurrent and adjuvant bevacizumab and temozolomide.78 Median survival was 19 months, with 5% radionecrosis and the intriguing finding that methylation status of O6-DNA methylguanine-methyltransferase (MGMT) was not significant for survival; hypofractionation may overcome the negative impact of absence of hypermethylation. However, a similar HF-SRS prospective trial of 25 to 40 Gy in 5 fractions with 5-mm margins with concurrent and adjuvant temozolomide, presented in abstract form, found that MGMT maintained its prognostic significance.95 Additionally, patients with radionecrosis had a 33-month survival compared with 11 months without.
Although these emerging data exploring dose escalation through hypofractionation are promising, the conclusion of the 2005 evidence-based review of the American Society for Radiation Oncology (ASTRO) on SRS for newly diagnosed GBM—that there is insufficient evidence to support using SRS/HF-SRS off protocol—is still relevant today.82 Overall, there are no randomized data to compare single-fraction SRS to 5–6 fraction regimens as a boost to standard fractionated treatment or as primary treatment of newly diagnosed GBM.
Recurrent GBM
The earliest reports of SRS for glioma were for recurrent GBM.96–98 Multiple small prospective99,100 and retrospective82,83,101 studies reveal a median survival of 6–18 months following salvage SRS. Similar outcomes are seen with HF-SRS83,102,103 with a 6–14 month median survival.
Radionecrosis is a concern with salvage SRS/HF-SRS within previously irradiated brain. Existing data are unclear whether HF-SRS has a different rate of radiation necrosis compared with single-fraction SRS, as toxicity reporting is not consistent between studies. Reports note an incidence of radionecrosis of 099 to 44%,101 while others report reoperation rates of 31%–57%,98,104 and some note grade 3–4 CNS toxicity, but not necessarily radiation necrosis. Overall, the heterogeneity in time to salvage treatment and patient and irradiation characteristics between these trials precludes direct comparison of outcomes to determine whether a difference exists between SRS and HF-SRS.
These high rates of necrosis led investigators to study the addition of bevacizumab to SRS/HF-SRS for recurrent GBM.80,105,106 Randomized data show bevacizumab to improve preexisting radiation necrosis.107 Retrospective data of SRS for recurrent GBM suggest a radionecrosis rate of 5%80 to 9%105 with bevacizumab compared with 19%80 to 43%105 without. Although a phase II trial of SRS or HF-SRS in recurrent GBM noted no hemorrhages with bevacizumab and only a grade 3 headache as the highest toxicity,108 other trials found increased toxicity without survival benefit with bevacizumab and hypofractionated radiotherapy in newly diagnosed GBM.109 Given the concern for radiation necrosis with the shortened fractionation of SRS/HF-SRS, as well as a potential benefit of bevacizumab, RTOG 1205 recently completed a phase III trial in recurrent GBM of bevacizumab with or without 35 Gy in 10 fractions, based on retrospective data.81
Overall, there is a suggestion that HF-SRS and bevacizumab may improve radiation necrosis rates compared with single-fraction SRS. On the other hand, the efficacy of these regimens would also be expected to differ widely based on the calculated BED,110 as shown in Table 2. At this time, given the heterogeneity of patient and treatment characteristics and lack of phase III data, there is insufficient evidence to support one treatment scheme over any other.111 Given that recurrent GBM often has indistinct borders, a problem compounded by the fact that these lesions typically appear within previously irradiated fields, novel imaging techniques, such as the use of MR-based apparent diffusion coefficient mapping, may prove useful in improving target delineation.112
Table 2.
Example planning target volumes (PTVs) and dose fractionation regimens for re-irradiation of recurrent GBM
| Institution | PTV | Dose Regimen | BED (Gy 2) | BED (Gy 10) |
|---|---|---|---|---|
| Memorial Sloan Kettering69 ,91 | CE T1 MRI volume + 5 mm | 6 Gy/day × 5 days | 120 | 48 |
| Duke100 | CE T1 MRI volume + 1 mm | <2 cm*: 24 Gy once | 312 | 81.6 |
| 2–3 cm: 18 Gy once | 180 | 50.4 | ||
| 3–5 cm: 5 Gy/ day × 5 days | 87.5 | 37.5 | ||
| Thomas Jefferson72 | CE T1 MRI volume only | 3.5 Gy/day × 10 days | 96.3 | 47.3 |
| RTOG 1205 | CE T1 MRI volume + 3–8 mm | 3.5 Gy/day × 10 days | 96.3 | 47.3 |
Abbreviation: CE = contrast enhancing.
Current Clinical Trials
Clinical trials of HF-SRS for brain metastases and benign tumors registered at ClinicalTrials.gov are presented in Table 3. To illustrate the opportunities to better define the role of HF-SRS in the treatment of brain lesions, it is worthwhile to discuss several of these trials. For example, the Stanford study (NCT00928226) asks the question, “What is the maximum tolerated dose (MTD) of HF-SRS for large brain metastases treated using a 3 fraction regimen?” Eligible patients have 1 to 4 brain metastases, one of which is 4.2–33.5 cm3 (equivalent to a uniform sphere 2–4 cm in diameter), intact and unresectable. The primary outcome is MTD with secondary measures of acute and late toxicity, quality of life, local control, appearance of distant metastases in the brain and overall survival. Patients are treated on 3 consecutive days to doses of 24, 27, 30, or 33 Gy (8–11 Gy/fraction) using a 3 + 3 dose escalation scheme. Preliminary results have been presented in abstract form.
Table 3.
Clinical trials of HF-SRS registered with ClinicalTrials.gov
| Trial | Institution | Principal Investigator | ClinicalTrials.gov Identifier | Primary Outcome |
|---|---|---|---|---|
| Brain Metastases | ||||
| Phase I/II Study of Fractionated Stereotactic Radiosurgery to Treat Large Brain Metastases | Stanford University | Scott Soltys | NCT00928226 | Determine MTD of SRS given in 3 fractions for brain metastases 4.2–14.1 cm3 and 14.2–33.5 cm3 |
| Fractionated Stereotactic Radiotherapy (FSRT) in Treatment of Brain Metastases | Moffitt Cancer Center | Solmaz Sahebjam | NCT02187822 | Determine MTD of TPI 287 given concurrently with FSRT to treat brain metastases |
| Hypofractionated Stereotactic Radiosurgery in Treating Patients With Large Brain Metastasis | Emory | Ian Crocker | NCT01705548 | Determine MTD of 5-fraction SRS for brain metastases, 3–6 cm diameter |
| Perfexion Brain Metastasis (HF-SRT) | Princess Margaret Hospital | Caroline Chung | NCT00805103 | Determine MTD of HF-SRS for recurrent brain metastases (at least 1 >2 cm diameter) post WBRT |
| Fractionated Stereotactic Radiosurgery with Concurrent Bevacizumab for Brain Metastases: A Phase I Dose- escalation Trial | National Taiwan University Hospital | Chia-Chun Wang | NCT02672995 | Determine MTD of 3-fraction SRS + bevacizumab for brain metastases, 1.5–3.5 cm diameter |
| Frameless Fractionated Stereotactic Radiation Therapy (FSRT) for Brain Mets Study | MD Anderson | Amit Garg | NCT02798029 | Incidence of failure based on imaging for each lesion (up to 5 cm diameter) after 3–5 fraction SRS |
| Fractionated Stereotactic Radiosurgery for Large Brain Metastases | University of Pittsburgh | Dwight Heron | NCT02054689 | MTD for 3-fraction SRS for brain metastases, 3-5 cm diameter |
| Hypofractionated Stereotactic Radiation Therapy of Brain Metastases: Evaluation of WBRT | Institut de Cancérologie de Lorraine | Phillipe Royer | NCT02913534 | Overall survival of patients with 1–3 brain metastases treated with HF-FSRT |
| Glioblastoma | ||||
| Phase I/II Study of Temozolomide and Hypofractionated Radiotherapy for Newly Diagnosed Supratentorial GBM | Stanford University | Scott Soltys | NCT01120639 | Determine MTD of 5-fraction SRS with 5 mm margins with temozolomide for newly diagnosed GBM |
| Benign Histologies | ||||
| Multisession SRS for Optic Nerve Sheath Meningiomas (ONSMsmSRS) | Istituto Neurologico Carlo Besta | Laura Fariselli | NCT02594709 | Visual function outcome in ONSM treated with 5-fraction SRS |
| 1 versus 3 fraction SRS for Patients with Neurinomas (ACOUNEU) | Istituto Neurologico Carlo Besta | Laura Fariselli | NCT02055859 | Hearing preservation in patients with acoustic neuromas randomized to 1- versus 3-fraction SRS |
Abbreviations: TPI 287 = a third-generation taxane; ONSM = optic nerve sheath meningiomas
A randomized study from Istituto Neurologico Carlo Besta seeks to determine whether single-fraction SRS or 3-fraction HF-SRS is superior in terms of hearing preservation for patients with acoustic neuromas. The primary endpoint is preservation of hearing defined audiometrically. Secondary endpoints are neurotoxicity (particularly trigeminal and facial nerve damage) and tumor control. Patients are randomized to either single-fraction SRS (11–13 Gy) or 3-fraction HF-SRS (6–7 Gy fraction, 18–21 Gy total). A total of 102 patients are planned for accrual through 2018.
Given the need to identify optimal treatment parameters and appropriate patient populations for HF-SRS in the treatment of large brain lesions, all eligible patients should consider enrollment on a clinical trial.
Conclusion
Hypofractionated SRS appears to offer a superior balance of efficacy and toxicity in patients with large tumors and tumors located close to critical normal organs, compared with single-fraction SRS. This benefit comes at a cost of increased treatment fractions required. Further work is required to identify the most appropriate applications for HF-SRS based on tumor and patient characteristics. It would be worthwhile to establish the optimal scheme for total dose and dose/fraction in HF-SRS as a function of tumor diameter and location. Moreover, given the rapid progress in targeted and immunomodulating treatments for cancer, these outcomes with HF-SRS need to be evaluated in the setting of concurrent and adjuvant systemic therapies. The benefits and toxicities of combined HF-SRS and immunomodulating therapy is of particular interest, given the purported role of HF-SRS in stimulating the immune response. Given the known toxicity of high-dose, single-fraction SRS in treating large lesions, a randomized trial of single-fraction SRS versus HF-SRS could not be conducted with equipoise. Thus, clinical trials to optimize HF-SRS must rely on well-constructed and well-analyzed prospective trials of sufficient size and duration to determine the toxicity and efficacy of HF-SRS for the treatment of large and/or critically located lesions.
Conflict of interest statement. This work was supported by Varian Medical Systems to J.P.K.; and Elekta to S.S.L.; Travel grant from Varian Medical Systems and Accuray.
References
- 1. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 2. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104(6):907–912. [DOI] [PubMed] [Google Scholar]
- 3. Ahmad SS, Duke S, Jena R, Williams MV, Burnet NG. Advances in radiotherapy. BMJ. 2012;345:e7765. [DOI] [PubMed] [Google Scholar]
- 4. Sahgal A. Technological advances in brain and spine radiosurgery. Technol Cancer Res Treat. 2012;11(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 6. Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol. 1989;56(6):1045–1048. [DOI] [PubMed] [Google Scholar]
- 7. Withers HR. The Four R’s of Radiotherapy. New York: Academic Press; 1975. [Google Scholar]
- 8. Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49(20):4825–4835. [DOI] [PubMed] [Google Scholar]
- 9. Hanin LG, Zaider M. Cell-survival probability at large doses: an alternative to the linear-quadratic model. Phys Med Biol. 2010;55(16):4687–4702. [DOI] [PubMed] [Google Scholar]
- 10. Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):847–852. [DOI] [PubMed] [Google Scholar]
- 11. Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown JM, Carlson DJ, Brenner DJ. Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. In reply to Rao et al. Int J Radiat Oncol Biol Phys. 2014;89(3):693–694. [DOI] [PubMed] [Google Scholar]
- 13. Kirkpatrick JP, Brenner DJ, Orton CG. Point/Counterpoint. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Med Phys. 2009;36(8):3381–3384. [DOI] [PubMed] [Google Scholar]
- 14. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. [DOI] [PubMed] [Google Scholar]
- 16. Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–243. [DOI] [PubMed] [Google Scholar]
- 17. Song CW, Lee YJ, Griffin RJ, et al. Indirect tumor cell death after high-dose hypofractionated irradiation: implications for stereotactic body radiation therapy and stereotactic radiation surgery. Int J Radiat Oncol Biol Phys. 2015;93(1):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperduto PW, Song CW, Kirkpatrick JP, Glatstein E. A hypothesis: indirect cell death in the radiosurgery era. Int J Radiat Oncol Biol Phys. 2015;91(1):11–13. [DOI] [PubMed] [Google Scholar]
- 19. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirkpatrick JP, Marks LB, Mayo CS, Lawrence YR, Bhandare N, Ryu S. Estimating normal tissue toxicity in radiosurgery of the CNS: application and limitations of QUANTEC. J Radiosurgery and SBRT. 2011;1:95–102. [PMC free article] [PubMed] [Google Scholar]
- 23. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 24. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. [DOI] [PubMed] [Google Scholar]
- 25. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311–327. [DOI] [PubMed] [Google Scholar]
- 26. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soltys SG, Kirkpatrick JP, Laack NN, Kavanagh BD, Breneman JC, Shih HA. Is less, more? The evolving role of radiation therapy for brain metastases. Int J Radiat Oncol Biol Phys. 2015;92(5):963–966. [DOI] [PubMed] [Google Scholar]
- 28. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118(9):2486–2493. [DOI] [PubMed] [Google Scholar]
- 31. Kirkpatrick JP, Wang Z, Sampson JH, et al. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91(1):100–108. [DOI] [PubMed] [Google Scholar]
- 32. Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sneed PK, Mendez J, Vemer-van den Hoek JG, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123(2):373–386. [DOI] [PubMed] [Google Scholar]
- 34. Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ, Jr, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81(1):18–24. [DOI] [PubMed] [Google Scholar]
- 36. Fahrig A, Ganslandt O, Lambrecht U, et al. Hypofractionated stereotactic radiotherapy for brain metastases—results from three different dose concepts. Strahlenther Onkol. 2007;183(11):625–630. [DOI] [PubMed] [Google Scholar]
- 37. Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109(1):91–98. [DOI] [PubMed] [Google Scholar]
- 38. Follwell MJ, Khu KJ, Cheng L, et al. Volume specific response criteria for brain metastases following salvage stereotactic radiosurgery and associated predictors of response. Acta Oncol. 2012;51(5):629–635. [DOI] [PubMed] [Google Scholar]
- 39. Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 2011;81(2):483–489. [DOI] [PubMed] [Google Scholar]
- 40. Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115(4):890–898. [DOI] [PubMed] [Google Scholar]
- 41. Ling DC, Vargo JA, Wegner RE, et al. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery. 2015;76(2):150–156; discussion 156–157;quiz 157. [DOI] [PubMed] [Google Scholar]
- 42. Manning MA, Cardinale RM, Benedict SH, et al. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(3):603–608. [DOI] [PubMed] [Google Scholar]
- 43. Minniti G, Esposito V, Clarke E, et al. Fractionated stereotactic radiosurgery for patients with skull base metastases from systemic cancer involving the anterior visual pathway. Radiat Oncol. 2014;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minniti G, Esposito V, Clarke E, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys. 2013;86(4):623–629. [DOI] [PubMed] [Google Scholar]
- 45. Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 46. Rajakesari S, Arvold ND, Jimenez RB, et al. Local control after fractionated stereotactic radiation therapy for brain metastases. J Neurooncol. 2014;120(2):339–346. [DOI] [PubMed] [Google Scholar]
- 47. Wang CC, Floyd SR, Chang CH, et al. Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neurooncol. 2012;106(3):601–610. [DOI] [PubMed] [Google Scholar]
- 48. Wegner RE, Leeman JE, Kabolizadeh P, et al. Fractionated stereotactic radiosurgery for large brain metastases. Am J Clin Oncol. 2015;38(2):135–139. [DOI] [PubMed] [Google Scholar]
- 49. Larson DA, Flickinger JC, Loeffler JS. The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys. 1993;25(3):557–561. [DOI] [PubMed] [Google Scholar]
- 50. Mansouri A, Guha D, Klironomos G, Larjani S, Zadeh G, Kondziolka D. Stereotactic radiosurgery for intracranial meningiomas: current concepts and future perspectives. Neurosurgery. 2015;76(4):362–371. [DOI] [PubMed] [Google Scholar]
- 51. Sheehan JP, Cohen-Inbar O, Ruangkanchanasetr R, et al. Post-radiosurgical edema associated with parasagittal and parafalcine meningiomas: a multicenter study. J Neurooncol. 2015;125(2):317–324. [DOI] [PubMed] [Google Scholar]
- 52. Flannery TJ, Kano H, Lunsford LD, et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. 2010;112(5):957–964. [DOI] [PubMed] [Google Scholar]
- 53. Lee JY, Niranjan A, McInerney J, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65–72. [DOI] [PubMed] [Google Scholar]
- 54. Sheehan JP, Starke RM, Kano H, et al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg. 2014;120(6):1268–1277. [DOI] [PubMed] [Google Scholar]
- 55. Adler JR, Jr, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2008;62(Suppl 2):733–743. [DOI] [PubMed] [Google Scholar]
- 56. Bria C, Wegner RE, Clump DA, et al. Fractionated stereotactic radiosurgery for the treatment of meningiomas. J Cancer Res Ther. 2011;7(1):52–57. [DOI] [PubMed] [Google Scholar]
- 57. Navarria P, Pessina F, Cozzi L, et al. Hypofractionated stereotactic radiation therapy in skull base meningiomas. J Neurooncol. 2015;124(2):283–289. [DOI] [PubMed] [Google Scholar]
- 58. Han JH, Kim DG, Chung HT, et al. Gamma knife radiosurgery for skull base meningiomas: long-term radiologic and clinical outcome. Int J Radiat Oncol Biol Phys. 2008;72(5):1324–1332. [DOI] [PubMed] [Google Scholar]
- 59. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426–1433. [DOI] [PubMed] [Google Scholar]
- 61. Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(4):985–997. [DOI] [PubMed] [Google Scholar]
- 62. van de Langenberg R, Hanssens PE, Verheul JB, et al. Management of large vestibular schwannoma. Part II. Primary gamma knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115(5):885–893. [DOI] [PubMed] [Google Scholar]
- 63. Karam SD, Tai A, Strohl A, et al. Frameless fractionated stereotactic radiosurgery for vestibular schwannomas: a single-institution experience. Front Oncol. 2013;3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys. 2003;56(5):1390–1396. [DOI] [PubMed] [Google Scholar]
- 65. Morimoto M, Yoshioka Y, Kotsuma T, et al. Hypofractionated stereotactic radiation therapy in three to five fractions for vestibular schwannoma. Jpn J Clin Oncol. 2013;43(8):805–812. [DOI] [PubMed] [Google Scholar]
- 66. Song DY, Williams JA. Fractionated stereotactic radiosurgery for treatment of acoustic neuromas. Stereotact Funct Neurosurg. 1999;73(1–4):45–49. [DOI] [PubMed] [Google Scholar]
- 67. Tsai JT, Lin JW, Lin CM, et al. Clinical evaluation of CyberKnife in the treatment of vestibular schwannomas. Biomed Res Int. 2013;2013:297093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vivas EX, Wegner R, Conley G, et al. Treatment outcomes in patients treated with CyberKnife radiosurgery for vestibular schwannoma. Otol Neurotol. 2014;35(1):162–170. [DOI] [PubMed] [Google Scholar]
- 69. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 70. Panet-Raymond V, Souhami L, Roberge D, et al. Accelerated hypofractionated intensity-modulated radiotherapy with concurrent and adjuvant temozolomide for patients with glioblastoma multiforme: a safety and efficacy analysis. Int J Radiat Oncol Biol Phys. 2009;73(2):473–478. [DOI] [PubMed] [Google Scholar]
- 71. Minniti G, Lanzetta G, Scaringi C, et al. Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2012;83(1):93–99. [DOI] [PubMed] [Google Scholar]
- 72. Perry JR, Laperriere N, O’Callaghan CJ, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062-22061, TROG 08.02, NCT00482677). J Clin Oncol. 2016;34(suppl;):abstr LBA2. [Google Scholar]
- 73. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 74. Terasaki M, Eto T, Nakashima S, et al. A pilot study of hypofractionated radiation therapy with temozolomide for adults with glioblastoma multiforme. J Neurooncol. 2011;102(2):247–253. [DOI] [PubMed] [Google Scholar]
- 75. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 76. Floyd NS, Woo SY, Teh BS, et al. Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;58(3):721–726. [DOI] [PubMed] [Google Scholar]
- 77. Chen C, Damek D, Gaspar LE, et al. Phase I trial of hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;81(4):1066–1074. [DOI] [PubMed] [Google Scholar]
- 78. Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roa W, Kepka L, Kumar N, et al. International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 80. Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsao MN, Mehta MP, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for malignant glioma. Int J Radiat Oncol Biol Phys. 2005;63(1):47–55. [DOI] [PubMed] [Google Scholar]
- 83. Redmond KJ, Mehta M. Stereotactic radiosurgery for glioblastoma. Cureus. 2015;7(12):e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shrieve DC, Alexander E, 3rd, Black PM, et al. Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long-term outcome. J Neurosurg. 1999;90(1):72–77. [DOI] [PubMed] [Google Scholar]
- 85. Masciopinto JE, Levin AB, Mehta MP, Rhode BS. Stereotactic radiosurgery for glioblastoma: a final report of 31 patients. J Neurosurg. 1995;82(4):530–535. [DOI] [PubMed] [Google Scholar]
- 86. Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853–860. [DOI] [PubMed] [Google Scholar]
- 87. Loeffler JS, Alexander E, 3rd, Shea WM, et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992;10(9):1379–1385. [DOI] [PubMed] [Google Scholar]
- 88. Baumert BG, Lutterbach J, Bernays R, Davis JB, Heppner FL. Fractionated stereotactic radiotherapy boost after post-operative radiotherapy in patients with high-grade gliomas. Radiother Oncol. 2003;67(2):183–190. [DOI] [PubMed] [Google Scholar]
- 89. Cardinale RM, Schmidt-Ullrich RK, Benedict SH, Zwicker RD, Han DC, Broaddus WC. Accelerated radiotherapy regimen for malignant gliomas using stereotactic concomitant boosts for dose escalation. Radiat Oncol Investig. 1998;6(4):175–181. [DOI] [PubMed] [Google Scholar]
- 90. Nieder C, Nestle U, Walter K, Niewald M, Schnabel K. Hypofractionated stereotactic radiotherapy for malignant glioma: a phase I/II study. Journal of Radiosurgery. 1999;2(2):107–111. [Google Scholar]
- 91. Hulshof MC, Schimmel EC, Andries Bosch D, González González D. Hypofractionation in glioblastoma multiforme. Radiother Oncol. 2000;54(2):143–148. [DOI] [PubMed] [Google Scholar]
- 92. Perry JR, Laperriere N, O’Callaghan CJ, et al. A phase III randomized controlled trial of short-course radiotherapy with or without concomitant and adjuvant temozolomide in elderly patients with glioblastoma (CCTG CE.6, EORTC 26062-22061, TROG 08.02, NCT00482677). JCO. 2016;34:(suppl; abstr LBA2). [Google Scholar]
- 93. Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol. 2012;85(1017):e770–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lipani JD, Jackson PS, Soltys SG, Sato K, Adler JR. Survival following CyberKnife radiosurgery and hypofractionated radiotherapy for newly diagnosed glioblastoma multiforme. Technol Cancer Res Treat. 2008;7(3):249–255. [DOI] [PubMed] [Google Scholar]
- 95. Azoulay M, Ho CK, Fujimoto DK, et al. A phase I/II trial of 5 fraction stereotactic radiosurgery with 5-mm margins with concurrent and adjuvant temozolomide in newly diagnosed supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2016;96(2S):E131–E132. [DOI] [PubMed] [Google Scholar]
- 96. Shrieve DC, Alexander E, 3rd, Wen PY, et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36(2):275–282; discussion 282–284. [DOI] [PubMed] [Google Scholar]
- 97. Lederman G, Arbit E, Odaimi M, Lombardi E, Wrzolek M, Wronski M. Fractionated stereotactic radiosurgery and concurrent taxol in recurrent glioblastoma multiforme: a preliminary report. Int J Radiat Oncol Biol Phys. 1998;40(3):661–666. [DOI] [PubMed] [Google Scholar]
- 98. Hall WA, Djalilian HR, Sperduto PW, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13(7):1642–1648. [DOI] [PubMed] [Google Scholar]
- 99. Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hudes RS, Corn BW, Werner-Wasik M, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43(2):293–298. [DOI] [PubMed] [Google Scholar]
- 101. Koga T, Maruyama K, Tanaka M, et al. Extended field stereotactic radiosurgery for recurrent glioblastoma. Cancer. 2012;118(17):4193–4200. [DOI] [PubMed] [Google Scholar]
- 102. Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111(2):187–194. [DOI] [PubMed] [Google Scholar]
- 103. Fields EC, Damek D, Gaspar LE, et al. Phase I dose escalation trial of vandetanib with fractionated radiosurgery in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(1):51–57. [DOI] [PubMed] [Google Scholar]
- 104. Regine WF, Patchell RA, Strottmann JM, Meigooni A, Sanders M, Young AB. Preliminary report of a phase I study of combined fractionated stereotactic radiosurgery and conventional external beam radiation therapy for unfavorable gliomas. Int J Radiat Oncol Biol Phys. 2000;48(2):421–426. [DOI] [PubMed] [Google Scholar]
- 105. Park KJ, Kano H, Iyer A, et al. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012;107(2):323–333. [DOI] [PubMed] [Google Scholar]
- 106. Hundsberger T, Brügge D, Putora PM, Weder P, Weber J, Plasswilm L. Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol. 2013;112(1):133–139. [DOI] [PubMed] [Google Scholar]
- 107. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys. 2013;86(5):873–879. [DOI] [PubMed] [Google Scholar]
- 109. Carlson JA, Reddy K, Gaspar LE, et al. Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV) for newly diagnosed glioblastoma multiforme (GBM): a comparison of two prospective phase II trials. J Neurooncol. 2015;123(2):251–257. [DOI] [PubMed] [Google Scholar]
- 110. Kirkpatrick JP, Sampson JH. Recurrent malignant gliomas. Semin Radiat Oncol. 2014;24(4):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. Radiation therapy for glioblastoma: executive summary of an American society for radiation oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2016;6(4):217–225. [DOI] [PubMed] [Google Scholar]
- 112. Jeong D, Malalis C, Arrington JA, Field AS, Choi JW, Kocak M. Mean apparent diffusion coefficient values in defining radiotherapy planning target volumes in glioblastoma. Quant Imaging Med Surg. 2015;5(6):835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]